Predicting Intestinal and Hepatic First-Pass Metabolism of Orally Administered Testosterone Undecanoate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Prediction of Hepatic First-Pass Metabolism
2.3. Prediction of Intestinal First-Pass Metabolism
2.3.1. Preparation of Caco-2 Cell Homogenate
2.3.2. Hydrolysis Experiments
2.4. Analytical Methods
2.4.1. Sample Preparation
2.4.2. Chromatographic Conditions
2.4.3. In silico Prediction of Human Effective Permeability
2.5. Statistical Analysis
3. Results
3.1. Hepatic First-Pass Metabolism
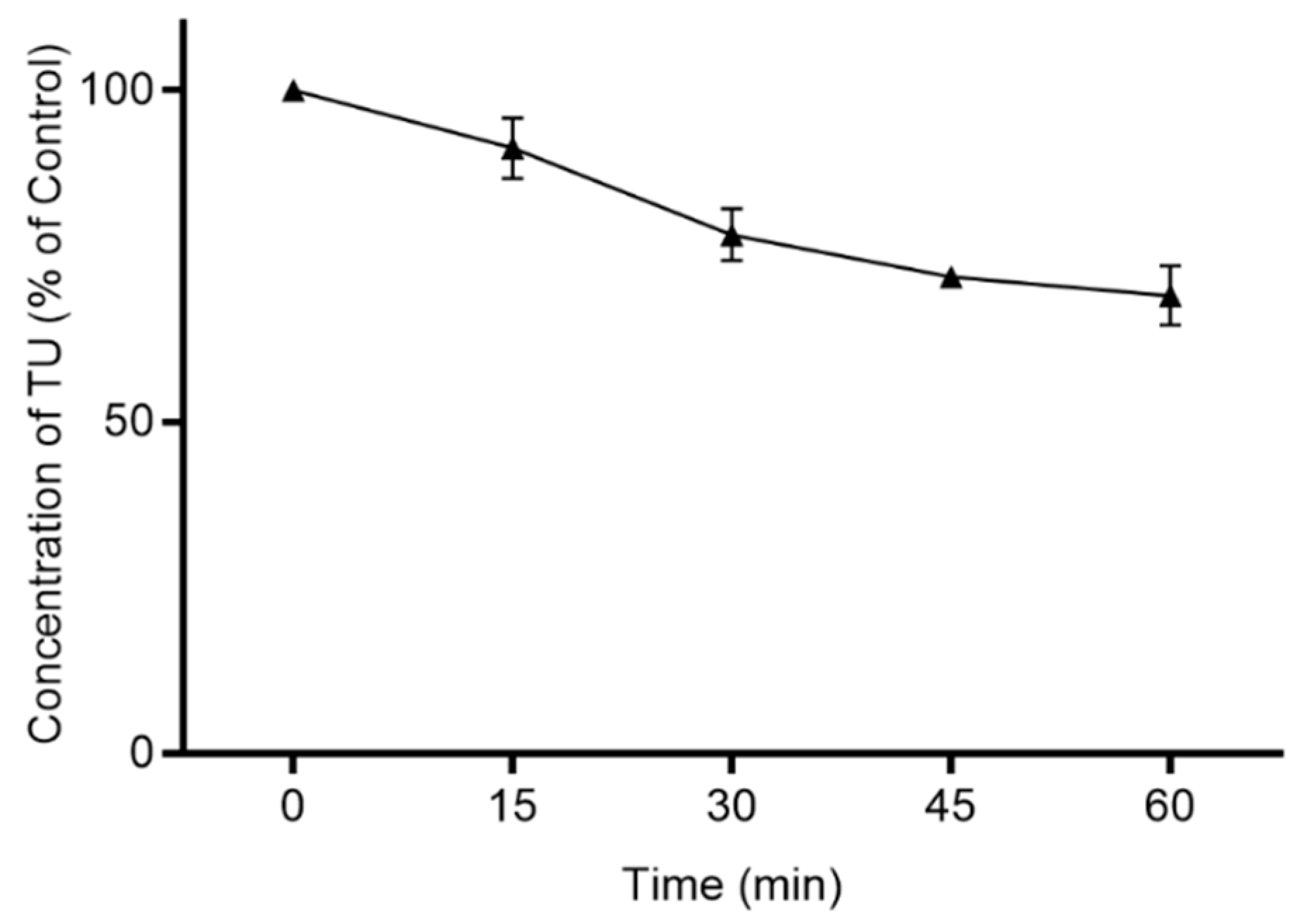
3.2. Intestinal First-Pass Metabolism
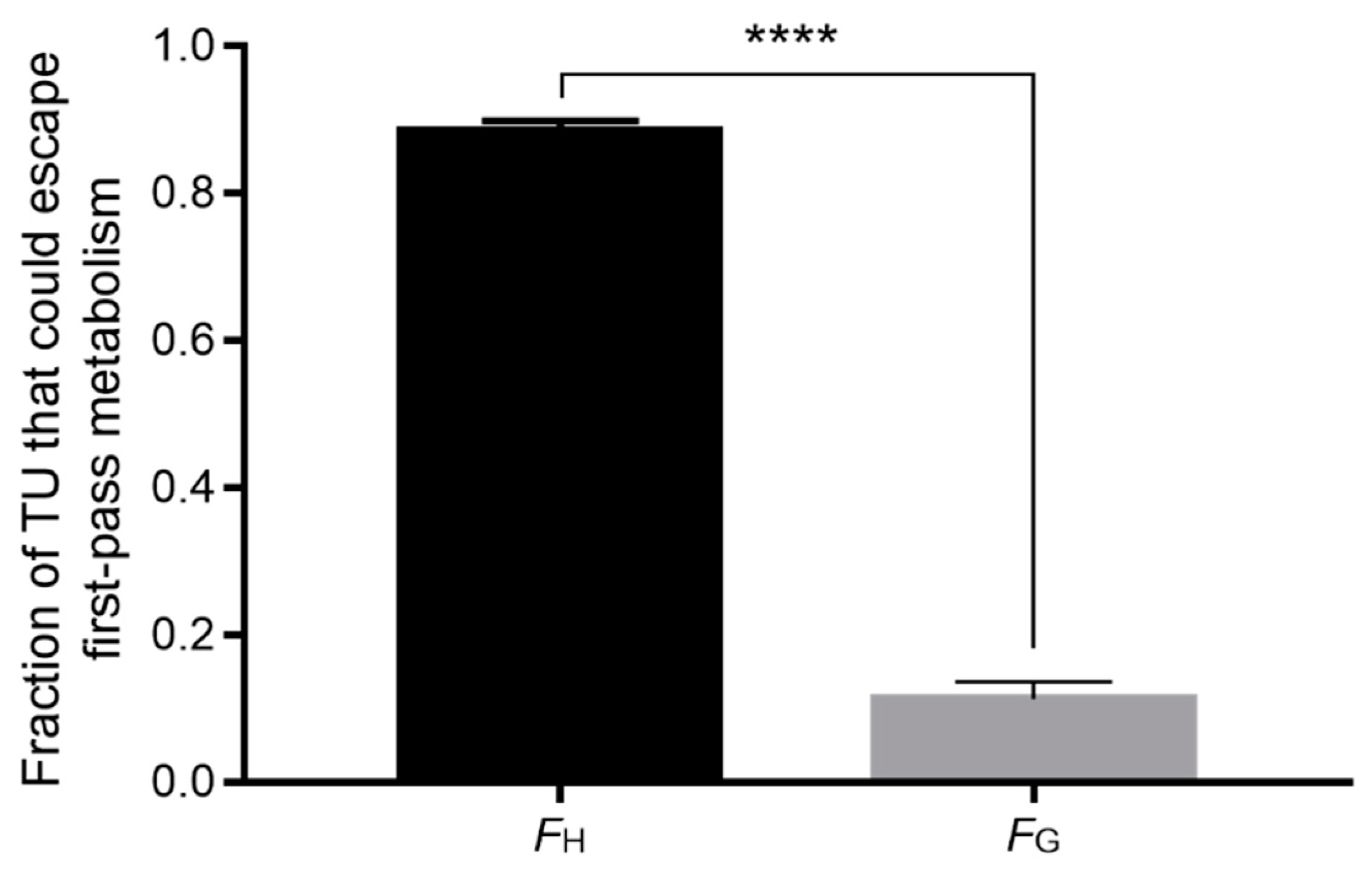
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Täuber, U.; Schröder, K.; Düsterberg, B.; Matthes, H. Absolute bioavailability of testosterone after oral administration of testosterone-undecanoate and testosterone. Eur. J. Drug Metab. Pharmacokinet. 1986. [Google Scholar] [CrossRef]
- Hoberman, J. Testosterone Dreams: Rejuvenation, Aphrodisia, Doping; University of California Press: Berkeley, CA, USA, 2005; ISBN 9780520939783. [Google Scholar]
- Frey, H.; Aakvaag, A.; Saanum, D.; Falch, J. Bioavailability of oral testosterone in males. Eur. J. Clin. Pharmacol. 1979. [Google Scholar] [CrossRef]
- Stella, V.; Borchardt, R.; Hageman, M.; Oliyai, R.; Maag, H.; Tilley, J. Prodrugs: Challenges and Rewards; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; ISBN 0387497854. [Google Scholar]
- Lachance, S.; Dhingra, O.; Bernstein, J.; Gagnon, S.; Savard, C.; Pelletier, N.; Boudreau, N.; Lévesque, A. Importance of measuring testosterone in enzyme-inhibited plasma for oral testosterone undecanoate androgen replacement therapy clinical trials. Future Sci. OA 2015. [Google Scholar] [CrossRef]
- Kempegowda, P.; Quinn, L.M.; Chandan, J.S.; Shepherd, L.; Kauser, S.; Rahim, A.; Bates, A. Long-term testosterone undecanoate replacement therapy: Impact of ethnicity. Clin. Endocrinol. 2020. [Google Scholar] [CrossRef]
- Coert, A.; Geelen, J.; De Visser, J.; Van Der Vies, J. The pharmacology and metabolism of testosterone undecanoate (TU), a new orally active androgen. Acta Endocrinol. 1975. [Google Scholar] [CrossRef] [PubMed]
- Geelen, J.; Coert, A.; Meijer, R.; Van Der Vies, J. Comparison of the metabolism of testosterone undecanoate and testosterone in the gastrointestinal wall of the rat in vitro and in vivo. Acta Endocrinol. 1977. [Google Scholar] [CrossRef]
- Shackleford, D.M.; Faassen, W.A.; Houwing, N.; Lass, H.; Edwards, G.A.; Porter, C.J.H.; Charman, W.N. Contribution of lymphatically transported testosterone undecanoate to the systemic exposure of testosterone after oral administration of two andriol formulations in conscious lymph duct-cannulated dogs. J. Pharmacol. Exp. Ther. 2003. [Google Scholar] [CrossRef]
- Horst, H.J.; Höltje, W.J.; Dennis, M.; Coert, A.; Geelen, J.; Voigt, K.D. Lymphatic absorption and metabolism of orally administered testosterone undecanoate in man. Klin. Wochenschr. 1976. [Google Scholar] [CrossRef]
- Nieschlag, E.; Mauss, J.; Coert, A.; Kićović, P. Plasma androgen levels in men after oral administration of testosterone or testosterone undecanoate. Eur. J. Endocrinol. 1975, 79, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Bagchus, W.M.; Hust, R.; Maris, F.; Schnabel, P.G.; Houwing, N.S. Important effect of food on the bioavailability of oral testosterone undecanoate. Pharmacotherapy 2003. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.Y.; Dudley, R.E.; Hull, L.; Leung, A.; Christenson, P.; Wang, C.; Swerdloff, R.; Amory, J.K. Steady-state pharmacokinetics of oral testosterone undecanoate with concomitant inhibition of 5α-reductase by finasteride. Int. J. Androl. 2011. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.; Alfadhli, E.; Htun, M.; Dudley, R.; Faulkner, S.; Hull, L.; Leung, A.; Bross, R.; Longstreth, J.; Swerdloff, R.; et al. Dietary fat modulates the testosterone pharmacokinetics of a new self-emulsifying formulation of oral testosterone undecanoate in hypogonadal men. J. Androl. 2012. [Google Scholar] [CrossRef]
- Zgair, A.; Wong, J.C.M.; Lee, J.B.; Mistry, J.; Sivak, O.; Wasan, K.M.; Hennig, I.M.; Barrett, D.A.; Constantinescu, C.S.; Fischer, P.M.; et al. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am. J. Transl. Res. 2016, 8, 3448. [Google Scholar] [PubMed]
- Brocks, D.R.; Davies, N.M. Lymphatic drug absorption via the enterocytes: Pharmacokinetic simulation, modeling, and considerations for optimal drug development. J. Pharm. Pharm. Sci. 2018. [Google Scholar] [CrossRef]
- Lee, J.B.; Kim, T.H.; Feng, W.; Choi, H.G.; Zgair, A.; Shin, S.; Yoo, S.D.; Gershkovich, P.; Shin, B.S. Quantitative Prediction of Oral Bioavailability of a Lipophilic Antineoplastic Drug Bexarotene Administered in Lipidic Formulation Using a Combined In Vitro Lipolysis/Microsomal Metabolism Approach. J. Pharm. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Benito-Gallo, P.; Marlow, M.; Zann, V.; Scholes, P.; Gershkovich, P. Linking in Vitro Lipolysis and Microsomal Metabolism for the Quantitative Prediction of Oral Bioavailability of BCS II Drugs Administered in Lipidic Formulations. Mol. Pharm. 2016. [Google Scholar] [CrossRef] [PubMed]
- Obach, R.S. The importance of nonspecific binding in in vitro matrices, its impact on enzyme kinetic studies of drug metabolism reactions, and implications for in vitro-in vivo correlations. Drug Metab. Dispos. 1996, 24, 1047–1049. [Google Scholar]
- Gertz, M.; Kilford, P.J.; Houston, J.B.; Galetin, A. Drug Lipophilicity and Microsomal Protein Concentration as Determinants in the Prediction of the Fraction Unbound in Microsomal Incubations. Drug Metab. Dispos. 2008, 36, 535–542. [Google Scholar] [CrossRef]
- Austin, R.P.; Barton, P.; Cockroft, S.L.; Wenlock, M.C.; Riley, R.J. The influence of nonspecific microsomal binding on apparent intrinsic clearance, and its prediction from physicochemical properties. Drug Metab. Dispos. 2002. [Google Scholar] [CrossRef]
- Hallifax, D.; Houston, J.B. Binding of drugs to hepatic microsomes: Comment and assessment of current prediction methodology with recommendation for improvement. Drug Metab. Dispos. 2006, 34, 724–726. [Google Scholar] [CrossRef]
- Advanced Chemistry Developmen Inc. ACD/I-Lab Version 12.1.0.50375. 2014. Available online: www.acdlabs.com (accessed on 10 June 2020).
- Gao, H.; Yao, L.; Mathieu, H.W.; Zhang, Y.; Maurer, T.S.; Troutman, M.D.; Scott, D.O.; Ruggeri, R.B.; Lin, J. In silico modeling of nonspecific binding to human liver microsomes. Drug Metab. Dispos. 2008. [Google Scholar] [CrossRef]
- Yang, C.X.; Li, Y.F.; Huang, C.Z. Determination of Total Protein Content in Human Serum Samples with Fast Red Vr by Resonance Light Scattering Technique. Anal. Lett. 2002, 35, 1945–1957. [Google Scholar] [CrossRef]
- Watanabe, R.; Esaki, T.; Kawashima, H.; Natsume-Kitatani, Y.; Nagao, C.; Ohashi, R.; Mizuguchi, K. Predicting Fraction Unbound in Human Plasma from Chemical Structure: Improved Accuracy in the Low Value Ranges. Mol. Pharm. 2018. [Google Scholar] [CrossRef] [PubMed]
- Barter, Z.; Bayliss, M.; Beaune, P.; Boobis, A.; Carlile, D.; Edwards, R.; Brian Houston, J.; Lake, B.; Lipscomb, J.; Pelkonen, O.; et al. Scaling Factors for the Extrapolation of In Vivo Metabolic Drug Clearance from In Vitro Data: Reaching a Consensus on Values of Human Micro-somal Protein and Hepatocellularity Per Gram of Liver. Curr. Drug Metab. 2006. [Google Scholar] [CrossRef] [PubMed]
- McGinnity, D.F.; Soars, M.G.; Urbanowicz, R.A.; Riley, R.J. Evaluation of fresh and cryopreserved hepatocytes as in vitro drug metabolism tools for the prediction of metabolic clearance. Drug Metab. Dispos. 2004. [Google Scholar] [CrossRef]
- Davies, B.; Morris, T. Physiological Parameters in Laboratory Animals and Humans. Pharm. Res. 1993, 10, 1093–1095. [Google Scholar] [CrossRef]
- Tsume, Y.; Amidon, G.L. Selection of Suitable Prodrug Candidates for in vivo Studies via in vitro Studies; The correlation of Prodrug Stability in Between Cell Culture Homogenates and Human Tissue Homogenates. J. Pharm. Pharm. Sci. 2012. [Google Scholar] [CrossRef]
- Lee, J.B.; Zgair, A.; Malec, J.; Kim, T.H.; Kim, M.G.; Ali, J.; Qin, C.; Feng, W.; Chiang, M.; Gao, X.; et al. Lipophilic activated ester prodrug approach for drug delivery to the intestinal lymphatic system. J. Control. Release 2018, 286. [Google Scholar] [CrossRef]
- Gertz, M.; Houston, J.B.; Galetin, A. Physiologically based pharmacokinetic modeling of intestinal first-pass metabolism of CYP3A substrates with high intestinal extraction. Drug Metab. Dispos. 2011. [Google Scholar] [CrossRef]
- Yang, J.; Jamei, M.; Yeo, K.; Tucker, G.; Rostami-Hodjegan, A. Prediction of Intestinal First-Pass Drug Metabolism. Curr. Drug Metab. 2007. [Google Scholar] [CrossRef]
- Granger, D.N.; Richardson, P.D.I.; Kvietys, P.R.; Mortillaro, N.A. Intestinal blood flow. Gastroenterology 1980, 78, 837–863. [Google Scholar] [CrossRef]
- Zgair, A.; Wong, J.C.M.; Sabri, A.; Fischer, P.M.; Barrett, D.A.; Constantinescu, C.S.; Gershkovich, P. Development of a simple and sensitive HPLC-UV method for the simultaneous determination of cannabidiol and δ9-tetrahydrocannabinol in rat plasma. J. Pharm. Biomed. Anal. 2015. [Google Scholar] [CrossRef] [PubMed]
- Krishna, G.; Chen, K.J.; Lin, C.C.; Nomeir, A.A. Permeability of lipophilic compounds in drug discovery using in-vitro human absorption model, Caco-2. Int. J. Pharm. 2001. [Google Scholar] [CrossRef]
- Pond, S.M.; Tozer, T.N. First-Pass Elimination Basic Concepts and Clinical Consequences. Clin. Pharmacokinet. 1984, 9, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Azzouni, F.; Godoy, A.; Li, Y.; Mohler, J. The 5 alpha-reductase isozyme family: A review of basic biology and their role in human diseases. Adv. Urol. 2012. [Google Scholar] [CrossRef]
- Junge, W.; Krisch, K.; Conney, A. The carboxylesterases/amidases of mammalian liver and their possible significance. Crit. Rev. Toxicol. 1975. [Google Scholar] [CrossRef]
- Rautio, J.; Mannhold, R.; Kubinyi, H. Prodrugs and Targeted Delivery: Towards Better ADME Properties. Methods Princ. Med. Chem. 2011, 47, 292. [Google Scholar]
- Gupta, D.; Gupta, S.V.; Lee, K.D.; Amidon, G.L. Chemical and enzymatic stability of amino acid prodrugs containing methoxy, ethoxy and propylene glycol linkers. Mol. Pharm. 2009, 6, 1604–1611. [Google Scholar] [CrossRef]
- Imai, T.; Imoto, M.; Sakamoto, H.; Hashimoto, M. Identification of esterases expressed in Caco-2 cells and effects of their hydrolyzing activity in predicting human intestinal absorption. Drug Metab. Dispos. 2005. [Google Scholar] [CrossRef]
- Hosokawa, M. Structure and catalytic properties of carboxylesterase isozymes involved in metabolic activation of prodrugs. Molecules 2008, 13, 412–431. [Google Scholar] [CrossRef]
- Satoh, T.; Hosokawa, M. Structure, function and regulation of carboxylesterases. Chem. Biol. Interact. 2006, 162, 195–211. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zgair, A.; Dawood, Y.; Ibrahem, S.M.; Back, H.-m.; Kagan, L.; Gershkovich, P.; Lee, J.B. Predicting Intestinal and Hepatic First-Pass Metabolism of Orally Administered Testosterone Undecanoate. Appl. Sci. 2020, 10, 7283. https://doi.org/10.3390/app10207283
Zgair A, Dawood Y, Ibrahem SM, Back H-m, Kagan L, Gershkovich P, Lee JB. Predicting Intestinal and Hepatic First-Pass Metabolism of Orally Administered Testosterone Undecanoate. Applied Sciences. 2020; 10(20):7283. https://doi.org/10.3390/app10207283
Chicago/Turabian StyleZgair, Atheer, Yousaf Dawood, Suhaib M. Ibrahem, Hyun-moon Back, Leonid Kagan, Pavel Gershkovich, and Jong Bong Lee. 2020. "Predicting Intestinal and Hepatic First-Pass Metabolism of Orally Administered Testosterone Undecanoate" Applied Sciences 10, no. 20: 7283. https://doi.org/10.3390/app10207283
APA StyleZgair, A., Dawood, Y., Ibrahem, S. M., Back, H.-m., Kagan, L., Gershkovich, P., & Lee, J. B. (2020). Predicting Intestinal and Hepatic First-Pass Metabolism of Orally Administered Testosterone Undecanoate. Applied Sciences, 10(20), 7283. https://doi.org/10.3390/app10207283






