Ultrasonic Regeneration Studies on Activated Carbon Loaded with Isopropyl Alcohol
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Pretreatment and Analysis of Activated Carbon
2.2. Adsorption of IPA by Activated Carbon
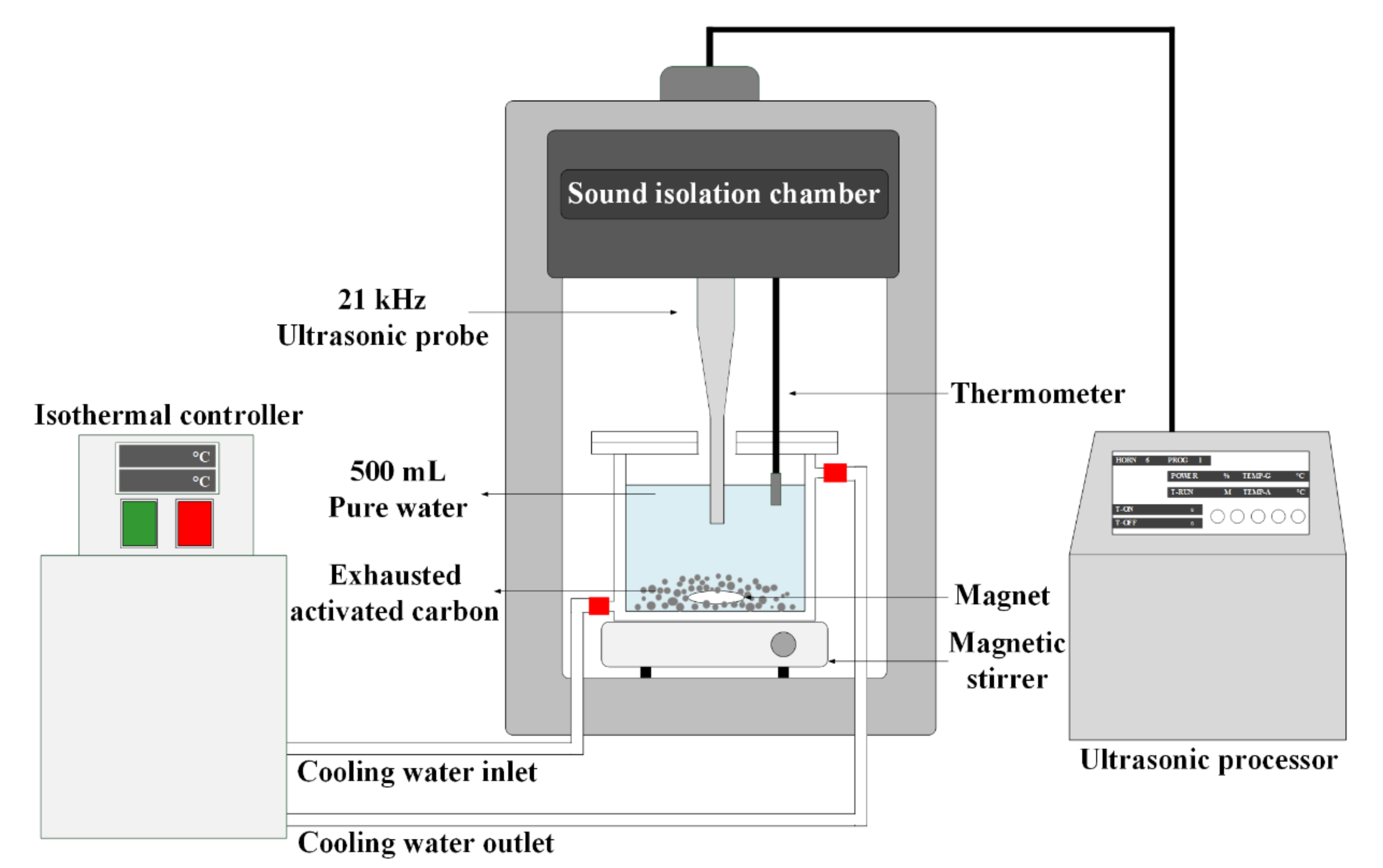
2.3. Ultrasonic Regeneration of IPA-Saturated Activated Carbon
3. Results
3.1. Properties of the Activated Carbon
3.2. Adsorption of Isopropyl Alcohol
3.3. Ultrasonic Regeneration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McGuire, G.E. Semiconductor Materials and Process Technology Handbook; Noyes Publications: Park Ridge, NJ, USA, 1988. [Google Scholar]
- Wu, J.J.; Yang, J.S.; Muruganandham, M.; Wu, C.C. The Oxidation Study of 2-propanol using Ozone-based Advanced Oxidation Processes. Sep. Purif. Technol. 2008, 62, 39–46. [Google Scholar] [CrossRef]
- Sudaryanto, Y.; Hartono, S.B.; Irawaty, W.; Hindarso, H.; Ismadji, S. High Surface Area Activated Carbon Prepared from Cassava Peel by Chemical Activation. Bioresour. Technol. 2009, 97, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Marketandmarket. Activated Carbon Market by Type (Powdered, Granular, Others (Pelletized, Bead)), Application (Liquid Phase (Water Treatment, Foods & Beverages, Pharmaceutical & Medical), Gaseous Phase (Industrial, Automotive)), Region—Global Forecast to 2021. 2017. Available online: https://www.marketsandmarkets.com/Market-Reports/activated-carbon-362.html (accessed on 23 October 2020).
- Arena, N.; Lee, J.; Clift, R. Life Cycle Assessment of Activated Carbon Production from Coconut Shells. J. Clean. Prod. 2016, 125, 68–77. [Google Scholar] [CrossRef]
- Zanella, O.; Tssaro, I.C.; Féris, L.A. Desorption-and Decomposition-Based Techniques for the Regeneration of Activated Carbon. Chem. Eng. Technol. 2014, 37, 1447–1459. [Google Scholar] [CrossRef]
- Álvarez, P.M.; Beltrán, F.J.; Gómez-Serrano, V.; Jaramillo, J.; Rodríguez, E.M. Comparison between Thermal and Ozone Regenerations of Spent Activated Carbon Exhausted with Phenol. Water Res. 2004, 38, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, Ö.; Cecen, F. Effect of Activation Type on Bioregeneration of Various Activated Carbons Loaded with Phenol. Chem. Technol. Biotechnol. 2006, 81, 1081–1092. [Google Scholar] [CrossRef]
- Zhang, H.P.; Ye, L.Y.; Zhong, H. Regeneration of Phenol-saturated Activated Carbon in an Electrochemical Reactor. Chem. Technol. Biotechnol. 2002, 77, 1246–1250. [Google Scholar] [CrossRef]
- Berenguer, R.; Marco-Lozar, J.P.; Quijada, C.; Cazorla-Amorós, D.; Morallón, E. Electrochemical Regeneration and Porosity Recovery of Phenol-saturated Granular Activated Carbon in an Alkaline Medium. Carbon 2010, 48, 2734–2745. [Google Scholar] [CrossRef]
- Liu, S.X.; Sun, C.L.; Zhang, S.R. Photocatalytic Regeneration of Exhausted Activated Carbon Saturated with Phenol. Bull. Environ. Contam. Toxicol. 2004, 73, 1017–1024. [Google Scholar] [CrossRef]
- Juang, R.S.; Lin, S.H.; Cheng, C.H. Liquid-phase Adsorption and Desorption of Phenol onto Activated Carbons with Ultrasound. Ultrason. Sonochem. 2006, 13, 251–260. [Google Scholar] [CrossRef]
- Hao, H.W.; Wu, M.S.; Chen, Y.F.; Yin, Y.W.; Lu, Z.L. Cavitation-Induced Pyrolysis of Toxic Chlorophenol by High-Frequency Ultrasonic Irradiation. Environ. Toxicol. 2003, 18, 413–417. [Google Scholar] [CrossRef]
- Teo, K.C.; Xu, Y.R.; Yang, C. Sonochemical Degradation for Toxic Halogenated Organic Compounds. Ultrason. Sonochem. 2001, 8, 241–246. [Google Scholar] [CrossRef]
- Lim, J.L.; Okada, M. Regeneration of Granular Activated Carbon Using Ultrasound. Ultrason. Sonochem. 2005, 12, 277–282. [Google Scholar] [CrossRef]
- Jing, G.H.; Zhou, Z.M.; Song, L.; Dong, M.X. Ultrasound Enhanced Adsorption and Desorption of Chromium (VI) on Activated Carbon and Polymeric Resin. Desalination. 2011, 279, 423–427. [Google Scholar] [CrossRef]
- Ince, N.H.; Tezcanli, G.; Belen, R.K.; Apikyan, I.G. Ultrasound as a Catalyzer of Aqueous Reaction Systems: The State of the Art and Environmental Applications. Appl. Catal. B-Environ. 2001, 29, 167–176. [Google Scholar] [CrossRef]
- Breitbach, M.; Bathen, D. Influence of Ultrasound on Adsorption Processes. Ultrason. Sonochem. 2001, 8, 277–283. [Google Scholar] [CrossRef]
- Adewuyi, Y.G. Sonochemistry: Environmental Science and Engineering Applications. Ind. Eng. Chem. Res. 2001, 40, 4681–4715. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Djeribi, R.; Naffrechoux, E. Desorption of Metal Ions from Activated Carbon in the Presence of Ulreasound. Ind. Eng. Chem. Res. 2005, 44, 4737–4744. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E.; Suptil, J.; Fachinger, C. Ultrasonic Desorption of p-Chlorophenol from Granular Activated Carbon. Chem. Eng. J. 2005, 106, 153–161. [Google Scholar] [CrossRef]
- Saoudi, F.; Hamdaoui, O. Innovative Technique for 4-chlorophenol Desorption from Granular Activated Carbon by Low Frequency Ultrasound: Influence of Operational Parameters. Micropor. Mesopor. Mater. 2011, 141, 69–76. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E.; Tifouti, L.; Pétrier, C. Effects of Ultrasound on Adsorption-Desorption of P-chlorophenol on Granular Activated Carbon. Ultrason. Sonochem. 2003, 10, 109–114. [Google Scholar] [CrossRef]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the Characterization of Acidic and Basic Surface Sites on Carbons by Various Techniques. Carbon 1999, 37, 1215–1221. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-García, M.A.; Moreno-Castilla, C. Activated Carbon Surface Modifications by Adsorption of Bacteria and Their Effect on Aqueous Lead Adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef]
- Liu, Q.S.; Zheng, T.; Wang, P.; Jiang, J.P.; Li, N. Adsorption Isotherm, Kinetic and Mechanism Studies of Some Substituted Phenols on Activated Carbon Fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, B.; Lin, Y.; Guan, Y. Aromatic and Hydrophobic Surfaces of Wood-derived Biochar Enhance Perchlorate Adsorption via Hydrogen Bonding to Oxygen-containing Organic Groups. Environ. Sci. Technol. 2014, 48, 279–288. [Google Scholar] [CrossRef]
- Horn, R.S.; Tseng, I.C. Regeneration of Granular Activated Carbon Saturated with Acetone and Isopropyl Alcohol Via a Recirculation Process Under H2O2/UV Oxidation. J. Hazard. Mater. 2008, 154, 366–372. [Google Scholar] [CrossRef]
- Lin, S.H.; Wang, C.S. Recovery of Isopropyl Alcohol from Waste Solvent of a Semiconductor Plant. J. Hazard. Mater. 2004, 106B, 161–168. [Google Scholar] [CrossRef]
- Liu, C.; Sun, Y.; Wang, D.; Sun, Z.; Chen, M.; Zhou, Z.; Chen, W. Performance and Mechanism of Low-frequency Ultrasound to Regenerate the Biological Activated Carbon. Ultrason. Sonochem. 2017, 34, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Hamdaoui, O.; Naffrechoux, E. An Investigation of the Mechanisms of Ultrasonically Enhanced Desorption. AIChE J. 2007, 53, 363–373. [Google Scholar] [CrossRef]
- Rege, S.U.; Yang, R.T.; Cain, C.A. Desorption by Ultrasound: Phenol on Activated Carbon and Polymeric Resin. AIChE J. 1998, 44, 1519–1527. [Google Scholar] [CrossRef]
- Hinoue, M.; Ishimatsu, S.; Fueta, Y.; Hori, H. A New Desorption Method for Removing Organic Solvents from Activated Carbon using Surfactant. J. Occup. Health 2017, 59, 194–200. [Google Scholar] [CrossRef] [PubMed]








| Type of Activated Carbon | Element Content (wt. %) | ||||||
|---|---|---|---|---|---|---|---|
| C | O | Mg | P | Si | K | Cl | |
| Original Activated Carbon | 89.60 ± 1.66 | 9.26 ± 2.23 | 0.18 ± 0.17 | 0.24 ± 0.25 | 0.24 ± 0.24 | 0.34 ± 0.22 | 0.14 ± 0.12 |
| Fresh Activated Carbon | 92.80 ± 1.15 | 6.40 ± 0.90 | - a | - a | 0.08 ± 0.14 | 0.56 ± 0.50 | 0.08 ± 0.14 |
| Activated Carbon | Micropore Volume (cm3/g) | BET Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Mean Pore Diameter (nm) |
|---|---|---|---|---|
| Original Activated Carbon | 243.06 ± 0.36 | 1057.9 ± 1.59 | 0.423 ± 0.001 | 1.6 ± 0.002 |
| Fresh Activated Carbon | 250.57 ± 0.38 | 1090.6 ± 1.64 | 0.435 ± 0.001 | 1.6 ± 0.002 |
| Activated Carbon after First Regeneration Cycle | 227.65 ± 0.34 | 990.9 ± 1.49 | 0.395 ± 0.001 | 1.6 ± 0.002 |
| Activated Carbon after Second Regeneration Cycle | 227.88 ± 0.34 | 991.9 ± 1.49 | 0.394 ± 0.001 | 1.6 ± 0.002 |
| Activated Carbon after Third Regeneration Cycle | 223.86 ± 0.34 | 974.4 ± 1.46 | 0.385 ± 0.001 | 1.6 ± 0.002 |
| Activated Carbon after Fourth Regeneration Cycle | 217.93 ± 0.32 | 948.5 ± 1.42 | 0.377 ± 0.001 | 1.6 ± 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, H.-Y.; Moed, N.M.; Ku, Y.; Lee, H.-Y. Ultrasonic Regeneration Studies on Activated Carbon Loaded with Isopropyl Alcohol. Appl. Sci. 2020, 10, 7596. https://doi.org/10.3390/app10217596
Hong H-Y, Moed NM, Ku Y, Lee H-Y. Ultrasonic Regeneration Studies on Activated Carbon Loaded with Isopropyl Alcohol. Applied Sciences. 2020; 10(21):7596. https://doi.org/10.3390/app10217596
Chicago/Turabian StyleHong, Hsuan-Yi, Niels Michiel Moed, Young Ku, and Hao-Yeh Lee. 2020. "Ultrasonic Regeneration Studies on Activated Carbon Loaded with Isopropyl Alcohol" Applied Sciences 10, no. 21: 7596. https://doi.org/10.3390/app10217596
APA StyleHong, H.-Y., Moed, N. M., Ku, Y., & Lee, H.-Y. (2020). Ultrasonic Regeneration Studies on Activated Carbon Loaded with Isopropyl Alcohol. Applied Sciences, 10(21), 7596. https://doi.org/10.3390/app10217596







