Sensor Micro and Nanoparticles for Microfluidic Application
Abstract
1. Introduction
2. Construction Principles of Sensor Particles
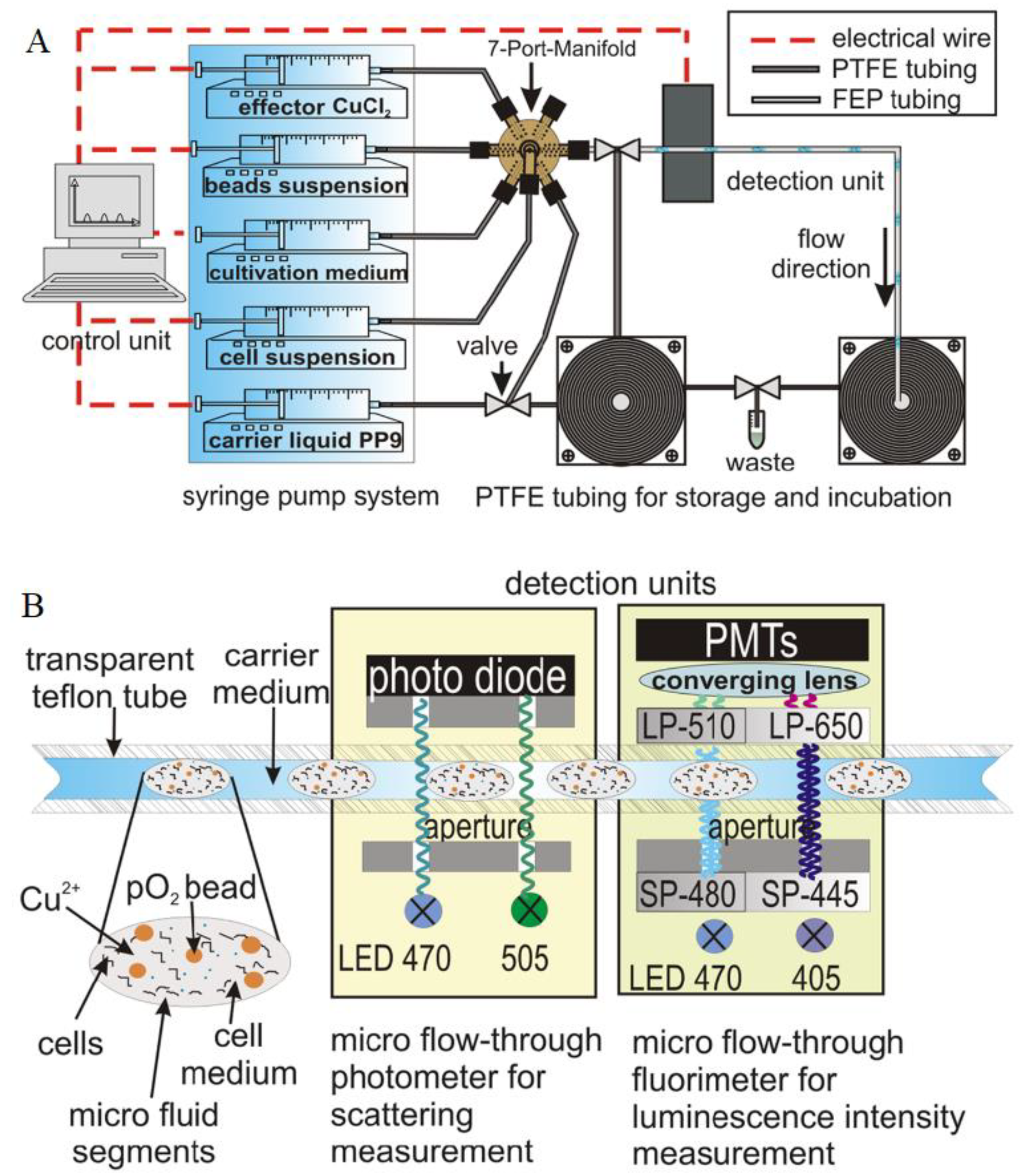
2.1. Preparation of Sensor Particles in Microfluidic Systems
2.2. Polymer and Hydrogel Particles with Embedded Sensor Dyes
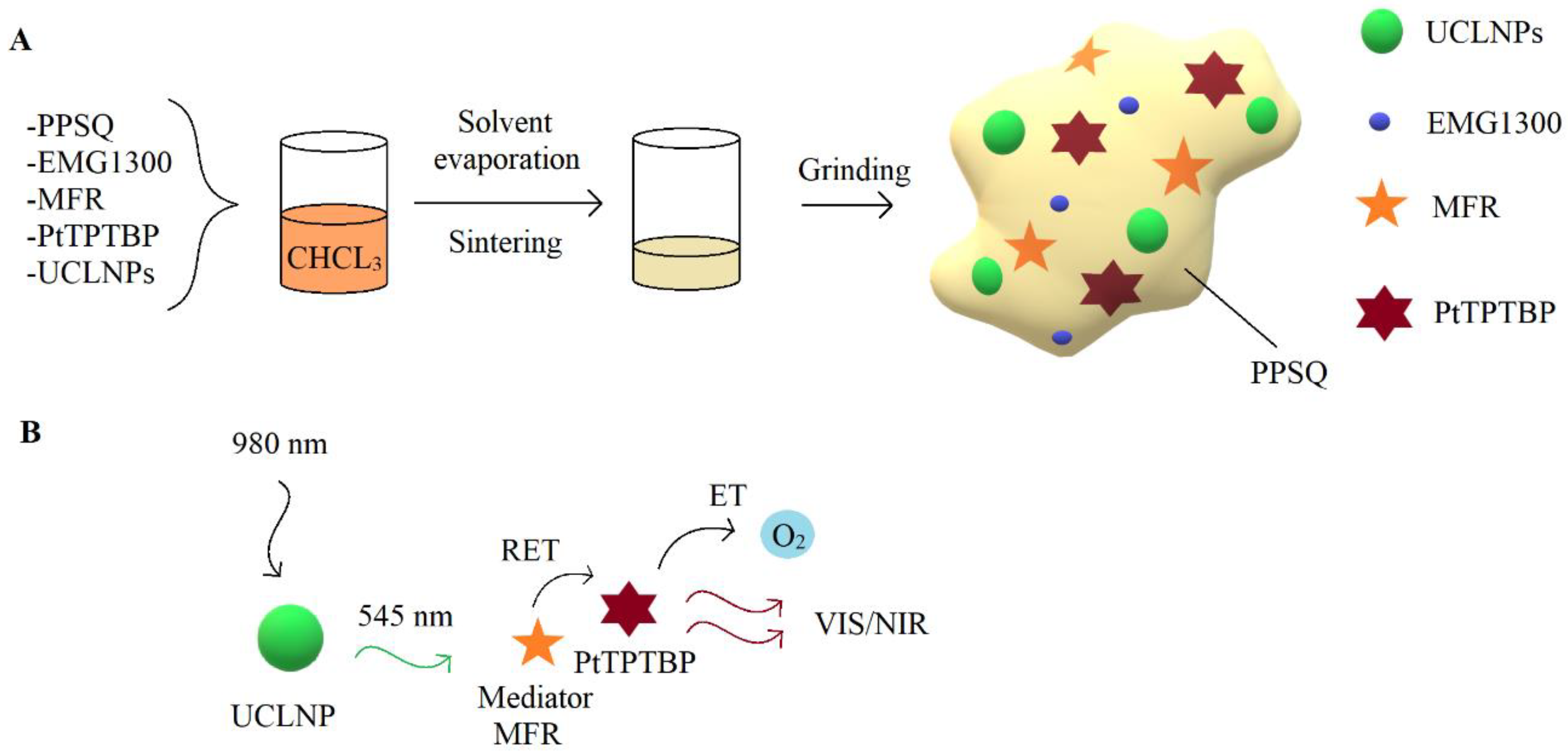
2.3. Preparation of Composite Particles
2.4. Preparation of Microcapsules
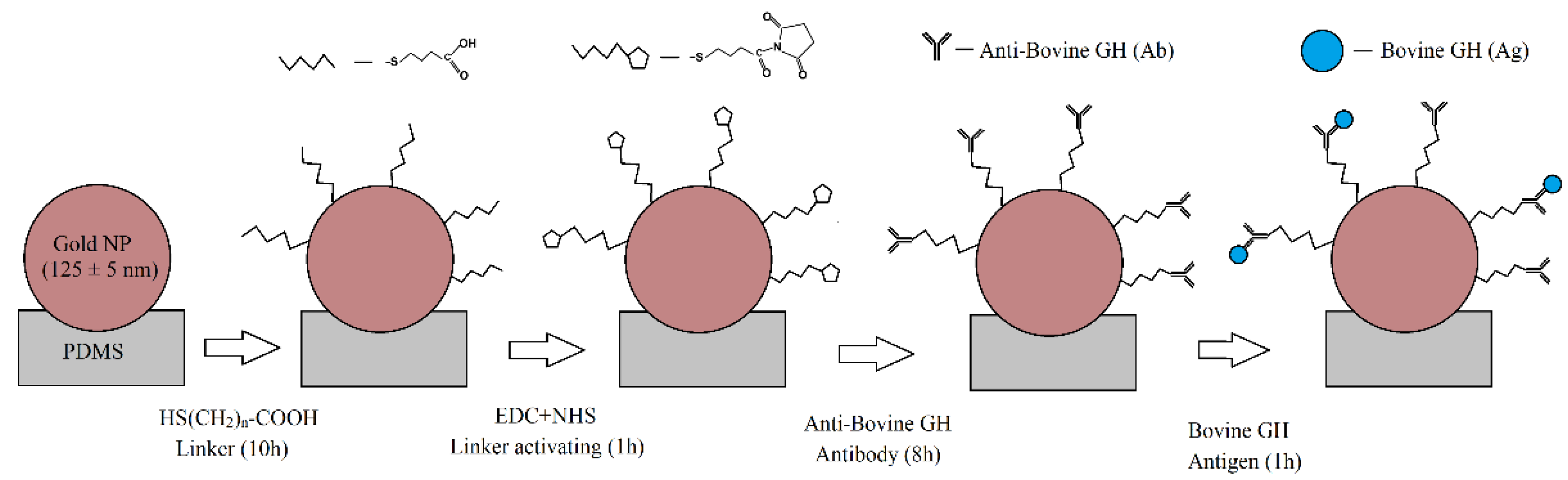
2.5. Metal Particles for LSPR-Based Sensing
3. Application of Sensor Particles for Temperature, pH and Oxygen Measurements in Microfluidic Systems
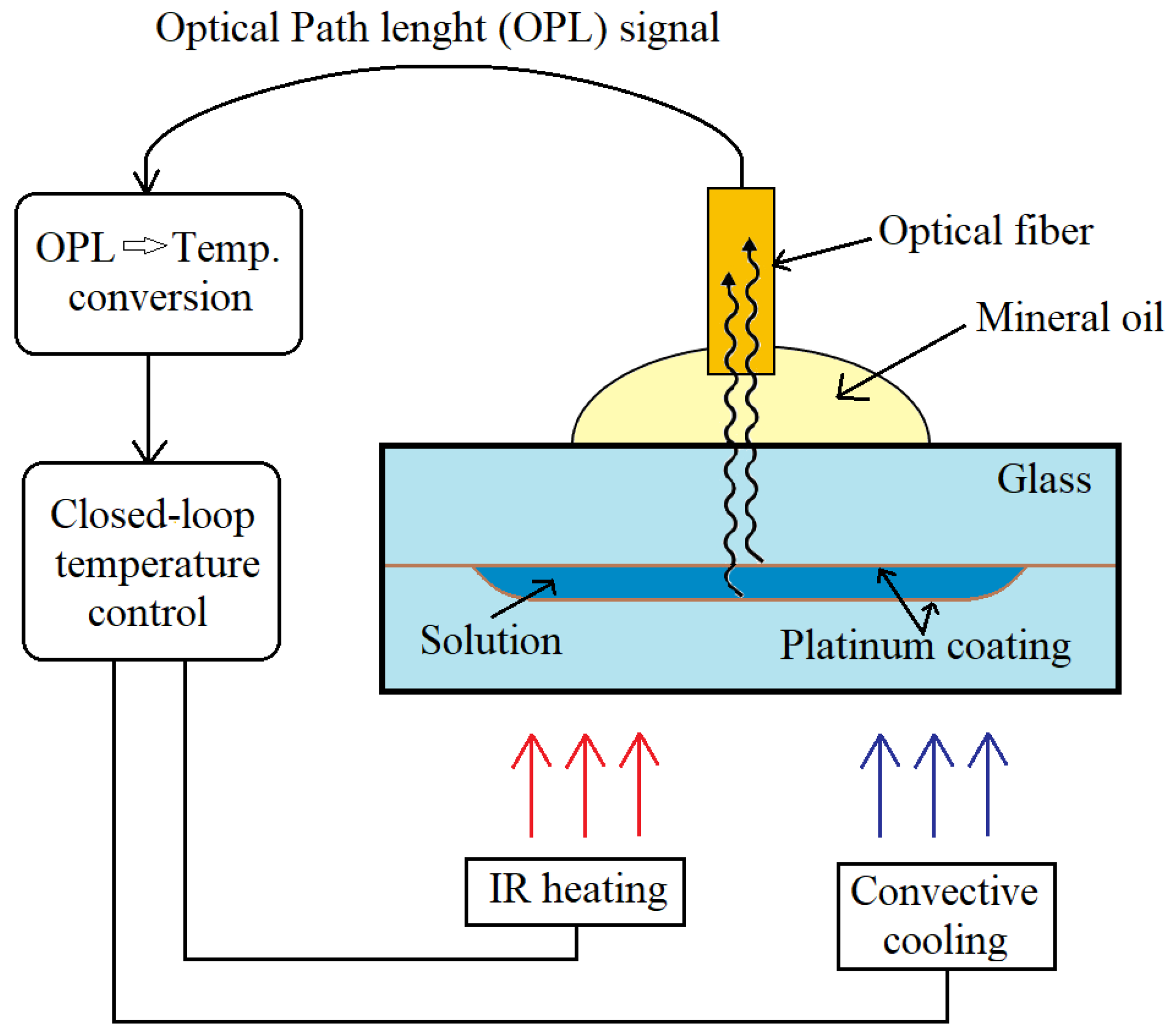
3.1. Temperature Sensing
3.2. pH Sensing
3.3. Oxygen Sensing
3.4. Particle-Base Imaging of Oxygen Distribution
3.5. Combination of Particle-Based Oxygen Sensing with Flow Velocimetry
3.6. Combination of Oxygen and pH Sensing
4. Microfluidic Systems for Chemosensing
4.1. Particle-Based Gas Sensing
4.2. Detection of Hydrogenperoxide
4.3. Detection of Chloride Ions by Using Fluorescent Microparticles
4.4. Sensor Particles for Detection of Environmental Pollution
5. Microfluidic Systems for Biomedical Applications
5.1. Colorimetric Particle Sensor for Microbiology and Biomedical Measurements
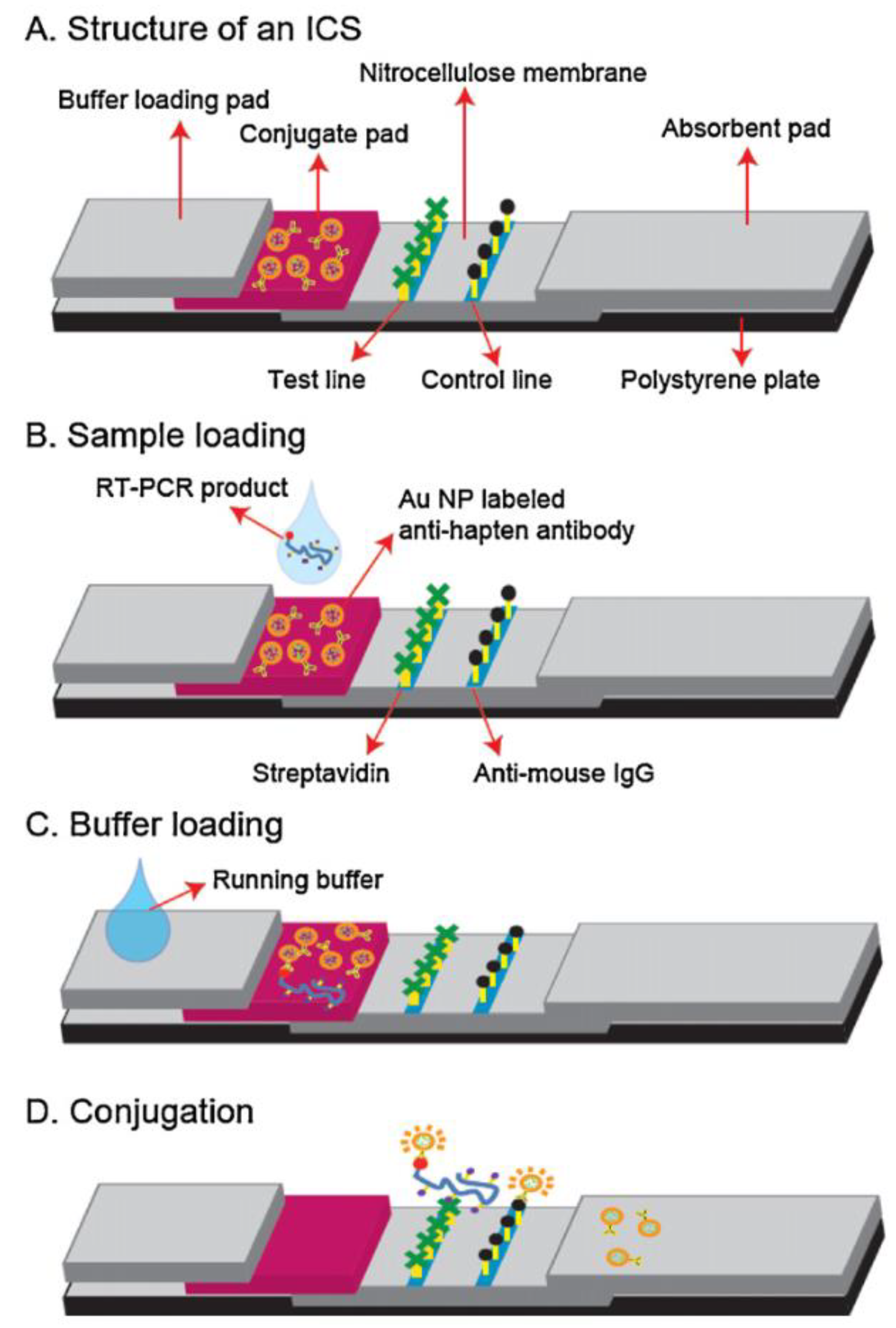
5.2. Paper-Based Bioanalytical Systems Using Sensor Particles
5.3. Plasmonic Nanoparticles in DNA Amplification Assays
5.4. Particle-Supported Electrochemical Detection Systems
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kohler, M.A. Mobile Microspies: Particles for Sensing and Communication, 1st ed.; Jenny Stanford Publishing: Beijing, China, 2018. [Google Scholar]
- Ma, J.; Lee, S.M.-Y.; Yi, C.; Li, C.-W. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications—A review. Lab Chip 2017, 17, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Kumacheva, E.; Garstecki, P. Microfluidic Reactors for Polymer Particles; Wiley-Blackwell: Oxford, UK, 2011. [Google Scholar]
- Kim, J. Joining plasmonics with microfluidics: From convenience to inevitability. Lab Chip 2012, 12, 3611. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-X.; Zeng, Q.; Zhang, M.; Hong, L.-Y.; Zhao, Y.-F.; Liu, W.; Guo, S.-S.; Zhao, X.-Z. Valve-based microfluidic droplet micromixer and mercury (II) ion detection. Sens. Actuators A Phys. 2011, 172, 546–551. [Google Scholar] [CrossRef]
- Cao, J.; Nagl, S.; Kothe, E.; Köhler, J.M. Oxygen sensor nanoparticles for monitoring bacterial growth and characterization of dose–response functions in microfluidic screenings. Microchim. Acta 2015, 182, 385–394. [Google Scholar] [CrossRef]
- Kung, C.-T.; Gao, H.; Lee, C.-Y.; Wang, Y.-N.; Dong, W.; Ko, C.-H.; Wang, G.; Fu, L.-M. Microfluidic synthesis control technology and its application in drug delivery, bioimaging, biosensing, environmental analysis and cell analysis. Chem. Eng. J. 2020, 399, 125748. [Google Scholar] [CrossRef]
- Serra, C.A.; Chang, Z. Microfluidic-Assisted Synthesis of Polymer Particles. Chem. Eng. Technol. 2008, 31, 1099–1115. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Tong, L.; Xia, Y. Seed-Mediated Synthesis of Silver Nanocrystals with Controlled Sizes and Shapes in Droplet Microreactors Separated by Air. Langmuir 2013, 29, 15719–15725. [Google Scholar] [CrossRef]
- Gonidec, M.; Puigmartí-Luis, J. Continuous- versus Segmented-Flow Microfluidic Synthesis in Materials Science. Crystals 2018, 9, 12. [Google Scholar] [CrossRef]
- Uson, L.; Sebastian, V.; Arruebo, M.; Santamaria, J. Continuous microfluidic synthesis and functionalization of gold nanorods. Chem. Eng. J. 2016, 285, 286–292. [Google Scholar] [CrossRef]
- Knauer, A.; Thete, A.; Li, S.; Romanus, H.; Csáki, A.; Fritzsche, W.; Köhler, J.M. Au/Ag/Au double shell nanoparticles with narrow size distribution obtained by continuous micro segmented flow synthesis. Chem. Eng. J. 2011, 166, 1164–1169. [Google Scholar] [CrossRef]
- Wacker, J.B.; Lignos, I.; Parashar, V.K.; Gijs, M.A.M. Controlled synthesis of fluorescent silica nanoparticles inside microfluidic droplets. Lab Chip 2012, 12, 3111. [Google Scholar] [CrossRef] [PubMed]
- Gashti, M.P.; Asselin, J.; Barbeau, J.; Boudreau, D.; Greener, J. A microfluidic platform with pH imaging for chemical and hydrodynamic stimulation of intact oral biofilms. Lab Chip 2016, 16, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, H.; Li, W.; Tian, Y.; Li, S.; Zhao, J.; Li, Y. PtOEP/PS composite particles based on fluorescent sensor for dissolved oxygen detection. Mater. Lett. 2016, 172, 112–115. [Google Scholar] [CrossRef]
- Kronfeld, K.-P.; Ellinger, T.; Köhler, J.M. Microfluidically prepared sensor particles for determination of chloride by fluorescence quenching of matrix-embedded lucigenin. SN Appl. Sci. 2020, 2, 366. [Google Scholar] [CrossRef]
- Lafleur, J.P.; Senkbeil, S.; Jensen, T.G.; Kutter, J.P. Gold nanoparticle-based optical microfluidic sensors for analysis of environmental pollutants. Lab Chip 2012, 12, 4651. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Boegli, M.; Bruzas, I.; Lum, W.; Sagle, L. Patterned Plasmonic Nanoparticle Arrays for Microfluidic and Multiplexed Biological Assays. Anal. Chem. 2015, 87, 11407–11414. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, S.; Xiao, X.; Li, S.; Gao, F.; Zhang, Z.; Du, J. Fabricating metal nanoparticle arrays at specified and localized regions of microfluidic chip for LSPR sensing. Microelectron. Eng. 2016, 151, 7–11. [Google Scholar] [CrossRef]
- Chen, J.-S.; Chen, P.-F.; Lin, H.T.-H.; Huang, N.-T. A Localized Surface Plasmon Resonance (LSPR) sensor integrated automated microfluidics for multiplex inflammatory biomarker detection. Analyst 2020, 145, 7654–7661. [Google Scholar] [CrossRef]
- Aigner, D.; Ungerböck, B.; Mayr, T.; Saf, R.; Klimant, I.; Borisov, S.M. Fluorescent materials for pH sensing and imaging based on novel 1,4-diketopyrrolo-[3,4-c]pyrrole dyes. J. Mater. Chem. C 2013, 1, 5685. [Google Scholar] [CrossRef]
- Borisov, S.M.; Herrod, D.L.; Klimant, I. Fluorescent poly(styrene-block-vinylpyrrolidone) nanobeads for optical sensing of pH. Sens. Actuators B Chem. 2009, 139, 52–58. [Google Scholar] [CrossRef]
- Ehgartner, J.; Strobl, M.; Bolivar, J.M.; Rabl, D.; Rothbauer, M.; Ertl, P.; Borisov, S.M.; Mayr, T. Simultaneous Determination of Oxygen and pH Inside Microfluidic Devices Using Core–Shell Nanosensors. Anal. Chem. 2016, 88, 9796–9804. [Google Scholar] [CrossRef] [PubMed]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Köhler, J.M.; März, A.; Popp, J.; Knauer, A.; Kraus, I.; Faerber, J.; Serra, C. Polyacrylamid/Silver Composite Particles Produced via Microfluidic Photopolymerization for Single Particle-Based SERS Microsensorics. Anal. Chem. 2013, 85, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Visaveliya, N.; Köhler, J.M. Microfluidic Assisted Synthesis of Multipurpose Polymer Nanoassembly Particles for Fluorescence, LSPR, and SERS Activities. Small 2015, 11, 6435–6443. [Google Scholar] [CrossRef]
- Hassan, N.; Cabuil, V.; Abou-Hassan, A. Continuous Multistep Microfluidic Assisted Assembly of Fluorescent, Plasmonic, and Magnetic Nanostructures. Angew. Chem. Int. Ed. 2013, 52, 1994–1997. [Google Scholar] [CrossRef]
- Scheucher, E.; Wilhelm, S.; Wolfbeis, O.S.; Hirsch, T.; Mayr, T. Composite particles with magnetic properties, near-infrared excitation, and far-red emission for luminescence-based oxygen sensing. Microsyst. Nanoeng. 2015, 1, 15026. [Google Scholar] [CrossRef][Green Version]
- Chen, S.-P.; Yu, X.-D.; Xu, J.-J.; Chen, H.-Y. Gold nanoparticles-coated magnetic microspheres as affinity matrix for detection of hemoglobin A1c in blood by microfluidic immunoassay. Biosens. Bioelectron. 2011, 26, 4779–4784. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Rogach, A.L.; Garstka, M.; Springer, S.; Parak, W.J.; Muñoz-Javier, A.; Kreft, O.; Skirtach, A.G.; Susha, A.S.; Ramaye, Y.; et al. Multifunctionalized Polymer Microcapsules: Novel Tools for Biological and Pharmacological Applications. Small 2007, 3, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Amoah, A.; Schellhaas, U.; Winterhalter, M.; Springer, S.; Kolesnikova, T.A. “To Catch or Not to Catch”: Microcapsule-Based Sandwich Assay for Detection of Proteins and Nucleic Acids. Adv. Funct. Mater. 2016, 26, 6015–6024. [Google Scholar] [CrossRef]
- Kolesnikova, T.A.; Kiragosyan, G.; Le, T.H.N.; Springer, S.; Winterhalter, M. Protein A Functionalized Polyelectrolyte Microcapsules as a Universal Platform for Enhanced Targeting of Cell Surface Receptors. ACS Appl. Mater. Interfaces 2017, 9, 11506–11517. [Google Scholar] [CrossRef]
- Sun, J.; Xianyu, Y.; Jiang, X. Point-of-care biochemical assays using gold nanoparticle-implemented microfluidics. Chem. Soc. Rev. 2014, 43, 6239–6253. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-S.; Fan, S.-K. Microfluidic Surface Plasmon Resonance Sensors: From Principles to Point-of-Care Applications. Sensors 2016, 16, 1175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Y.; Hsieh, Y.-H.; Hsu, C.-Y.; Xi, J.; Lin, K.-J.; Jiang, X. Towards a high-throughput label-free detection system combining localized-surface plasmon resonance and microfluidics. Lab Chip 2012, 12, 3012. [Google Scholar] [CrossRef] [PubMed]
- Csáki, A.; Stranik, O.; Fritzsche, W. Localized surface plasmon resonance based biosensing. Expert Rev. Mol. Diagn. 2018, 18, 279–296. [Google Scholar] [CrossRef] [PubMed]
- SadAbadi, H.; Badilescu, S.; Packirisamy, M.; Wüthrich, R. Integration of gold nanoparticles in PDMS microfluidics for lab-on-a-chip plasmonic biosensing of growth hormones. Biosens. Bioelectron. 2013, 44, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Doherty, B.; Csáki, A.; Thiele, M.; Zeisberger, M.; Schwuchow, A.; Kobelke, J.; Fritzsche, W.; Schmidt, M.A. Nanoparticle functionalised small-core suspended-core fibre—a novel platform for efficient sensing. Biomed. Opt. Express 2017, 8, 790. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, H.; Sumana, G.; Marquette, C.A. A label-free ultrasensitive microfluidic surface Plasmon resonance biosensor for Aflatoxin B1 detection using nanoparticles integrated gold chip. Food Chem. 2020, 307, 125530. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhao, G.; Xiao, Y.; Zhao, J.; Zhang, Z.; Geng, B. Facile Growth of High-Yield Gold Nanobipyramids Induced by Chloroplatinic Acid for High Refractive Index Sensing Properties. Sci. Rep. 2016, 6, 36706. [Google Scholar] [CrossRef]
- Huang, C.; Bonroy, K.; Reekmans, G.; Laureyn, W.; Verhaegen, K.; De Vlaminck, I.; Lagae, L.; Borghs, G. Localized surface plasmon resonance biosensor integrated with microfluidic chip. Biomed. Microdevices 2009, 11, 893–901. [Google Scholar] [CrossRef]
- Frederix, F.; Friedt, J.-M.; Choi, K.-H.; Laureyn, W.; Campitelli, A.; Mondelaers, D.; Maes, G.; Borghs, G. Biosensing Based on Light Absorption of Nanoscaled Gold and Silver Particles. Anal. Chem. 2003, 75, 6894–6900. [Google Scholar] [CrossRef]
- Steinbrück, A.; Stranik, O.; Csaki, A.; Fritzsche, W. Sensoric potential of gold–silver core–shell nanoparticles. Anal. Bioanal. Chem. 2011, 401, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55. [Google Scholar] [CrossRef]
- Thiele, M.; Soh, J.Z.E.; Knauer, A.; Malsch, D.; Stranik, O.; Müller, R.; Csáki, A.; Henkel, T.; Köhler, J.M.; Fritzsche, W. Gold nanocubes—Direct comparison of synthesis approaches reveals the need for a microfluidic synthesis setup for a high reproducibility. Chem. Eng. J. 2016, 288, 432–440. [Google Scholar] [CrossRef]
- Wieduwilt, T.; Zeisberger, M.; Thiele, M.; Doherty, B.; Chemnitz, M.; Csaki, A.; Fritzsche, W.; Schmidt, M.A. Gold-reinforced silver nanoprisms on optical fiber tapers—A new base for high precision sensing. APL Photonics 2016, 1, 066102. [Google Scholar] [CrossRef]
- Ebert, S.; Travis, K.; Lincoln, B.; Guck, J. Fluorescence ratio thermometry in a microfluidic dual-beam laser trap. Opt. Express 2007, 15, 15493. [Google Scholar] [CrossRef]
- Leslie, D.C.; Seker, E.; Bazydlo, L.A.L.; Strachan, B.C.; Landers, J.P. Platinum nanoparticle-facilitated reflective surfaces for non-contact temperature control in microfluidic devices for PCR amplification. Lab Chip 2012, 12, 127–132. [Google Scholar] [CrossRef]
- Geitenbeek, R.G.; Vollenbroek, J.C.; Weijgertze, H.M.H.; Tregouet, C.B.M.; Nieuwelink, A.-E.; Kennedy, C.L.; Weckhuysen, B.M.; Lohse, D.; Van Blaaderen, A.; Van Den Berg, A.; et al. Luminescence thermometry for in situ temperature measurements in microfluidic devices. Lab Chip 2019, 19, 1236–1246. [Google Scholar] [CrossRef]
- Petrov, A.I.; Volodkin, D.V.; Sukhorukov, G.B. Protein-Calcium Carbonate Coprecipitation: A Tool for Protein Encapsulation. Biotechnol. Prog. 2008, 21, 918–925. [Google Scholar] [CrossRef]
- Kreft, O.; Javier, A.M.; Sukhorukov, G.B.; Parak, W.J. Polymer microcapsules as mobile local pH-sensors. J. Mater. Chem. 2007, 17, 4471. [Google Scholar] [CrossRef]
- Poehler, E.; Herzog, C.; Suendermann, M.; Pfeiffer, S.A.; Nagl, S. Development of microscopic time-domain dual lifetime referencing luminescence detection for pH monitoring in microfluidic free-flow isoelectric focusing. Eng. Life Sci. 2015, 15, 276–285. [Google Scholar] [CrossRef]
- Sun, S.; Ungerböck, B.; Mayr, T. Imaging of oxygen in microreactors and microfluidic systems. Methods Appl. Fluoresc 2015, 3, 034002. [Google Scholar] [CrossRef] [PubMed]
- Becker, W. Fluorescence lifetime imaging—techniques and applications. J. Microsc. 2012, 247, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Horka, M.; Sun, S.; Ruszczak, A.; Garstecki, P.; Mayr, T. Lifetime of Phosphorescence from Nanoparticles Yields Accurate Measurement of Concentration of Oxygen in Microdroplets, Allowing One To Monitor the Metabolism of Bacteria. Anal. Chem. 2016, 88, 12006–12012. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y.; Iwamoto, Y.; Tsukada, K. Micro/nano particle-based oxygen sensing film for monitoring respiration of cells cultured in a microfluidic device. Jpn. J. Appl. Phys. 2019, 58, SDDK03. [Google Scholar] [CrossRef]
- Lasave, L.C.; Borisov, S.M.; Ehgartner, J.; Mayr, T. Quick and simple integration of optical oxygen sensors into glass-based microfluidic devices. RSC Adv. 2015, 5, 70808–70816. [Google Scholar] [CrossRef]
- Ungerböck, B.; Pohar, A.; Mayr, T.; Plazl, I. Online oxygen measurements inside a microreactor with modeling of transport phenomena. Microfluid. Nanofluidics 2013, 14, 565–574. [Google Scholar] [CrossRef]
- Borisov, S.M.; Klimant, I. Luminescent nanobeads for optical sensing and imaging of dissolved oxygen. Microchim. Acta 2009, 164, 7–15. [Google Scholar] [CrossRef]
- Ungerböck, B.; Fellinger, S.; Sulzer, P.; Abel, T.; Mayr, T. Magnetic optical sensor particles: A flexible analytical tool for microfluidic devices. Analyst 2014, 139, 2551–2559. [Google Scholar] [CrossRef]
- Kim, H.D.; Yi, S.J.; Kim, K.C. Simultaneous measurement of dissolved oxygen concentration and velocity field in microfluidics using oxygen-sensitive particles. Microfluid. Nanofluidics 2013, 15, 139–149. [Google Scholar] [CrossRef]
- Zhou, X.; Lee, S.; Xu, Z.; Yoon, J. Recent Progress on the Development of Chemosensors for Gases. Chem. Rev. 2015, 115, 7944–8000. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef] [PubMed]
- Tofighi, G.; Degler, D.; Junker, B.; Müller, S.; Lichtenberg, H.; Wang, W.; Weimar, U.; Barsan, N.; Grunwaldt, J.-D. Microfluidically synthesized Au, Pd and AuPd nanoparticles supported on SnO2 for gas sensing applications. Sens. Actuators B Chem. 2019, 292, 48–56. [Google Scholar] [CrossRef]
- Salve, M.; Mandal, A.; Amreen, K.; Pattnaik, P.K.; Goel, S. Greenly synthesized silver nanoparticles for supercapacitor and electrochemical sensing applications in a 3D printed microfluidic platform. Microchem. J. 2020, 157, 104973. [Google Scholar] [CrossRef]
- Zheng, L.; Cai, G.; Wang, S.; Liao, M.; Li, Y.; Lin, J. A microfluidic colorimetric biosensor for rapid detection of Escherichia coli O157:H7 using gold nanoparticle aggregation and smart phone imaging. Biosens. Bioelectron. 2019, 124–125, 143–149. [Google Scholar] [CrossRef]
- Nagatani, N.; Yamanaka, K.; Ushijima, H.; Koketsu, R.; Sasaki, T.; Ikuta, K.; Saito, M.; Miyahara, T.; Tamiya, E. Detection of influenza virus using a lateral flow immunoassay for amplified DNA by a microfluidic RT-PCR chip. Analyst 2012, 137, 3422. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Chen, Y.; Choi, J.Y.; Kim, W.-J.; Dae, H.-M.; Jung, J.; Seo, T.S. Integrated microdevice of reverse transcription-polymerase chain reaction with colorimetric immunochromatographic detection for rapid gene expression analysis of influenza A H1N1 virus. Biosens. Bioelectron. 2012, 33, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; McCord, B. A four-channel paper microfluidic device with gold nanoparticles and aptamers for seized drugs. Anal. Biochem. 2020, 595, 113619. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Li, J.; Qiang, W.; Sun, L.; Li, H.; Xu, D. Microfluidic chip-based silver nanoparticles aptasensor for colorimetric detection of thrombin. Talanta 2016, 150, 81–87. [Google Scholar] [CrossRef]
- Evans, E.; Moreira Gabriel, E.F.; Benavidez, T.E.; Tomazelli Coltro, W.K.; Garcia, C.D. Modification of microfluidic paper-based devices with silica nanoparticles. Analyst 2014, 139, 5560–5567. [Google Scholar] [CrossRef]
- Tsai, T.-T.; Huang, C.-Y.; Chen, C.-A.; Shen, S.-W.; Wang, M.-C.; Cheng, C.-M.; Chen, C.-F. Diagnosis of Tuberculosis Using Colorimetric Gold Nanoparticles on a Paper-Based Analytical Device. ACS Sens. 2017, 2, 1345–1354. [Google Scholar] [CrossRef]
- Tseng, S.-C.; Yu, C.-C.; Wan, D.; Chen, H.-L.; Wang, L.A.; Wu, M.-C.; Su, W.-F.; Han, H.-C.; Chen, L.-C. Eco-Friendly Plasmonic Sensors: Using the Photothermal Effect to Prepare Metal Nanoparticle-Containing Test Papers for Highly Sensitive Colorimetric Detection. Anal. Chem. 2012, 84, 5140–5145. [Google Scholar] [CrossRef] [PubMed]
- Reuter, C.; Urban, M.; Arnold, M.; Stranik, O.; Csáki, A.; Fritzsche, W. 2-LED-µSpectrophotometer for Rapid On-Site Detection of Pathogens Using Noble-Metal Nanoparticle-Based Colorimetric Assays. Appl. Sci. 2020, 10, 2658. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J.-H.; Rudi Strickler, J.; Chang, W.-J.; Gunasekaran, S. Nickel nanoparticle–chitosan-reduced graphene oxide-modified screen-printed electrodes for enzyme-free glucose sensing in portable microfluidic devices. Biosens. Bioelectron. 2013, 47, 530–538. [Google Scholar] [CrossRef] [PubMed]
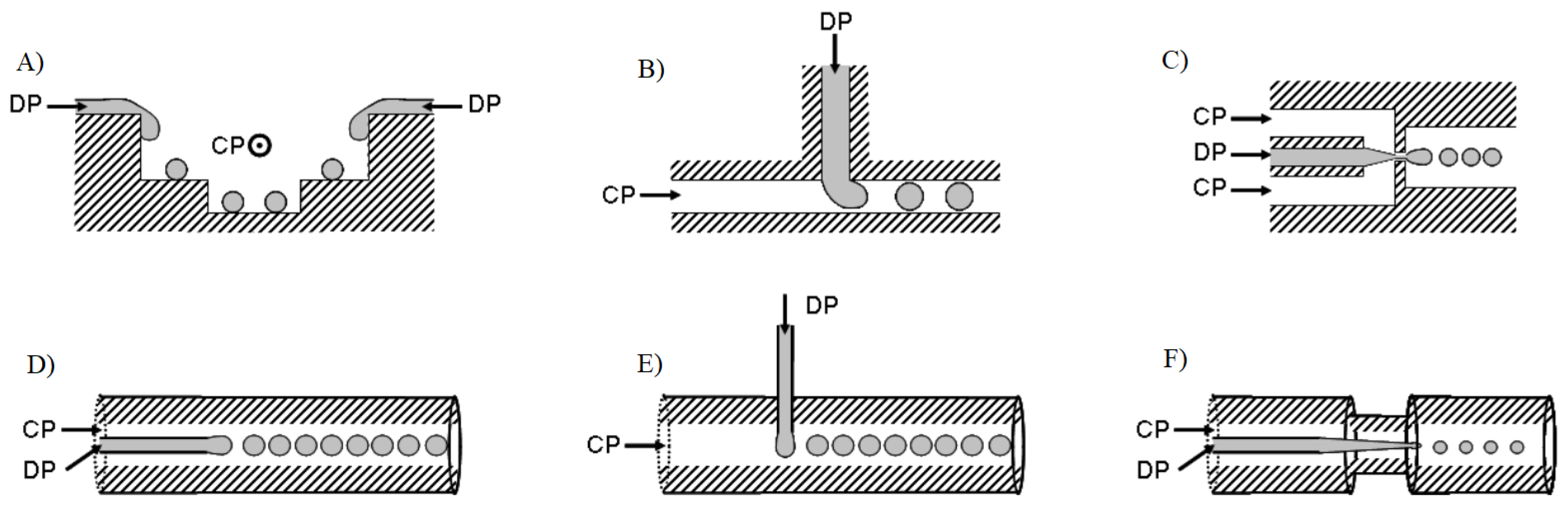
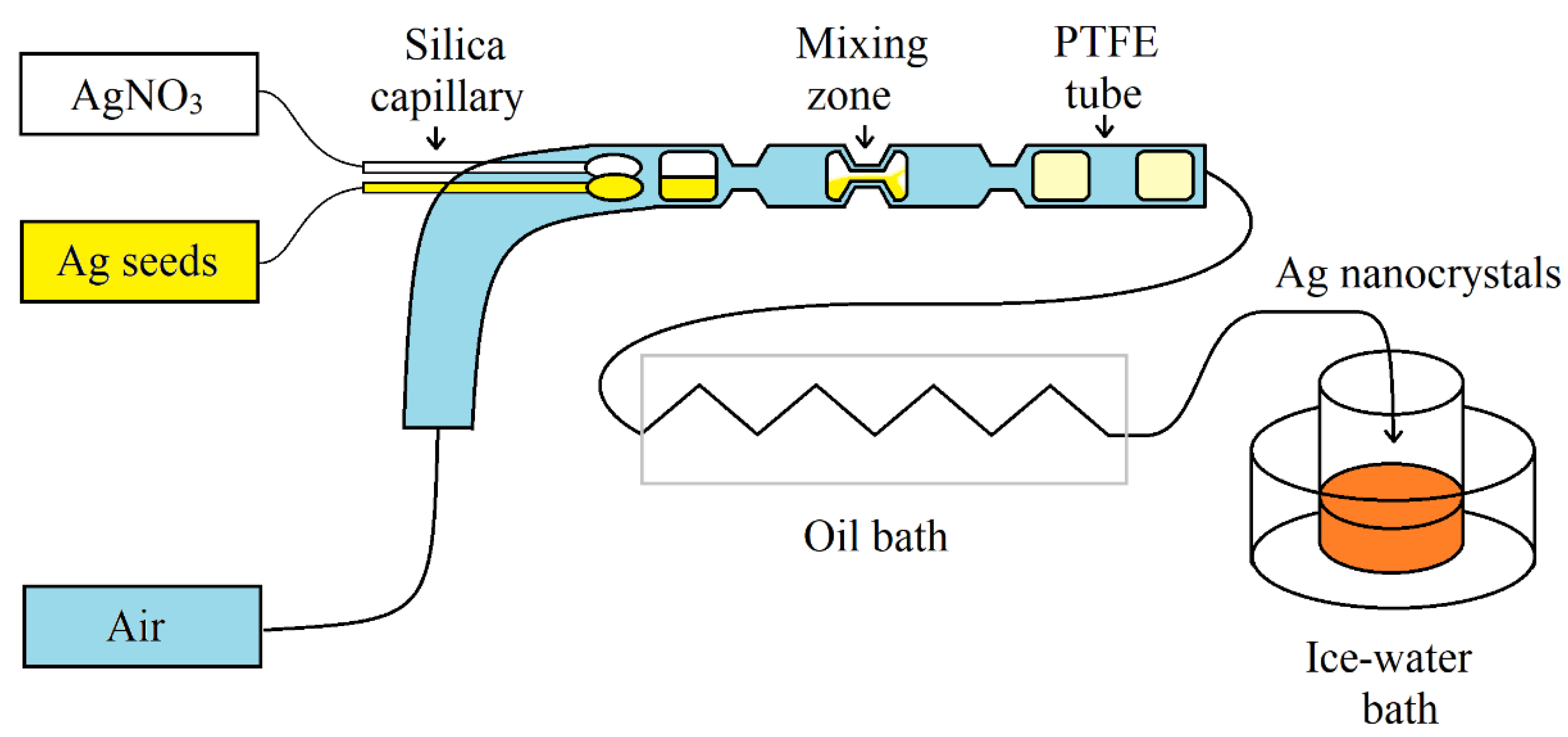
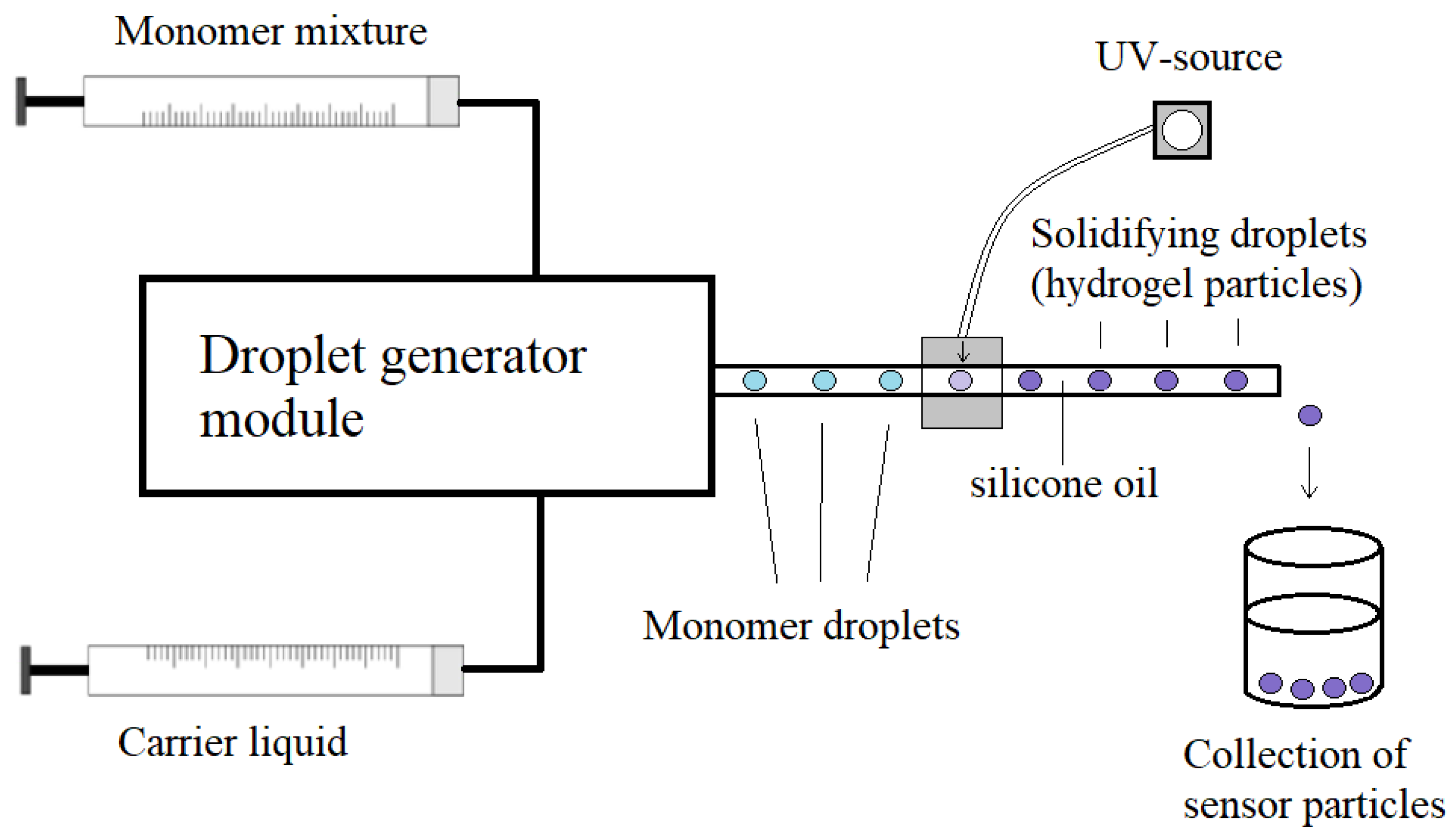

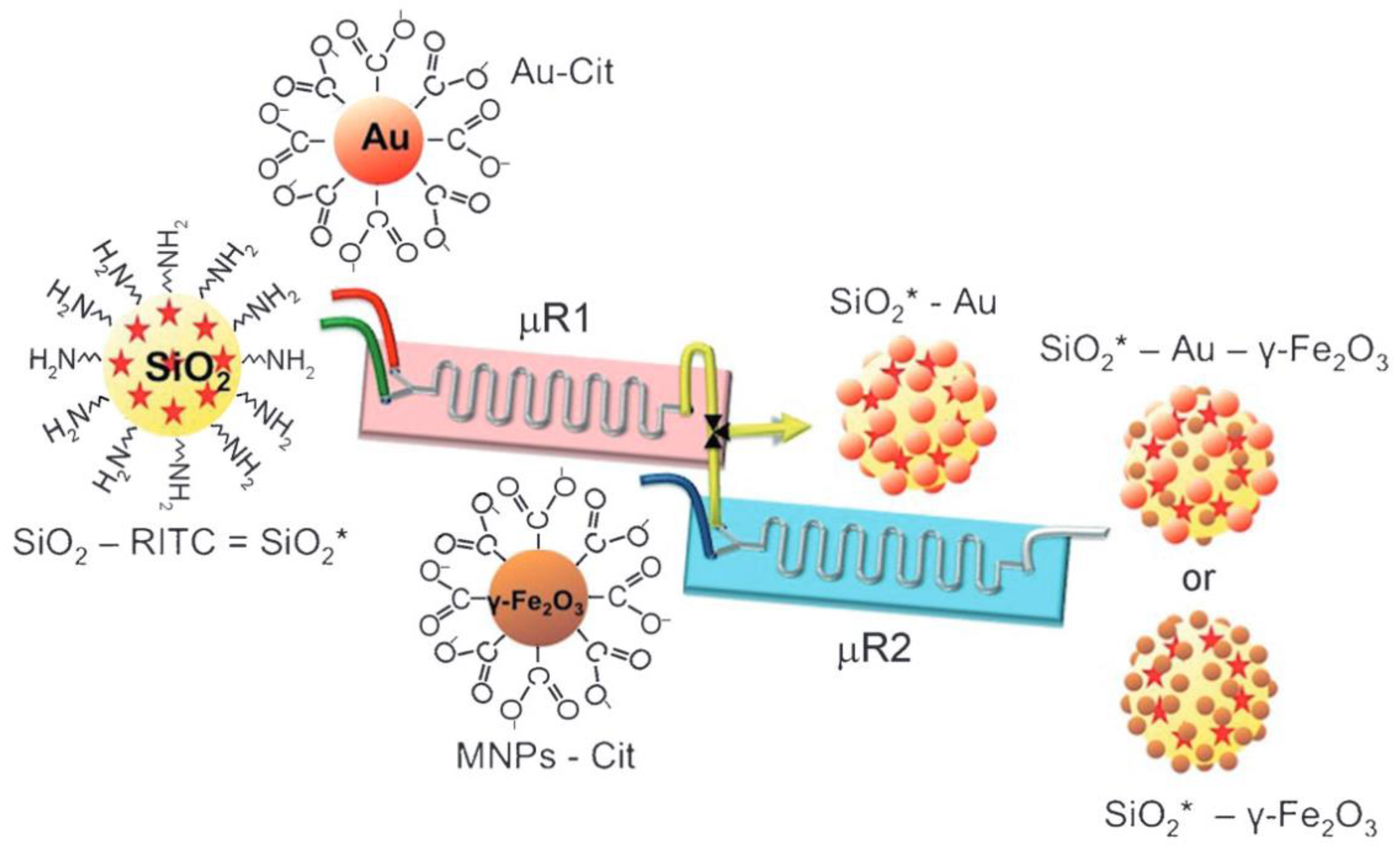

| Synthesis Type | Advantages | Challenges |
|---|---|---|
| Microfluidic synthesis |
|
|
| Conventional bulk synthesis |
|
|
| Fabrication Method | Particle Type | Main Advantage | Ref. |
|---|---|---|---|
| Continuous single-phase microfluidic synthesis | Gold nanorods | Improved reproducibility and quality, 100 times reduced consumption of SH-PEG, ability to tune halide concentration at various stages of synthesis. | [11] |
| Continuous micro segmented flow synthesis | Au/Ag core/shell | Efficient reactants mixing in droplets, high homogeneity, narrow size distribution, smaller particle diameter. | [12] |
| Microdroplet-based synthesis | Silica nanoparticles | Improvement of nanoparticle size uniformity (down to 3% relative standard deviation), ability to tune particle size by adjusting reaction time or reagent concentrations | [13] |
| Gas–liquid droplet- based synthesis | Silver nanocrystals | tunable edge lengths of Ag nanocubes from 30 to 100 nm | [9] |
| Batch synthesis | Silver core–silica shell nanoparticles | Used as the immobilization matrix for pH-sensitive dyes | [14] |
| Surfactant-free emulsion polymerization | Polystyrene particles | Good size distribution, used as the immobilization matrix for oxygen-sensitive dyes | [6,15] |
| Continuous flow synthesis | Polyacrylamide hydrogel particles | Narrow size distribution (standard deviation 2–3%), used as the immobilization matrix for lucigenin | [16] |
| Frens–Turkevich method | Gold nanoparticles | 17 nm size particles were functionalized with Rhodamine 6G | [17] |
| Hole mask colloidal lithography | nanoparticle arrays | Scalable and inexpensive fabrication of uniform nanoparticle arrays, ability to tune size of nanoparticles | [18,19] |
| Physical vapor deposition followed by a rapid thermal annealing (RTA) | Gold nanostructures | Simple and rapid fabrication | [20] |
| Application (Analyte) | Particle Type | Size | Fluidic System | Main Advantage | Ref. |
|---|---|---|---|---|---|
| Anti-gp41 antibody | AuNPs | - | Microfluidic chip | Fast and simple label-free sensing | [35] |
| Bovine growth hormone | AuNPs | 125 nm | PDMS microfluidic chip | Low limit of detection (185 pM) | [37] |
| Detecting small quantities of specific target molecules | AuNPs | 34 nm | Nanoparticle-functionalized suspended-core fiber | Low sample volume and a real-time monitoring of analyte | [38] |
| Aflatoxin B1 | AuNPs | 20 nm | - | Incorporation of AuNPs on Au chip caused 3-times higher sensitivity and 6-times reduced limit of the detection. | [39] |
| Streptavidin | AuNPs | 130 nm | 7-channel microfluidic devices | Simultaneous measurements of 6 different protein pairs | [18] |
| Biotin | AuNPs | 14–43 nm | Microfluidic chip | Decreased reaction time and lower reagent consumption. | [41] |
| IgG or CRP antibody | Au nanostuctures | 108 nm | Four-channel microfluidic device | Reduced assay time, only 60 μL of sample consumption, limit of a detection of IgG or CRP antibody was 10 ng/mL | [20] |
| Detecting small quantities of specific target molecules | Au nanocubes | 55–80 nm | Microfluidic DFM-reactor | Produced more homogenous and smaller Au seeds used for formation of Au nanocubes, whose sensitivity was twice better than AuNP | [45] |
| Lab-on-a-chip systems | Ag nanoprism | 50 nm | Cross-shape microchannel device | Low cost, high-efficient fabrication of localized silver NP array in a line-shape and a cross-shape microchannels. | [19] |
| Analyte | Detection Method | Particle Type | Size | Application | Fluidic System | Main Advantage | Ref. |
|---|---|---|---|---|---|---|---|
| Temp. | Extrinsic Fabry-Perot interferometry | Pt NPs | 10–100 nm | Polymerase chain reaction | Microfluidic device | Universal and non-contact temperature sensing with high resolution | [48] |
| Temp. | Luminescence thermometry | NaYF4:Yb,Er | 25–35 nm | Polymerase chain reaction | PDMS/glass chip | Measurements of temperature up to 120 °C with high accuracy (0.34 °C) and improved spatial resolution (up to 9 μm) | [49] |
| pH | Fluorescence | Ag@SiO2 + FiTC NPs | 110 nm | Streptococus salivarius biofilms | Y-channel microfluidic device | Good biocompatibility and photostability led to carrying out the experiment longer than one week | [14] |
| pH | Fluorescence | SNARF-1 dextran-loaded CaCO3 | 4–6 µm | breast cancer cells and fibroblasts | Microfluidic device | pH monitoring in living cells, rapid and efficient microparticles separation from the sample by applying a magnetic field | [51] |
| pH | Time-domain dual lifetime referencing (t-DLR) | Ru(dpp)3PAN | - | conalbumin, myoglobin and β-Lactoglobulin A, | Microfluidic free-flow isoelectric focusing chip | Working range from pH 4 to 8, rapid response times in the millisecond range | [52] |
| O2 | Phosphorescence | PS-PtTPTBP | 350 nm | bacterial cultivation | Segmented flow | Simultaneous, real-time and non-invasive measurement of dissolved oxygen, cellular autofluorescence, and cell density in nl-sized droplets | [6] |
| O2 | Frequency-domain lifetime imaging | PSPVP- PtTPTBPF | 180 nm | monitoring the growth of E. coli and M. smegmatis. | Microfluidic Cell Culturing Device | Accurate determination of the absolute oxygen concentration in inside microdroplets with resolution of 0.07–0.12 μM | [55] |
| O2 | Phosphorescence lifetime imaging | PS-(Pd-OEP) | 0.6–1.6 µm | 4T1 cells | Microfluidic device | Good stability over a long period of time due to formed sensing film, which prevented oxygen-sensitive dye direct contact with living cells. | [56] |
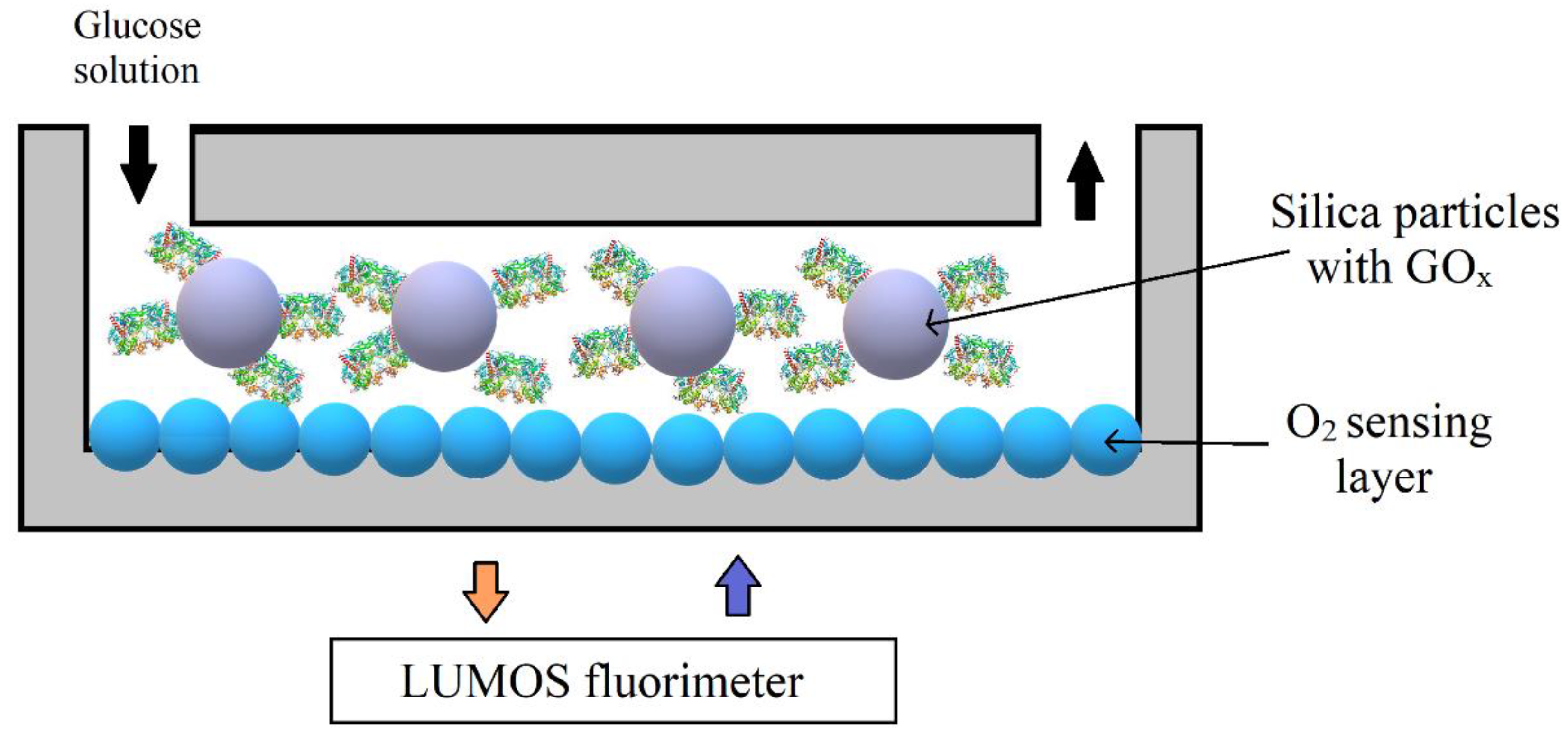
| O2 | Phosphorescence lifetime imaging | Pt (II) benzoporphyrin grafted conjugated polymer NPs | 30–100 nm | Glucose oxidase enzymatic activity | Microfluidic glass chips | Less than 0.2 s response time, optimum sensitivity, excellent stability, possibility to reuse reactor multiple times | [57] |
| O2 | Epifluorescence microscopy | PSPVP- Ir(Cs)2(acac) | 245 nm | Glucose oxidation reaction | Y-channel microfluidic device | High measurement accuracy (0.03) | [58] |
| O2 | ratiometric luminescence imaging | MOSePs- Ir(Cs)2(acac) | 160 nm | E. coli respiratory activity | microfluidic device | Ability to easily form and move sensor spots along the microfluidic channel by applying magnet | [60] |
| O2 | Time-resolved particle image velocimetry (µ-PIV) | PS- PtOEP | 2.5 µm | DI water | Y-channel microfluidic device | Simultaneous determination of velocity and dissolved oxygen concentration, low photobleaching, high accuracy with 0.5% random error. | [61] |
| pH/oxygen | Frequency-domain lifetime measurement | PSPVP- PtTPTBPF, PSPVP- Aza-BODIP | 180 nm | Penicillin G | Y-channel microfluidic device | Simultaneous detection of oxygen and pH, high stability in aqueous media, pH resolution was 0.03–0.1 pH units, resolution of oxygen was 0.02–0.32 mg/l | [23] |
| Application | Detection Method | Particle Type | Size | Fluidic System | Main Advantage | Ref. |
|---|---|---|---|---|---|---|
| Gas sensing | Diffuse reflectance infrared Fourier-transformed spectroscopy (DRIFTS) | Au, Pd, AuPd NPs | 6–9 nm | Microfluidic chip | Investigated four gas samples, high sensitivity for CO detection | [64] |
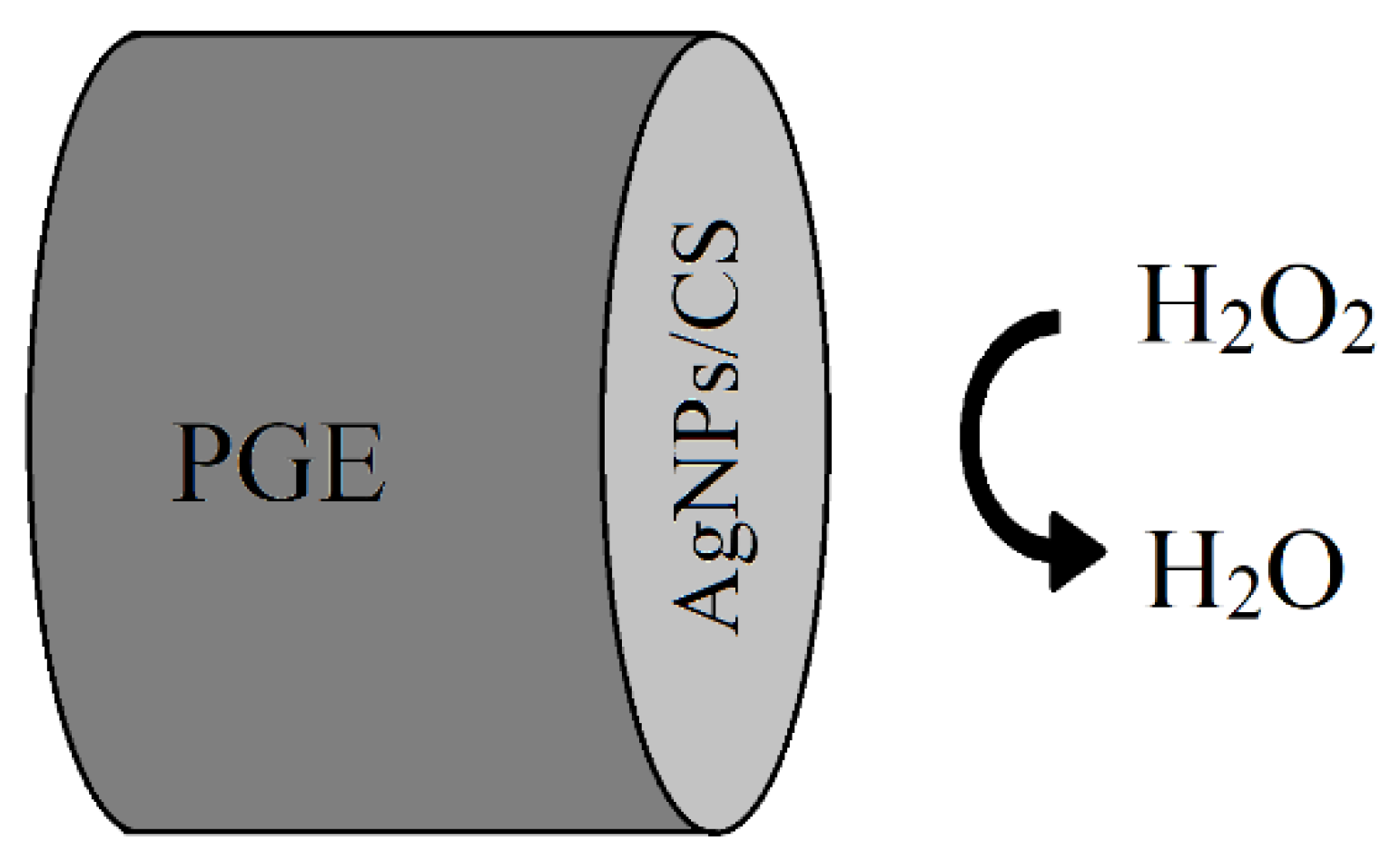
| H2O2 | Cyclic voltammetry (CV) | AgNPs | - | 3D Microfluidic channel | Green synthesis of AgNPs using marigold flowers extract, disposable, low-cost device for H2O2 detection in cosmetic and medical samples with limit of the detection of 0.52 µM | [65] |
| Chloride ion | Fluorescence | PAM- Lucigenin | 800 μm | Microfluidic generation of hydrogel particles | Determination of chloride concentrations up to 50 mM, only 10 μL of sample consumption | [16] |
| Hg/Ziram | Fluorescence | AuNPs | 17–23 nm | Microfluidic chip | Automated sample preparation step, limit of detection was 0.6 µg/L for mercury and 16 µg/L for ziram, wide and linear detection range from 4 µg/L to 200 µg/L | [17] |
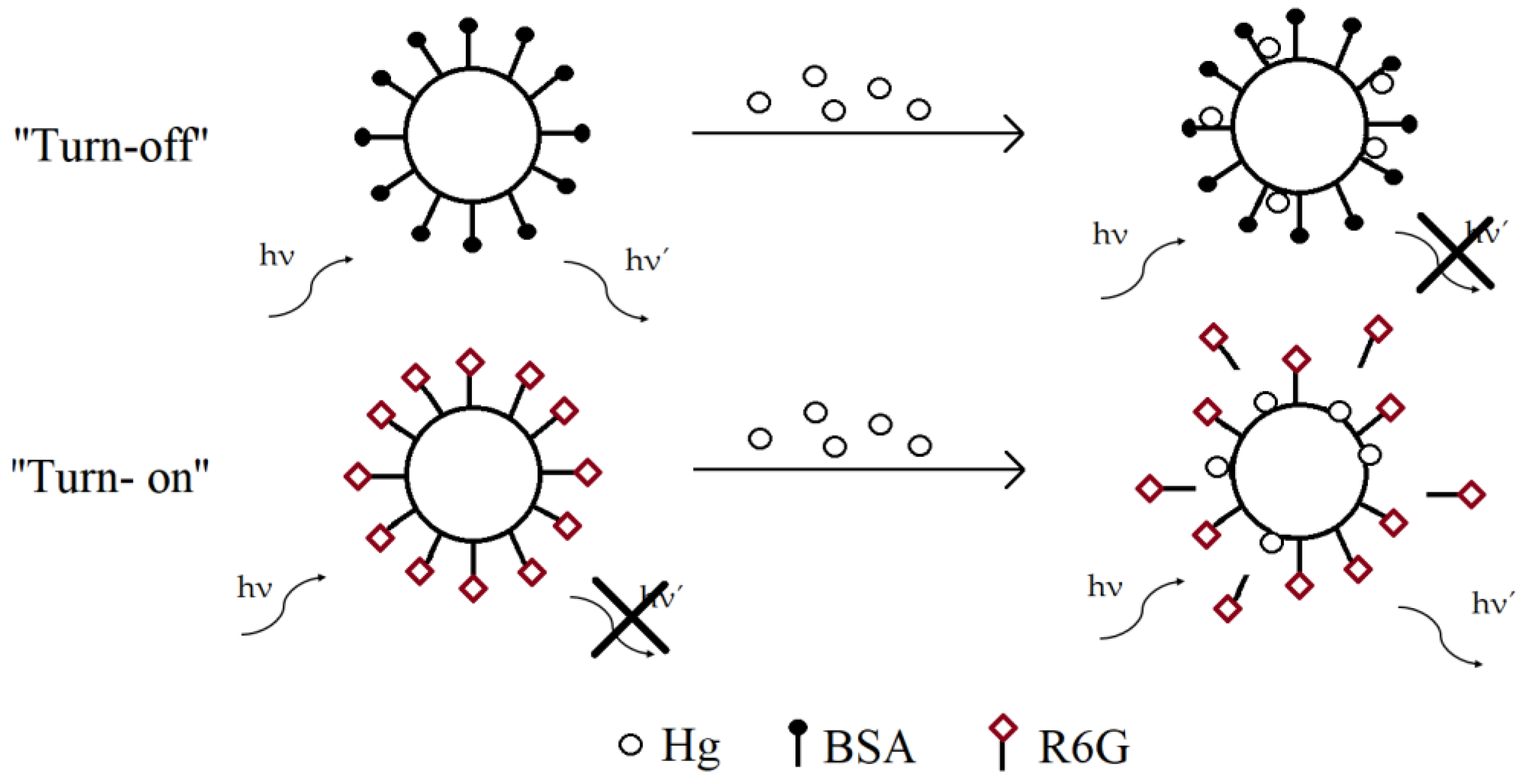
| Mercury (II) ion | Fluorescence | AuNPs-RB | - | Valve-based microfluidic device | Sample encapsulation into nl or pl droplets, automated and effective mixing of reagents, application for clinical diagnosis or environmental test | [5] |
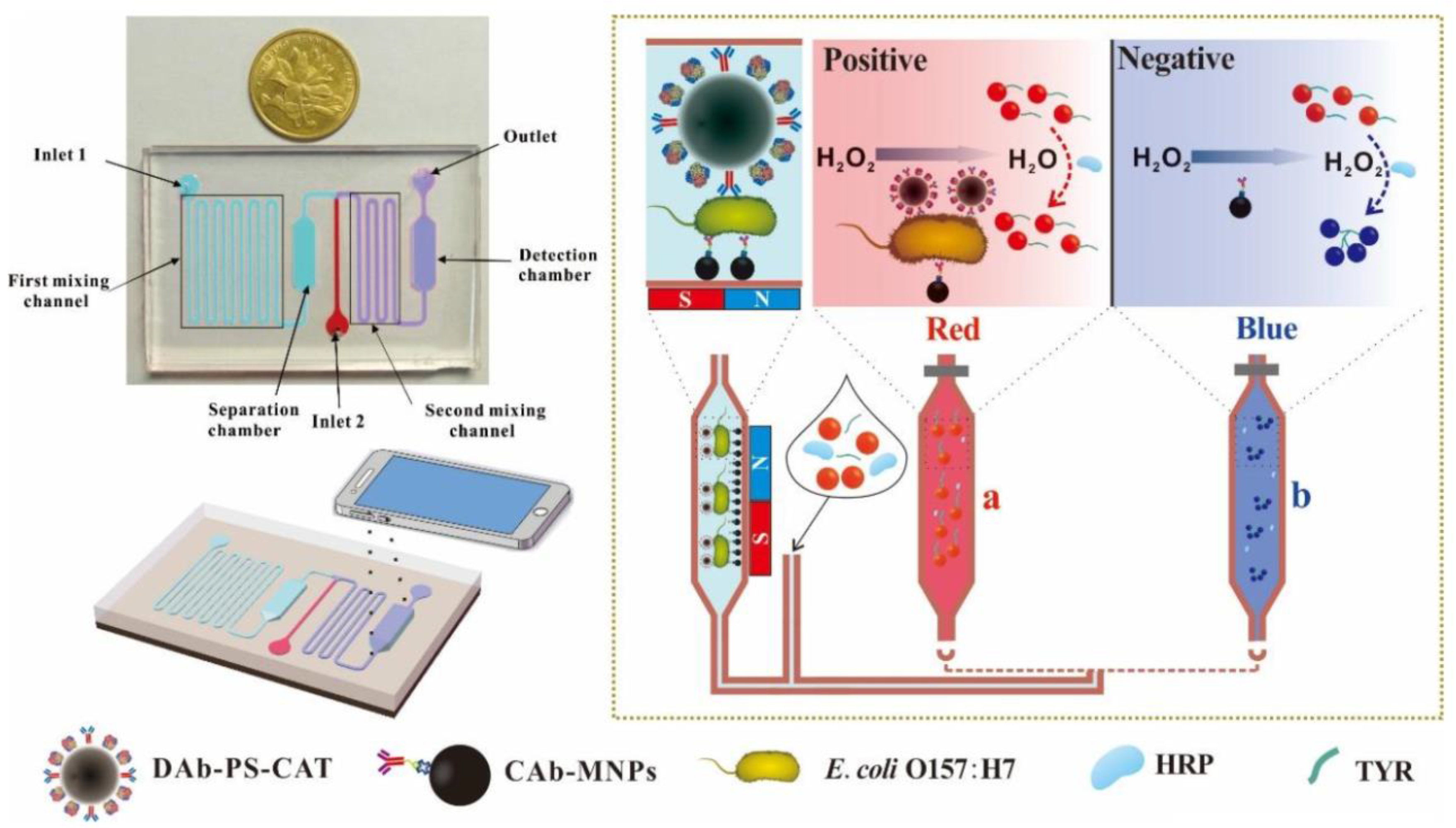
| E. coli O157:H7 | Colorimetric assay | AuNPs | 19 nm | Microfluidic device | Limit of detection was 50 CFU/mL, good sensitivity and specificity for E. coli in chicken samples | [66] |
| Influenza virus | Colorimetric assay | AuNPs | 40 nm | Microfluidic RT-PCR chip | Replication of influenza virus RNA by a continuous-flow RT-PCR chip within 15–20 min, rapid sample measurement within 1 min, naked-eye detection | [67] |
| Influenza virus | Colorimetric assay | AuNPs | 72 nm | RT-PCR-ICS microdevice | Identification by the naked eye, rapid and simple analysis within 2.5 h, 100 times improved sensitivity in comparison to gel electrophoresis | [68] |
| Cocaine, codeine, methamphetamine | Colorimetric assay | AuNPs | 20–40 nm | four-channel paper microfluidic device | Simultaneous, sensitive and specific detection with limit of detection of 4 μg for cocaine, codeine and methamphetamine drugs. | [69] |
| Thrombin | Colorimetric assay | AgNPs | 35 nm | PDMS microfluidic device | Simple, straightforward and highly specific protein analysis by the naked eye and a flatbed scanner, low sample cost, good linear relationship with the detection limit of 20 pM | [70] |
| Enzymatic reactions | Colorimetric assay | SiO2 NPs | - | microfluidic paper-based device | Improved color intensity and color uniformity by applying SiO2 nanoparticles, semiquantitative detection of lactate, glucose, and glutamate in artificial urine samples within 30 min. | [71] |
| Tuberculosis | Colorimetric assay | AuNPs | 13 nm | paper-based analytical device | Accurate, simple, low-cost and rapid analysis within 1 h, only 200 μL sample consumption, limit of detection was 0.0195 ng/mL | [72] |
| Cysteine | Colorimetric assay | Au, Ag NPs | - | paper-based microfluidic sensor | Rapid fabrication time, identification by the naked eye, eco-friendly, low cost, portable test paper can be burned after usage | [73] |
| Legionella pneumophila | Colorimetric assay | AuNPs | 13 nm | 2-LED- μSpectrophotometer | low-cost, easy to use and specific assay, detection limit of 0.5 pg/μL DNA | [74] |
| Glucose | Cyclic voltammetry | CS-RGO–NiNPs | 32 nm | PDMS microfluidic device | Linear detection range up to 9 mM with limit of detection of 4.1 µM glucose | [75] |
| Hemoglobin A1c | Electrophoresis | Au/chitosan/Fe3O4 NP | 80 nm | Microfluidic device | Rapid detection within 30 min, low reagent consumption, good accuracy with limit of detection of 0.025 µg/mL | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazetyte-Stasinskiene, R.; Köhler, J.M. Sensor Micro and Nanoparticles for Microfluidic Application. Appl. Sci. 2020, 10, 8353. https://doi.org/10.3390/app10238353
Mazetyte-Stasinskiene R, Köhler JM. Sensor Micro and Nanoparticles for Microfluidic Application. Applied Sciences. 2020; 10(23):8353. https://doi.org/10.3390/app10238353
Chicago/Turabian StyleMazetyte-Stasinskiene, Raminta, and Johann Michael Köhler. 2020. "Sensor Micro and Nanoparticles for Microfluidic Application" Applied Sciences 10, no. 23: 8353. https://doi.org/10.3390/app10238353
APA StyleMazetyte-Stasinskiene, R., & Köhler, J. M. (2020). Sensor Micro and Nanoparticles for Microfluidic Application. Applied Sciences, 10(23), 8353. https://doi.org/10.3390/app10238353






