Simulation of Chronic Intoxication in Rats Exposed to Cadmium and Mercury
Abstract
1. Introduction
2. Experimental Section
2.1. Exposure Methods
- Animal survival
- Weight changes
- Food intake
- Water intake
- Number of births (litters), number of born pups, and number of raised pups (live pups assessed on the 21st day after delivery);
- Number of born pups per litter and number of raised pups per litter
- Average daily dose (ADD);
- Total dose received in 52 weeks in mg·kg−1 of live weight of a rat;
- The amount of toxic metal received in relation to the LD50 dose.
2.2. Statistical Analysis
3. Results
3.1. General Experiment
3.2. Reproductive Experiment
3.3. Toxicological Experiment
4. Discussion
4.1. Toxicological Parameters
4.2. Physiological Parameters
4.3. Reproduction Parameters
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Piršelová, B.; Galuščáková, Ľ.; Lengyelová, L. Hormetic response of plants to metals and metalloids. Chem. Listy 2018, 112, 317–323. [Google Scholar]
- Kimáková, T. Toxická ortuť v organizme človeka. Via Pract. 2018, 15, 222–225. [Google Scholar]
- Calabrese, E.J. Cancer biology and hormesis: Human tumor cell lines commonly display hormeti (biphasic) dose responses. Crit. Rev. Toxicol. 2005, 35, 463–592. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Calabrese, E.J. Hormesis A Revolution in Biology, Toxicology and Medicine, 1st ed.; Humana Press: Totowa, NJ, USA; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–213. [Google Scholar]
- Zhou, F.; Yin, G.; Gao, Y.; Ouyang, L.; Liu, S.; Jia, Q.; Yu, H.; Zha, Z.; Wang, K.; Xie, J.; et al. Insights into cognitive deficits caused by low-dose toxic heavy metal mixtures and their remediation through a postnatal enriched environment in rats. J. Hazard. Mater. 2020, 388, 122081. [Google Scholar] [CrossRef]
- Ahmed, N.F.; Sadek, K.M.; Soliman, M.K.; Khalil, R.H.; Khafaga, A.F.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A. Moringa oleifera leaf extract repairs the oxidative misbalance following sub-chronic exposure to sodium fluoride in nile tilapia Oreochromis niloticus. Animals 2020, 10, 626. [Google Scholar] [CrossRef]
- Budnik, L.T.; Baur, X. The assessment of environmental and occupational exposure to hazardous substances by biomonitoring. Dtsch. Arztebl. Int. 2009, 106, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Checkoway, H.; Savitz, D.A.; Heyer, N.J. Assessing the effects of nondifferential misclassification of exposures in occupational studies. Appl. Occup. Environ. Hyg. 1991, 6, 528–533. [Google Scholar] [CrossRef]
- Ništiar, F.; Rácz, O.; Lukačinová, A.; Hubková, B.; Nováková, J.; Lovásová, E.; Sedláková, E. Age dependency on some physiological and biochemical parameters of male Wistar rats in controlled environment. J. Environ. Sci. Health 2012, 47, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Anke, M.; Dorn, W.; Müller, M.; Seifert, M. Recent progress in exploring the essentiality of the ultratrace element cadmium to the nutrition of animals and man. Biomed. Res. Trace Elem. 2005, 16, 198–202. [Google Scholar]
- Lafuente, A.; Marquez, N.; Perez-Lorenzo, M.; Pazo, D.; Esquifino, A. Cadmium Effects on Hypothalamic-Pituitary-Testicular Axis in Male Rats. Exp. Biol. Med. 2001, 226, 605–611. [Google Scholar] [CrossRef]
- Abou-Kassem, D.E.; Abd El-Hack, M.E.; Taha, A.E.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A. Detoxification impacts of ascorbic acid and clay on laying japanese quail fed diets polluted by various levels of cadmium. Animals 2020, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Hijová, E.; Ništiar, F.; Lovásová, E.; Šipolová, A. Ascorbic acid malondialdehyde in rats after exposure to mercury. Trace Elem. Electrolytes 2003, 20, 244–248. [Google Scholar] [CrossRef]
- Kotsonis, F.; Klaasen, C.D. Toxicity and distribution of cadmium administered to rats at sublethal doses. Toxicol. Appl. Pharmacol. 1977, 41, 667–680. [Google Scholar] [CrossRef]
- Donaldson, M.L.; Gubler, C.J. Biochemical effects of mercury poisoning in rats. Am. J. Clin. Nutr. 1978, 31, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Lukačínová, A.; Rácz, O.; Lovászová, E.; Ništiar, F. Effect of lifetime low dose exposure to heavy metals on selected serum proteins of Wistar rats during three subsequent generations. Ecotoxicol. Environ. Saf. 2011, 74, 1747–1755. [Google Scholar] [CrossRef]
- Almášiová, V.; Holovská, K.; Cigánková, V.; Ništiar, F. Effect of lifetime lowdose exposure to cadmium on lipid metabolism of wistar rats. J. Microbiol. Biotechnol. Food Sci. 2012, 2, 293–303. [Google Scholar]
- Baumans, V. Environmental enrichment for laboratory rodents and rabbits: Requirements of rodents, rabbits, and research. ILAR J. 2005, 46, 162–170. [Google Scholar] [CrossRef]
- Ništiar, F.; Beňačka, R.; Lovásová, E.; Rácz, O.; Lukačínová, A. Selected physiological and biochemical indices at lifetime low-dose heavy metal exposure in rats. In Proceedings of the 28th Symposium Industrial Toxicology, Štrba, Slovakia, 1–4 June 2008; Slovak University of Technology: Bratislava, Slovakia, 2008; pp. 162–172. [Google Scholar]
- Bergman, F.; Chaimovity, M.; Gutman, Y.; Zerachia, A. Effect of mercury derivates, implanted into the hypothalamus, on the water intake of albino rats. Br. J. Pharmacol. Chemother. 1968, 32, 483–492. [Google Scholar] [CrossRef]
- Counter, S.A.; Buchanan, L.H. Mercury exposure in children: A review. Toxicol. Appl. Pharmacol. 2004, 198, 209–230. [Google Scholar] [CrossRef]
- Wirth, J.J.; Mijal, R.S. Adverse effects of low level heavy metal exposure on male reproductive function. Syst. Biol. Reprod. Med. 2010, 56, 147–167. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormetic-dose response relationships in immunology: Occurrence, quantitative features of the dose response, mechanisti foundations, and clinica implications. Crit. Rev. Toxicol. 2008, 35, 89–295. [Google Scholar] [CrossRef]
- Shibutani, M.; Mitsumori, K.; Satoh, S.; Hiratsuka, H.; Satoh, M.; Sumiyoshi, M.; Nishijima, M.; Katsuki, Y.; Suzuki, J.; Nakagawa, J.; et al. Relationship between toxicity and cadmium accumulation in rats given low amounts of cadmium chloride or cadmium-polluted rice for 22 months. J. Toxicol. Sci. 2001, 26, 337–358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Toman, R.; Massányi, P.; Lukáč, N.; Mojžišová, M. Effect of cadmium on the growth of some rat organs after long-term oral administration in feed. In Proceedings of the Risk Factors in the Food Chain III, Nitra, Slovakia, 3 December 2003; Massányi, P., Toman, R., Lukáč, N., Eds.; Slovak University of Agriculture in Nitra: Nitra, Slovakia, 2003; Volume 67, pp. 162–164. [Google Scholar]
- Santana, M.G.; Moraes, R.; Bernardi, M.M. Toxicity of cadmium in Japanese quail: Evaluation of body weight, hepatic and renal function, and cellular immune response. Environ. Res. 2005, 99, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Predes, F.S.; Diamante, M.A.S.; Dolder, H. Testis response to low doses of cadmium in Wistar rats. Int. J. Exp. Pathol. 2010, 91, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Toman, R.; Massanyi, P.; Zakrzewski, M.; Stawarz, R.; Hluchy, S.; Kulac, N.; Mindek, S.; Formicki, G. Effect of cadmium on blood parameters of pheasants (Phasianus colchicus) alter long-term administration in drinkng water. J. Physiol. Pharmacol. 2006, 57, 235. [Google Scholar]
- Toxicological Profile for Cadmium. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf (accessed on 1 August 2019).
- Alloway, B.J. Soil processes and the behaviour of metals. In Heavy Metals in Soils, 2nd ed.; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Toxicological Profile for Mercury. Available online: https://www.atsdr.cdc.gov/toxprofiles/mercury_organic_addendum.pdf (accessed on 1 August 2019).
- Thompson, J.; Bannigan, J. Cadmium: Toxic Effects on the Reproductive System and the Embryo. Reprod. Toxicol. 2008, 25, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Cadmium in Drinking-Water. Available online: https://apps.who.int/iris/bitstream/handle/10665/75364/WHO_SDE_WSH_03.04_80_eng.pdf?sequence=1&isAllowed=y (accessed on 1 August 2019).
- Massanyi, P.; Toman, R.; Valent, M.; Čupka, P. Evaluation of selected parameters of a metabolic profile and levels of cadmium in reproductive organs of rabbits after an experimental administration. Acta Physiol. Hung. 1995, 83, 267–273. [Google Scholar]
- Dostál, M.; Soukupová, D. Development toxicity of cadmium in mice. I. Embryotoxic effects. Funct. Dev. Morphol. 1991, 1–2, 3–9. [Google Scholar]
- Lukačínová, A.; Beňačka, R.; Lovásová, E.; Rácz, O.; Ništiar, F. Reproduction parameters in low dose chronic exposure with heavy metals in rats. Pol. J. Environ. Stud. 2008, 17, 911–915. [Google Scholar]
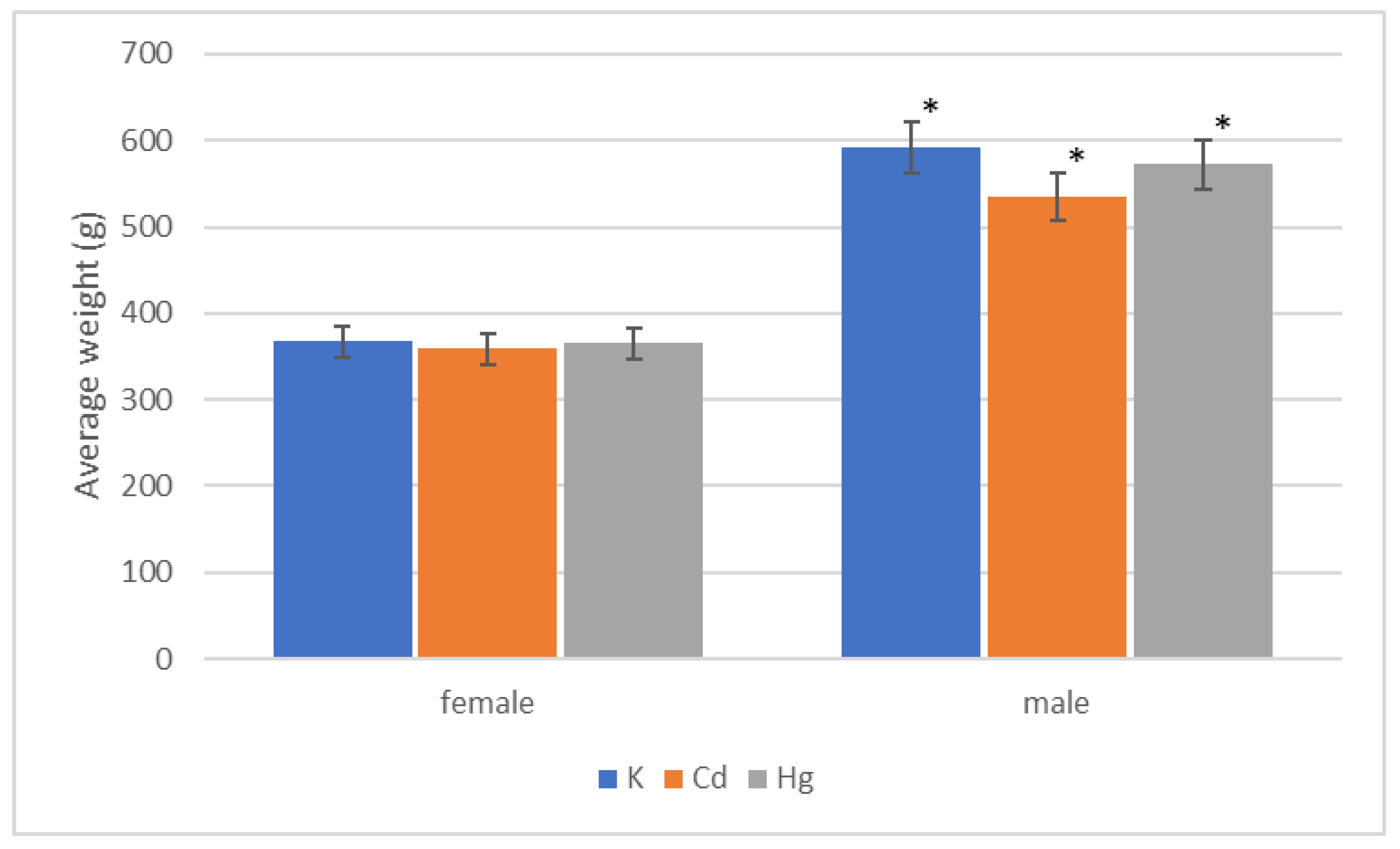
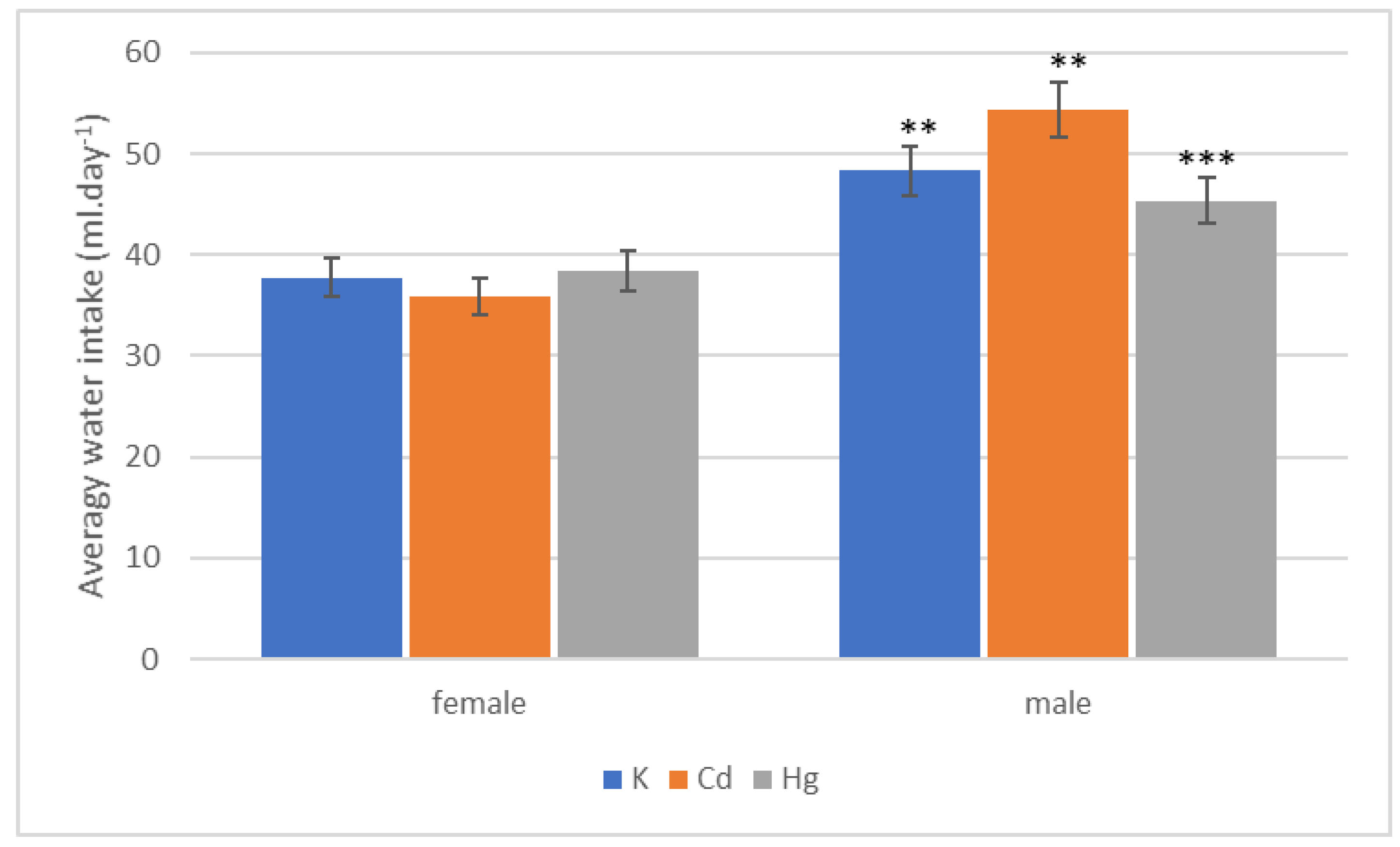
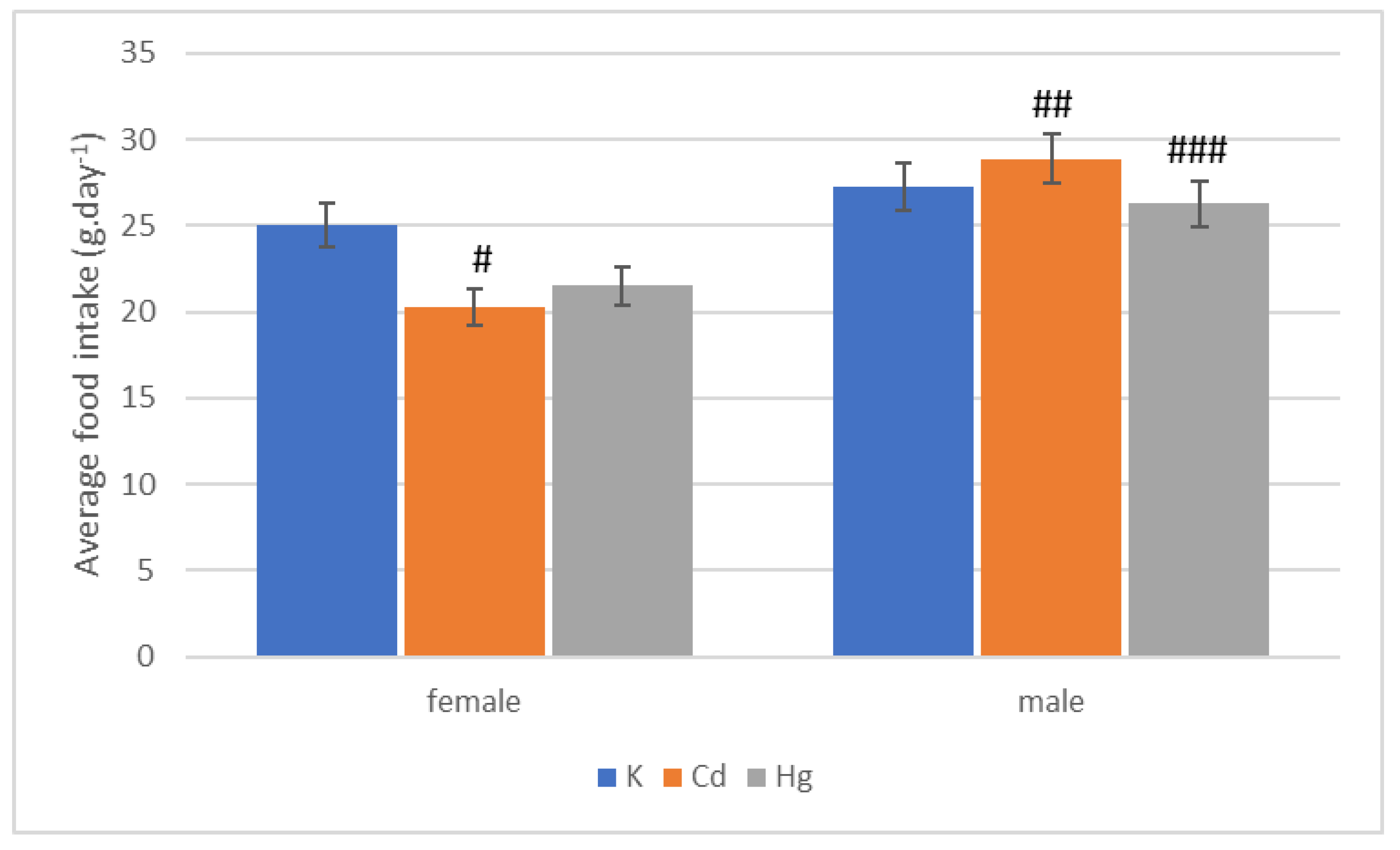


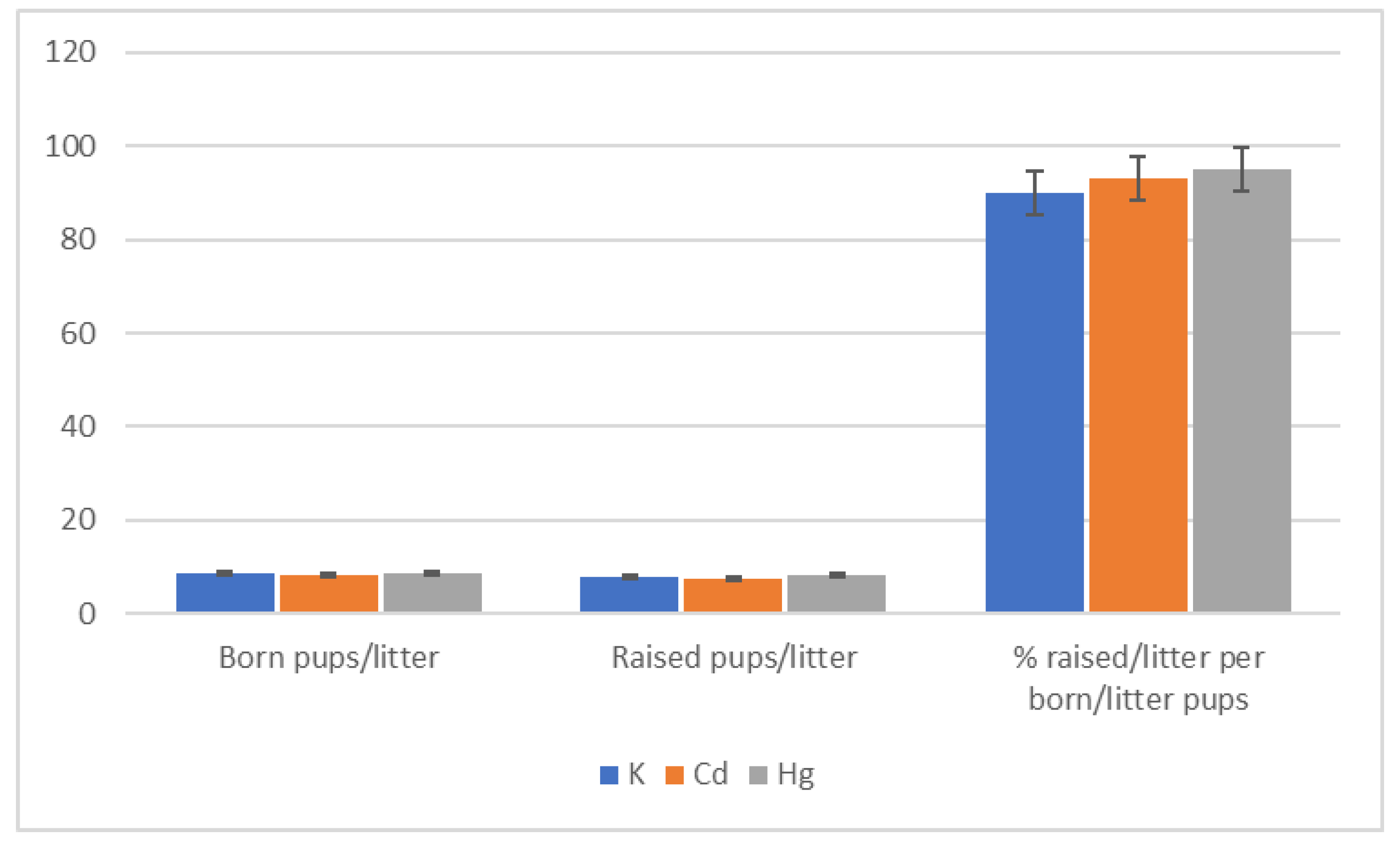
| Group | Start | End of 1st Quarter | End of 2nd Quarter | End of 3rd Quarter | End of 4th Quarter |
|---|---|---|---|---|---|
| K (g) | 70.62 ± 1.47 | 272.50 ± 9.77 | 327.50 ± 6.39 | 333.75 ± 8.85 | 367.14 ± 12.09 |
| Cd (g) | 72.50 ± 1.33 | 258.75 ± 8.11 | 306.25 ± 13.75 | 307.50 ± 15.32 | 358.75 ± 21.50 |
| Hg (g) | 81.25 ± 3.98 | 277.50 ± 6.19 | 331.25 ± 11.56 | 335.00 ± 6.81 | 365.00 ± 14.01 |
| Group | Start | End of 1st Quarter | End of 2nd Quarter | End of 3rd Quarter | End of 4th Quarter |
|---|---|---|---|---|---|
| K (g) | 73.12 ± 1.87 | 401.25 ± 6.92 | 488.57 ± 8.84 | 512.85 ± 17.00 | 592.85 ± 10.84 |
| Cd (g) | 77.5 ± 5.34 | 396.25 ± 9.62 | 492.50 ± 9.77 | 492.550 ± 9.77 | 535.00 ± 14.63 |
| Hg (g) | 86.87 ± 3.12 | 395.00 ± 13.75 | 471.25 ± 13.28 | 491.25 ± 16.84 | 572.50 ± 23.43 |
| Group | 1st Quarter | 2nd Quarter | 3rd Quarter | 4th Quarter | Whole |
|---|---|---|---|---|---|
| K (mL·day−1) | 36.18 ± 1.16 | 38.31 ± 1.08 | 39.40 ± 0.50 | 38.84 ± 0.83 | 37.76 ± 0.76 |
| Cd (mL·day−1) | 34.51 ± 1.48 | 32.79 ± 0.99 | 36.01 ± 1.28 | 41.94 ± 1.45 | 35.86 ± 1.12 |
| Hg (mL·day−1) | 38.54 ± 1.32 | 35.67 ± 1.97 | 38.44 ± 2.78 | 42.32 ± 2.94 | 38.42 ± 1.82 |
| Group | 1st Quarter | 2nd Quarter | 3rd Quarter | 4th Quarter | Whole |
|---|---|---|---|---|---|
| K (mL·day−1) | 44.97 ± 0.58 | 50.85 ± 4.152 | 50.17 ± 5.01 | 48.85 ± 5.19 | 48.35 ± 3.49 |
| Cd (mL·day−1) | 51.98 ± 1.91 | 53.25 ± 0.750 | 54.04 ± 0.69 | 60.04 ± 1.93 | 54.45 ± 0.95 |
| Hg (mL·day−1) | 45.91 ± 1.86 | 41.25 ± 1.025 | 46.80 ± 1.92 | 48.55 ± 3.61 | 45.39 ± 2.22 |
| Group | 1st Quarter | 2nd Quarter | 3rd Quarter | 4th Quarter | Whole |
|---|---|---|---|---|---|
| K (g·day−1) | 27.51 ± 1.32 | 26.49 ± 2.87 | 30.16 ± 4.36 § | 16.82 ± 0.54 | 25.06 ± 2.14 |
| Cd (g·day−1) | 24.45 ± 0.52 | 19.36 ± 0.62 | 20.71 ± 0.56 § | 17.95 ± 0.55 | 20.28 ± 0.52 |
| Hg (g·day−1) | 27.17 ± 0.62 | 19.01 ± 0.36 | 22.75 ± 0.55 | 17.92 ± 0.46 | 21.5 ± 0.39 |
| Group | 1st Quarter | 2nd Quarter | 3rd Quarter | 4th Quarter | Whole |
|---|---|---|---|---|---|
| K (g·day−1) | 30.57 ± 0.25 | 27.17 ± 0.93 | 28.72 ± 0.54 | 22.92 ± 0.62 | 27.22 ± 0.28 |
| Cd (g·day−1) | 32.26 ± 0.22 | 27.81 ± 0.53 | 31.24 ± 0.62 | 24.72 ± 0.54 | 28.86 ± 0.18 |
| Hg (g·day−1) | 31.24 ± 0.96 | 24.11 ± 0.42 | 28.19 ± 0.98 | 22.18 ± 0.82 | 26.25 ± 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimboláková, I.; Kimáková, T.; Pavolová, H.; Bakalár, T.; Kudelas, D.; Seňová, A. Simulation of Chronic Intoxication in Rats Exposed to Cadmium and Mercury. Appl. Sci. 2020, 10, 8066. https://doi.org/10.3390/app10228066
Cimboláková I, Kimáková T, Pavolová H, Bakalár T, Kudelas D, Seňová A. Simulation of Chronic Intoxication in Rats Exposed to Cadmium and Mercury. Applied Sciences. 2020; 10(22):8066. https://doi.org/10.3390/app10228066
Chicago/Turabian StyleCimboláková, Iveta, Tatiana Kimáková, Henrieta Pavolová, Tomáš Bakalár, Dušan Kudelas, and Andrea Seňová. 2020. "Simulation of Chronic Intoxication in Rats Exposed to Cadmium and Mercury" Applied Sciences 10, no. 22: 8066. https://doi.org/10.3390/app10228066
APA StyleCimboláková, I., Kimáková, T., Pavolová, H., Bakalár, T., Kudelas, D., & Seňová, A. (2020). Simulation of Chronic Intoxication in Rats Exposed to Cadmium and Mercury. Applied Sciences, 10(22), 8066. https://doi.org/10.3390/app10228066







