Effects of a Combination of Elderberry and Reishi Extracts on the Duration and Severity of Respiratory Tract Infections in Elderly Subjects: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Study Products
2.3. Study Outcomes and Data Collection
2.4. Statistical Analysis
3. Results
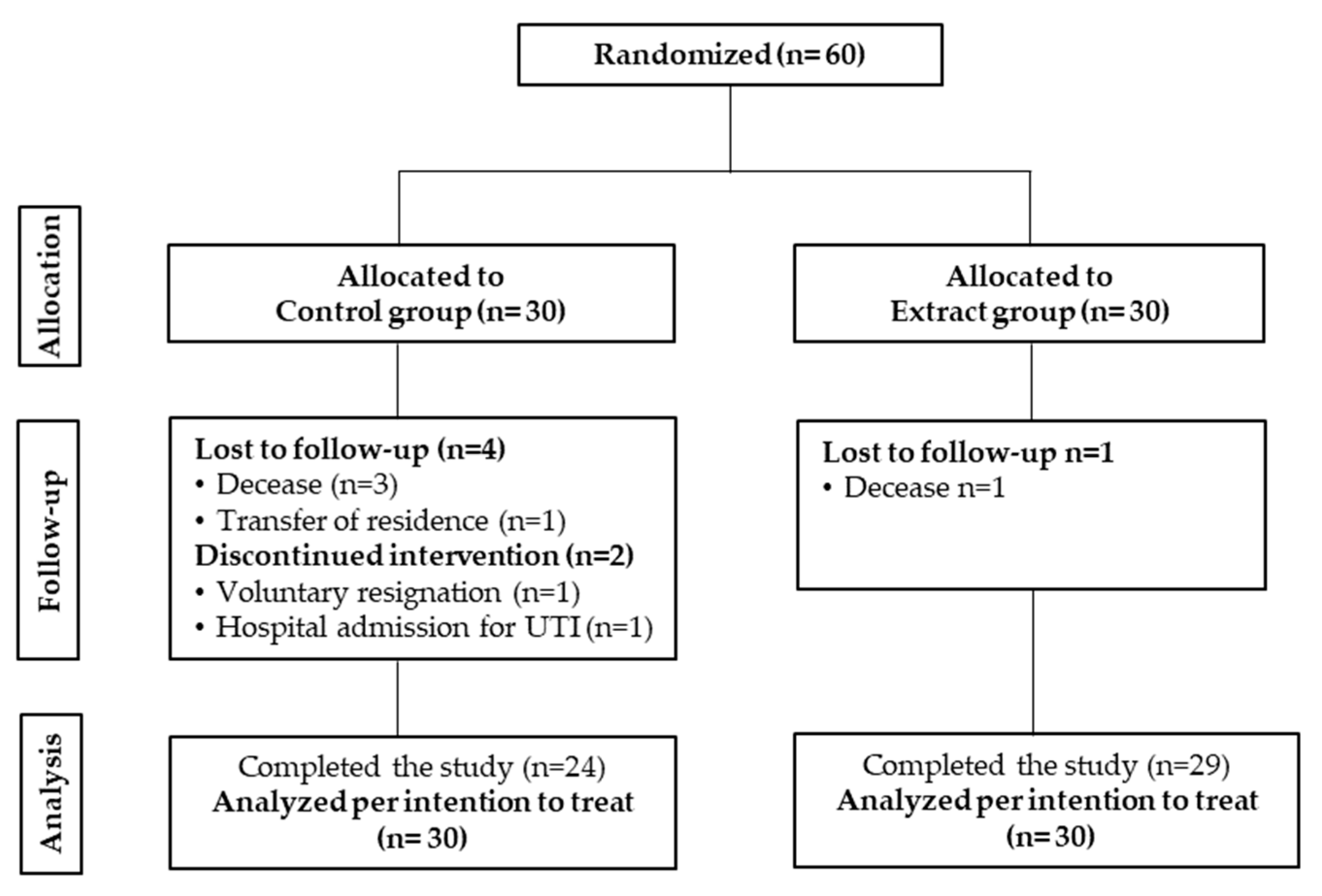
3.1. Study Data, Compliance and Baseline Characteristics of the Subjects
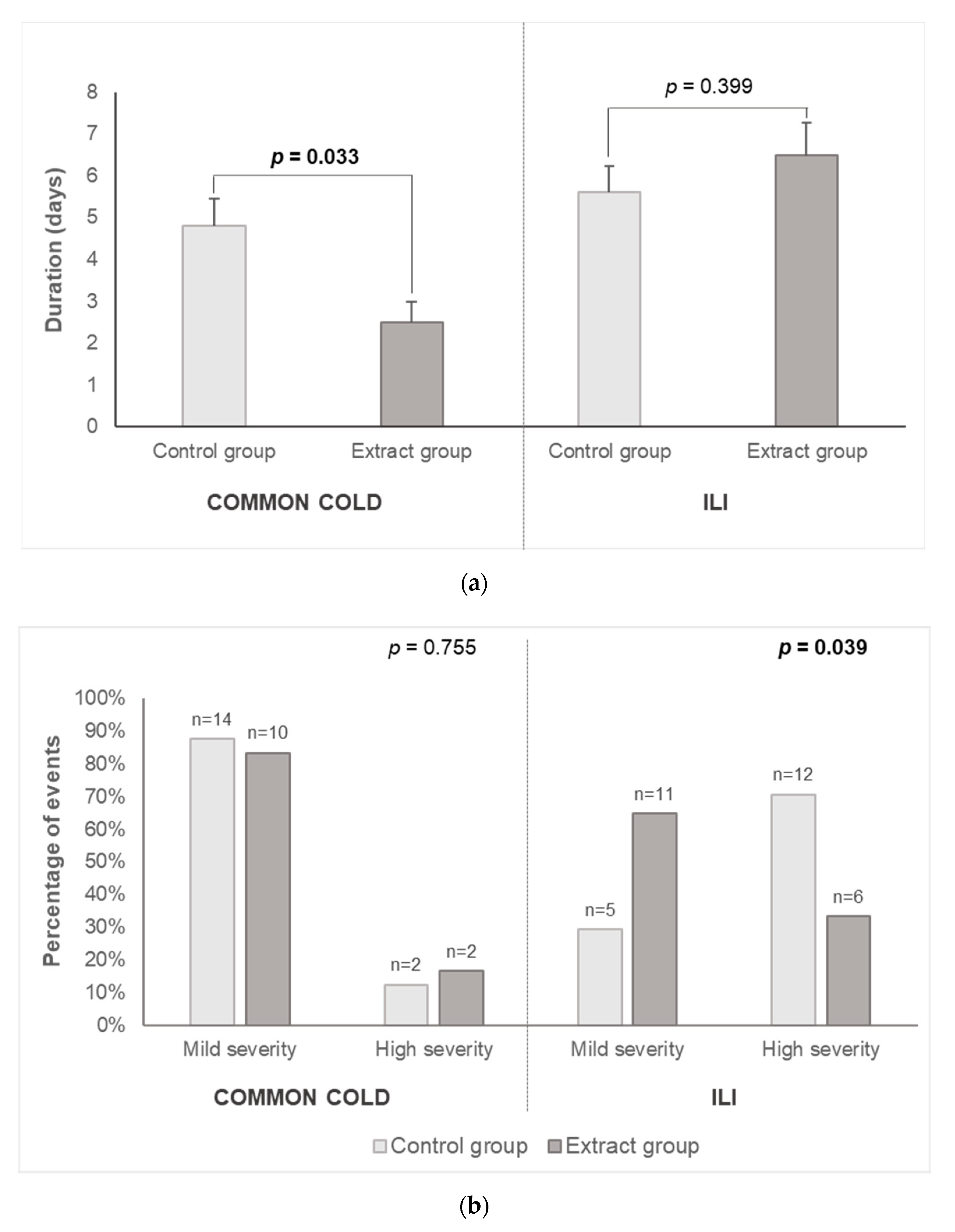
3.2. Respiratory Tract Infections Incidence and Duration and Severity of Related Symptoms
3.3. Safety Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicholson, K.G.; Abrams, K.R.; Batham, S.; Medina, M.J.; Warren, F.C.; Barer, M.; Bermingham, A.; Clark, T.W.; Latimer, N.; Fraser, M.; et al. Randomised controlled trial and health economic evaluation of the impact of diagnostic testing for influenza, respiratory syncytial virus and Streptococcus pneumoniae infection on the management of acute admissions in the elderly and high-risk 18- to 64-year-olds. Health Technol. Assess. 2014, 18, 1–274. [Google Scholar] [PubMed]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Petersen, E.; Koopmans, M.; Go, U.; Hamer, D.H.; Petrosillo, N.; Castelli, F.; Storgaard, M.; Al Khalili, S.; Simonsen, L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020, 20, e238–e244. [Google Scholar] [CrossRef]
- Castle, S.C. Clinical relevance of age-related immune dysfunction. Clin. Infect. Dis. 2000, 31, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Dewan, S.K.; Zheng, S.; Xia, S.; Bill, K. Senescent remodeling of the immune system and its contribution to the predisposition of the elderly to infections. Chin. Med. J. 2012, 125, 3325–3331. [Google Scholar] [PubMed]
- Masse, S.; Capai, L.; Falchi, A. Epidemiology of Respiratory Pathogens among Elderly Nursing Home Residents with Acute Respiratory Infections in Corsica, France, 2013–2017. Biomed Res. Int. 2017, 2017, 1423718. [Google Scholar] [CrossRef]
- Childs, A.; Zullo, A.R.; Joyce, N.R.; McConeghy, K.W.; van Aalst, R.; Moyo, P.; Bosco, E.; Mor, V.; Gravenstein, S. The burden of respiratory infections among older adults in long-term care: A systematic review. BMC Geriatr. 2019, 19, 210. [Google Scholar] [CrossRef]
- Rainwater-Lovett, K.; Chun, K.; Lessler, J. Influenza outbreak control practices and the effectiveness of interventions in long-term care facilities: A systematic review. Influenza Other Respir. Viruses 2014, 8, 74–82. [Google Scholar] [CrossRef]
- Licciardi, P.V.; Underwood, J.R. Plant-derived medicines: A novel class of immunological adjuvants. Int. Immunopharmacol. 2011, 11, 390–398. [Google Scholar] [CrossRef]
- Shahzad, F.; Anderson, D.; Najafzadeh, M. The Antiviral, Anti-Inflammatory Effects of Natural Medicinal Herbs and Mushrooms and SARS-CoV-2 Infection. Nutrients 2020, 12, 2573. [Google Scholar] [CrossRef]
- Roxas, M.; Jurenka, J. Colds and influenza: A review of diagnosis and conventional, botanical, and nutritional considerations. Altern. Med. Rev. 2007, 12, 25–48. [Google Scholar] [PubMed]
- Hawkins, J.; Baker, C.; Cherry, L.; Dunne, E. Black elderberry (Sambucus nigra) supplementation effectively treats upper respiratory symptoms: A meta-analysis of randomized, controlled clinical trials. Complement. Ther. Med. 2019, 42, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Harnett, J.; Oakes, K.; Carè, J.; Leach, M.; Brown, D.; Cramer, H.; Pinder, T.-A.; Steel, A.; Anheyer, D. The effects of Sambucus nigra berry on acute respiratory viral infections: A rapid review of clinical studies. Adv. Integr. Med. 2020, 7, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, E.; Hayashi, K.; Katayama, H.; Hayashi, T.; Obata, A. Anti-influenza virus effects of elderberry juice and its fractions. Biosci. Biotechnol. Biochem. 2012, 76, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-T.; Buswell, J.A. Ganoderma lucidum (Curt.: Fr.) P. karst.(Aphyllophoromycetideae)—A mushrooming medicinal mushroom. Int. J. Med. Mushrooms 1999, 1, 139–146. [Google Scholar] [CrossRef]
- Wasser, S.P.; Coates, P.; Blackman, M.; Cragg, G.; Levine, M.; Moss, J.; White, J. Reishi or ling zhi (Ganoderma lucidum). In Encyclopedia of Dietary Supplements; Marcel Dekker: New York, NY, USA, 2005; pp. 680–690. [Google Scholar]
- Xu, Z.; Chen, X.; Zhong, Z.; Chen, L.; Wang, Y. Ganoderma lucidum Polysaccharides: Immunomodulation and Potential Anti-Tumor Activities. Am. J. Chin. Med. 2011, 39, 15–27. [Google Scholar] [CrossRef]
- Ko, H.-H.; Hung, C.-F.; Wang, J.-P.; Lin, C.-N. Antiinflammatory triterpenoids and steroids from Ganoderma lucidum and G. tsugae. Phytochemistry 2008, 69, 234–239. [Google Scholar] [CrossRef]
- Mohammed, A.; Tanko, Y.; Mohammed, K.A.; Yaro, A.H. Studies of analgesic and anti-inflammatory effects of aqueous extract of Ganoderma lucidum in mice and wister rats. IOSR J. Pharm. Biol. Sci. 2012, 4, 54–57. [Google Scholar]
- Yu, A.; Wong, C.-H.; Jan, J.T.; Cheng, Y.-S.E. Anti-Viral Effect of an Extract of Ganoderma Lucidum 2008. U.S. Patent Application, No. 11/859,729, 21 September 2006. [Google Scholar]
- Rauš, K.; Pleschka, S.; Klein, P.; Schoop, R.; Fisher, P. Effect of an Echinacea-Based Hot Drink Versus Oseltamivir in Influenza Treatment: A Randomized, Double-Blind, Double-Dummy, Multicenter, Noninferiority Clinical Trial. Curr. Ther. Res. Clin. Exp. 2015, 77, 66–72. [Google Scholar] [CrossRef]
- Influenza Case Definitions (2017) Stockholm: ECDC. Available online: http://ecdc.europa.eu/en/healthtopics/influenza/surveillance/Pages/influenza_case_definitions.aspx (accessed on 20 October 2020).
- Eccles, R. Understanding the symptoms of the common cold and influenza. Lancet Infect. Dis. 2005, 5, 718–725. [Google Scholar] [CrossRef]
- Henao, S.L.D.; Urrego, S.A.; Cano, A.M.; Higuita, E.A. Randomized Clinical Trial for the Evaluation of Immune Modulation by Yogurt Enriched with β-Glucans from Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes), in Children from Medellin, Colombia. Int. J. Med. Mushrooms 2018, 20, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Zakay-Rones, Z.; Varsano, N.; Zlotnik, M.; Manor, O.; Regev, L.; Schlesinger, M.; Mumcuoglu, M. Inhibition of several strains of influenza virus in vitro and reduction of symptoms by an elderberry extract (Sambucus nigra L.) during an outbreak of influenza B Panama. J. Altern. Complement. Med. 1995, 1, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Zakay-Rones, Z.; Thom, E.; Wollan, T.; Wadstein, J. Randomized study of the efficacy and safety of oral elderberry extract in the treatment of influenza A and B virus infections. J. Int. Med. Res. 2004, 32, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Kong, F. Pilot clinical study on a proprietary elderberry extract: Efficacy in addressing influenza symptoms. Online J. Pharmacol. Pharmacokinet. 2009, 5, 32–43. [Google Scholar]
- Tiralongo, E.; Wee, S.S.; Lea, R.A. Elderberry supplementation reduces cold duration and symptoms in air-travellers: A randomized, double-blind placebo-controlled clinical trial. Nutrients 2016, 8, 182. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Vlachojannis, J.E.; Cameron, M.; Chrubasik, S. A systematic review on the sambuci fructus effect and efficacy profiles. Phytother. Res. 2010, 24, 1–8. [Google Scholar] [CrossRef]
- Barak, V.; Halperin, T.; Kalickman, I. The effect of Sambucol, a black elderberry-based, natural product, on the production of human cytokines: I. Inflammatory cytokines. Eur. Cytokine Netw. 2001, 12, 290–296. [Google Scholar]
- Barak, V.; Birkenfeld, S.; Halperin, T.; Kalickman, I. The effect of herbal remedies on the production of human inflammatory and anti-inflammatory cytokines. Isr. Med Assoc. J. IMAJ 2002, 4, 919–922. [Google Scholar]
- Roschek, B.; Fink, R.C.; McMichael, M.D.; Li, D.; Alberte, R.S. Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochemistry 2009, 70, 1255–1261. [Google Scholar] [CrossRef]
- Swaminathan, K.; Müller, P.; Downard, K.M. Substituent effects on the binding of natural product anthocyanidin inhibitors to influenza neuraminidase with mass spectrometry. Anal. Chim. Acta 2014, 828, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Bang, T.H.; Ohnuki, K.; Sawai, T.; Sawai, K.; Shimizu, K. Inhibition of neuraminidase by Ganoderma triterpenoids and implications for neuraminidase inhibitor design. Sci. Rep. 2015, 5, 13195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Amen, Y.M.; Ohnuki, K.; Shimizu, K. Anti-influenza effects of Ganoderma lingzhi: An animal study. J. Funct. Foods 2017, 34, 224–228. [Google Scholar] [CrossRef]
- Hughes, D.A. Dietary antioxidants and human immune function. Nutr. Bull. 2000, 25, 35–41. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Lin, Z.; Deng, A. Antioxidative and Free Radical Scavenging Activity of Ganoderma (Lingzhi). In Ganoderma and Health; Springer: Singapore, 2019; pp. 271–297. [Google Scholar]
- Ivanova, D.; Tasinov, O.; Kiselova-Kaneva, Y. Improved lipid profile and increased serum antioxidant capacity in healthy volunteers after Sambucus ebulus L. fruit infusion consumption. Int. J. Food Sci. Nutr. 2014, 65, 740–744. [Google Scholar] [CrossRef]
- Hunter, D.C.; Skinner, M.A.; Wolber, F.M.; Booth, C.L.; Loh, J.M.S.; Wohlers, M.; Stevenson, L.M.; Kruger, M.C. Consumption of gold kiwifruit reduces severity and duration of selected upper respiratory tract infection symptoms and increases plasma vitamin C concentration in healthy older adults. Br. J. Nutr. 2012, 108, 1235–1245. [Google Scholar] [CrossRef]
- Tang, W.; Gao, Y.; Chen, G.; Gao, H.; Dai, X.; Ye, J.; Chan, E.; Huang, M.; Zhou, S. A Randomized, Double-Blind and Placebo-Controlled Study of a Ganoderma lucidum Polysaccharide Extract in Neurasthenia. J. Med. Food 2005, 8, 53–58. [Google Scholar] [CrossRef]
- Chu, Q.-P.; Wang, L.-E.; Cui, X.-Y.; Fu, H.-Z.; Lin, Z.-B.; Lin, S.-Q.; Zhang, Y.-H. Extract of Ganoderma lucidum potentiates pentobarbital-induced sleep via a GABAergic mechanism. Pharmacol. Biochem. Behav. 2007, 86, 693–698. [Google Scholar] [CrossRef]
- Cui, X.-Y.; Cui, S.-Y.; Zhang, J.; Wang, Z.-J.; Yu, B.; Sheng, Z.-F.; Zhang, X.-Q.; Zhang, Y.-H. Extract of Ganoderma lucidum prolongs sleep time in rats. J. Ethnopharmacol. 2012, 139, 796–800. [Google Scholar] [CrossRef]
| Control Group (n = 30) | Extract Group (n = 30) | p between Groups | |
|---|---|---|---|
| Age (years) | 82.7 ± 9.2 | 85.9 ± 7.8 | 0.155 |
| Sex | 0.739 | ||
| Men | 5 (16.7%) | 6 (20%) | |
| Women | 25 (83.3%) | 24 (60%) | |
| Weight (kg) | 68.1 ± 13.9 | 63.6 ± 10.9 | 0.176 |
| BMI (kg/m2) | 26.1 ± 4.6 | 25.4 ± 3.8 | 0.478 |
| Smoking habit | 0.109 | ||
| Current smoker | 4 (13.3%) | 2 (6.7%) | |
| Former smoker | 1 (3.3%) | 6 (20%) | |
| No smokers | 25 (83.3%) | 22 (73.3%) | |
| Physical activity | 0.519 | ||
| Very low | 23 (76.7%) | 25 (83.3%) | |
| Low | 7 (23.3%) | 5 (16.7%) | |
| Systolic BP (mm Hg) | 126.2 ± 14.7 | 130.3 ± 10.4 | 0.218 |
| Diastolic BP (mm Hg) | 70.7 ± 10.2 | 71.8 ± 9.4 | 0.667 |
| Sleeping medication | 10 (33.3%) | 8 (26.7%) | 0.389 |
| Hypertension medication | 19 (63.3%) | 18 (60.0%) | 0.518 |
| Control Group IR (SD) | Extract Group IR (SD) | IR Ratio (95% CI) | IR Decrease, % | p Value c | |
|---|---|---|---|---|---|
| Common cold | 0.321 (0.188) | 0.240 (0.135) | 0.750 (0.320–1.759) | 25.0 | 0.508 |
| ILI a | 0.494 (0.188) | 0.523 (0.174) | 1.059 (0.514–2.182) | −0.06 | 0.978 |
| Respiratory infections b | 0.921 (0.273) | 0.834 (0.224) | 0.906 (0.523–1.570) | 9.4 | 0.725 |
| Control Group IR (SD) | Extract Group IR (SD) | IR Ratio (95% CI) | IR Decrease, % | p-Value d | |
|---|---|---|---|---|---|
| Cough | 0.558 (0.202) | 0.552 (0.177) | 0.989 (0.495–1.976) | 1.1 | 0.976 |
| Nasal congestion | 0.478 (0.200) | 0.357 (0.141) | 0.747 (0.334–1.6719 | 25.3 | 0.478 |
| Sore throat | 0.356 (0.211) | 0.108 (0.073) | 0.302 (0.095–0.963) | 69.8 | 0.043 |
| Fever | 0.244 (0.112) | 0.128 (0.717) | 0.528 (0.52–1.834) | 47.2 | 0.315 |
| Headache | 0.128 (0.937) | 0.102 (0.070) | 0.798 (0.266–2.396) | 21.2 | 0.688 |
| Muscle/Bone Pain | 0.146 (0.92) | 0.138 (0.794) | 0.941 (0.327–2.713) | 5.9 | 0.911 |
| Tiredness/Malaise | 0.180 (0.101) | 0.342 (0.155) | 1.897 (0.755–4.770) | −89.7 | 0.173 |
| Local Respiratory Symptoms a | 1.466 (0.352) | 1.031 (0.239) | 0.703 (0.440–1.124) | 29.7 | 0.141 |
| Systemic Symptoms b | 0.754 (0.233) | 0.656 (.189) | 0.870 (0.500–1.511) | 23 | 0.62 |
| Total Symptoms c | 2.185 (0.410) | 1.808 (0.316) | 0.828 (0.584–1.174) | 27.2 | 0.289 |
| Control Group | Extract Group | |||
|---|---|---|---|---|
| Nausea IR (SD) a | 0.100 (0.057) | 0.166 (0.074) | ||
| Lack of appetite IR (SD) a | 0.666 (0.149) | 0.633 (0.145) | ||
| Sleeping disturbance IR (SD) a | 0.899 (0.325) | 0.372 (0.152) * | ||
| Baseline | End of intervention | Baseline | End of intervention | |
| Weight (kg) b | 68.4 ± 14.2 | 67.3 ± 13.4 | 64.2 ± 10.6 | 63.8 ± 9.5 |
| Systolic BP (mm Hg) b | 126.8 ± 15 | 127.8 ± 10.7 | 130.6 ± 10.4 | 126.5 ± 9.1 |
| Diastolic BP (mm Hg) b | 70.5 ± 10.7 | 72 ± 8.1 | 72 ± 9.5 | 68.3 ± 8.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gracián-Alcaide, C.; Maldonado-Lobón, J.A.; Ortiz-Tikkakoski, E.; Gómez-Vilchez, A.; Fonollá, J.; López-Larramendi, J.L.; Olivares, M.; Blanco-Rojo, R. Effects of a Combination of Elderberry and Reishi Extracts on the Duration and Severity of Respiratory Tract Infections in Elderly Subjects: A Randomized Controlled Trial. Appl. Sci. 2020, 10, 8259. https://doi.org/10.3390/app10228259
Gracián-Alcaide C, Maldonado-Lobón JA, Ortiz-Tikkakoski E, Gómez-Vilchez A, Fonollá J, López-Larramendi JL, Olivares M, Blanco-Rojo R. Effects of a Combination of Elderberry and Reishi Extracts on the Duration and Severity of Respiratory Tract Infections in Elderly Subjects: A Randomized Controlled Trial. Applied Sciences. 2020; 10(22):8259. https://doi.org/10.3390/app10228259
Chicago/Turabian StyleGracián-Alcaide, Carlos, Jose A. Maldonado-Lobón, Elisabeth Ortiz-Tikkakoski, Alejandro Gómez-Vilchez, Juristo Fonollá, Jose L. López-Larramendi, Mónica Olivares, and Ruth Blanco-Rojo. 2020. "Effects of a Combination of Elderberry and Reishi Extracts on the Duration and Severity of Respiratory Tract Infections in Elderly Subjects: A Randomized Controlled Trial" Applied Sciences 10, no. 22: 8259. https://doi.org/10.3390/app10228259
APA StyleGracián-Alcaide, C., Maldonado-Lobón, J. A., Ortiz-Tikkakoski, E., Gómez-Vilchez, A., Fonollá, J., López-Larramendi, J. L., Olivares, M., & Blanco-Rojo, R. (2020). Effects of a Combination of Elderberry and Reishi Extracts on the Duration and Severity of Respiratory Tract Infections in Elderly Subjects: A Randomized Controlled Trial. Applied Sciences, 10(22), 8259. https://doi.org/10.3390/app10228259





