Abstract
The apricot storability is one of the largest challenges, which the apricot industry has to face all over the world; therefore, finding options for prolonging fruit quality during cold storage (CS) and shelf-life (SL) will help to decrease postharvest losses of apricot. The aim of this apricot fruit work was to study the temporal changes and correlations of 10 quality parameters (quality losses, antioxidant properties and enzyme activities) in the postharvest treatments of methyl jasmonate (MeJA) and salicylic acid (SA) under 1 °C CS (7, 14 and 21 days) and 25 °C SL (4 and 8 days after the 21-day CS) treatments. MeJA and SA significantly decreased the quality loss of chilling injury (CI) and fruit decay (FD) at all dates for both storage conditions. MeJA- and SA-treated fruits increased total antioxidant capacity (TAC), total soluble phenolic compounds (TSPC) and carotenoids contents (TCC) at all dates of both storage treatments. In contrast, the ascorbic acid content (AAC) increased only until days 14 and 4 in the CS and SL treatments, respectively. Among enzyme activity parameters, the activities of phenylalanine ammonia-lyase (PAL), peroxidase and superoxide dismutase (SOD) were significantly increased in the MeJA and SA treatments in all dates of both storage treatments. Catalase (CAT) activity increased in the SA and control treatments, while it decreased in the MeJA treatment in both storage conditions. In both the MeJA and the SA treatments, six pair-variables (FD vs. CI, PAL vs. CAT, PAL vs. SOD, TAC vs. SOD, TAC vs. FD, and AAC vs. CI) were significant in Pearson correlation and regression analyses among the 45 parameters pairs. Principal component analyses explained 89.3% of the total variance and PC1 accounted for 55.6% of the variance and correlated with the CI, FD, TAC, TSPC, TCC, PAL and SOD, indicating strong connections among most parameters. In conclusion, MeJA and SA are practically useful and inexpensive techniques to maintain quality attributes of CI, FD, TAC, TSPC, TCC, PAL, POD and SOD in apricot fruit during both CS and SL conditions.
1. Introduction
Minerals, vitamins, and antioxidant materials in fruit crops are essential sources for human health care [1,2]. The amount and longevity of these sources in stone fruits are highly dependent on the pre- and postharvest quality of the fruit e.g., [3,4,5]. Among stone fruit species, the post-harvest life of apricot (Prunus armeniaca) is short due to its climacteric nature [5]. Apricot fruit can keep its quality for 15–30 days after harvest if the fruit is stored at low temperature under cold storage (CS), e.g., [6,7,8,9], and this quality can be kept for up to a further 5 days at 25 °C under shelf-life (SL) conditions [10]. However, longer storage at low temperatures can cause symptoms of chilling injury and/or fruit decay incidence in the apricot fruit e.g., [5,7,11].
Jasmonic acid (JA) and salicylic acid (SA) have crucial roles in the growth of plants and in the regulation to various plant stresses e.g., [12,13], as well as in plant defense mechanisms, e.g., [14].
Methyl jasmonate (MeJA), as a natural plant growth regulator, e.g., [12,13,15,16,17], plays essential roles in many physiological mechanisms of plants, and it increases postharvest quality of horticultural crops including tropical fruits, such as avocado, papaya, grapefruit, mango, guava, pomegranates and loquat [16,18,19,20,21,22,23,24], and temperate fruits, such as apple, peach and cherry [25,26,27,28]. These studies demonstrated that MeJA reduced incidences of fruit decay and chilling injury but increased the amount of phenolic and antioxidant contents as well as defense-related enzymes in fruits during CS. One apricot study evaluated the effect of MeJA on the sensory quality and physico-chemical parameters of fruit during storage [8], but treatment effects on chilling injury, fruit decay, antioxidant capacity, phenolic contents and enzyme activities were not reported.
Salicylic acid (SA), together with its derivatives, is often applied to improve the quality of horticultural crops during storage [29,30,31,32,33,34]. Salicylic acid was shown to improve fruit quality and to decrease chilling injury and quality loss in some fruit crops, such as on peach [29,31,35], banana [30], loquat [36] and apricot [8,9,37]. The three apricot studies showed that SA improved some quality features of apricot fruit after harvest [8,9,37], but changes in enzyme activities under CS and the degree of quality loss and the variation of antioxidant properties under SL conditions were not investigated.
Only two studies carried out comparisons between MeJA and SA on evaluating quality features of cherry and apricot during storage [8,26] but such a comparative study on chilling injury, fruit decay, antioxidant capacity, phenolic contents and enzyme activities was not prepared for apricot under CS or SL storage conditions.
Several biological and physiological connections exist among the plant quality measurements, which can be expressed by presenting the correlation between the corresponding measurements such as between phenolic compounds, antioxidant properties and enzyme activity e.g., [38,39,40,41], which help to understand the background of physiological processes in plant organs. However, no attempt has been made to determine the potential inter-correlations between chilling injury, fruit decay, the parameters of antioxidant capacity, phenolic contents and enzyme activities for SA and MeJA in various storage conditions for apricot fruit.
The objective of this work was to evaluate and compare the effect of MeJA and SA on 10 fruit quality parameters of apricot under 1 °C CS (7, 14 and 21 days) and 25 °C SL (4 and 8 days after the 21 day CS) treatments. The 10 measured fruit quality parameters were classified into three groups: quality losses (chilling injury and fruit decay indices), antioxidant properties (total antioxidant capacity, total soluble phenol, total carotenoid and ascorbic acid content) and enzyme activities (phenylalanine ammonia-lyase (PAL), peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT)). The inter-correlations among the 10 fruit quality parameters for treatments of SA and MeJA were also determined in the two storage conditions in order to clarify the physiological changes in apricot fruit during CS and SL storage.
2. Materials and Methods
2.1. Fruit Samples and Treatments
For all experiments, the cultivar ‘Bergarouge’ was used from a commercial apricot orchard (Nord-cot Ltd., Boldogkőváralja, Hungary). This cultivar has been planted in large areas in Hungary in the past few years. The mature fruit is firmer than most other cultivars, but the storage ability of this cultivar is similar to other commonly grown apricot cultivars in Hungary. The fruits were harvested 98 days from the anthesis (on 5 July) for the experiment. The fruits were packed in paper bags and transported to the laboratory on the same day of harvest. Healthy and uniform fruits (40 mm, equally colored, equally matured and equally shaped) were used for the experiment.
The concentrations of 0.2 mmol L−1 MeJA and 2 mmol L−1 SA were used for the experiments together with an untreated control (water treated). Three units of apricot fruits were used as MeJA, SA, and untreated control treatments. Each unit contained 150 fruits (3 treatments × 150 fruits = 450 fruits. Each unit was dipped into solutions of 0.2 mmol L−1 MeJA, 2 mmol L−1 SA, and distilled water (untreated control), respectively, for 15 min. Then, each treatment was divided into 2 further units (2 units × 75 fruits). The first unit, the CS treatment, was incubated at 1 °C and 95% RH; and then fruits were observed on days 7, 14 and 21. The second unit, the SL treatment, was incubated at 1°C and 95% RH for 21 days, then held at 25 °C for 4 and 8 days. All treatments were replicated three times (3 replicates × 450 fruits = 1350 fruits), and 2 repeats of the experiment were used (2 repeats × 1350 fruits).
2.2. Quality Loss Parameters
The two selected quality loss parameters were chilling injury (CI) and fruit decay (FD), which were evaluated for both CS and SL treatments at each assessment date.
2.2.1. Chilling Injury
Symptoms of CI occurred as flesh browning on 10 assessed fruits per treatments per assessment dates. Fruits were cut double parallel to the axial diameter and then the degree of CI symptoms was visually detected on the cut surface of fruits. The degree of flesh browning was classified into five categories as described by Wang et al. [31]: 0, flesh browning is not visible; 1, flesh browning is less than 25%; 2, the flesh browning is between ≥25% and <50%; 3, the flesh browning is between ≥50% and <75%; and 4, the flesh browning is more than ≥75%. Then an index of CI was calculated as CI index = Σ[(CI category) × (fruit number at the CI category)]/(4 × total fruit number).
2.2.2. Fruit Decay
A fruit surface with superficial browning was considered as a fruit decay symptom. The degree of FD symptoms was classified into five categories as described by Wang et al. [31]: 0, superficial browning is not visible; 1, the superficial browning is less than 25%; 2, the superficial browning is between ≥25% and <50%; 3, the superficial browning is between ≥50% and <75%; and 4, the superficial browning is more than ≥75%. Then an index of FD was calculated as FD index = Σ[(FD category) × (fruit number in the FD category)]/(4 × total fruit number).
2.3. Antioxidant Capacity and Related Parameters
Antioxidant capacity and related parameters were assessed at the CS and the SL treatments and at each assessment date. All reagents and materials for the analyses were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.3.1. Fruit Extract
Samples of apricot fruit were placed in the laboratory on the day of harvest, then the fruit were frozen at −18 °C. The extracts were prepared by homogenizing the fresh fruit for 5 min in 95% ethanol (1:3 w/v). Then, the homogenates were blended for 30 min at room temperature, and then centrifuged at 1500× g for 5 min. The supernatants were concentrated in an evaporator. The extract obtained from each fruit was adjusted to the same volume by reconstituting the samples in the water. The adjusted ratio was 0.20 mL extract/gram of fruit.
2.3.2. Total Antioxidant Capacity
The total antioxidant capacity (TAC) was measured spectrophotometrically by applying the ferric reducing antioxidant power (FRAP) method as described by Benzie and Strain [42]. Briefly, the FRAP reagent was prepared early before the measurements by adding 2.5 mL of a 10 mmol L−1 TPTZ (Sigma) solution into 40 mmol L−1 HCl plus 2.5 mL of 20 mmol L−1 FeCl3 and 25 mL of 0.3 mol L−1 acetate buffer, pH 3.6, and then it was warmed up to 37 °C. The 0.04 mL sample supernatants were mixed with 0.2 mL of distilled water and 1.8 mL of FRAP reagent. The measurement used the reduction of the ferric-tripyridyltriazine complex [Fe3+-TPTZ] to the ferrous form [Fe2+-TPTZ] in acidic buffer (pH = 3.6). Fe2+-TPTZ has an intensive color and this was monitored by measuring the absorption change at 593 nm. Then TAC was given as mg equivalents of ascorbic acid (AA) for 1 g fresh weight (FW) (mg AA g−1 FW).
2.3.3. Total Soluble Phenol Content
An Folin-Ciocalteu’s (FC) reagent was applied to measure the amount of total soluble phenols (TSP) in the extracted fruits. The FC assay was used according to the study of Singleton and Rossi [43] in order to determine the TSP content as follows. The FC reagent was pre-diluted 10 times with distilled water, and 1.8 mL of the reagent was mixed with 40 μL of fruit extract solution and incubated for 5 min at 25 °C. Then, 1.2 mL of (7.5% w/v) sodium carbonate was mixed with the solutions. After 1 h at 25 °C, the absorbance of the samples was determined at 760 nm with a Hitachi UV2800 spectrophotometer (Tokyo, Japan). In order to express TSP in gallic acid equivalents (GAE), a calibration curve (R2 = 0.995) was prepared with 20, 40, 60, 80 and 100 mg L−1 solutions of gallic acid. Then the content of TSP was given as mg GAEs for 100 g FW sample (GAE 100 g−1 FW).
2.3.4. Total Carotenoids Content
Total carotenoids content (TCC) was measured according to the study of Akin et al. [44] with a few modifications. In brief, 100 mL of methanol/petroleum ether (1:9, v/v) was homogenized with 5 g of fruit extract. Then this solution was poured into a separating funnel. The petroleum ether layer was filtrated through sodium sulphate and transferred to a flask. Then, TCC was measured with a Hitachi UV2800 spectrophotometer (Tokyo, Japan) at 450 nm. An extinction coefficient of 2500 was used to evaluate carotenoid content, and TCC was given as β-carotene equivalents (mg β-carotene 100 g−1 FW) according to Gross [45].
2.3.5. Ascorbic Acid Content
The ascorbic acid content (AAC) was determined spectrophotometrically using the dinitrophenylhydrazine (DNPH) method as described by Terada et al. [46]. Ten milligrams of each sample (2 mL extract) were added into a 100 mL flask contain 50 mL acetic acid solution, 5 drops of bromine water was added until the solution became colored, and then thiourea solution drops were added to it until a clear solution was obtained. Then 2, 4-dinitrophenyl hydrazine solution was added, and we completed the solution with acetic acid. The AAC was given as mg per 100 g FW.
2.4. Activity of Enzymes
Enzyme activities were measured using Sigma-Aldrich reagents (St. Louis, MO, USA) for all enzyme assessments.
2.4.1. PAL Activity
The activity of PAL was determined as described by Assis et al. [47]. The enzyme extract was prepared as 10 g of flesh from 10 fruits were homogenized in 25 mL of 50 mmol L−1 sodium borate buffer (5 mmol β-mercaptoethanol + 0.5 g polyvinyl pyrrolidone, PVPP; pH 8.8,). Then the enzyme extract (1 mL) together with L-phenylalanine (1 mL; 20 mmol L−1) and sodium borate buffer (2 mL; 50 mmol L−1) was incubated at 37 °C for 1 h. During the incubation, PAL catalyzes the reaction of the deamination of L-phenylalanine to trans-cinnamic acid. Then the reaction was stopped with HCl (1 mL; 1 mol L−1), and the activity of PAL was given by measuring the amounts of trans-cinnamic acid spectrophotometrically at 290 nm. The raw enzyme preparation, together with L-phenylalanine, was not incubated and used as a blank sample. Each analysis was replicated three times and the activity of PAL was given as nmol cinnamic acid h−1 mg−1 protein.
2.4.2. POD Activity
The activity of POD samples was measured with the method described by Chance and Maehly [48]. Briefly, enzyme extract (0.5 mL) was mixed with 2 mL buffer containing 100 mmol L−1 sodium phosphate (pH 6.4) and 8 mmol L−1 guaiacol, and then the solution was placed into the incubator for 5 min at 30 °C. Then 1 mL of H2O2 (24 mmol L−1) was added to the sample and the increasing absorbance was determined at 460 nm five times at 30, 60, 90, 120 and 150 s. Then, activity of POD was given as unit per gram FW per minute (U mg−1 FW min−1).
2.4.3. SOD Activity
Supernatants were prepared for SOD activity measurements as frozen fruit tissues (1 g) were mixed with sodium phosphate buffer (5 mL; 50 mmol L−1, pH 7.8) at 4 °C and then the supernatants was centrifuged (12,000× g for 20 min; 4 °C). The activity of SOD in these supernatants was measured as described by Rao et al. [49]. The 3 mL reaction mixture contained the following ingredients: sodium phosphate (50 mmol L−1, pH 7.8), methionine (14 mmol L−1), EDTA (3 µmol L−1), nitro-blue-tetrazolium (NBT, 1 µmol L−1), riboflavin (60 µmol L−1) and crude enzyme extract (0.1 mL). Then the absorbance was detected at 560 nm for blue formazan. The quantity of enzyme that causes the 50% inhibition of NBT reduction was used as the SOD activity unit. The activity of SOD in the samples was given as U mg−1 protein.
2.4.4. CAT Activity
Catalase activity was measured as described by Abassi et al. [50] using the A and the B buffer solutions. In this process, 1 mL buffer A was added to a 50 μL enzyme extract in a cuvette, and 1 mL buffer B was added to a 50 μL enzyme extract in another cuvette. The change in the optical density in the cuvettes was measured spectrophotometrically at 240 nm for 45 s and 1 min when the extract was placed into the cuvettes. The differences between the values measured at 45 s and 1 min were defined as the activity of CAT. The activity of CAT in the samples was given as U mg-1 protein.
2.5. Statistical Analysis
2.5.1. ANOVA
A completely randomized design (CRD) was used to accomplish the experiments. Experimental data were analyzed by ANOVA using an SPSS program (SPSS Inc., Chicago, IL, USA). The effects of treatment (MeJA, SA, and untreated control), storage condition (CS and SL) and their interactions were evaluated on all parameters of quality losses, antioxidant properties and enzyme activities. Duncan’s multiple range tests used separated means at p < 0.05 level.
2.5.2. Correlation and Regression Analysis among Parameters
In order to quantify the relationship between the fruit quality parameters, Pearson’s correlation coefficients were determined for the relationships of the two quality loss parameters, the four antioxidant properties and the four enzyme activities in all combinations (Tables 2 and 3). Correlation analyses were performed separately for the two chemical (SA and MeJA) treatments. Statistical calculations using Pearson’s correlation analysis were performed by Genstat 5 Release 4.1 (Lawes Agricultural Trust, IACR, Rothamsted, UK). Then, the best correlated variables were plotted and linear regression analyses were used in order to evaluate the strongest relationships among quality loss parameters, antioxidant properties and enzyme activities. The regression slopes were tested by a t-test in order to determine whether they are different between MeJA and SA treatments at α = 0.05.
2.5.3. Principal Component Analysis
We conducted a standardized principal component analysis (PCA) based on the correlation matrix with the CI, FD, TAC, TSPC, TCC, AAC, PAL, POD, SOD, and CAT variables. All variables were standardized with transforming the values to z-scores. Model fit was tested with the root mean square residual (RMSR) [51]. Principal components (PCs) were visualized in biplot diagram. PCA was conducted with R 4.03 [52] with the psych [53], FactoMiner [54] and factoextra [55] packages (R Core Team, Vienna, Austria).
3. Results
3.1. Quality Loss Parameters
3.1.1. Chilling Injury
The degree of CI index was larger with the progression of assessment times in all chemical and storage treatments (Table 1). However, fruit pre-treated with MeJA and SA had lower CI indices at p < 0.05 compared to water-treated fruit for both storage treatments. Fruit treated with MeJA showed significantly lower CI index in the CS treatments at day 21 and in the SL treatments at day 8 compared to corresponding SA treatments.

Table 1.
Indices of CI (chilling injury) and FD (fruit decay) values for the treatments of methyl jasmonate (MeJA, 0.2 mmol L−1) and salicylic acid (SA, 2 mmol L−1) on cultivar ‘Bergarouge’ apricot fruit at three cold storage (CS) dates (days 7, 14 and 21) and at two assessment dates (days 4 and 8) of shelf-life (SL) treatment.
3.1.2. Fruit Decay
Non-treated apricot fruit showed a great increase in FD index in both storage treatments reaching 96 and 100% after 21 and 8 days, respectively (Table 1). MeJA and SA treatments reduced superficial browning symptoms at p < 0.05 compared with control treatments. The FD indices were not significantly different between the treatments of SA and MeJA in either CS or SL treatments.
3.2. Antioxidant Capacity and Related Parameters
3.2.1. Total Antioxidant Capacity
The TAC of fruit increased in the MeJA and SA treatments until day 14 and day 4 in the CS and SL treatments, respectively, and then TAC is reduced (Figure 1A,B), while the TAC of control fruits decreased continuously under both CS and SL conditions (Figure 1A,B). Antioxidant capacity for control fruits was lower at p < 0.05 than the corresponding MeJA- and SA-treated fruits in both storage treatments. However, TAC values showed no significant differences between MeJA and SA treatments in either CS or SL treatments.
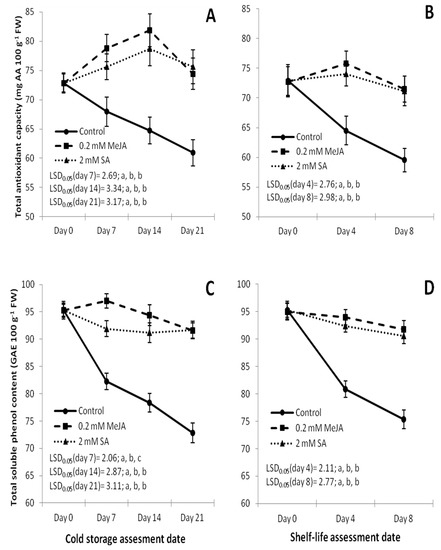

Figure 1.
Effect of methyl jasmonate (MeJA, 0.2 mmol L−1) and salicylic acid (SA, 2 mmol L−1) on TSP (total soluble phenol) content, TAC (total antioxidant capacity) total carotenoids and AAC (ascorbic acid content) in cv. ‘Bergarouge’ in cold storage treatments (A,C,E,G) at 1 °C on the assessment days 7, 14 and 21, and in shelf-life (SL) treatments (B,D,F,H) at 25 °C on the assessment days 4 and 8. Standard deviation (SD) values are given for error bars. LSD0.05 values were used to assess differences among the control, MeJA and SA treatments at p < 0.05. Control means water treated fruits. The first letter given after each LSD0.05 value belongs to control treatments, second letter to MeJA; and third letter to SA treatments. Different letters represent significant differences among the treatments at p < 0.05.
3.2.2. Total Soluble Phenol Content
The content of TSP was similar in the MeJA and SA treatments in both storage treatments, while the TSP content of control fruits decreased continuously in both treatments (Figure 1C,D). The TSP content was higher in the treatments of MeJA and SA at p < 0.05 compared to the control treatment in both storage treatments (Figure 1C,D).
3.2.3. Total Carotenoids Content
The contents of total carotenoids increased in both chemical (MeJA and SA) treatments until day 14 and day 4 in the CS and SL treatments, respectively, and then it started to decrease (Figure 1E,F), except for MeJA treatments for CS where the increase stopped at day 7. Salycilic acid treatments showed the highest total carotenoids content which was significantly higher at days 14 and 21 in the CS and at day 4 in the SL treatments compared to either the control or MeJA treatments (Figure 1E,F). In the control treatments, carotenoids slightly increased at days 7 and 14 in the CS and at day 4 in the SL treatments (Figure 1E,F), but these increases were negligible and non-significant compared to the increase in carotenoids in either MeJA or SA treatments.
3.2.4. Ascorbic Acid Content
AAC increased in the control fruits until days 14 and day 4 in the CS and SL treatments, respectively, then it sharply decreased (Figure 1G,H). AAC in the MeJA- and SA-treated fruits decreased continuously in both storage conditions (Figure 1G,H). However, by the last assessment dates (day 21 and day 8 for the CS and SL treatments, respectively), SA showed the highest ascorbic acid content, followed by the MeJA and then the control treatments, showing significant differences (p < 0.05) from each other (Figure 1G,H).
3.3. Enzyme Activity
3.3.1. PAL Activity
The activity of PAL increased with assessment dates in the MeJA and SA treated fruits while it decreased in the water treated fruits in the two storage treatments (Figure 2A,B). The PAL activity was the highest in MeJA treatments followed by SA and control treatments in both storage treatments. The three treatments were different at p < 0.05 from each other at days 14 and 21 in the CS and at day 8 in the SL treatments.


Figure 2.
Effect of methyl jasmonate (MeJA; 0.2 mmol L−1) and salicylic acid (SA, 2 mmol L−1) on phenylalanine ammonia-lyase (PAL), peroxidase (POD), superoxide dismutase (SOD), and catalase activity (CAT) in cv. ‘Bergarouge’ in cold storage (CS) treatments (A,C,E,G) at 1 °C on the assessment days 7, 14 and 21, and in shelf-life (SL) treatments (B,D,F,H) at 25 °C on the assessment days 4 and 8. Other information is given in Figure 1 (symbols, error bars and LSD0.05 values).
3.3.2. POD Activity
POD activity increased in the MeJA and SA treatments until day 14 and until day 8 in the CS and SL treatments, respectively, then it decreased (Figure 2C,D). POD activity was inconsistent for the control treatment in both storage conditions. The activity of POD in the MeJA and SA treatments was similar, but it was higher at p < 0.05 in all assessment dates compared to the fluctuating levels of POD activity in the control treatments in both the CS and the SL treatments (Figure 2C,D).
3.3.3. SOD Activity
Activity of SOD increased in the chemical (MeJA and SA) treatments until day 14 and day 4 in the CS and SL treatments, respectively, and then enzyme activity started to decrease (Figure 2E,F), except for MeJA in the SL treatment, where the increase was continuous. A reduction in SOD activity was detected in the water-treated fruits in both CS and SL treatments (Figure 2E,F). Activity of SOD in the control treatments was lower at p < 0.05 than the corresponding MeJA and SA treatments in both CS and SL conditions. SOD activity was high in MeJA treatments, which was higher at p < 0.05 than the SA treatments at day 14 in the CS and at day 8 at the SL treatment.
3.3.4. CAT Activity
CAT activity increased in the SA and control treatments at all assessment dates in both storage treatments (Figure 2G,H). CAT activity in the MeJA-treated fruits decreased continuously in both CS and SL treatments, except for MeJA treatments between days 14 and 21 under CS conditions (Figure 2G,H). Activity of CAT in the MeJA treatments was lower at p < 0.05 compared to in the corresponding SA or control treatments in both CS and SL conditions. CAT activity was the highest in SA treatments, which was higher at p < 0.05 compared to the MeJA treatments at days 14 and 21 in the CS and at days 4 and 8 in the SL treatment (Figure 2G,H).
3.4. Relationship between Fruit Quality Parameters
Among the 45 pair-variables, Pearson’s correlation coefficients of 23 and 9 pair-variables correlated significantly (at p < 0.05) in both chemical (MeJA and SA) treatments, respectively (Table 2 and Table 3). Among these significantly pair-variables, six pair-variables were significant in both the MeJA and the SA. Among these six pair-variables, four were correlated positively (FD versus (vs.) CI, PAL vs. CAT, PAL vs. SOD, and total antioxidant content vs. SOD) and two negatively (total antioxidant content vs. FD and ascorbic acid content vs. CI), indicating connections among fruit quality loss, antioxidant parameters and enzyme activities (Table 2 and Table 3).

Table 2.
Pearson correlation coefficients (r) and corresponding significance levels (p) amongst 10 fruit quality parameters in the methyl jasmonate (MeJA) treatments (0.2 mmol L−1) on cv. ‘Bergarouge’ apricot. Data were combined for the assessment dates of days 7, 14 and 21 of cold storage (CS) and for the assessment dates of days 4 and 8 of shelf-life (SL) treatment.

Table 3.
Pearson correlation coefficients (r) and corresponding significance levels (p) amongst 10 fruit quality parameters in salicylic acid (SA) treatments (2 mmol L−1) on cv. ‘Bergarouge’ apricot. Data were combined for the assessment dates of days 7, 14 and 21 of cold storage (CS) and for the assessment dates of days 4 and 8 of shelf-life (SL) treatment.
Thus, the relationships of the six pair-variables were further revealed by regression analysis (Figure 3). The linear regression analysis showed significant relationships for all six of the pair-variables with r = 0.716–0.932, p = 0.03–0.001 and with r = 0.703–0.889, p = 0.03–0.001 for the MeJA and SA treatments, respectively, but the slopes for all six of the pair-variables were not different between the MeJA and the SA treatments (p = 0.654–0.116 according to a t-test).

Figure 3.
Relationships between fruit decay and chilling injury index, PAL (phenylalanine ammonia-lyase) and CAT (catalase activity), PAL and SOD (superoxide dismutase), total antioxidant content and SOD, total antioxidant content and fruit decay, and ascorbic acid content and chilling injury index in treatments of methyl jasmonate (MeJA, 0.2 mmol L−1) and salicylic acid (SA, 2 mmol L−1).
PCA explained the 89.3% of the total variance and the PCs had been justified. The RMSR was 0.05 indicating good fit. PC1 accounted for 55.6% of the variance and correlated with the CI, FD, TAC, TSPC, TCC (Carotenoids), PAL, and SOD. PC2 accounted for 19.7% of the variance and correlated with the POD and AAC. PC3 accounted for 14.0% of the variance and correlated with the CAT. The 95 ellipses of treatments indicated that the control group overlapped with the treatments (Figure 4); however, the difference was significant in the case of PC1 (F = 13, df = 2, p > 0.001) and non-significant for PC2 (F = 1.518, df = 2, p = 0.251). Accordingly, variables of PC1 were more efficient in discriminating the treatments than PC2, where the control and treated samples had similar values.
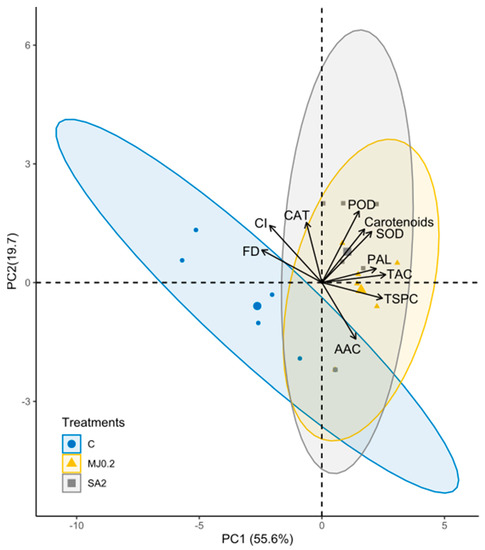
Figure 4.
Biplot of principal component analyses (PCA) conducted on the measured fruit quality parameters: CI = chilling injury index, FD = fruit decay index, CAT = catalyse activity, SOD = superoxide dismutase activity, POD = peroxidase activity, PAL = phenylalanine ammonia-lyase activity, AAC = ascorbic acid content, Carotenoids = total carotenoid content, TSPC = total soluble phenol content and TAC = total antioxidant capacity. (C: control; MJ0.2: MeJA, 0.2 mmol L−1; SA2: SA, 2 mmol L−1); 95% ellipses).
4. Discussion
4.1. Quality Loss Parameters
In agreement with this study, MeJA treatment was demonstrated to decrease CI for fruits such as papaya, peach, pomegranates, and loquat, e.g., [20,22,23,24,27]. MeJA was shown to reduce CI throughout, promoting the expression of heat-shock proteins and regulating arginine metabolism [56]. Furthermore, CI reduction on the MeJA-treated fruits was also likely to be connected to the increase in polyamines (such as putrescine or spermidine) in the cells [15,34]. These polyamines maintain the ratio between the unsaturated and the saturated fatty acid, which have a key role in the membrane integrity and fluidity [57]. Acclimation to low temperatures is highly related to the increase in this fatty acid ratio in the membrane lipids [58], which could result in lower CI symptoms on the MeJA-treated fruits.
In SA treatments, previous studies on pomegranates and peach reported that SA and its derivates decreased CI in postharvest treatments [22,31,58], which is in agreement with our apricot study. The possible mechanism of the effect of SA on CI was studied by Ding et al. [59] and they indicated that MeSA probably had little direct effect on chilling damage, but it could provide indirectly a reduction in CI by inducing some defense-mechanism responses in the cells.
MeJA and MeSA treatments were also compared in the study of Sayyari et al. [22], but in contrast with this study, the authors concluded that differences among MeJA and MeSA treatments were non-significant for CI. The reasons for the different result are likely to be the differences among applied concentrations, fruit crops and SA derivates used in the two studies. Overall, our results indicate that both compounds (MeJA and SA) could be suitable in reducing CI in apricot fruit.
In accordance with our results, some studies also revealed that SA can decrease fruit decay on peach and cherry [26,60]. Salicylic acid is likely to induce the defense resistance system in fruit tissues, which result in lower FD compared to untreated fruits. In the case of MeJA, our results of the FD reduction by MeJA are also in agreement with the results of previous studies on grapefruit, peach, mango and strawberry. The study of Droby et al. [18] on grapefruit showed that fruit treated with different MeJA concentrations (1 to 50 mmol L−1) had less FD compared to untreated fruit. They reported that the MeJA treatments had no direct toxicity effect on the fungus (Penicillium digitatum), which causes FD. They suggested that MeJA decreased FD indirectly by enhancing the natural resistance mechanisms of the fruit to fungus infection [18]. The effect of SA and MeJA in reducing FD was also reported by Yao and Tian [26]. The authors demonstrated that the sweet cherry fruit treated with SA (2 mM) or with MeJA (0.2 mM) had lower fruit damage caused by Monilinia fructicola than in the control treatment. The authors reported that SA and MeJA had direct inhibitory effects on the mycelial growth of M. fructicola. González-Aguilar et al. [19] and Ayala-Zavala et al. [61] showed that MeJA treatments reduced fruit decay in mango and strawberry, respectively, during both CS and SL periods. The possible mechanism of MeJA in reducing fruit decay may involve direct inhibition on the pathogen and/or indirect mechanism through induced resistance. The observed suppression of fruit decay of apricot in this study supported the hypothesis that MeJA can prolong the postharvest health of the fruit.
No previous studies compared the joint effect of MeJA and SA on fruit decay and we showed a similar efficacy for both compounds. We demonstrated significant correlation, significant regression and significant PCA (in PC1) relationships between FD and CI in both MeJA and SA treatments (Table 2 and Table 3, Figure 3 and Figure 4), which were not demonstrated by previous studies. The possible reason for the correlative connection between FD and CI may be that FD or CI can be reduced by similar mechanisms such as the activation of the defense mechanism in the fruit tissues by both MeJA and SA compounds. Overall, MeJA and SA treatments could be useful compounds for a joint reduction in chilling injury and fruit decay without adversely affecting fruit quality.
4.2. Antioxidant Capacity and Related Parameters
Similarly to our apricot study, antioxidant capacity including total phenol contents significantly increased by MeJA in CS and/or SL treatments in strawberry, peach and pomegranate fruits compared to untreated fruits [22,28,61].
In SA treatments, Sayyari et al. [22] demonstrated that SA significantly increased the TAC and TSP content of pomegranate fruits compared to control fruit. Previous studies showed that phenolic compounds, as an antioxidant substance, usually accumulated or at least maintained their levels under cold stress [62], which was the case in the SA and the MeJA treatments but not in the control treatments of this apricot study. The applications of MeJA or SA were able to keep the total phenol contents or were able to increase the antioxidant capacity during the storage periods (Figure 2). Our results indicate that postharvest applications of both MeJA and SA compounds have the potential to improve fruit health by reducing not only CI and FD but increasing the TAC of fruit tissues.
Ascorbic acid content of apricot fruit progressively increases during fruit maturity stages [63]. Our results demonstrate that SA- and/or MeJA-treated fruit had lower ascorbic acid contents at the beginning of storage. Reports on the effect of MeJA on AAC of fruits are not consistent as, for instance, González-Aguilar et al. [21] demonstrated that MeJA treatments did not change AAC, while Jin et al. [28] demonstrated that MeJA significantly influenced vitamin C levels of fruits after CS and SL treatments on peach compared to control treatments. In SA treatments, Sayyari et al. [58] demonstrated that AAC reduced significantly in the control and SA treatments at 0.7 and 1.4 mmol L−1, while the AAC of the fruits was not changed in the dose of 2 mmol L−1. Similarly, ascorbic acid levels remained the same by the end of storage in the SA treatments at 1 mmol L−1 in peaches and 2 mmol L−1 in oranges [31,64].
Previous fruit studies showed significant positive correlations among parameters of antioxidant capacity and phenol contents under storage conditions, e.g., [31,65]. One study [31] provided these correlations in postharvest application of SA and this study investigated these correlations for MeJA and SA jointly. For instance, the reduction in fruit decay was associated with increasing antioxidant capacity for both SA and MeJA treatments, which corroborated well with the significant correlation coefficients, the significant regression, and the significant PCA (in PC1) relationships between FD and TAC (Table 2 and Table 3, Figure 3 and Figure 4). In the case of CI vs. AAC pair-variable, the increasing ascorbic acid level in the fruit tissues was connected to a decreasing level of chilling injury, which was confirmed by the significant correlation coefficients and also by the significant regression relationship between CI and AAC for both MeJA and SA compounds (Table 2 and Table 3, Figure 3).
4.3. Enzyme Activity
The key enzyme of phenyl alanine-lyase is included in the biosynthesis of phenolics such as flavonoids and phenols [66]. Previous studies showed that the high amounts of phenols and anthocyanins in apple and grape fruits resulted in an increased the level of PAL [67,68]. In this study, MeJA-treated fruits showed higher TAC and PAL activity compared to the water-treated fruits (Figure 1 and Figure 2). These results indicate that antioxidant capacity increased by MeJA may also induce PAL activity, thus promoting phenolic metabolism. This result agrees with the studies of Yao and Tian [26]. Meng et al. [27] and Sayyari et al. [58] also demonstrated that MeJA increased the activity of PAL in fruits of sweet cherry, peach and pomegranate. In the case of SA, two studies [26,69] reported that SA had an essential role in systematic acquired resistance (SAR) induction by promoting defense and antioxidant enzymes such as PAL, which was also supported by our results in apricot fruit.
PAL and POD enzymes are involved in the biosynthesis of lignin; as a consequence, the activities of these two enzymes affect the fruit lignin content. Cao et al. [23] demonstrated that MeJA treatment promoted activities of PAL and POD as well as the lignin content of loquat fruit, which might also account for a lower decay index, which was also demonstrated in this apricot study (Table 1, Figure 2). Qin et al. [69] demonstrated that SA also increased the activities of POD and PAL, in agreement with this study (Figure 2). Yang et al. [35] showed that SA treatments prevent fruit from softening by increasing the activities of PAL and POD and the lignin content in fruit resulting in a higher firmness and a lower weight loss.
In addition, induction of systemic resistance to biotic or abiotic stresses can lead to direct activation of defense-related proteins. POD has an essential role in the structure components of cell walls and lignin formation. An increase in POD activity was reported for SA-treated (4 mM) mandarin fruit which was related to low FD [70]. Previous studies, e.g., [26,71,72], and this study also confirmed that either MeJA or SA treatment increased POD activity and decreased fruit decay (Figure 2; Table 1).
The SOD enzyme has several roles in plant cells, such as detoxifying ROS and lowering chilling injury [73,74]. SOD activity was high in chilling tolerant mandarin fruit during CS at 2.5 °C for 8 weeks [73]. Previous studies e.g., [23,24,75] showed that MeJA significantly increased the activity of SOD in loquat, peach and strawberry fruit in the storehouse compared to the control treatments, while Huang et al. [64] showed a similar effect of SA treatment for orange fruit. Our apricot study was in agreement with the above previous studies on SA or MeJA results on SOD activity. However, here we provided for the first time a comparison between MeJA and SA treatments on SOD activity which showed that SOD activity was generally higher in the MeJA treatments than in the SA treatments.
CAT is a key enzyme in the catalyzation of H2O2 into H2O and O2, and therefore it has a function to remove active oxygen species (AOS) from the cell during stress [64]. In agreement with our results, MeJA was reported to maintain high CAT activity in strawberry plants [75] and in loquat fruit during the CS period [23] compared to non-treated fruit. However, SA treatment’s effects on CAT activity were not uniform in previous studies. Huang et al. [64] showed that SA pretreatment reduced CAT activities compared to the non-treated fruit. However, similarly to our apricot study, Mo et al. [76] demonstrated that SA treatments increased CAT activity in apple fruit compared to the non-treated control. This might be associated with the result of the study of Tian et al. [77] that SA can increase the CAT gene activity, i.e., the transcription and translation. In addition, not only CAT, but also ascorbate peroxidase is a key enzyme of fruits in the removal of H2O2. Although this peroxidase was not investigated in this study, future data on ascorbate peroxidase activity also contribute to the better understanding of the mechanism of the antioxidant capacity and of the defense systems involved.
No previous fruit studies investigated correlations among enzyme activity parameters for MeJA and SA jointly under postharvest treatments. However, this study confirmed that an increase in CAT was associated with increasing PAL activity for both SA and MeJA treatments, which corroborated well with the significant positive correlation coefficients and regression relationships between CAT and PAL (Table 2 and Table 3, Figure 3). A similar relationship was detected for the pair-variable of SOD vs. PAL, where the increasing superoxide dismutase level in the fruit tissues was connected to an increasing level of phenylalanine ammonia-lyase, which was confirmed by significant correlation coefficients and also by significant regression as well as by significant PCA (in PC1) relationship between SOD and PAL for both MeJA and SA compounds (Table 2 and Table 3, Figure 3 and Figure 4).
Overall, our results on the activities of enzymes may imply that the four enzymes can promote various functions in the defense system and these may be jointly induced by MeJA and/or SA in apricot fruit. PAL, POD, SOD and CAT enzymes are substantially induced by the given MeJA and SA concentrations, and as a result, the storability of apricot fruit is increased.
5. Conclusions
This was the first study to investigate the effect of MeJA and SA jointly on quality losses, antioxidant properties and enzyme activities of apricot fruit in CS and SL conditions.
Our results show that treatments of both SA and MeJA decreased the CI and FD of apricot fruit in CS and SL periods. Both elicitors ensured high total antioxidant capacity, total polyphenolic and carotenoids contents, and enhanced the enzyme activity of PAL, POD, SOD and CAT in CS and SL conditions; as a result, the storability of apricot fruit is increased. Among the 45 pair-variables, Pearson’s correlation coefficients of 23 and 9 pair-variables correlated significantly in the treatments of MeJA and SA, respectively. Among these pair-variables, six pair-variables (FD vs. CI, PAL vs. CAT, PAL vs. SOD, TAC vs. SOD, TAC vs. FD, and AAC vs. CI) were significant in both the MeJA and the SA, indicating connections among fruit quality loss, antioxidant parameters and enzyme activities. The relationship between the six pair-variables was further confirmed by linear regression analysis with r = 0.716–0.932, p = 0.03–0.001 and with r = 0.703–0.889, p = 0.03–0.001 for the MeJA and SA treatments. PCA explained 89.3% of the total variance and PC1 accounted for 55.6% of the variance and correlated with the CI, FD, TAC, TSPC, TCC, PAL, and SOD variables. Variables of PC1 were efficient in discriminating the treatments. The treatments of MeJA and SA are practically useful and inexpensive techniques to maintain several quality attributes of apricot fruit during both CS and SL conditions.
Author Contributions
Conceptualization, A.E., I.J.H., A.A., Z.S. and J.N.; methodology, A.E.; A.H. and A.A.; software, S.S.; validation, A.E., I.J.H., A.H. and S.S.; formal analysis, I.J.H. and A.E.; investigation, A.E.; resources, I.J.H.; data curation, A.E.; writing—original draft preparation, A.E. and I.J.H.; writing—review and editing, S.S., J.N., Z.S. and B.M.; visualization, A.E. and I.J.H.; supervision, I.J.H.; project administration, B.M.; funding acquisition, I.J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hungarian Scientific Research Funds (K 131478), by a János Bolyai Research Fellowship awarded to I. J. Holb, by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP-4.2.4.A/2-11/1-2012-0001 ‘National Excellence Program’ under project number A2-SZJ-TOK-13-0061, and by The research was financed by the Thematic Excellence Programme of the Ministry for Innovation and Technology in Hungary, within the framework of the Space Sciences thematic programme of the University of Debrecen, and by the Thematic Excellence Programme of the Ministry for Innovation and Technology in Hungary (ED_18-1-2019-0028), within the framework of the climate change thematic programme of the University of Debrecen.
Acknowledgments
We also thank to Gyümölcsért Ltd.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ercisli, S.; Akbulut, M.; Ozdemir, O.; Sengul, M.; Orhan, E. Phenolic and antioxidant diversity among persimmon (Diospyrus kaki L.) genotypes in Turkey. Int. J. Food Sci. Nutr. 2008, 59, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Ercisli, S.; Tosun, M.; Karlidag, H.; Dzubur, A.; Hadziabulic, S.; Aliman, Y. Color and antioxidant characteristics of some fresh fig (Ficus carica L.) genotypes from Northeastern Turkey. Plant Foods Hum. Nutr. 2012, 67, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Crisosto, C.H.; Mitchell, F.G.; Johnson, R.S. Factors in fresh market stone fruit quality. Postharv. News Inform. 1995, 6, 17N–21N. [Google Scholar]
- Hacıseferoğulları, H.; Gezer, I.; Özcan, M.M.; Asma, B.M. Post-harvest chemical and physical–mechanical properties of some apricot varieties cultivated in Turkey. J. Food Eng. 2007, 79, 364–373. [Google Scholar] [CrossRef]
- Muzzaffar, S.; Bhat, M.M.; Wani, T.A.; Wani, I.A.; Masoodi, F.A. Postharvest Biology and Technology of Apricot. In Postharvest Biology and Technology of Temperate Fruits; Mir, S., Shah, M., Mir, M., Eds.; Springer: Berlin, Germany, 2018. [Google Scholar]
- Infante, R.; Meneses, C.; Defilippi, B.G. Effect of harvest maturity stage on the sensory quality of ‘Palsteyn’ apricot (Prunus armeniaca L.) after cold storage. J. Hortic. Sci. Biotechnol. 2008, 83, 828–832. [Google Scholar] [CrossRef]
- Stanley, J.; Marshall, R.; Ogwaro, J.; Feng, R.; Wohlers, M.; Woolf, A. Postharvest storage temperatures impact significantly on apricot fruit quality. Acta Hortic. 2010, 880, 525–532. [Google Scholar] [CrossRef]
- Ezzat, A.; Ammar, A.; Szabó, Z.; Holb, I. Salicylic acid treatment saves quality and enhances antioxidant properties of apricot fruit. Hortic. Sci. 2017, 44, 73–81. [Google Scholar]
- Ezzat, A.; Ammar, A.; Szabó, Z.; Nyéki, J.; Holb, I.J. Postharvest treatments with methyl jasmonate and salicylic acid for maintaining physico-chemical characteristics and sensory quality properties of apricot fruit during cold storage and shelf-life. Pol. J. Food Nutr. Sci. 2017, 67, 159–166. [Google Scholar] [CrossRef]
- Egea, M.I.; Matrinez-Madrid, P.; Sanchez-Bel, M.A.; Romojaro, F. The influence of electron-beam ionization on ethylene metabolism and quality parameter in apricot (Prunus armeniaca L., cv ‘Builda’). Swiss Soc. Food Sci. Technol. 2007, 40, 1027–1035. [Google Scholar]
- Crisosto, C.H.; Mitchell, F.G.; Zhiguo, J. Susceptibility to chilling injury of peach, nectarine, and plum cultivars grown in California. HortScience 1999, 34, 1116–1118. [Google Scholar] [CrossRef]
- Turner, J.G.; Ellis, C.; Devoto, A. The jasmonate signal pathway. Plant Cell Suppl. 2002, 14, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Delker, C.; Stenzel, I.; Hause, B.; Miersch, O.; Feussner, I.; Wasternack, C. Jasmonate biosynthesis in Arabidopsis thaliana—Enzymes, products, regulation. Plant Biol. 2006, 8, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Wang, S.Y.; Buta, J.G. Methyl jasmonate reduces chilling injury in Cucurbita pepo through its regulation of abscisic acid and polyamine levels. Environ. Exp. Bot. 1994, 34, 427–432. [Google Scholar] [CrossRef]
- Meir, S.; Philosoph-Hadas, S.; Lurie, S.; Droby, S.; Akerman, M.; Zauberman, G.; Shapiro, B.; Cohen, E.; Fuchs, Y. Reduction of chilling injury in stored avocado, grapefruit, and bell pepper by methyl jasmonate. Can. J. Bot. 1996, 74, 870–874. [Google Scholar] [CrossRef]
- Jong-Joo, C.; Do, C.Y. Methyl jasmonate as a vital substance in plants. Trends Gen. 2003, 19, 409–413. [Google Scholar]
- Droby, S.; Porat, R.; Cohen, L.; Weiss, B.; Shapira, B.; Philosoph-Hadas, S.; Meir, S. Suppressing green mold decay in grape fruit with postharvest jasmonates application. J. Am. Soc. Hortic. Sci. 1999, 124, 184–188. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Buta, J.G.; Wang, C.Y. Methyl jasmonate reduces chilling injury symptoms and enhances colour development of ‘Kent’ mangoes. J. Sci. Food Agric. 2001, 81, 1244–1249. [Google Scholar] [CrossRef]
- González Aguilar, G.A.; Buta, J.G.; Wang, C.Y. Methyl jasmonate and modified atmosphere packaging (MAP) reduce decay and maintain post harvest quality of papaya ‘Sunrise’. Postharv. Biol. Technol. 2003, 28, 361–370. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Tizuado-Hernándoz, M.E.; Zavaleeta-Gatica, R.; Martínez-Téllez, M.A. Methyl jasmonate treatments reduce chilling injury and activate the defense response of guava fruits. Biochem. Biophysiol. Res. Commun. 2004, 313, 694–701. [Google Scholar] [CrossRef]
- Sayyari, M.; Babalar, M.; Kalantari, S.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, M. Vapour treatments with methyl salicylate or methyl jasmonate alleviated chilling injury and enhanced antioxidant potential during postharvest storage of pomegranates. Food Chem. 2011, 124, 964–970. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Yang, Z.; Wang, K.; Rui, H. Effect of methyl jasmonate on quality and antioxidant activity of postharvest loquat fruit. J. Sci. Food Agric. 2009, 89, 2064–2070. [Google Scholar] [CrossRef]
- Jin, P.; Duan, Y.; Wang, L.; Wang, J.; Zheng, Y. Reducing chilling injury of loquat fruit by combined treatment with hot air and methyl jasmonate. Food Bioprocess Technol. 2014, 7, 2259–2266. [Google Scholar] [CrossRef]
- Fan, X.; Mattheis, J.P.; Fellman, J.K. Responses of apples to postharvest jasmonate treatments. J. Am. Soc. Hortic. Sci. 1998, 123, 421–425. [Google Scholar] [CrossRef]
- Yao, H.J.; Tian, S.P. Effects of pre- and postharvest application of SA or MeJA on inducing disease resistance of sweet cherry fruit in storage. Postharv. Biol. Technol. 2005, 35, 253–262. [Google Scholar] [CrossRef]
- Meng, X.; Han, J.; Wang, Q.; Tian, S.P. Changes in physiology and quality of peach fruits treated by methyl jasmonate under low temperature stress. Food Chem. 2009, 114, 1028–1035. [Google Scholar] [CrossRef]
- Jin, P.; Wang, K.; Shang, H.; Tong, J.; Zheng, Y. Low temperature conditioning combined with methyl jasmonate treatment reduces chilling injury of peach fruit. J. Sci. Food Agric. 2009, 89, 1690–1696. [Google Scholar] [CrossRef]
- Li, L.P.; Han, T. The effects of salicylic acid in the storage of peach. Food Sci. 1999, 7, 61–63. [Google Scholar]
- Srivastava, M.K.; Dwivedi, U.N. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 2000, 158, 87–96. [Google Scholar] [CrossRef]
- Wang, L.; Chena, S.; Kong, W.; Li, S.; Archbold, D. Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peaches during cold storage. Postharv. Biol. Technol. 2006, 41, 244–251. [Google Scholar] [CrossRef]
- Shafiee, M.; Taghavi, T.S.; Babalar, M. Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid, and calcium dipping) improved postharvest fruit quality of strawberry. Sci. Hortic. 2010, 124, 40–45. [Google Scholar] [CrossRef]
- Valero, D.; Diaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillen, F.; Martinez-Romero, D.; Serrano, M. Postharvest treatments with salicylic acid, acetylsalicylic acid or oxalic acid delayed ripening and enhanced bioactive compounds and antioxidant capacity in sweet cherry. J. Agric. Food Chem. 2011, 59, 5483–5489. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Z.; Su, Y.; Liu, D.; Ye, X. Effect of salicylic acid treatment on postharvest quality, antioxidant activities, and free polyamines of asparagus. J. Food Sci. 2011, 76, S126–S132. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Cao, S.; Zheng, Y.; Jiang, Y. Combined salicyclic acid and ultrasound treatments for reducing the chilling injury on peach fruit. J. Agric. Food Chem. 2012, 60, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.X.; Li, L.P.; Chen, K.S. Acetyl salicylic acid alleviates chilling injury of postharvest loquat (Eriobotrya japonica Lindl.) fruit. Eur. Food Res. Technol. 2005, 223, 533–539. [Google Scholar] [CrossRef]
- Satraj, A.; Masud, T.; Abassi, K.S.; Mahmood, T.; Ali, A. Effect of different concentrations of salicylic acid on keeping quality of apricot cv. ‘Habi’ at ambient storage. J. Biol. Food Sci. Res. 2013, 2, 66–78. [Google Scholar]
- Maisuthisakul, P.; Pasuk, S.; Ritthiruangdejc, P. Relationship between antioxidant properties and chemical composition of some Thai plants. J. Food Compos. Anal. 2008, 21, 229–240. [Google Scholar] [CrossRef]
- Du, G.; Li, M.; Ma, F.; Lian, D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Yusoff, N.A.; Eldeen, I.M.; Seow, E.M.; Sajak, A.A.B.; Supriatno Ooi, K.L. Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.). J. Food Compos. Anal. 2011, 24, 1–10. [Google Scholar] [CrossRef]
- Ulewicz-Magulska, B.; Wesolowski, M. Total phenolic contents and antioxidant potential of herbs used for medical and culinary purposes. Plant Foods Hum. Nutr. 2019, 74, 61–67. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of ’antioxidant power’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid ‘reagents’. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Akin, E.B.; Karabulut, I.; Topcu, A. Some compositional properties of main Malatya apricot (Prunus armeniaca, L.) varieties. Food Chem. 2008, 107, 939–948. [Google Scholar] [CrossRef]
- Gross, J. Carotenoids: Pigments in Fruits; Academic: London, UK, 1987. [Google Scholar]
- Terada, M.; Watanabe, Y.; Kunitoma, M.; Hayashi, E. Differential rapid analysis of ascorbic acid and ascorbic acid 2-sulfate by dinitrophenilhydrazine method. Ann. Clinic. Biochem. 1978, 84, 604–608. [Google Scholar] [CrossRef]
- Assis, J.S.; Maldonado, R.; Mnoz, T.; Escribano, M.I.; Merodio, C. Effect of high carbon dioxid concentration on PAL activity and phenol content in ripening cherimoya fruit. Postharv. Biol. Technol. 2001, 23, 33–39. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Method Enzymol. 1995, 2, 764–817. [Google Scholar]
- Rao, M.V.; Paliyath, G.; Ormord, D.P. Ultraviolet B and ozon induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef]
- Abassi, N.A.; Kushad, M.M.; Endress, S. Active oxygen-scavenging enzymes activities in developing apple flowers and fruits. Sci. Hortic. 1998, 74, 183–194. [Google Scholar] [CrossRef]
- Basto, M.; Pereira, J.M. An SPSS R-menu for ordinal factor analysis. J. Stat. Softw. 2012, 46, 1–29. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 10 May 2020).
- Revelle, W. Psych: Procedures for Personality and Psychological Research; Northwestern University: Evanston, IL, USA, 2015; Available online: http://CRAN.R-project.org/package=psych (accessed on 12 June 2015).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 30294. Available online: https://www.jstatsoft.org/article/view/v025i01 (accessed on 25 October 2008).
- Kassambara, A.; Mundt, A. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.6. 2019. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 1 June 2019).
- Zhang, X.; Sheng, J.; Li, F.; Meng, D.; Shen, L. Methyl jasmonate alters arginine catabolism and improves postharvest chilling tolerance in cherry tomato fruit. Postharv. Biol. Technol. 2012, 64, 160–167. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Rahemi, M.; Castillo, S.; Martínez-Romero, D.; Serrano, M.; Valero, D. Pre-storage application of polyamines by pressure or immersion improves shelf-life of pomegranate stored at chilling temperature by increasing endogenous polyamine levels. Postharv. Biol. Technol. 2007, 44, 26–33. [Google Scholar] [CrossRef]
- Sayyari, M.; Babalar, M.; Kalantari, S.; Serrano, M.; Valero, D. Effect of salicylic acid treatment on reducing chilling injury in stored pomegranates. Postharv. Biol. Technol. 2009, 53, 152–154. [Google Scholar] [CrossRef]
- Ding, C.K.; Wang, C.Y.; Gross, K.C.; Smith, D.L. Jasmonate and salicylate induce the expression of pathogenesis related-proteingenes and increase resistance to chilling injury in tomato fruit. Planta 2002, 214, 895–900. [Google Scholar] [CrossRef]
- Chan, Z.; Tian, S. Induction of H2O2-metabolizing enzymes and total protein synthesis by antagonistic yeast and salicylic acid in harvested sweet cherry fruit. Postharv. Biol. Technol. 2006, 39, 314–320. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G.A. Methyl jasmonate in conjunction with ethanol treat- ment increases antioxidant capacity, volatile compounds and post-harvest life of strawberry fruit. Eur. Food Res. Technol. 2005, 221, 731–738. [Google Scholar] [CrossRef]
- Kondo, S.; Kittikorn, M.; Kanlayanarat, S. Preharvest antioxidant activities of tropical fruit and the effect of low temperature storage on antioxidants and jasmonates. Postharv. Biol. Technol. 2005, 36, 309–318. [Google Scholar] [CrossRef]
- Hegedűs, A.; Pfeiffer, N.; Abrankó, L.; Blázovics, A.; Pedryc, A.; Stefanovits-Bányai, E. Accumulation of antioxidants in apricot fruit through ripening: Characterization of a genotype with enhanced functional properties. Biol. Res. 2011, 44, 339–344. [Google Scholar] [CrossRef]
- Huang, R.-H.; Liu, J.-H.; Lu, Y.-M.; Xia, R.-X. Effect of salicylic acid on the antioxidant system in the pulp of ‘Cara cara’ navel orange (Citrus sinensis (L.) Osbeck) at different storage temperatures. Postharv. Biol. Technol. 2008, 47, 168–175. [Google Scholar] [CrossRef]
- Ezzat, A.; El-Sherif, A.R.; Doaa Elgear, D.; Szabó, S.Z.; Holb, I.J. A comparison of fruit and leaf parameters of apple in three orchard training systems. Zemdirb. Agric. 2020, 107, 373–382. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.G.; Yuan, Y.B.; Lieu, C.L.; Xin, S.H. Relationships among phenylalanine ammonia-lyase activity, simple phenol concentrations and anthocyanin accumulation in apple. Sci. Hortic. 1995, 61, 215–226. [Google Scholar] [CrossRef]
- Hiratsuka, S.; Onodera, H.; Kawai, Y.; Kubo, T.; Itoh, H.; Wada, R. Enzyme activity changes during anthocyanin synthesis in ’Olympia’ grape berries. Sci. Hortic. 2001, 90, 255–264. [Google Scholar] [CrossRef]
- Qin, G.Z.; Tian, S.P.; Xu, Y.; Wan, Y.K. Enhancement of biocontrol efficacy of antagonistic yeasts by salicylic acid in sweet cherry fruit. Physiol. Mol. Plant. Pathol. 2003, 62, 147–154. [Google Scholar] [CrossRef]
- Haider, S.T.A.; Ahmad, S.; Khan, S.; Anjum, M.A.; Nasir, M.; Naz, S. Effects of salicylic acid on postharvest fruit quality of “Kinnow” mandarin under cold storage. Sci. Hortic. 2020, 259, 108843. [Google Scholar] [CrossRef]
- Wills, R.; McGlasson, B.; Graham, D.; Joyce, D. Postharvest, An Introduction to the Physiology and Handling of Fruit and Vegetables and Ornamentals, 4th ed.; University of New South Wales Press Ltd.: Sydney, Australia, 1998; pp. 125–156. [Google Scholar]
- Peng, L.; Jiang, Y. Exogenous salicylic acid inhibits browning of fresh-cut Chinese water chestnut. Food Chem. 2006, 94, 535–540. [Google Scholar] [CrossRef]
- Sala, J.M. Involvement of oxidative stress in chilling injury in cold-stored mandarin fruits. Postharv. Biol. Technol. 1998, 13, 255–261. [Google Scholar] [CrossRef]
- Wang, S.Y.; Bowman, L.; Ding, M. Methyl jasmonate enhances antioxidant activity and flavonoid content in blackberries (Rubus sp.) and promotes antiproliferation of human cancer cells. Food Chem. 2008, 107, 1261–2619. [Google Scholar] [CrossRef]
- Wang, S.Y. Methyl jasmonate reduces water stress in strawberry. J. Plant Growth Regul. 1999, 18, 127–134. [Google Scholar] [CrossRef]
- Mo, Y.; Gong, D.; Liang, L.; Han, R.; Xie, J.; Li, W. Enhanced preservation effects of sugar apple fruits by salicylic acid treatment during post-harvest storage. J. Sci. Food Agric. 2008, 88, 2693–2699. [Google Scholar] [CrossRef]
- Tian, S.; Qin, G.; Li, B.; Wang, Q.; Meng, X. Effects of salicylic acid on disease resistance and postharvest decay control of fruits. Stewart Postharv. Rev. 2007, 6, 1–7. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).