Osmolality of Components and Their Application in the Design of Functional Recovery Drinks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Powder Preparation
2.2.2. Osmolality
2.2.3. The Soluble Solids Content
2.2.4. The pH
2.2.5. The Antioxidant Activity
2.2.6. The Total Content of Polyphenols
2.2.7. Vitamin C
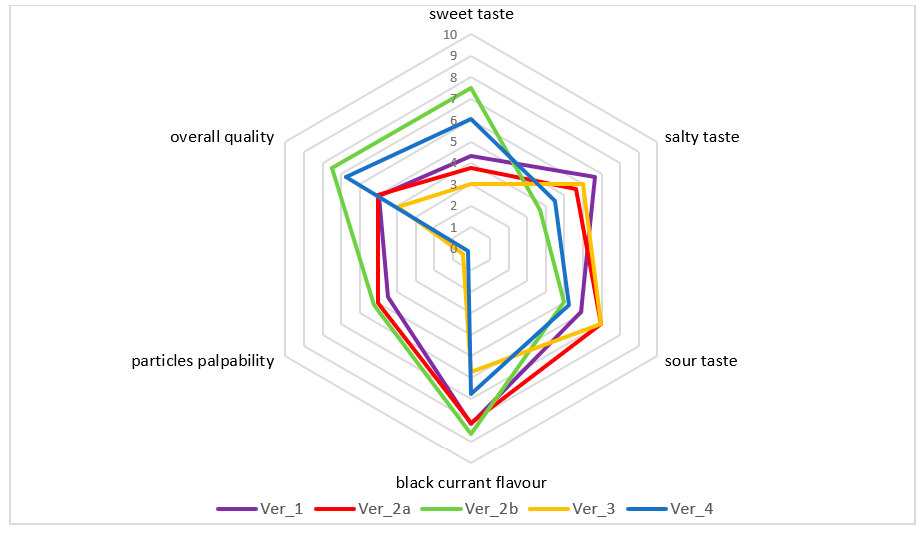
2.2.8. The Sensory Analysis
2.2.9. The Statistical Analysis of the Data Obtained
3. Results and Discussion
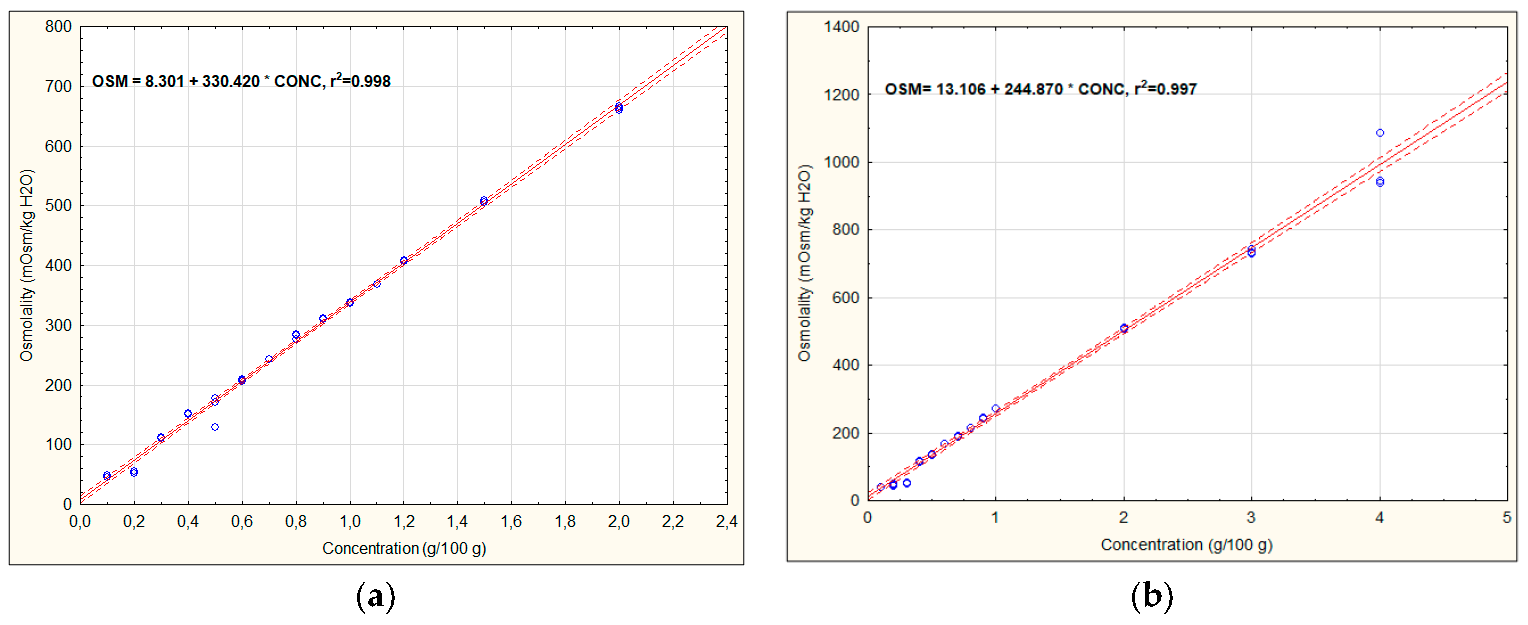
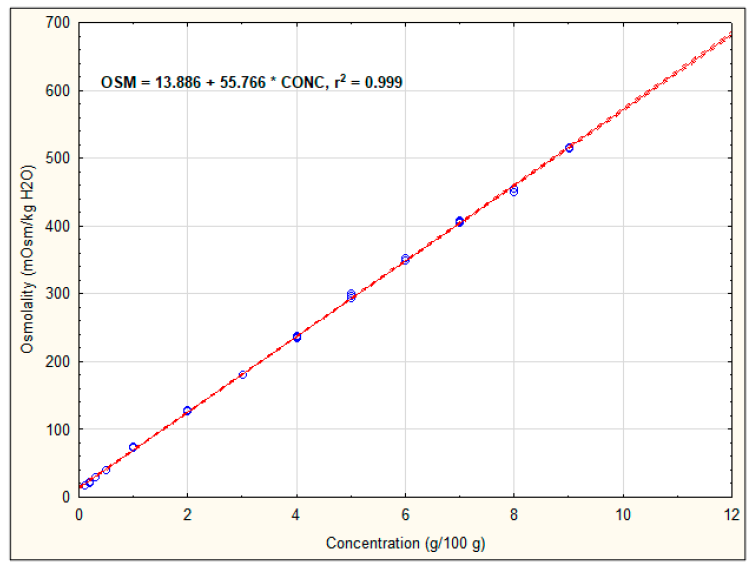
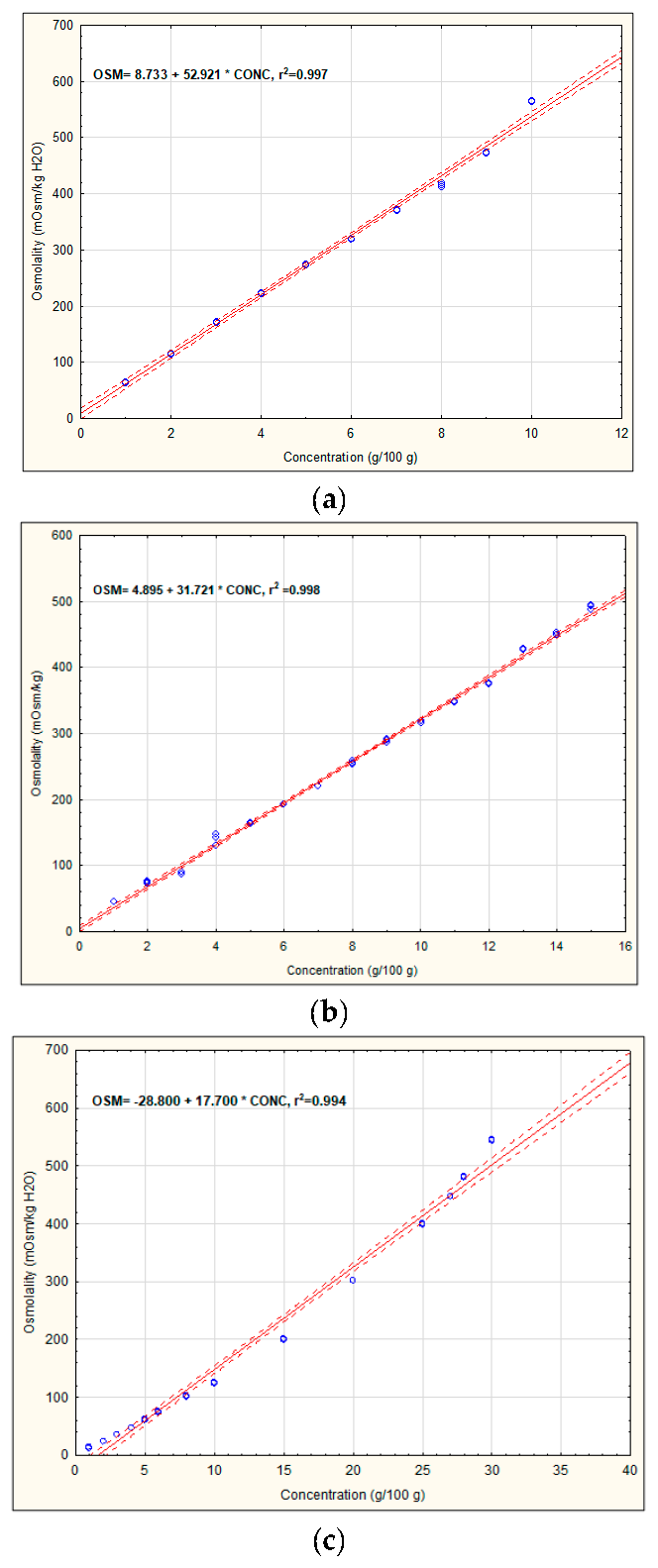
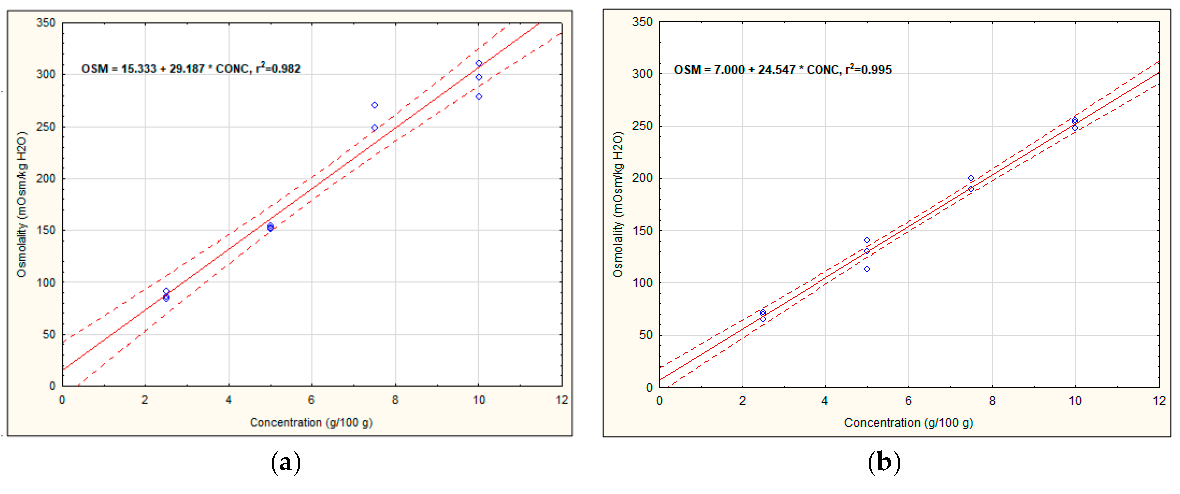
3.1. Osmolality of Recovery Drinks’ Components
3.2. Determining the Composition and Physico-Chemical Properties of Recovery Drinks
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sadowska, A.; Świderski, F.; Rakowska, R.; Waszkiewicz-Robak, B.; Żebrowska-Krasuska, M.; Dybkowska, E. Beverage osmolality as a marker for maintaining appropriate body hydration. Rocz. Panstw. Zakl. Hig. 2017, 68, 167–173. [Google Scholar] [PubMed]
- Faria, D.K.; Mendes, M.E.; Sumita, N.M. The measurement of serum osmolality and its application to clinical practice and laboratory: Literature review. J. Bras. Patol. Med. Lab. 2017, 53, 38–45. [Google Scholar] [CrossRef]
- Pound, C.M.; Blair, B. Energy and sports drinks in children and adolescents. Paed. Child Health-Can. 2017, 22, 406–410. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics; Committee on Nutrition and the Council on Sports Medicine and Fitness. Sports drinks and energy drinks for children and adolescents: Are they appropriate? Pediatrics 2011, 127, 1182–1189. [Google Scholar] [CrossRef]
- Campbell, B.; Wilborn, C.; La Bounty, P.; Taylor, L.; Nelson, M.T.; Greenwood, M.; Ziegenfuss, T.N.; Lopez, H.L.; Hoffman, J.R.; Stout, J.R.; et al. International Society of Sports Nutrition position stand: Energy drinks. J. Int. Soc. Sports Nutr. 2013, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, J.; Barber, J.; Strowbridge, C.; Hayward, S.; Huang, R.; Godefroy, S.B. Energy drinks: An assessment of the potential health risks in the Canadian context. Int. Food Risk Anal. J. 2013, 3, 1–29. [Google Scholar] [CrossRef]
- Brouns, F.; Kovacs, E. Functional drinks for athletes rewiev. Trends Food Sci. Technol. 1997, 8, 414–421. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Snow, R.J.; Stathis, C.G.; Hargreaves, M.; Carey, M.F. Effect of heat stress on muscle energy metabolism during exercise. J. Appl. Physiol. 1994, 77, 2827–2831. [Google Scholar] [CrossRef]
- Nielsen, B. Temperature Regulation; Effects of sweat loss during prolonged exercise. Acta Physiol. Stand. 1986, 128, 105–109. [Google Scholar]
- Sawka, M.N. Physiological consequences of hypohydration: Exercise performance and thermoregulation. Med. Sci. Sports Exerc. 1992, 24, 657–670. [Google Scholar] [CrossRef]
- Mettler, S.; Rusch, C.; Colombani, P.C. Osmolality and pH of sport and other drinks available in Switzerland. Schweiz. Z. Sportmed. Sporttraumatol. 2006, 54, 92–95. [Google Scholar]
- Evans, G.H.; Shirreffs, S.M.; Maughan, R.J. Postexercise rehydration in man: The effects of osmolality and carbohydrate content of ingested drinks. Nutrition 2009, 25, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Leiper, J.B. Intestinal water absorption—implications for the formula-tion of rehydration solutions. Int. J. Sport Med. 1998, 19, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Farthing, M.J. Oral rehydration: An evolving solution. J. Pediatr. Gastro-Enterol. Nutr. 2002, 34, 64–67. [Google Scholar] [CrossRef]
- Hiller, W.D.B. Dehydration and hyponatremia during triathlons. Med. Sci. Sports Exerc. 1989, 21, 222–237. [Google Scholar]
- Noakes, T.D.; Norman, R.J.; Buck, R.H.; Godlonton, J.; Stevenson, K.; Pittaway, D. The incidence of hyponatremia during prolonged ultraendurance Exercise. Med. Sci. Sports Exerc. 1990, 22, 165–170. [Google Scholar]
- Noakes, T.D. Hyponatremia during endurance running: A Physiological and Clinical Interpretation. Med. Sci. Sports Exerc. 1993, 24, 403–405. [Google Scholar] [CrossRef]
- Gisoffi, C.V.; Summers, R.W.; Schedl, H.P.; Bleiler, T.L. Intestinal water absorption from select carbohydrate solutions in humans. J. Appl. Physiol. 1992, 73, 2142–2150. [Google Scholar] [CrossRef]
- Hawley, J.A.; Dennis, S.C.; Noakes, T.D. Oxidation of carbohydrate ingested during prolonged endurance exercise. Sports Med. 1992, 14, 27–42. [Google Scholar] [CrossRef]
- Rehrer, N.; Wagenmakers, A.J.; Beckers, E.J.; Halliday, D.; Leiper, J.B.; Brouns, F.; Maughan, R.J.; Westerterp, K.; Saris, W.H. Gastric emptying, absorption, and carbohydrate oxidation during prolonged exercise. Appl. Physiol. 1992, 72, 468–475. [Google Scholar] [CrossRef]
- Wagenmakers, A.J.M.; Brouns, F.; Saris, W.H.; Halliday, D. Oxidation rates of orally ingested carbohydrates during prolonged exercise in men. Appl. Physiol. 1993, 75, 2774–2780. [Google Scholar] [CrossRef] [PubMed]
- Urdampilleta, A.; Gómez-Zorita, S.; Soriano, J.M.; Martínez-Sanz, J.M.; Medina, S.; Gil-Izquierdo, A. Hydration and chemical ingredients in sport drinks: Food safety in the European context. Nutr Hosp. 2015, 31, 1889–1899. [Google Scholar] [PubMed]
- PN-EN 12147:2000. Fruit and Vegetable Juices-Determination of Titrable Acidity; Polish Committee for Standardization: Warszawa, Poland, 2000. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radicalcation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics withphosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- ISO PN-EN ISO 13299:2016-05. Sensory Analysis-Methodology-General Guidelines for Determining the Sensory Profile; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- PN-EN ISO 8586:2014-03. Sensory Analysis-General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Sensory Evaluation Experts; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- PN-EN ISO 8589:2010/A1:2014-07. Sensory Analysis-General Guidelines for Designing a Sensory Analysis Laboratory; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Santos, L.S.; Ferreira-Bonomo, R.C.; Lemos, A.R.; da Costa Ilheu Fontain, R.; Santos, W.O.; Veloso, C.M.; Souza, M.A. Influence of concentration and type of sugar additives on the osmolality and acceptance of coconut water. UNOPAR Cient Ciênc Biol. Saúde 2011, 13, 29–32. [Google Scholar]
- Sadowska, A.; Rakowska, R.; Świderski, F.; Kulik, K.; Hallmann, E. Properties and microstructure of blackcurrantpowders prepared using a new method offluidized-bed jet milling and drying versus otherdrying methods. CyTA J. Food. 2019, 17, 439–446. [Google Scholar] [CrossRef]
- Regulation No 1169/2011 of the European Parliament and of the Council (UE) of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union 2011, 20, 168–213.
- Rao, L.J.M.; Ramalakshmi, K. (Eds.) Ingredients of soft drinks. In Recent Trends in Soft Beverages; Woodhead Publishing: India, New Delhi, 2011; pp. 189–209. [Google Scholar]
| Ingredient (g/100 mL) | Version 1 | Version 2a | Version 2b | Version 3 | Version 4 |
|---|---|---|---|---|---|
| Lyophilized powder of blackcurrant | 3 | 4 | 4 | - | - |
| Spray-dried powder of blackcurrant | - | - | - | 7 | 6 |
| Glucose | 5 | 5 | - | 1 | 3 |
| Sucrose | - | - | 5 | - | - |
| NaCl | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 |
| KCl | 0.025 | 0.025 | 0.025 | 0.025 | 0.025 |
| Water | 91.775 | 90.875 | 90.875 | 91.875 | 90.875 |
| Beverage | Osmolalities Calculated by Regression Equations | Measured Osmolality |
|---|---|---|
| Version 1 | OSM = (15.33 + 29.19 × 3) + (8.73 + 52.92 × 5) + (8.30 + 330.42 × 0.2) + (13.11 + 244.87 × 0.025) = 469.85 | 557.67 ± 0.58 d |
| Version 2a | OSM = (15.33 + 29.19 × 4) + (8.73 + 52.92 × 5) + (8.30 + 330.42 × 0.1) + (13.11 + 244.87 × 0.025) = 465.99 | 564.00 ± 1.00 e |
| Version 2b | OSM = (15.33 + 29.19 × 4) + (4.90 + 31.72 × 5) + (8.30 + 30.42 × 0.1) + (13.11 + 244.87 × 0.025) = 326.16 | 425.57 ± 0.58 b |
| Version 3 | OSM = (7.00 + 24.55 × 7) + (8.73+ 52.92 × 1) + (8.30 + 330.42 × 0.1) + (13.11 + 244.87 × 0.025) = 301.35 | 401.33 ± 0.58 a |
| Version 4 | OSM = (7.00 + 24.55 × 6) + (8.73 + 52.92 × 3) + (8.30 + 330.42 × 0.1) + (13.11 + 244.87 × 0.025) = 382.35 | 436.33 ± 0.58 c |
| The Tested Physico-Chemical Parameters | Version 1 | Version 2a,b | Version 3 | Version 4 |
|---|---|---|---|---|
| Soluble solids content (Bx) | 7.50 ± 0.00 a | 7.50 ± 0.25 a | 8.00 ± 0.46 a | 8.50 ± 0.20 b |
| Acidity | 0.34 ± 0.04 b | 0.53 ± 0.01 a | 0.55 ± 0.07 a | 0.36 ± 0.04 b |
| pH | 3.01 ± 0.02 b | 3.01 ± 0.01 b | 2.95 ± 0.03 a | 2.99 ± 0.02 ab |
| Bioactive Compound and Antioxidant Properties | Version 1 | Version 2a,b | Version 3 | Version 4 |
|---|---|---|---|---|
| Vitamin C (mg/100 g) | 25.83 ± 0.3 a | 34.45 ± 0.7 b | 35.62 ± 1.0 b | 26.71 ± 0.4 a |
| Total polyphenols (mg GAE/100 g) | 43.11 ± 0.2 a | 57.47 ± 0.1 c | 62.50 ± 0.1 d | 46.86 ± 0.8 b |
| Antioxidant properties (mmol TEAC/100 g) | 1.27 ± 0.03 a | 1.69 ± 0.05 c | 2.13 ± 0.07 d | 1.60 ± 0.01 b |
| Sweet Taste | Salty Taste | Sour Taste | Blackcurrant Flavor | Particles Palpability | Overall Quality | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ver | a | b | c | Ver | a | b | c | Ver | a | b | c | Ver | a | b | Ver | a | b | Ver | a | b |
| 3 | *** | 2b | *** | 2b | *** | 3 | *** | 4 | *** | 2a | *** | |||||||||
| 2a | *** | *** | 4 | *** | *** | 4 | *** | *** | 4 | *** | 3 | *** | 1 | *** | ||||||
| 1 | *** | *** | 2a | *** | *** | 1 | *** | *** | 1 | *** | 1 | *** | 3 | *** | *** | |||||
| 4 | *** | 3 | *** | *** | 2a | *** | *** | 2b | *** | 2b | *** | 4 | *** | *** | ||||||
| 2b | *** | 1 | *** | 3 | *** | 2a | *** | 2a | *** | 2b | *** | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska, A.; Świderski, F.; Laskowski, W. Osmolality of Components and Their Application in the Design of Functional Recovery Drinks. Appl. Sci. 2020, 10, 7663. https://doi.org/10.3390/app10217663
Sadowska A, Świderski F, Laskowski W. Osmolality of Components and Their Application in the Design of Functional Recovery Drinks. Applied Sciences. 2020; 10(21):7663. https://doi.org/10.3390/app10217663
Chicago/Turabian StyleSadowska, Anna, Franciszek Świderski, and Wacław Laskowski. 2020. "Osmolality of Components and Their Application in the Design of Functional Recovery Drinks" Applied Sciences 10, no. 21: 7663. https://doi.org/10.3390/app10217663
APA StyleSadowska, A., Świderski, F., & Laskowski, W. (2020). Osmolality of Components and Their Application in the Design of Functional Recovery Drinks. Applied Sciences, 10(21), 7663. https://doi.org/10.3390/app10217663





