A Review on Sampling Techniques and Analytical Methods for Microbiota of Cultural Properties and Historical Architecture
Abstract
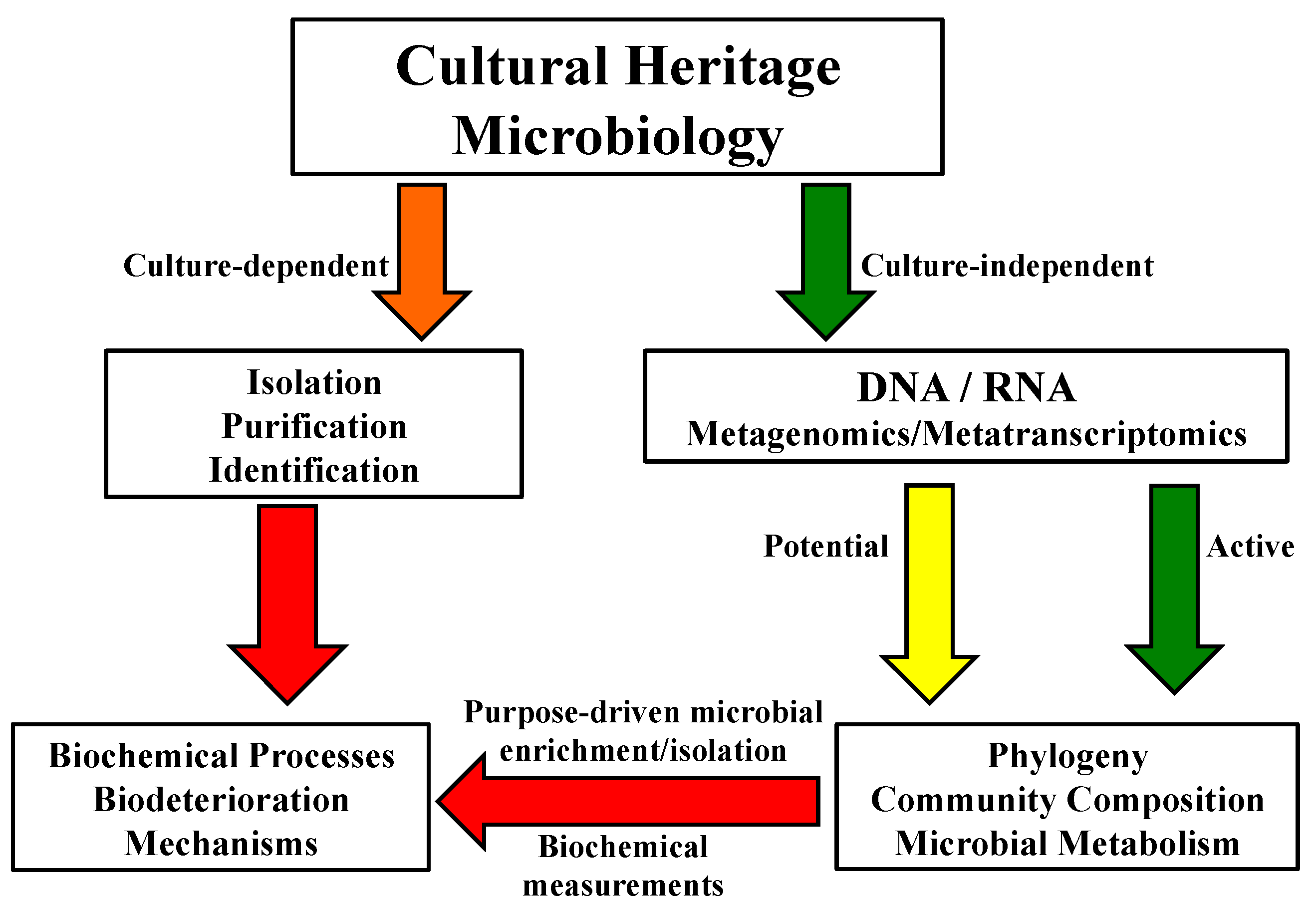
1. Introduction
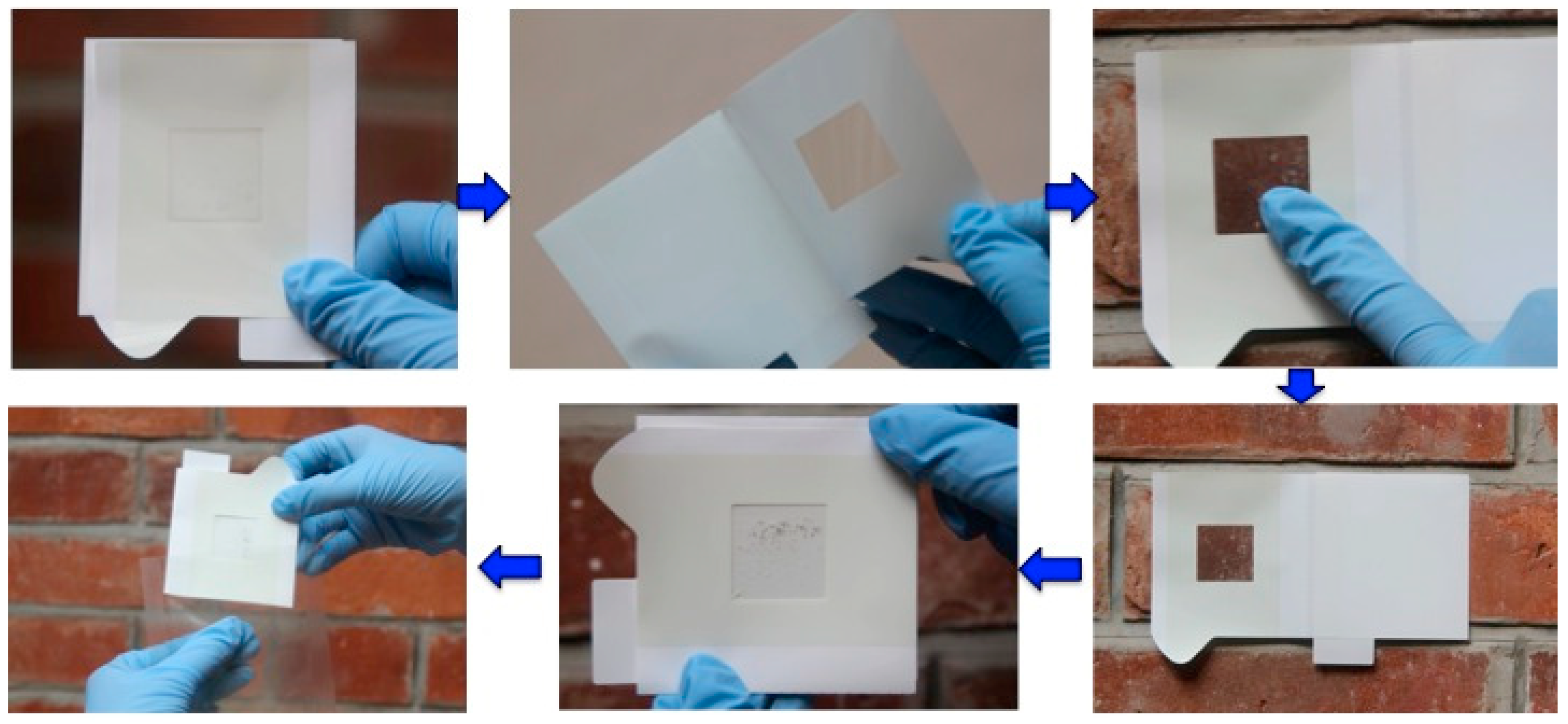
2. Sampling Techniques
3. Microbiome Analysis by Culture-Independent Methods
3.1. High Through-Put Sequencing
3.2. Genomic DNA vs. RNA
4. Biochemical Functional Approaches
5. Summary and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ertan, T.; Egercioglu, Y. The Impact of UNESCO World Heritage List on Historic Urban City Centers and Its Place in Urban Regeneration: The Case of Melaka, Malaysia and Tire, Turkey. Procedia Soc. Behav. Sci. 2016, 216, 591–602. [Google Scholar] [CrossRef][Green Version]
- Silverman, H.; Ruggles, D.F. Cultural Heritage and Human Rights; Springer: New York, NY, USA, 2007; p. ix. 205p. [Google Scholar]
- Hilbert, G.S. Protection against Theft and Willful Damage + Museum Security. Museum 1985, 37, 115–118. [Google Scholar] [CrossRef]
- Martin-Sanchez, P.M.; Novakova, A.; Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. Use of Biocides for the Control of Fungal Outbreaks in Subterranean Environments: The Case of the Lascaux Cave in France. Environ. Sci. Technol. 2012, 46, 3762–3770. [Google Scholar] [CrossRef]
- Tanselle, G.T. Literature and Artifacts; Bibliographical Society of the University of Virginia: Charlottesville, VA, USA, 1998; p. xvii. 356p. [Google Scholar]
- Mckenna, G.A. Security and the Spirit of Cooperation—Protection Systems in Museums Large and Small. Mus. News 1979, 58, 7–9. [Google Scholar]
- Skaggs, D. An Ounce of Prevention—A Handbook on Disaster Contingency Planning for Archives, Libraries and Record Centers—Barton, Jp, Wellheiser, Jg. Am. Arch. 1986, 49, 471–472. [Google Scholar]
- Castanier, S.; Le Metayer-Levrel, G.; Perthuisot, J.P. Ca-carbonates precipitation and limestone genesis—The microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Magaudda, G. The recovery of biodeteriorated books and archive documents through gamma radiation: Some considerations on the results achieved. J. Cult. Herit. 2004, 5, 113–118. [Google Scholar] [CrossRef]
- Fernandes, P. Applied microbiology and biotechnology in the conservation of stone cultural heritage materials. Appl. Microbiol. Biotechnol. 2006, 73, 291–296. [Google Scholar] [CrossRef]
- Rives, V.; Garcia-Talegon, J. Decay and conservation of building stones on cultural heritage monuments. Mater. Sci. Forum 2006, 514–516, 1689–1694. [Google Scholar] [CrossRef]
- Mesquita, N.; Portugal, A.; Videira, S.; Rodriguez-Echeverria, S.; Bandeira, A.M.L.; Santos, M.J.A.; Freitas, H. Fungal diversity in ancient documents. A case study on the Archive of the University of Coimbra. Int. Biodeterior. Biodegrad. 2009, 63, 626–629. [Google Scholar] [CrossRef]
- Pinar, G.; Jimenez-Lopez, C.; Sterflinger, K.; Ettenauer, J.; Jroundi, F.; Fernandez-Vivas, A.; Gonzalez-Munoz, M.T. Bacterial Community Dynamics During the Application of a Myxococcus xanthus-Inoculated Culture Medium Used for Consolidation of Ornamental Limestone. Microb. Ecol. 2010, 60, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-D.; Kigawa, R.; Sato, Y.; Katayama, Y. Addressing the microbiological problems of cultural property and archive documents after earthquake and tsunami. Int. Biodeterior. Biodegrad. 2013, 85, 345–346. [Google Scholar] [CrossRef]
- Pinar, G.; Garcia-Valles, M.; Gimeno-Torrente, D.; Fernandez-Turiel, J.L.; Ettenauer, J.; Sterflinger, K. Microscopic, chemical, and molecular-biological investigation of the decayed medieval stained window glasses of two Catalonian churches. Int. Biodeterior. Biodegrad. 2013, 84, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Pinar, G.; Piombino-Mascali, D.; Maixner, F.; Zink, A.; Sterflinger, K. Microbial survey of the mummies from the Capuchin Catacombs of Palermo, Italy: Biodeterioration risk and contamination of the indoor air. FEMS Microbiol. Ecol. 2013, 86, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Nitterus, M. Ethanol as fungal sanitizer in paper conservation. Restaurator 2000, 21, 101–115. [Google Scholar] [CrossRef]
- Nitterus, M. Fungi in archives and libraries—A literary survey. Restaurator 2000, 21, 25–40. [Google Scholar] [CrossRef]
- Bohm, C.B.; Zehnder, K.; Domeisen, H.; Arnold, A. Climate control for the passive conservation of the Romanesque painted wooden ceiling in the church of Zillis (Switzerland). Stud. Conserv. 2001, 46, 251–268. [Google Scholar] [CrossRef]
- Morgan, G.C. The Materials of Cultural Heritage in their Environment. Mediev. Archaeol. 2007, 51, 400–401. [Google Scholar]
- Bastian, F.; Alabouvette, C. Lights and shadows on the conservation of a rock art cave: The case of Lascaux Cave. Int. J. Speleol. 2009, 38, 55–60. [Google Scholar] [CrossRef]
- Cappitelli, F.; Fermo, P.; Vecchi, R.; Piazzalunga, A.; Valli, G.; Zanardini, E.; Sorlini, C. Chemical-physical and Microbiological Measurements for Indoor Air Quality Assessment at the Ca’ Granda Historical Archive, Milan (Italy). Water Air Soil Pollut. 2009, 201, 109–120. [Google Scholar] [CrossRef]
- Gheyle, W.; Dossche, R.; Bourgeois, J.; Stichelbaut, B.; Van Eetvelde, V. Integrating Archaeology and Landscape Analysis for the Cultural Heritage Management of a World War I Militarised Landscape: The German Field Defences in Antwerp. Landsc. Res. 2014, 39, 502–522. [Google Scholar] [CrossRef]
- Adamo, A.M.; Giovannotti, M.; Magaudda, G.; Zappala, M.P.; Rocchetti, F.; Rossi, G. Effect of gamma rays on pure cellulose paper as a model for the study of a treatment of “biological recovery” of biodeteriorated books. Restaurator 1998, 19, 41–59. [Google Scholar] [CrossRef]
- Baer, N.S. Microclimate for cultural heritage. Stud. Conserv. 2000, 45, 143. [Google Scholar] [CrossRef]
- Allsopp, D.; Seal, K.J.; Gaylarde, C.C. Introduction to Biodeterioration, 2nd ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2004; p. xii. 237p. [Google Scholar]
- Albertano, P.; Bruno, L.; Bellezza, S. New strategies for the monitoring and control of cyanobacterial films on valuable lithic faces. Plant. Biosyst. 2005, 139, 311–322. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; Miller, A.Z.; Martin-Sanchez, P.M.; Hernandez-Marine, M. Uncovering the origin of the black stains in Lascaux Cave in France. Environ. Microbiol. 2012, 14, 3220–3231. [Google Scholar] [CrossRef]
- Scheerer, S.; Ortega-Morales, O.; Gaylarde, C. Microbial Deterioration of Stone Monuments-An Updated Overview. Adv. Appl. Microbiol. 2009, 66, 97–139. [Google Scholar] [CrossRef]
- Sterflinger, K.; Pinar, G. Microbial deterioration of cultural heritage and works of art—Tilting at windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef]
- Zhang, X.W.; Ge, Q.Y.; Zhu, Z.B.; Deng, Y.M.; Gu, J.-D. Microbiological community of the Royal Palace in Angkor Thom and Beng Mealea of Cambodia by Illumina sequencing based on 16S rRNA gene. Int. Biodeterior. Biodegrad. 2018, 134, 127–135. [Google Scholar] [CrossRef]
- Meng, H.; Katayama, Y.; Gu, J.-D. More wide occurrence and dominance of ammonia-oxidizing archaea than bacteria at three Angkor sandstone temples of Bayon, Phnom Krom and Wat Athvea in Cambodia. Int. Biodeterior. Biodegrad. 2017, 117, 78–88. [Google Scholar] [CrossRef]
- Kusumi, A.; Li, X.S.; Osuga, Y.; Kawashima, A.; Gu, J.-D.; Nasu, M.; Katayama, Y. Bacterial Communities in Pigmented Biofilms Formed on the Sandstone Bas-Relief Walls of the Bayon Temple, Angkor Thom, Cambodia. Microbes Environ. 2013, 28, 422–431. [Google Scholar] [CrossRef]
- Ettenauer, J.; Sterflinger, K.; Pinar, G. Cultivation and molecular monitoring of halophilic microorganisms inhabiting an extreme environment presented by a salt-attacked monument. Int. J. Astrobiol. 2010, 9, 59–72. [Google Scholar] [CrossRef]
- Pinar, G.; Ripka, K.; Weber, J.; Sterflinger, K. The micro-biota of a sub-surface monument the medieval chapel of St. Virgil (Vienna, Austria). Int. Biodeterior. Biodegrad. 2009, 63, 851–859. [Google Scholar] [CrossRef]
- Saarela, M.; Alakomi, H.L.; Suihko, M.L.; Maunuksela, L.; Raaska, L.; Mattila-Sandholm, T. Heterotrophic microorganisms in air and biofilm samples from Roman catacombs, with special emphasis on actinobacteria and fungi. Int. Biodeterior. Biodegrad. 2004, 54, 27–37. [Google Scholar] [CrossRef]
- Sterflinger, K. Fungi as geologic agents. Geomicrobiol. J. 2000, 17, 97–124. [Google Scholar] [CrossRef]
- Liu, X.B.; Koestler, R.J.; Warscheid, T.; Katayama, Y.; Gu, J.-D. Microbial deterioration and sustainable conservation of stone monuments and buildings. Nat. Sustain. 2020, 1–14. [Google Scholar] [CrossRef]
- Ma, Y.T.; Zhang, H.; Du, Y.; Tian, T.; Xiang, T.; Liu, X.D.; Wu, F.S.; An, L.Z.; Wang, W.F.; Gu, J.-D.; et al. The community distribution of bacteria and fungi on ancient wall paintings of the Mogao Grottoes. Sci. Rep. 2015, 5, 7752. [Google Scholar] [CrossRef]
- Liu, X.B.; Meng, H.; Wang, Y.L.; Katayama, Y.; Gu, J.-D. Water is a critical factor in evaluating and assessing microbial colonization and destruction of Angkor sandstone monuments. Int. Biodeterior. Biodegrad. 2018, 133, 9–16. [Google Scholar] [CrossRef]
- Saijo, Y.; Nakagi, Y.; Ito, T.; Sugioka, Y.; Endo, H.; Yoshida, T. Relation of Dampness to Sick Building Syndrome in Japanese Public Apartment Houses. Epidemiology 2009, 20, S150. [Google Scholar] [CrossRef][Green Version]
- Polo, A.; Cappitelli, F.; Brusetti, L.; Principi, P.; Villa, F.; Giacomucci, L.; Ranalli, G.; Sorlini, C. Feasibility of Removing Surface Deposits on Stone Using Biological and Chemical Remediation Methods. Microb. Ecol. 2010, 60, 1–14. [Google Scholar] [CrossRef]
- Dicus, D.H. One response to a collection-wide mold outbreak: How bad can it be, how good can it get? J. Am. Inst. Conserv. 2000, 39, 85–105. [Google Scholar] [CrossRef]
- Diaz-Herraiz, M.; Jurado, V.; Cuezva, S.; Laiz, L.; Pallecchi, P.; Tiano, P.; Sanchez-Moral, S.; Saiz-Jimenez, C. The Actinobacterial Colonization of Etruscan Paintings. Sci. Rep. 2013, 3, 1440. [Google Scholar] [CrossRef]
- American Institute for Conservation of Historic and Artistic Works. Strategic Plan; American Institute for Conservation of Historic and Artistic Works: Washington, DC, USA, 1990; 54p. [Google Scholar]
- Arai, H. Foxing caused by Fungi: Twenty-five years of study. Int. Biodeterior. Biodegrad. 2000, 46, 181–188. [Google Scholar] [CrossRef]
- Choi, S. Foxing on paper: A literature review. J. Am. Inst. Conserv. 2007, 46, 137–152. [Google Scholar] [CrossRef]
- Gates, G.A. Discovering the material secrets of art: Tools of cultural heritage science. Am. Ceram. Soc. Bull. 2014, 93, 20–27. [Google Scholar]
- Gu, J.-D. Microbiological deterioration and degradation of synthetic polymeric materials: Recent research advances. Int. Biodeterior. Biodegrad. 2003, 52, 69–91. [Google Scholar] [CrossRef]
- Duan, Y.L. The microbial community characteristics of ancient painted sculptures in Maijishan Grottoes, China (vol 12, 2017). PLoS ONE 2018, 13, e0179718. [Google Scholar] [CrossRef]
- Xu, H.B.; Tsukuda, M.; Takahara, Y.; Sato, T.; Gu, J.-D.; Katayama, Y. Lithoautotrophical oxidation of elemental sulfur by fungi including Fusarium solani isolated from sandstone Angkor temples. Int. Biodeterior. Biodegrad. 2018, 126, 95–102. [Google Scholar] [CrossRef]
- Cole, A.C.; Semmens, M.J.; LaPara, T.M. Stratification of activity and bacterial community structure in biofilms grown on membranes transferring oxygen. Appl. Environ. Microb. 2004, 70, 1982–1989. [Google Scholar] [CrossRef]
- Fdz-Polanco, F.; Mendez, E.; Uruena, M.A.; Villaverde, S.; Garcia, P.A. Spatial distribution of heterotrophs and nitrifiers in a submerged biofilter for nitrification. Water Res. 2000, 34, 4081–4089. [Google Scholar] [CrossRef]
- Stewart, P.S.; Camper, A.K.; Handran, S.D.; Huang, C.T.; Warnecke, M. Spatial distribution and coexistence of Klebsiella pneumoniae and Pseudomonas aeruginosa in biofilms. Microb. Ecol. 1997, 33, 2–10. [Google Scholar] [CrossRef]
- McNamara, C.J.; Perry, T.D.; Bearce, K.A.; Hernandez-Duque, G.; Mitchell, R. Epilithic and endolithic bacterial communities in limestone from a Maya archaeological site. Microb. Ecol. 2006, 51, 51–64. [Google Scholar] [CrossRef]
- Lan, W.S.; Li, H.; Wang, W.D.; Katayama, Y.; Gu, J.-D. Microbial Community Analysis of Fresh and Old Microbial Biofilms on Bayon Temple Sandstone of Angkor Thom, Cambodia. Microb. Ecol. 2010, 60, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Ishidoshiro, A.; Yoshida, Y.; Saika, T.; Senda, S.; Nasu, M. Development of an adhesive sheet for direct counting of bacteria on solid surfaces. J. Microbiol. Methods 2003, 53, 405–410. [Google Scholar] [CrossRef]
- Hu, H.L.; van den Brink, J.; Gruben, B.S.; Wosten, H.A.B.; Gu, J.-D.; de Vries, R.P. Improved enzyme production by co-cultivation of Aspergillus niger and Aspergillus oryzae and with other fungi. Int. Biodeterior. Biodegrad. 2011, 65, 248–252. [Google Scholar] [CrossRef]
- Hu, H.L.; Ding, S.P.; Katayama, Y.; Kusumi, A.; Li, S.X.; de Vries, R.P.; Wang, J.; Yu, X.Z.; Gu, J.-D. Occurrence of Aspergillus allahabadii on sandstone at Bayon temple, Angkor Thom, Cambodia. Int. Biodeterior. Biodegrad. 2013, 76, 112–117. [Google Scholar] [CrossRef]
- Kusumi, A.; Li, X.S.; Katayama, Y. Mycobacteria isolated from Angkor monument sandstones grow chemolithoautotrophically by oxidizing elemental sulfur. Front. Microbiol 2011, 2, 104. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Arai, H.; Shimoda, I.; Kuraishi, H.; Katayama, Y. Enumeration of Sulfur-Oxidizing Microorganisms on Deteriorating Stone of the Angkor Monuments, Cambodia. Microbes Environ. 2008, 23, 293–298. [Google Scholar] [CrossRef]
- Li, X.S.; Sato, T.; Ooiwa, Y.; Kusumi, A.; Gu, J.-D.; Katayama, Y. Oxidation of Elemental Sulfur by Fusarium solani Strain THIF01 Harboring Endobacterium Bradyrhizobium sp. Microb. Ecol. 2010, 60, 96–104. [Google Scholar] [CrossRef]
- Mitchell, R.; Gu, J.-D. Changes in the biofilm microflora of limestone caused by atmospheric pollutants. Int. Biodeterior. Biodegrad. 2000, 46, 299–303. [Google Scholar] [CrossRef]
- Hanada, S.; Hiraishi, A.; Shimada, K.; Matsuura, K. Chloroflexus Aggregans Sp-Nov, a Filamentous Phototrophic Bacterium Which Forms Dense Cell Aggregates by Active Gliding Movement. Int. J. Syst. Bacteriol. 1995, 45, 676–681. [Google Scholar] [CrossRef]
- Hanada, S.; Takaichi, S.; Matsuura, K.; Nakamura, K. Roseiflexus castenholzii gen. nov., sp nov., a thermophilic, filamentous, photosynthetic bacterium that lacks chlorosomes. Int. J. Syst. Evolut. Microbiol. 2002, 52, 187–193. [Google Scholar] [CrossRef]
- Pierson, B.K.; Giovannoni, S.J.; Stahl, D.A.; Castenholz, R.W. Heliothrix-Oregonensis, Gen-Nov, Sp-Nov, a Phototrophic Filamentous Gliding Bacterium Containing Bacteriochlorophyll-A. Arch. Microbiol. 1985, 142, 164–167. [Google Scholar] [CrossRef]
- Nubel, U.; Bateson, M.M.; Madigan, M.T.; Kuhl, M.; Ward, D.M. Diversity and distribution in hypersaline microbial mats of bacteria related to Chloroflexus spp. Appl. Environ. Microb. 2001, 67, 4365–4371. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Yamada, T.; Hanada, S.; Ohashi, A.; Harada, H.; Kamagata, Y. Anaerolinea thermophila gen. nov., sp nov and Caldilinea aerophila gen. nov., sp nov., novel filamentous thermophiles that represent a previously uncultured lineage of the domain Bacteria at the subphylum level. Int. J. Syst. Evolut. Microbiol. 2003, 53, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Keppen, O.I.; Tourova, T.P.; Kuznetsov, B.B.; Ivanovsky, R.N.; Gorlenko, V.M. Proposal of Oscillochloridaceae fam. nov, on the basis of a phylogenetic analysis of the filamentous anoxygenic phototrophic bacteria, and emended description of Oscillochloris and Oscillochloris trichoides in comparison with further new isolates. Int. J. Syst. Evolut. Microbiol. 2000, 50, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Laiz, L.; Pinar, G.; Lubitz, W.; Saiz-Jimenez, C. Monitoring the colonization of monuments by bacteria: Cultivation versus molecular methods. Environ. Microbiol. 2003, 5, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Kaarakainen, P.; Rintala, H.; Vepsalainen, A.; Hyvarinen, A.; Nevalainen, A.; Meklin, T. Microbial content of house dust samples determined with qPCR. Sci. Total Environ. 2009, 407, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Amann, R.I.; Ludwig, W.; Schleifer, K.H. Phylogenetic Identification and in-Situ Detection of Individual Microbial-Cells without Cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.R.; Brayton, P.R.; Grimes, D.J.; Roszak, D.B.; Huq, S.A.; Palmer, L.M. Viable but Non-Culturable Vibrio-Cholerae and Related Pathogens in the Environment—Implications for Release of Genetically Engineered Microorganisms. Nat. Biotechnol. 1985, 3, 817–820. [Google Scholar] [CrossRef]
- Rahman, I.; Shahamat, M.; Chowdhury, M.A.R.; Colwell, R.R. Potential virulence of viable but nonculturable Shigella dysenteriae type 1. Appl. Environ. Microb. 1996, 62, 115–120. [Google Scholar] [CrossRef]
- Rosado, T.; Mirao, J.; Candeias, A.; Caldeira, A.T. Microbial communities analysis assessed by pyrosequencing-a new approach applied to conservation state studies of mural paintings. Anal. Bioanal. Chem. 2014, 406, 887–895. [Google Scholar] [CrossRef]
- Riley, M.; Anilionis, A. Evolution of Bacterial Genome. Annu. Rev. Microbiol. 1978, 32, 519–560. [Google Scholar] [CrossRef]
- Otlewska, A.; Adamiak, J.; Gutarowska, B. Application of molecular techniques for the assessment of microorganism diversity on cultural heritage objects. Acta Biochim. Pol. 2014, 61, 217–225. [Google Scholar] [CrossRef]
- Adamiak, J.; Otlewska, A.; Tafer, H.; Lopandic, K.; Gutarowska, B.; Sterflinger, K.; Pinar, G. First evaluation of the microbiome of built cultural heritage by using the Ion Torrent next generation sequencing platform. Int. Biodeterior. Biodegrad. 2018, 131, 11–18. [Google Scholar] [CrossRef]
- Gutarowska, B.; Celikkol-Aydin, S.; Bonifay, V.; Otlewska, A.; Aydin, E.; Oldham, A.L.; Brauer, J.I.; Duncan, K.E.; Adamiak, J.; Sunner, J.A.; et al. Metabolomic and high-throughput sequencing analysis-modern approach for the assessment of biodeterioration of materials from historic buildings. Front. Microbiol. 2015, 6, 979. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Municchia, A.C.; Futagami, Y.; Kashiwadani, H.; Moon, K.H.; Caneva, G. Biological colonization patterns on the ruins of Angkor temples (Cambodia) in the biodeterioration vs bioprotection debate. Int. Biodeterior. Biodegrad. 2014, 96, 157–165. [Google Scholar] [CrossRef]
- Selbmann, L.; Onofri, S.; Zucconi, L.; Isola, D.; Rottigni, M.; Ghiglione, C.; Piazza, P.; Alvaro, M.C.; Schiaparelli, S. Distributional records of Antarctic fungi based on strains preserved in the Culture Collection of Fungi from Extreme Environments (CCFEE) Mycological Section associated with the Italian National Antarctic Museum (MNA). Mycokeys 2015, 10, 57–71. [Google Scholar] [CrossRef]
- Perito, B.; Cavalieri, D. Innovative metagenomic approaches for detection of microbial communities involved in biodeteriorattion of cultural heritage. IOP Conf. Ser. Mater. Sci. Eng. 2018, 364. [Google Scholar] [CrossRef]
- Ding, X.H.; Lan, W.S.; Wu, J.P.; Hong, Y.G.; Li, Y.L.; Ge, Q.; Urzi, C.; Katayama, Y.; Gu, J.-D. Microbiome and nitrate removal processes by microorganisms on the ancient Preah Vihear temple of Cambodia revealed by metagenomics and N-15 isotope analyses. Appl. Microbiol. Biotechnol. 2020, 1–15. [Google Scholar] [CrossRef]
- Meng, H.; Luo, L.; Chan, H.W.; Katayama, Y.; Gu, J.-D. Higher diversity and abundance of ammonia-oxidizing archaea than bacteria detected at the Bayon Temple of Angkor Thom in Cambodia. Int. Biodeterior. Biodegrad. 2016, 115, 234–243. [Google Scholar] [CrossRef]
- de Felice, B.; Pasquale, V.; Tancredi, N.; Scherillo, S.; Guida, M. Genetic fingerprint of microorganisms associated with the deterioration of an historical tuff monument in Italy. J. Genet. 2010, 89, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Ettenauer, J.; Pinar, G.; Sterflinger, K.; Gonzalez-Munoz, M.T.; Jroundi, F. Molecular monitoring of the microbial dynamics occurring on historical limestone buildings during and after the in situ application of different bio-consolidation treatments. Sci. Total Environ. 2011, 409, 5337–5352. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.G.; Phipps, D.; Ishiguro, K.; Ridgway, H.F. Use of a Fluorescent Redox Probe for Direct Visualization of Actively Respiring Bacteria. Appl. Environ. Microb. 1992, 58, 1801–1808. [Google Scholar] [CrossRef]
- Berthold, F.; Tarkkanen, V. Luminometer development in the last four decades: Recollections of two entrepreneurs. Luminescence 2013, 28, 1–6. [Google Scholar] [CrossRef]
- Rakotonirainy, M.S.; Arnold, S. Development of a new procedure based on the energy charge measurement using ATP bioluminescence assay for the detection of living mould from graphic documents. Luminescence 2008, 23, 182–186. [Google Scholar] [CrossRef]
- Lowenthal, D. The Past is a Foreign Country; Cambridge University Press: Cambridge Cambridgeshire, UK; New York, NY, USA, 1985; p. xxvii. 489p. [Google Scholar]
- Zhang, G.X.; Gong, C.J.; Gu, J.G.; Katayama, Y.; Someya, T.; Gu, J.-D. Biochemical reactions and mechanisms involved in the biodeterioration of stone world cultural heritage under the tropical climate conditions. Int. Biodeterior. Biodegrad. 2019, 143, 104723. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; Laiz, L. Occurrence of halotolerant/halophilic bacterial communities in deteriorated monuments. Int. Biodeterior. Biodegrad. 2000, 46, 319–326. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Rodriguez-Navarro, C.; Pinar, G.; Carrillo-Rosua, F.J.; Rodriguez-Gallego, M.; Gonzalez-Munoz, M.T. Consolidation of degraded ornamental porous limestone stone by calcium carbonate precipitation induced by the microbiota inhabiting the stone. Chemosphere 2007, 68, 1929–1936. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Lan, W.; Gu, J.-D. A Review on Sampling Techniques and Analytical Methods for Microbiota of Cultural Properties and Historical Architecture. Appl. Sci. 2020, 10, 8099. https://doi.org/10.3390/app10228099
Ding X, Lan W, Gu J-D. A Review on Sampling Techniques and Analytical Methods for Microbiota of Cultural Properties and Historical Architecture. Applied Sciences. 2020; 10(22):8099. https://doi.org/10.3390/app10228099
Chicago/Turabian StyleDing, Xinghua, Wensheng Lan, and Ji-Dong Gu. 2020. "A Review on Sampling Techniques and Analytical Methods for Microbiota of Cultural Properties and Historical Architecture" Applied Sciences 10, no. 22: 8099. https://doi.org/10.3390/app10228099
APA StyleDing, X., Lan, W., & Gu, J.-D. (2020). A Review on Sampling Techniques and Analytical Methods for Microbiota of Cultural Properties and Historical Architecture. Applied Sciences, 10(22), 8099. https://doi.org/10.3390/app10228099




