A Review of Validation Methods for the Intracranial Response of FEHM to Blunt Impacts
Abstract
1. Introduction
2. Experimental Procedures Used in FEHM Validation
2.1. Cadaver Experimentation
2.1.1. Intracranial Pressure Response
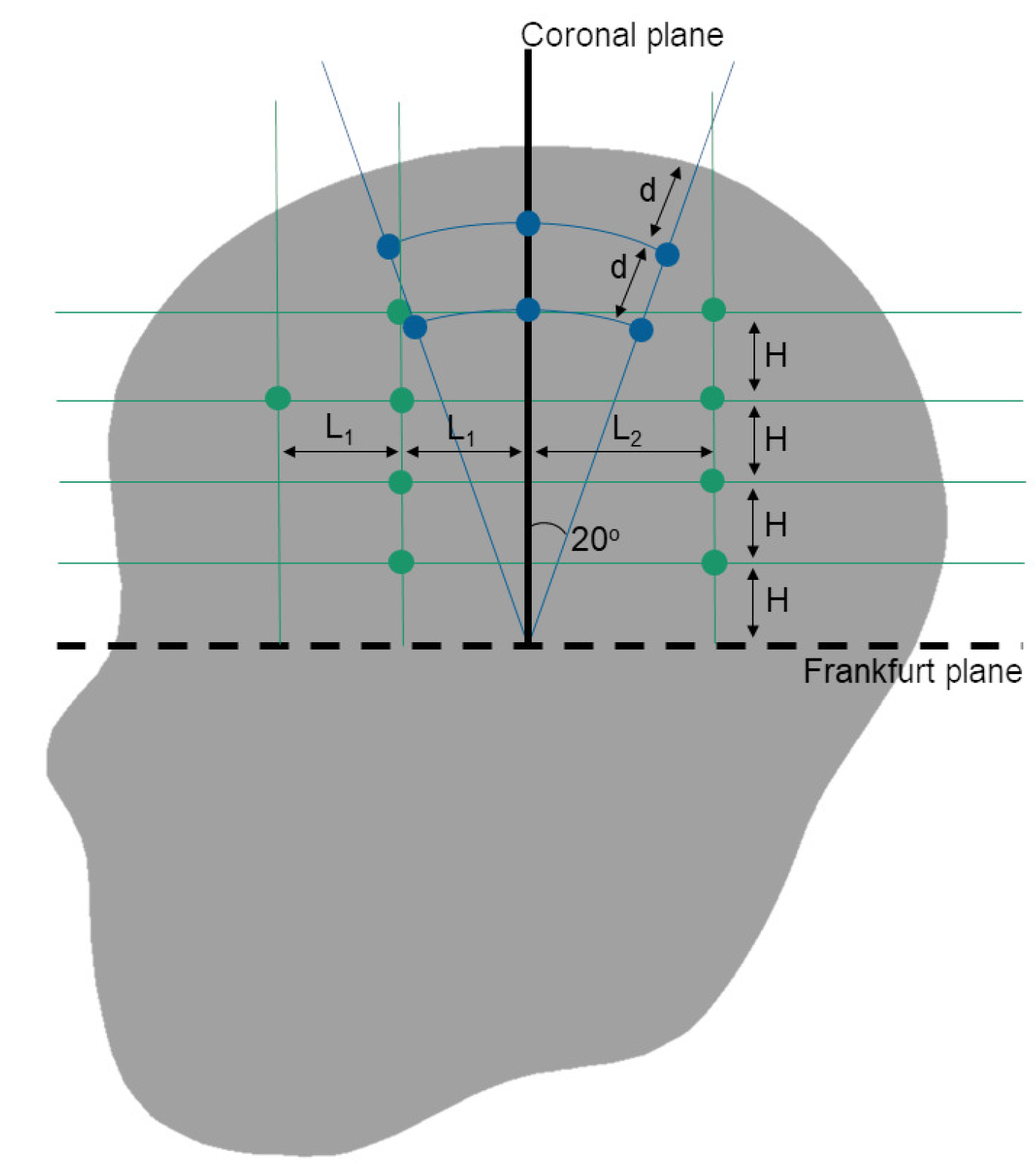
2.1.2. Relative Brain Displacement Response
2.2. In Vivo Experimentation
3. Prominent FEHM and Their Validation
3.1. Empirical Data Applied to FEHM Validation
3.2. Replication of Empirical Scenarios in Numerical Environment
3.3. Assessment of FEHM Performance and Validation
4. Further Research into Validation
4.1. Retrospective Validation and Comparison Investigations
4.2. Investigation into Validation Protocol
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Humphreys, I.; Wood, R.L.; Phillips, C.J.; Macey, S. ClinicoEconomics and Outcomes Research The costs of traumatic brain injury: A literature review. Clin. Outcomes Res. 2013, 5, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Voss, J.D.; Connolly, J.; Schwab, K.A.; Scher, A.I. Update on the Epidemiology of Concussion/Mild Traumatic Brain Injury. Curr. Pain Headache Rep. 2015, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill. Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Samaka, H.; Tarlochan, F. Finite Element (FE) Human Head Models / Literature Review. Int. J. Sci. Technol. Res. 2013, 2, 17–31. [Google Scholar]
- Tse, K.M.; Lim, S.P.; Tan, V.B.C.; Lee, H.P. A Review of Head Injury and Finite Element Head Models. Am. J. Eng. Technol. Soc. 2014, 1, 28–52. [Google Scholar]
- Dixit, P.; Liu, G.R. A Review on Recent Development of Finite Element Models for Head Injury Simulations. Arch. Comput. Methods Eng. 2017, 24, 979–1031. [Google Scholar] [CrossRef]
- Madhukar, A.; Ostoja-Starzewski, M. Finite Element Methods in Human Head Impact Simulations: A Review. Ann. Biomed. Eng. 2019, 47, 1832–1854. [Google Scholar] [CrossRef]
- Miller, K.; Kurtcuoglu, V. Biomechanics of the Brain; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Mao, H. Modeling the Head for Impact Scenarios. In Basic Finite Element Method as Applied to Injury Biomechanics; Yang, K.H., Ed.; Academic Press: Amsterdam, The Netherlands, 2018; Chapter 12; pp. 469–502. [Google Scholar] [CrossRef]
- van Dommelen, J.; Hrapko, M.; Peters, G. Mechanical Properties of Brain Tissue: Characterisation and Constitutive Modelling. In Mechanosensitivity of the Nervous System; Springer: Dordrecht, The Netherlands, 2009; pp. 249–279. [Google Scholar] [CrossRef]
- Bilston, L.E. Brain Tissue Mechanical Properties. In Biomechanics of the Brain; Springer: New York, NY, USA, 2011; pp. 69–89. [Google Scholar] [CrossRef]
- Mihai, L.A.; Chin, L.K.; Janmey, P.A.; Goriely, A. A comparison of hyperelastic constitutive models applicable to brain and fat tissues. J. R. Soc. Interface 2015, 12, 20150486. [Google Scholar] [CrossRef]
- Forte, A.E.; Gentleman, S.M.; Dini, D. On the characterization of the heterogeneous mechanical response of human brain tissue. Biomech. Model. Mechanobiol. 2017, 16, 907–920. [Google Scholar] [CrossRef]
- Budday, S.; Ovaert, T.C.; Holzapfel, G.A.; Steinmann, P.; Kuhl, E. Fifty Shades of Brain: A Review on the Mechanical Testing and Modeling of Brain Tissue. Arch. Comput. Methods Eng. 2019, 1, 3. [Google Scholar] [CrossRef]
- Shariyat, M.; Ashrafi, H.; Bandband, H. Brain Tissue Response Analysis Based on Several Hyperelastic Models, for Traumatic Brain Injury Assessment. Univers. J. Biomed. Eng. 2016, 4, 11–26. [Google Scholar] [CrossRef]
- Kleiven, S. Predictors for Traumatic Brain Injuries Evaluated through Accident Reconstructions. Stapp Car Crash J. 2007, 51, 81–114. [Google Scholar]
- Zhao, W.; Choate, B.; Ji, S. Material properties of the brain in injury-relevant conditions—Experiments and computational modeling. J. Mech. Behav. Biomed. Mater. 2018, 80, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Tse, K.M.; Chen, N.; Tan, L.B.; Zheng, Q.Q.; Yang, H.M.; Hu, M.; Pan, G.; Lee, H.P. Development of a finite element head model for the study of impact head injury. BioMed Res. Int. 2014, 2014, 408278. [Google Scholar] [CrossRef]
- Duckworth, H.; Ghajari, M. Modelling Brain Biomechanics Using a Hybrid Smoothed Particle Hydrodynamics and Finite Element Model. Ohio State University Injury Biomechanics Symposium. 2019. Available online: https://www.semanticscholar.org/paper/Modelling-Brain-Biomechanics-Using-a-Hybrid-and-Duckworth-Ghajari/768b54983f88554cfcb6218764d3583f0669973c (accessed on 9 October 2020).
- Zhou, Z.; Li, X.; Kleiven, S. Fluid–structure interaction simulation of the brain–skull interface for acute subdural haematoma prediction. Biomech. Model. Mechanobiol. 2019, 18, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Horgan, T.J.; Gilchrist, M.D. Influence of FE model variability in predicting brain motion and intracranial pressure changes in head impact simulations. Int. J. Crashworthiness 2004, 9, 401–418. [Google Scholar] [CrossRef]
- Chafi, M.S.; Dirisala, V.; Karami, G.; Ziejewski, M. A finite element method parametric study of the dynamic response of the human brain with different cerebrospinal fluid constitutive properties. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2009, 223, 1003–1019. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Chen, H. Finite-element study of cerebrospinal fluid in mitigating closed head injuries. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2012, 226, 499–509. [Google Scholar] [CrossRef]
- Jin, J.X.; Zhang, J.Y.; Song, X.W.; Hu, H.; Sun, X.Y.; Gao, Z.H. Effect of Cerebrospinal Fluid Modeled with Different Material Properties on a Human Finite Element Head Model. J. Mech. Med. Biol. 2015, 15, 1550027. [Google Scholar] [CrossRef]
- Atsumi, N.; Nakahira, Y.; Iwamoto, M. Development and validation of a head/brain FE model and investigation of influential factor on the brain response during head impact. Int. J. Veh. Saf. 2016, 9, 1–23. [Google Scholar] [CrossRef]
- Sahoo, D.; Deck, C.; Yoganandan, N.; Willinger, R. Influence of head mass on temporo-parietal skull impact using finite element modeling. Med. Biol. Eng. Comput. 2015, 53, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Kleiven, S. Can sulci protect the brain from traumatic injury? J. Biomech. 2009, 42, 2074–2080. [Google Scholar] [CrossRef] [PubMed]
- Drake, A.; Takhounts, E.G.; Hasija, V. Investigation of Parameters Affecting Brain Model Validation and Brain Strains Using the SIMon Finite Element Head Model. In Proceedings of the IRCOBI Conference, Antwerp, Belgium, 13–15 September 2017; pp. 410–431. [Google Scholar]
- Sáez, P.; Duñó, C.; Sun, L.Y.; Antonovaite, N.; Malvè, M.; Tost, D.; Goriely, A. Topological features dictate the mechanics of the mammalian brains. Int. J. Mech. Sci. 2020, 187, 105914. [Google Scholar] [CrossRef]
- Giudice, J.S.; Zeng, W.; Wu, T.; Alshareef, A.; Shedd, D.F.; Panzer, M.B. An Analytical Review of the Numerical Methods used for Finite Element Modeling of Traumatic Brain Injury. Ann. Biomed. Eng. 2019, 47, 1855–1872. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Urban, J.E.; Stitzel, J.D. Validation performance comparison for finite element models of the human brain. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 1273–1288. [Google Scholar] [CrossRef]
- Ji, S.; Zhao, W.; Li, Z.; McAllister, T.W. Head impact accelerations for brain strain-related responses in contact sports: A model-based investigation. Biomech. Model. Mechanobiol. 2014, 13, 1121–1136. [Google Scholar] [CrossRef]
- Talebanpour, A.; Smith, L. A Comparison between Simulated and Measured Human Brain Response under Mild Acceleration. In Proceedings of the 2017 ICROBI Conference, Antwerp, Belgium, 13–15 September 2017. [Google Scholar]
- Nahum, A.M.; Smith, R.; Ward, C.C. Intracranial Pressure Dynamics During Head Impact; SAE Technical Paper 770922; SAE: Warrendale, PA, USA, 1977. [Google Scholar] [CrossRef]
- Hardy, W.N.; Foster, C.D.; Mason, M.J.; Yang, K.H.; King, A.I.; Tashman, S. Investigation of Head Injury Mechanisms Using Neutral Density Technology and High-Speed Biplanar X-ray. Stapp Car Crash J. 2001, 45, 337–368. [Google Scholar]
- Hardy, W.N.; Mason, M.J.; Foster, C.D.; Shah, C.S.; Kopacz, J.M.; Yang, K.H.; King, A.I.; Bishop, J.; Bey, M.; Anderst, W.; et al. A study of the response of the human cadaver head to impact. Stapp Car Crash J. 2007, 51, 17–80. [Google Scholar] [PubMed]
- Nahum, A.M.; Smith, R.W. An Experimental Model for Closed Head Impact Injury; SAE Technical Paper 760825; SAE: Warrendale, PA, USA, 1976. [Google Scholar] [CrossRef]
- Trosseille, X.; Tarriére, C.; Lavaste, F.; Guillon, F.; Domont, A. Development of a F.E.M. of the Human Head According to a Specific Test Protocol; SAE Technical Paper 922527; SAE: Warrendale, PA, USA, 1992. [Google Scholar] [CrossRef]
- Guettler, A.J.; Ramachandra, R.; Bolte, J.; Hardy, W.N. Kinematics Response of the PMHS Brain to Rotational Loading of the Head: Development of Experimental Methods and Analysis of Preliminary Data; SAE Technical Paper 2018-01-0547; SAE: Warrendale, PA, USA, 2018. [Google Scholar] [CrossRef]
- Alshareef, A.; Giudice, J.S.; Forman, J.; Salzar, R.S.; Panzer, M.B. A novel method for quantifying human in situ whole brain deformation under rotational loading using sonomicrometry. J. Neurotrauma 2018, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Weickenmeier, J.; Kurt, M.; Ozkaya, E.; de Rooij, R.; Ovaert, T.C.; Ehman, R.L.; Butts Pauly, K.; Kuhl, E. Brain stiffens post mortem. J. Mech. Behav. Biomed. Mater. 2018, 84, 88–98. [Google Scholar] [CrossRef]
- Gadd, C.W. Use of a Weighted-Impulse Criterion for Estimating Injury Hazard; SAE Technical Paper 660793; SAE: Warrendale, PA, USA, 1966. [Google Scholar] [CrossRef]
- Hutchinson, J.; Kaiser, M.J.; Lankarani, H.M. The Head Injury Criterion (HIC) functional. Appl. Math. Comput. 1998, 96, 1–16. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, L.; Jiang, B.; Genthikatti, V.V.; Jin, X.; Zhu, F.; Makwana, R.; Gill, A.; Jandir, G.; Singh, A.; et al. Development of a Finite Element Human Head Model Partially Validated With Thirty Five Experimental Cases. J. Biomech. Eng. 2013, 135, 111002. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Kleiven, S.; Shah, C.S.; Hardy, W.N. A Reanalysis of Experimental Brain Strain Data: Implication for Finite Element Head Model Validation. Stapp Car Crash J. 2018, 62, 293–318. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Kleiven, S.; Hardy, W.N. Brain Strain from Motion of Sparse Markers. Stapp Car Crash J. 2019, 63, 1–27. [Google Scholar] [CrossRef]
- Kleiven, S. Evaluation of head injury criteria using a finite element model validated against experiments on localized brain motion, intracerebral acceleration, and intracranial pressure. Int. J. Crashworthiness 2006, 11, 65–79. [Google Scholar] [CrossRef]
- Gehre, C.; Gades, H.; Wernicke, P. Objective Rating of Signals Using Test and Simulation Responses. In Proceedings of the 21st International Technical Conference on the Enhanced Safety of Vehicles ESV, Stuttgart, Germany, 15–18 June 2009. [Google Scholar]
- Alshareef, A.; Giudice, S.; Forman, J.; Shedd, D.; Reynier, K.; Wu, T.; Panzer, M. Human Brain Deformation During Controlled Dynamic Rotation of the Head. J. Neurotrauma 2020, 37, 1546–1555. [Google Scholar] [CrossRef]
- Wu, T.; Alshareef, A.; Giudice, J.S.; Panzer, M.B. Explicit Modeling of White Matter Axonal Fiber Tracts in a Finite Element Brain Model. Ann. Biomed. Eng. 2019, 47, 1908–1922. [Google Scholar] [CrossRef]
- Anderson, E.D.; Giudice, J.S.; Wu, T.; Panzer, M.B.; Meaney, D.F. Predicting Concussion Outcome by Integrating Finite Element Modeling and Network Analysis. Front. Bioeng. Biotechnol. 2020, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Bayly, P.V.; Cohen, T.S.; Leister, E.P.; Ajo, D.; Leuthardt, E.C.; Genin, G.M. Deformation of the human brain induced by mild acceleration. J. Neurotrauma 2005, 22, 845–856. [Google Scholar] [CrossRef]
- Sabet, A.A.; Christoforou, E.; Zatlin, B.; Genin, G.M.; Bayly, P.V. Deformation of the human brain induced by mild angular head acceleration. J. Biomech. 2008, 41, 307–315. [Google Scholar] [CrossRef]
- Feng, Y.; Abney, T.M.; Okamoto, R.J.; Pless, R.B.; Genin, G.M.; Bayly, P.V. Relative brain displacement and deformation during constrained mild frontal head impact. J. R. Soc. Interface 2010, 7, 1677–1688. [Google Scholar] [CrossRef]
- Knutsen, A.K.; Magrath, E.; McEntee, J.E.; Xing, F.; Prince, J.L.; Bayly, P.V.; Butman, J.A.; Pham, D.L. Improved measurement of brain deformation during mild head acceleration using a novel tagged MRI sequence. J. Biomech. 2014, 47, 3475–3481. [Google Scholar] [CrossRef]
- Ji, S.; Zhao, W.; Ford, J.C.; Beckwith, J.G.; Bolander, R.P.; Greenwald, R.M.; Flashman, L.A.; Paulsen, K.D.; McAllister, T.W. Group-wise evaluation and comparison of white matter fiber strain and maximum principal strain in sports-related concussion. J. Neurotrauma 2015, 32, 441–454. [Google Scholar] [CrossRef]
- Saboori, P.; Sadegh, A. On the properties of brain sub arachnoid space and biomechanics of head impacts leading to traumatic brain injury. Adv. Biomech. Appl. 2014, 1, 253–267. [Google Scholar] [CrossRef]
- Saboori, P.; Walker, G. Brain Injury and Impact Characteristics. Ann. Biomed. Eng. 2019, 47, 1982–1992. [Google Scholar] [CrossRef]
- Puhulwelle Gamage, N.T.; Knutsen, A.K.; Pham, D.L.; Taberner, A.J.; Nash, M.P.; Nielsen, P.M. Abusive head trauma: Developing a computational adult head model to predict brain deformations under mild accelerations. In Computational Biomechanics for Medicine: From Algorithms to Models and Applications; Springer International Publishing: Berlin, Germany, 2017; pp. 147–157. [Google Scholar] [CrossRef]
- Chan, D.D.; Knutsen, A.K.; Lu, Y.C.; Yang, S.H.; Magrath, E.; Wang, W.T.; Bayly, P.V.; Butman, J.A.; Pham, D.L. Statistical Characterization of Human Brain Deformation during Mild Angular Acceleration Measured in Vivo by Tagged Magnetic Resonance Imaging. J. Biomech. Eng. 2018, 140. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.D.; Knutsen, A.K.; Xing, F.; Lu, Y.C.; Chan, D.; Pham, D.L.; Bayly, P.; Prince, J.L. 3-D Measurements of Acceleration-Induced Brain Deformation via Harmonic Phase Analysis and Finite-Element Models. IEEE Trans. Biomed. Eng. 2019, 66, 1456–1467. [Google Scholar] [CrossRef]
- Knutsen, A.K.; Gomez, A.D.; Gangolli, M.; Wang, W.T.; Chan, D.; Lu, Y.C.; Christoforou, E.; Prince, J.L.; Bayly, P.V.; Butman, J.A.; et al. In vivo estimates of axonal stretch and 3D brain deformation during mild head impact. Brain Multiphysics 2020, 100015. [Google Scholar] [CrossRef]
- Chen, Y.; Sutton, B.; Conway, C.; Broglio, S.P.; Ostoja-Starzewski, M. Brain Deformation Under Mild Impact: Magnetic Resonance Imaging-Based Assessment and Finite Element Study. Int. J. Numer. Anal. Model. Ser. B 2012, 3, 20–35. [Google Scholar]
- Chen, Y.; Ostoja-Starzewski, M. MRI-based finite element modeling of head trauma: Spherically focusing shear waves. Acta Mech. 2010, 213, 155–167. [Google Scholar] [CrossRef]
- Ganpule, S.; Daphalapurkar, N.P.; Ramesh, K.T.; Knutsen, A.K.; Pham, D.L.; Bayly, P.V.; Prince, J.L. A Three-Dimensional Computational Human Head Model That Captures Live Human Brain Dynamics. J. Neurotrauma 2017, 34, 2154–2166. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Daphalapurkar, N.P.; Knutsen, A.K.; Glaister, J.; Pham, D.L.; Butman, J.A.; Prince, J.L.; Bayly, P.V.; Ramesh, K.T. A 3D Computational Head Model Under Dynamic Head Rotation and Head Extension Validated Using Live Human Brain Data, Including the Falx and the Tentorium. Ann. Biomed. Eng. 2019, 47, 1923–1940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Z.; Liu, Y. The Material Point Method; Academic Press: Cambridge, MA, USA, 2017; p. 300. [Google Scholar] [CrossRef]
- Xing, F.; Woo, J.; Gomez, A.D.; Pham, D.L.; Bayly, P.V.; Stone, M.; Prince, J.L. Phase Vector Incompressible Registration Algorithm for Motion Estimation from Tagged Magnetic Resonance Images. IEEE Trans. Med Imaging 2017, 36, 2116–2128. [Google Scholar] [CrossRef] [PubMed]
- Badachhape, A.A.; Okamoto, R.J.; Durham, R.S.; Efron, B.D.; Nadell, S.J.; Johnson, C.L.; Bayly, P.V. The Relationship of Three-Dimensional Human Skull Motion to Brain Tissue Deformation in Magnetic Resonance Elastography Studies. J. Biomech. Eng. 2017, 139, 051002. [Google Scholar] [CrossRef] [PubMed]
- Badachhape, A.A.; Okamoto, R.J.; Johnson, C.L.; Bayly, P.V. Relationships between scalp, brain, and skull motion estimated using magnetic resonance elastography. J. Biomech. 2018, 73, 40–49. [Google Scholar] [CrossRef]
- Hiscox, L.V.; Johnson, C.L.; Barnhill, E.; McGarry, M.D.; Huston, J.; Van Beek, E.J.; Starr, J.M.; Roberts, N. Magnetic resonance elastography (MRE) of the human brain: Technique, findings and clinical applications. Phys. Med. Biol. 2016, 61, R401–R437. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, K.H.; Dwarampudi, R.; Omori, K.; Li, T.; Chang, K.; Hardy, W.N.; Khalil, T.B.; King, A.I. Recent Advances in Brain Injury Research: A New Human Head Model Development and Validation. Stapp Car Crash J. 2001, 45, 369–394. [Google Scholar]
- Viano, D.C.; Casson, I.R.; Pellman, E.J.; Zhang, L.; King, A.I.; Yang, K.H. Concussion in Professional Football: Brain Responses by Finite Element Analysis: Part 9. Neurosurgery 2005, 57, 891–916. [Google Scholar] [CrossRef]
- Horgan, T.J.; Gilchrist, M.D. The creation of three-dimensional finite element models for simulating head impact biomechanics. Int. J. Crashworthiness 2003, 8, 353–366. [Google Scholar] [CrossRef]
- Ho, J.; von Holst, H.; Kleiven, S. Automatic generation and validation of patient-specific finite element head models suitable for crashworthiness analysis. Int. J. Crashworthiness 2009, 14, 555–563. [Google Scholar] [CrossRef]
- Takhounts, E.G.; Ridella, S.A.; Hasija, V.; Tannous, R.E.; Campbell, J.Q.; Malone, D.; Danelson, K.; Stitzel, J.; Rowson, S.; Duma, S. Investigation of traumatic brain injuries using the next generation of simulated injury monitor (SIMon) finite element head model. Stapp Car Crash J. 2008, 52, 1–31. [Google Scholar]
- McAllister, T.W.; Ford, J.C.; Ji, S.; Beckwith, J.G.; Flashman, L.A.; Paulsen, K.; Greenwald, R.M. Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Ann. Biomed. Eng. 2012, 40, 127–140. [Google Scholar] [CrossRef]
- Tse, K.M.; Tan, L.B.; Lee, S.J.; Lim, S.P.; Lee, H.P. Development and validation of two subject-specific finite element models of human head against three cadaveric experiments. Int. J. Numer. Methods Biomed. Eng. 2014, 30, 397–415. [Google Scholar] [CrossRef]
- Willinger, R.; Kang, H.S.; Diaw, B. Three-dimensional human head finite-element model validation against two experimental impacts. Ann. Biomed. Eng. 1999, 27, 403–410. [Google Scholar] [CrossRef]
- Sahoo, D.; Deck, C.; Willinger, R. Development and validation of an advanced anisotropic visco-hyperelastic human brain FE model. J. Mech. Behav. Biomed. Mater. 2014, 33, 24–42. [Google Scholar] [CrossRef]
- Zhao, W.; Ruan, S.; Ji, S. Brain pressure responses in translational head impact: A dimensional analysis and a further computational study. Biomech. Model. Mechanobiol. 2015, 14, 753–766. [Google Scholar] [CrossRef]
- Garimella, H.T.; Kraft, R.H. Modeling the mechanics of axonal fiber tracts using the embedded finite element method. Int. J. Numer. Methods Biomed. Eng. 2017, 33, e2823. [Google Scholar] [CrossRef]
- Miller, L.E.; Urban, J.E.; Stitzel, J.D. Development and validation of an atlas-based finite element brain model. Biomech. Model. Mechanobiol. 2016, 15, 1201–1214. [Google Scholar] [CrossRef]
- Ghajari, M.; Hellyer, P.J.; Sharp, D.J. Computational modelling of traumatic brain injury predicts the location of chronic traumatic encephalopathy pathology. Brain J. Neurol. 2017, 140, 333–343. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Railkar, A.; Awamori, S.; Kokeguchi, A.; Amamori, I.; Katagiri, M.; Yoshii, K. Intracranial Brain Motion Measurement in Frontal Sled Tests by Using a New Anthropometric Test Dummy Head Capable of Direct Brain Motion Evaluation and Visualisation. In Proceedings of the ICROBI Conference, Antwerp, Belgium, 13–15 September 2017; pp. 285–295. [Google Scholar]
- Fernandes, F.A.O.; Alves de Sousa, R.J.; Ptak, M. Head Injury Simulation in Road Traffic Accidents; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Ratajczak, M.; Ptak, M.; Chybowski, L.; Gawdzińska, K.; Bedziński, R. Material and structural modeling aspects of brain tissue deformation under dynamic loads. Materials 2019, 12, 271. [Google Scholar] [CrossRef]
- Khanuja, T.; Unni, H.N. Intracranial pressure-based validation and analysis of traumatic brain injury using a new three-dimensional finite element human head model. J. Eng. Med. 2020, 2020, 3–15. [Google Scholar] [CrossRef]
- Hassan, M.; Taha, Z.; Hasanuddin, I.; Majeed, A.; Mustafa, H.; Othman, N.A. A simplified human head finite element model for brain injury assessment of blunt impacts. J. Mech. Eng. Sci. 2020, 14, 6538–6547. [Google Scholar] [CrossRef]
- Trotta, A.; Clark, J.M.; McGoldrick, A.; Gilchrist, M.D.; Annaidh, A.N. Biofidelic finite element modelling of brain trauma: Importance of the scalp in simulating head impact. Int. J. Mech. Sci. 2020, 173, 105448. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Z.; Kleiven, S. A detailed and personalizable head model with axons for brain injury prediction: Sensitivity of brain strain to inter-subject variability of the brain and white matter tract morphology. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ji Lab. The Worcester Head Injury Model. 2020. Available online: http://labs.wpi.edu/jilab/tbi/ (accessed on 9 October 2020).
- Cai, Z.; Xia, Y.; Bao, Z.; Mao, H. Creating a human head finite element model using a multi-block approach for predicting skull response and brain pressure. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Levadnyi, I.; Awrejcewicz, J.; Zhang, Y.; Goethel, M.F.; Gu, Y. Finite Element Analysis of Impact for Helmeted and Non-helmeted Head. J. Med Biol. Eng. 2018, 38, 587–595. [Google Scholar] [CrossRef]
- Taha, Z.; Hassan, M.H.A. Parametric Analysis of the Influence of Elastomeric Foam on the Head Response during Soccer Heading Manoeuvre. Procedia Eng. 2016, 147, 139–144. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, Z. Understanding how a sport-helmet protects the head from closed injury by virtual impact tests. Bio-Med. Mater. Eng. 2017, 28, 279–291. [Google Scholar] [CrossRef]
- Wu, J.Z.; Pan, C.S.; Wimer, B.M.; Rosen, C.L. Finite element simulations of the head-brain responses to the top impacts of a construction helmet: Effects of the neck and body mass. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 58–68. [Google Scholar] [CrossRef]
- Mohotti, D.; Fernando, P.L.; Zaghloul, A. Evaluation of possible head injuries ensuing a cricket ball impact. Comput. Methods Programs Biomed. 2018, 158, 193–205. [Google Scholar] [CrossRef]
- Ruan, J.S.; Khalil, T.; King, A.I. Human head dynamic response to side impact by finite element modeling. J. Biomech. Eng. 1991, 113, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, B.R.; Morgan, R.M.; Eppinger, R.H. Durability, repeatability and reproducibility of the NHTSA side impact dummy. In Proceedings of the 27th Stapp Car Crash Conference, Warrendale, PA, USA, 17–19 October 1983; pp. 299–310. [Google Scholar] [CrossRef]
- Kimpara, H.; Nakahira, Y.; Iwamoto, M.; Miki, K.; Ichihara, K.; Kawano, S.i.; Taguchi, T. Investigation of anteroposterior head-neck responses during severe frontal impacts using a brain-spinal cord complex FE model. Stapp Car Crash J. 2006, 50, 509–544. [Google Scholar] [PubMed]
- International Organisation for Standards. ISO/TR9790 Road Vehicles—Anthropomorphic Side Impact Dummy— Lateral Impact Response Requirements to Assess the Biofidelity of the Dummy; Technical Report; ISO:29828; ISO: Geneva, Switzerland, 1999. [Google Scholar]
- Vavalle, N.A.; Jelen, B.C.; Moreno, D.P.; Stitzel, J.D.; Gayzik, F.S. Traffic Injury Prevention An Evaluation of Objective Rating Methods for Full-Body Finite Element Model Comparison to PMHS Tests. Traffic Inj. Prev. 2013, 14, S87–S94. [Google Scholar] [CrossRef][Green Version]
- International Organisation for Standards. ISO/TR 16250:2013 Road Vehicles—Objective Rating Metrics for Dynamic Systems; Technical Report; ISO:56015; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- Giordano, C.; Kleiven, S. Development of an Unbiased Validation Protocol to Assess the Biofidelity of Finite Element Head Models used in Prediction of Traumatic Brain Injury. Stapp Car Crash J. 2016, 60, 363–471. [Google Scholar]
- Vavalle, N.A.; Davis, M.L.; Stitzel, J.D.; Gayzik, F.S. Quantitative Validation of a Human Body Finite Element Model Using Rigid Body Impacts. Ann. Biomed. Eng. 2015, 43, 2163–2174. [Google Scholar] [CrossRef]
- Park, G.; Kim, T.; Panzer, M.B.; Crandall, J.R. Validation of Shoulder Response of Human Body Finite-Element Model (GHBMC) Under Whole Body Lateral Impact Condition. Ann. Biomed. Eng. 2016, 44, 2558–2576. [Google Scholar] [CrossRef]
- Decker, W.; Koya, B.; Davis, M.L.; Gayzik, F.S. Modular use of human body models of varying levels of complexity: Validation of head kinematics. Traffic Inj. Prev. 2017, 18, S155–S160. [Google Scholar] [CrossRef]
- Katagiri, M.; Zhao, J.; Lee, S.; Moorhouse, K.; Kang, Y.S. Biofidelity Evaluation of GHBMC Male Occupant Models in Rear Impacts. In Proceedings of the ICROBI Conference, Florence, Italy, 11–13 September 2019; pp. 19–52. [Google Scholar]
- Giordano, C.; Kleiven, S. Evaluation of Axonal Strain as a Predictor for Mild Traumatic Brain Injuries Using Finite Element Modeling. Stapp Car Crash J. 2014, 58, 29–61. [Google Scholar] [CrossRef]
- Yang, K.H.; Mao, H. Modelling of the Brain for Injury Simulation and Prevention. In Biomechanics of the Brain; Springer: Cham, Switzerland, 2019; pp. 97–133. [Google Scholar] [CrossRef]
- Atsumi, N.; Nakahira, Y.; Tanaka, E.; Iwamoto, M. Human Brain Modeling with Its Anatomical Structure and Realistic Material Properties for Brain Injury Prediction. Ann. Biomed. Eng. 2018, 46, 736–748. [Google Scholar] [CrossRef]
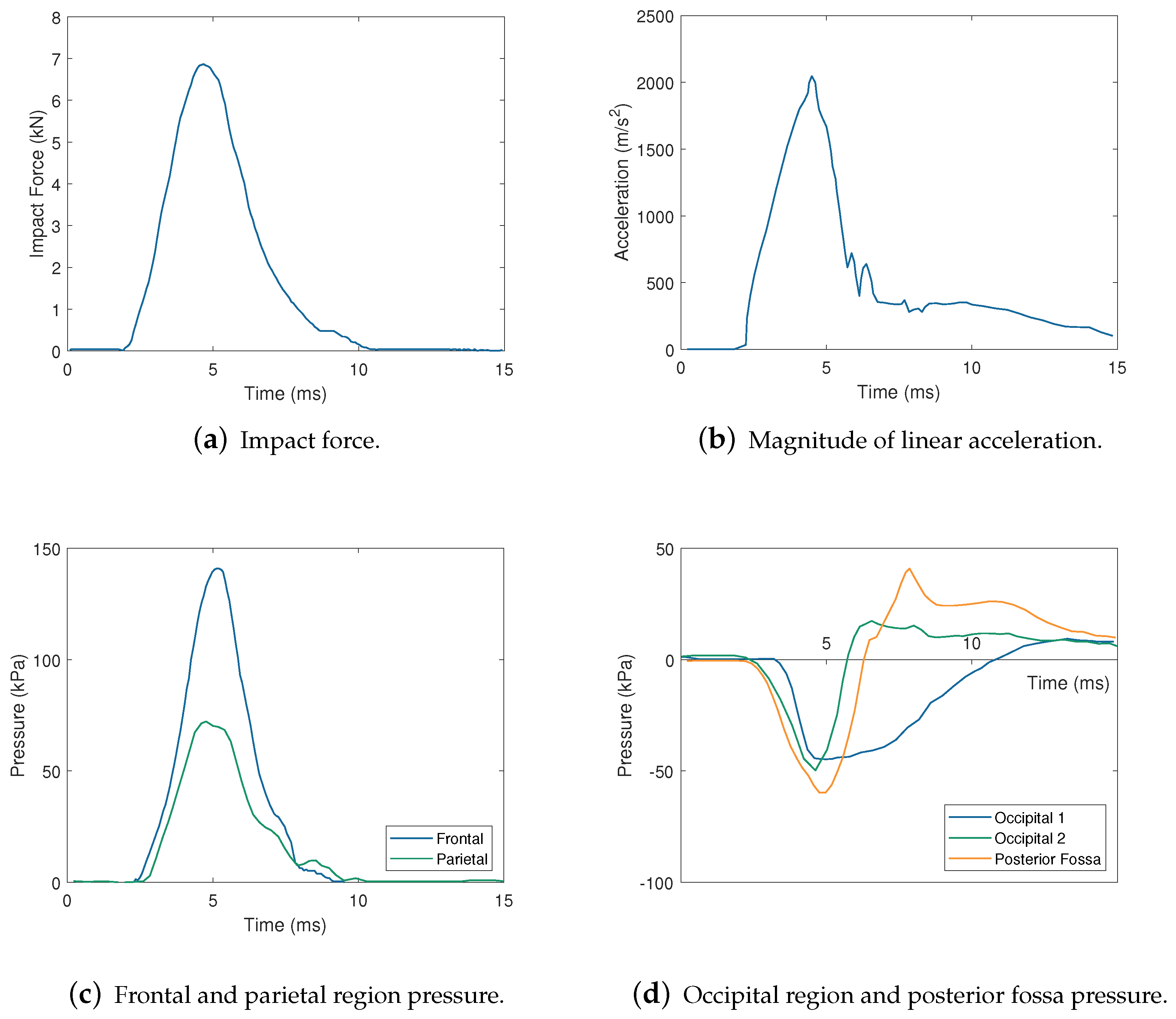
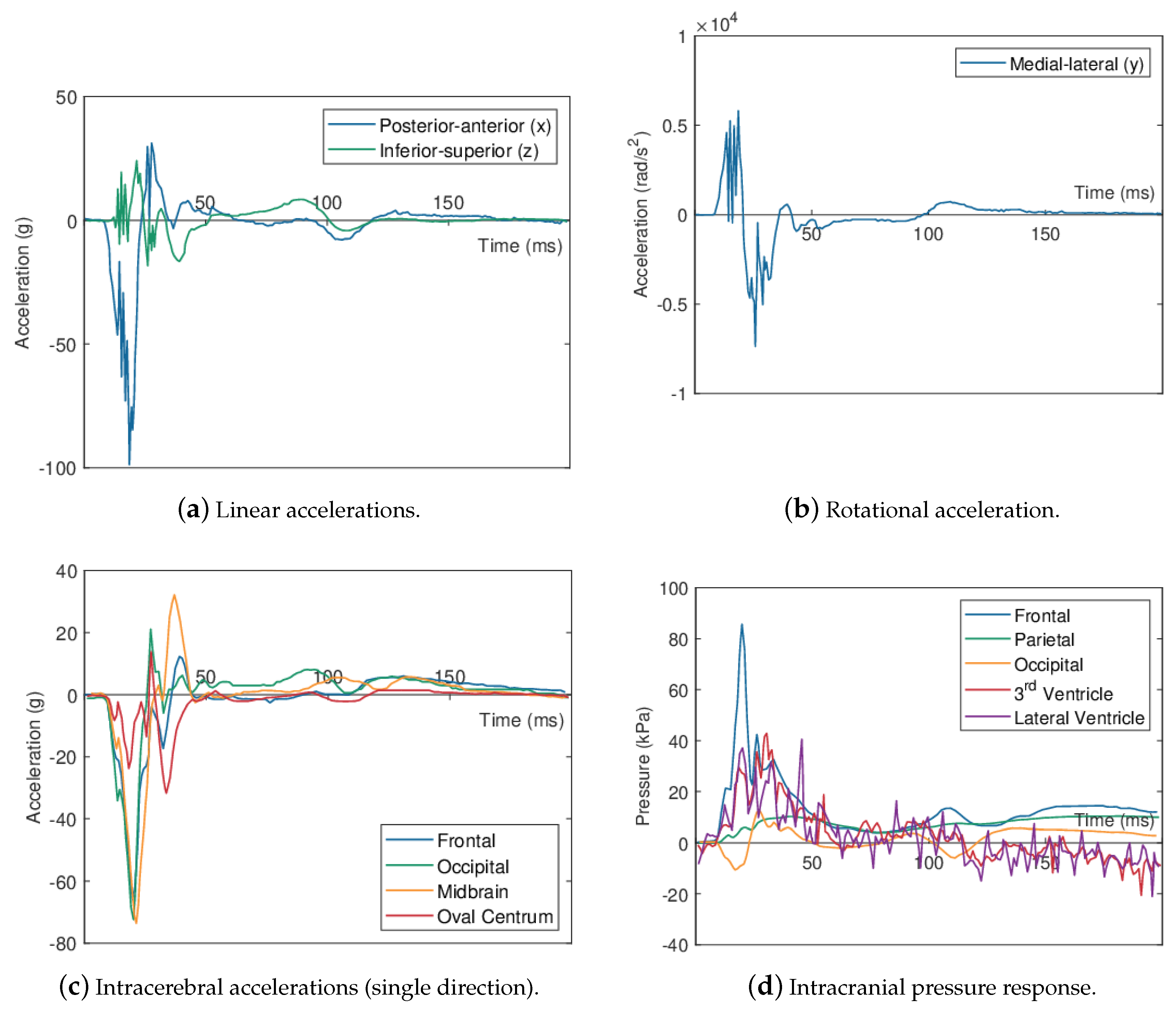
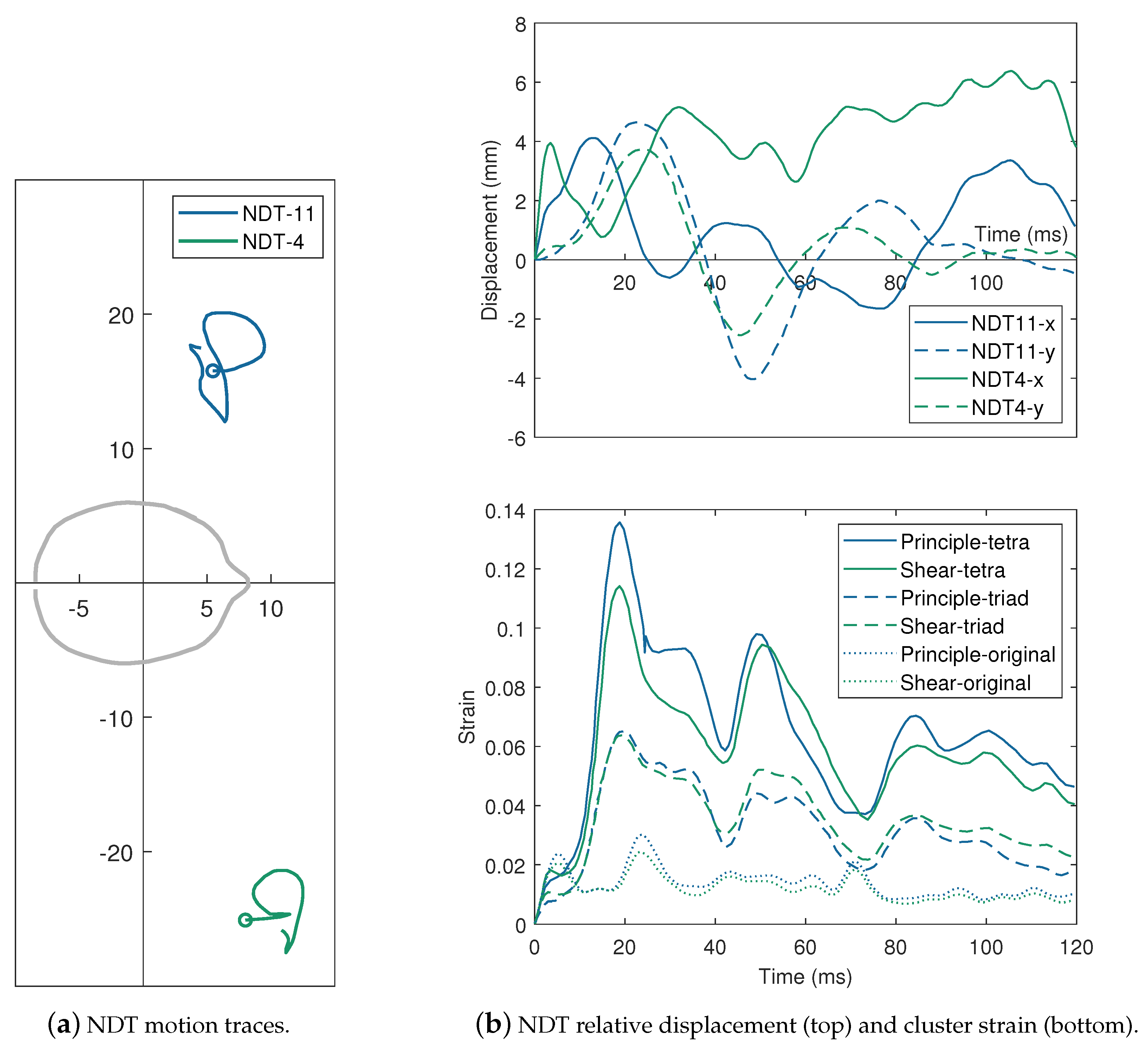
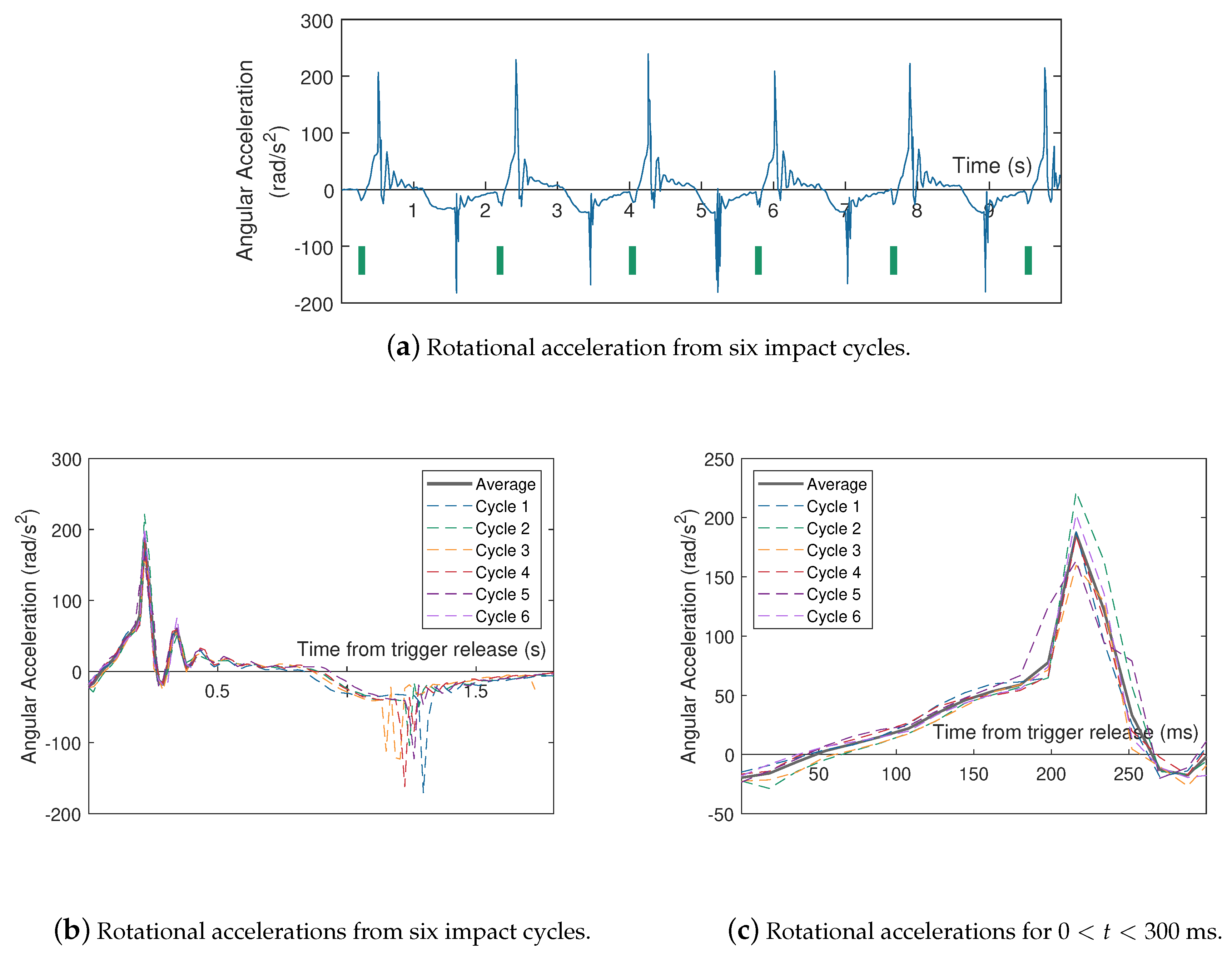
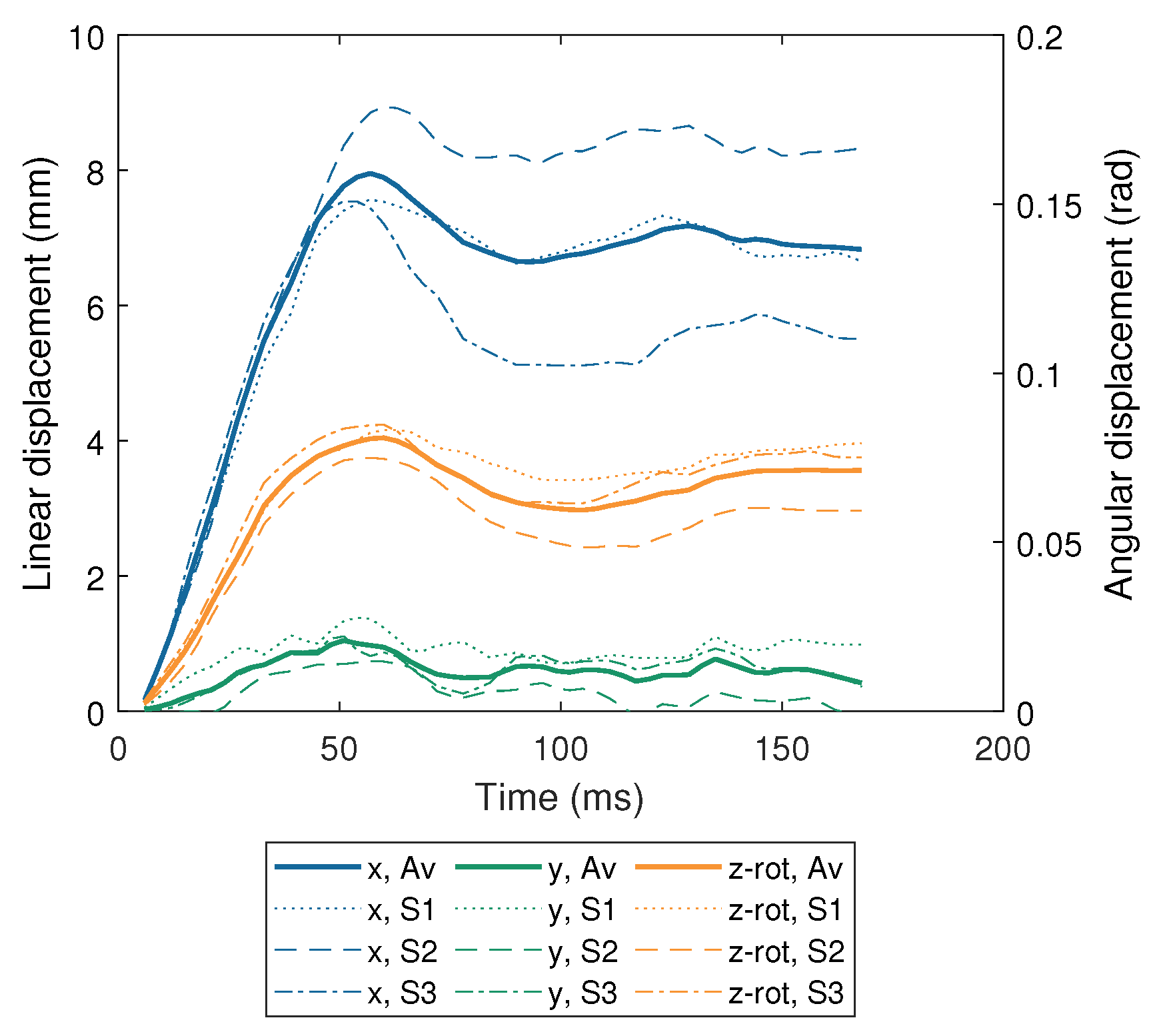
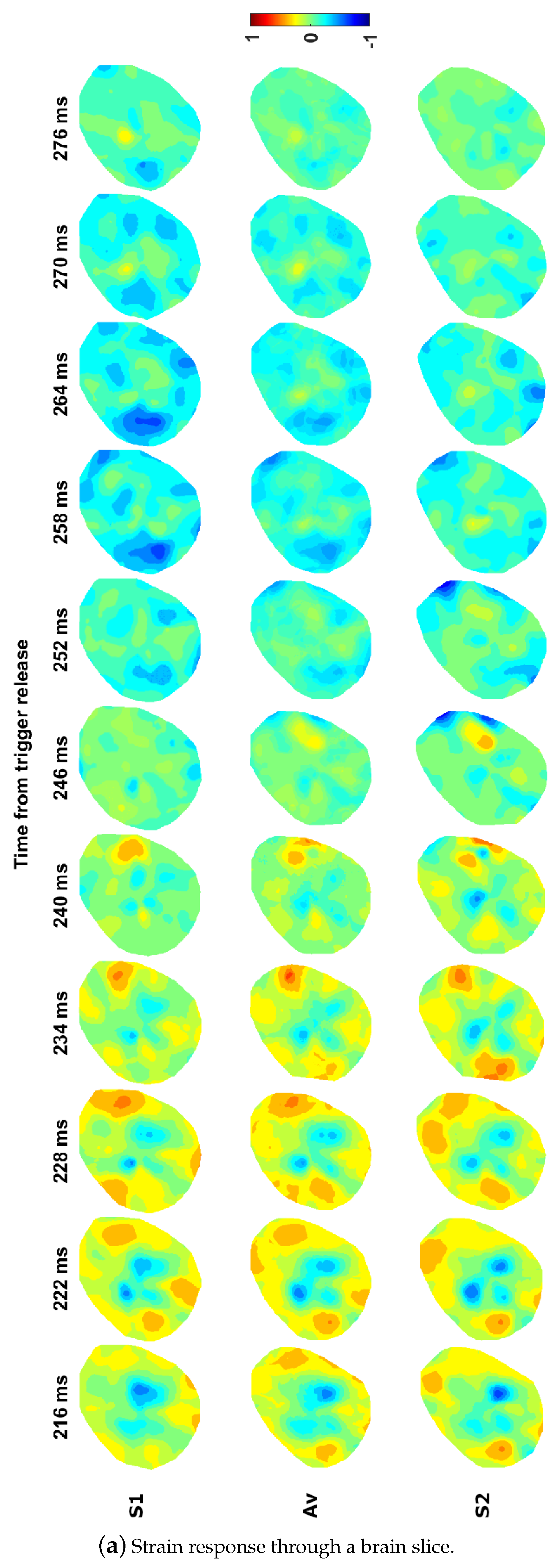
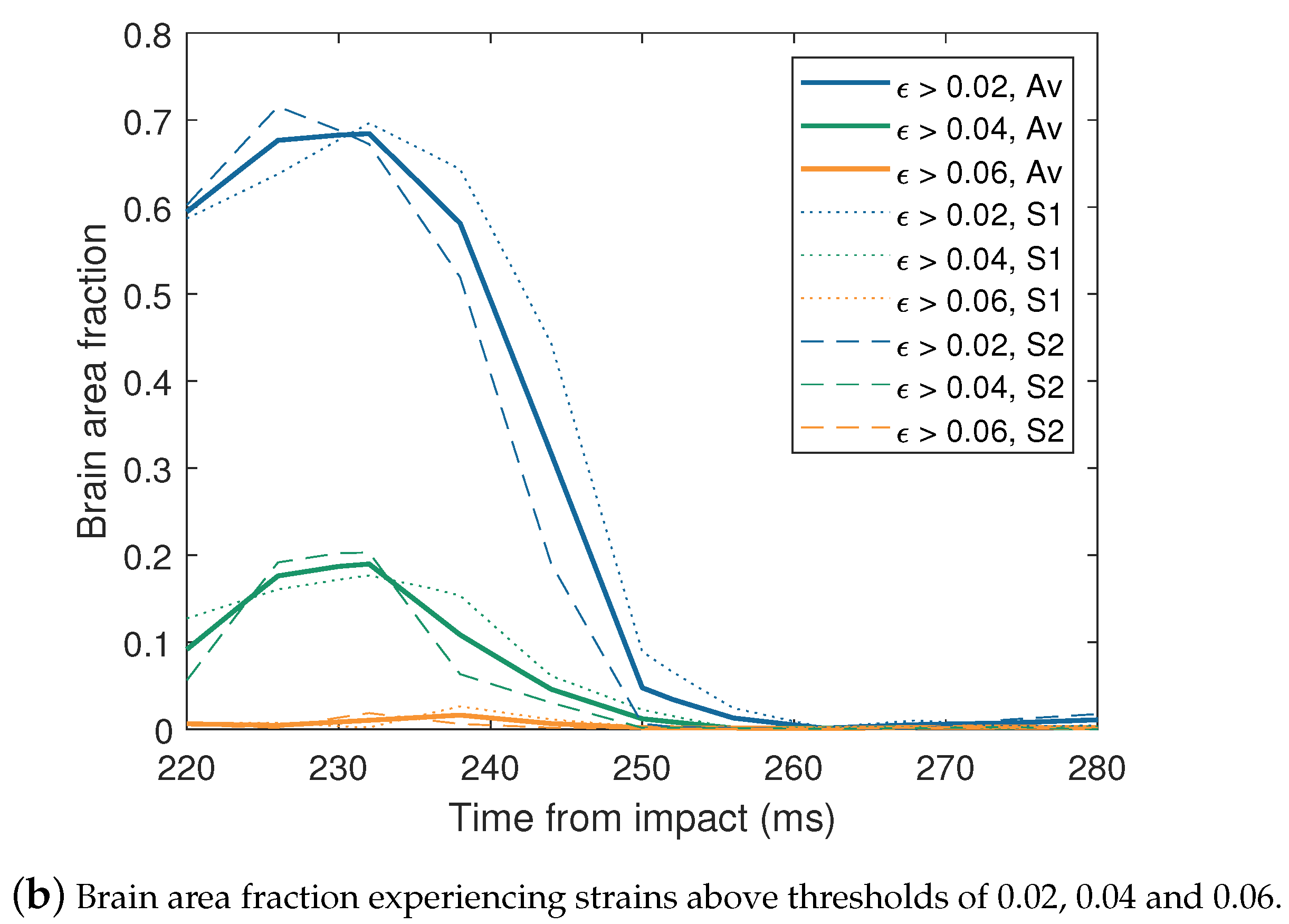
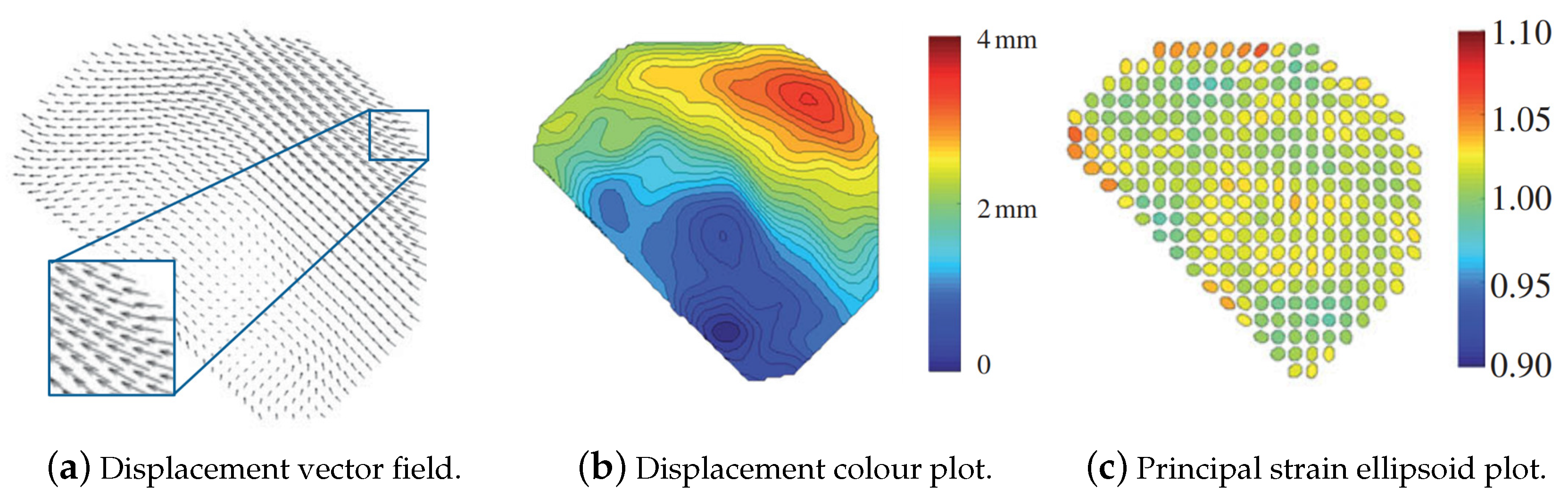
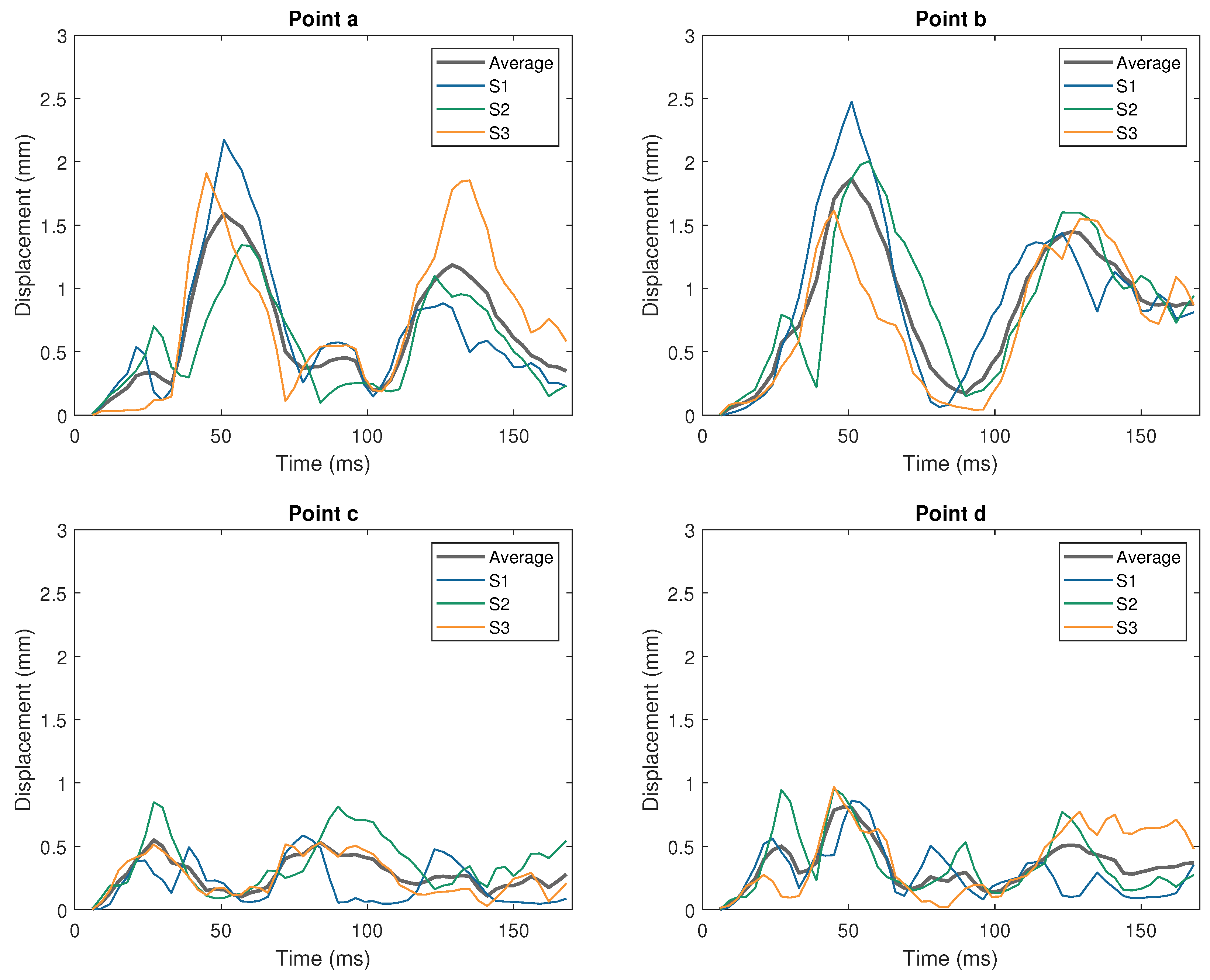
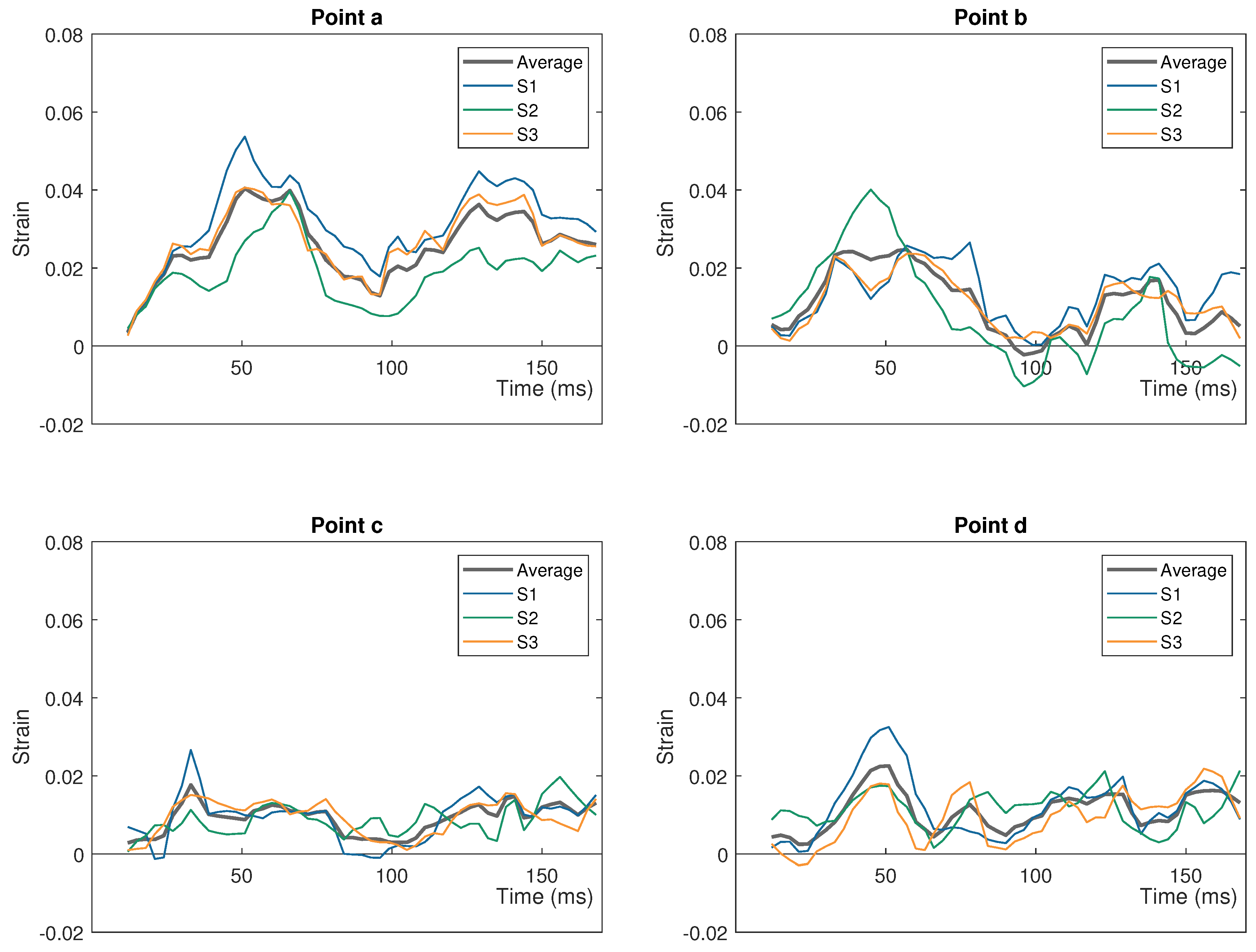
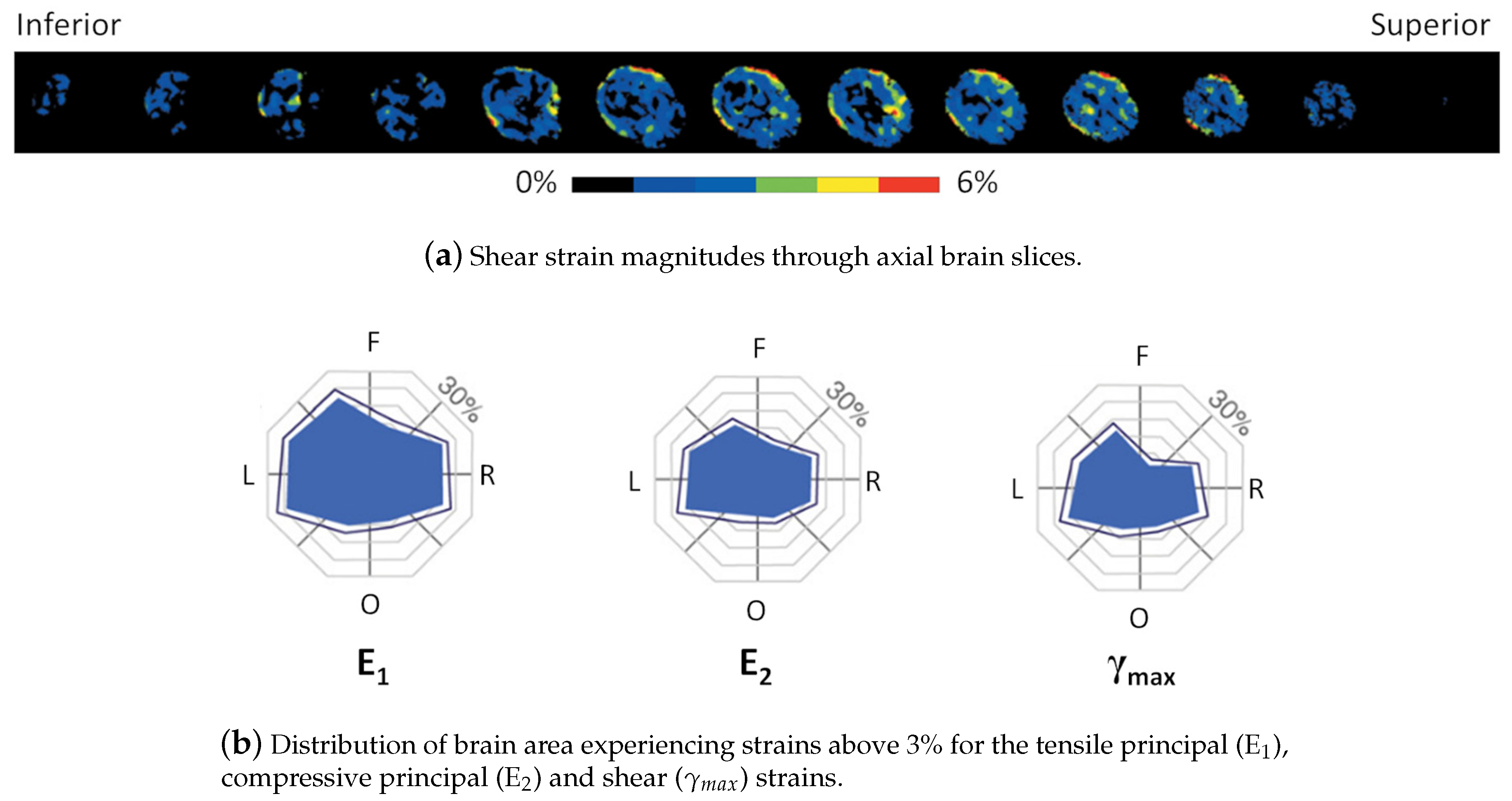
| Impactor Properties | Pressures (kPa) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment # | Mass (kg) | Velocity (m/s) | Impact Force (kN) | Acceleration (m/s) | Frontal | Parietal | Occipital 1 | Occipital 2 | Posterior Fossa | Carotid Siphon |
| Series 1 | ||||||||||
| 36 | 136 | 79 | − | |||||||
| 37 | 141 | 74 | − | |||||||
| 38 | 139 | 66 | − | − | ||||||
| 41 | 428 | 189 | 114 | − | 47 | |||||
| 42 | − | 9 | − | − | 73 | |||||
| 43 | 271 | 222 | 64 | − | 108 | |||||
| 44 | 102 | 20 | 15 | − | 115 | |||||
| 54 | 275 | 181 | 33 | − | 48 | |||||
| Series 2 | ||||||||||
| 46 | − | − | − | − | − | − | ||||
| 47 | − | − | − | − | − | − | ||||
| 48 | − | − | − | − | − | − | ||||
| 49 | − | − | − | − | − | − | ||||
| 50 | − | − | − | − | − | − | ||||
| 51 | − | − | − | − | − | − | ||||
| 52 | − | − | − | − | − | − | ||||
| Impact Properties | Acceleration | Pressure (kPa) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test ID | Impactor | Location | Velocity (m/s) | Linear x (g) | Linear z (g) | Rotational y (rad/s) | Frontal | Parietal | Occipital | Lateral Ventricle | Third Ventricle |
| MS408-1 | Plate | Thorax | 5 | 9 | 1765 | 7 | 12 | − | − | ||
| MS408-2 | Styrofoam | Forehead | 5 | 6 | 6 | 2 | − | − | |||
| MS408-3 | Wheel (1) | Face | 5 | 31 | 10 | 5 | 30 | − | − | ||
| MS428-1 | Styrofoam | Forehead | 6 | 16 | >60 | 12 | 30 | 25 | |||
| MS438-2 | Wheel (2) | Face | 7 | 27 | 88 | 11 | 32 | 35 | |||
| MS429-1 | Wheel (2) | Face | 7 | − | − | − | 85 | − | − | − | |
| Outputs Reported | |||||||
|---|---|---|---|---|---|---|---|
| Cadaver | Test | Impact Properties | Kinematics | NDT Motion | Relative Braint Displacement | Brain Strain | Brain Pressure |
| Hardy et al. [35] | |||||||
| C731 | T2 | Oa | 🗸 | ||||
| T3 | Oa | 🗸 | |||||
| C755 | T2 | Oa | 🗸 | 🗸 | 🗸 | ||
| T3 | Oa | 🗸 | 🗸 | 🗸 | |||
| T4 | Oa | 🗸 | |||||
| T5 | Fa | 🗸 | 🗸 | 🗸 | |||
| C383 | T1 | Fd | 🗸 | 🗸 | 🗸 | ||
| T2 | Fd | 🗸 | |||||
| T3 | Fd | 🗸 | 🗸 | 🗸 | |||
| T4 | Od | 🗸 | 🗸 | 🗸 | |||
| Hardy et al. [36] | |||||||
| C288 | T1 | Od | 🗸 | 🗸 | 🗸 | 🗸 | |
| T2 | Od | 🗸 | 🗸 | 🗸 | 🗸 | ||
| T3 | Od | 🗸 | 🗸 | 🗸 | 🗸 | 🗸 | |
| T4 | Od | 🗸 | 🗸 | 🗸 | |||
| C241 | T1 | Od | 🗸 | 🗸 | |||
| T2 | Od | 🗸 | 🗸 | ||||
| T3 | Od | 🗸 | 🗸 | ||||
| T4 | Od | 🗸 | 🗸 | ||||
| T5 | Od | 🗸 | 🗸 | 🗸 | |||
| T6 | Od | 🗸 | 🗸 | 🗸 | |||
| C015 | T1 | Od | |||||
| T2 | Od | ||||||
| C064 | T1 | Od | 🗸 | ||||
| T2 | Od | 🗸 | 🗸 | 🗸 | 🗸 | ||
| T3 | Od | ||||||
| T4 | Od | ||||||
| C380 | T1 | Td | 🗸 | 🗸 | 🗸 | 🗸 | 🗸 |
| T2 | Pd | 🗸 | 🗸 | 🗸 | 🗸 | 🗸 | |
| T3 | Td | 🗸 | 🗸 | 🗸 | 🗸 | 🗸 | |
| T4 | Td | 🗸 | 🗸 | 🗸 | 🗸 | 🗸 | |
| T5 | Pd | 🗸 | 🗸 | 🗸 | 🗸 | 🗸 | |
| T6 | Td | 🗸 | 🗸 | 🗸 | 🗸 | 🗸 | |
| C393 | T1 | Td | 🗸 | ||||
| T2 | Td | 🗸 | |||||
| T3 | Td | 🗸 | |||||
| T4 | Td | 🗸 | 🗸 | 🗸 | 🗸 | 🗸 | |
| Validation Tests | ||||||
|---|---|---|---|---|---|---|
| Name | Nahum | Trosseille | Hardy et al. [35] | Hardy et al. [36] | Other | References |
| WSUBIM | 36, 37, 38, 43, 44, 54 | MS428-2 | C291-T1, C383-T1 | [72,73] | ||
| UCDBTM V1.0 | 37 | MS428-2 | C383-T4 | [21,74] | ||
| KTH | 37 | MS428-2 | C291-T1, C383-T1, C755-T2 | [47] | ||
| KTH-voxel | C755-T2 | [75] | ||||
| SIMon | 37 | MS428-2 | C291-T1, C383-T1, C755-T2 | [76] | ||
| DSSM | C288-T1, C380-T5 | [77] | ||||
| GHBMC V1.0 | 36, 37, 38, 43, 44, 54 | MS428-2 | C383-T3, C755-T2 | C241-T1,T6, C380-T3,T4,T5, C393-T4 | [37] | [44] |
| Nanjing | 37 | MS428-2 | C383-T1 | [18,78] | ||
| SUFEHM | 37 | MS428-2 | C383-T1,T3,T4, C755-T2,T3,T5 | C288-T1,T2, C380-T2,T3,T5 | [79,80] | |
| Manhattan | [54] | [57,58] | ||||
| WHIM * | 37 | MS428-2 | C383-T1, C755-T2 | C393-T4 | [53] | [56,81] |
| THUMS | 37 | MS428-2 | C291-T1, C383-T1, C755-T2 | [25] | ||
| Pennsylvania | 37 | C383-T1, C755-T2 | C393-T4 | [82] | ||
| ABM | C291-T1, C383-T1, C755-T2 | [83] | ||||
| Imperial | None found published | [84] | ||||
| Tokyo | C755-T2, C383-T1 | [85] | ||||
| YEAHM | 37 | [86] | ||||
| Head | C755-T2 | [87] | ||||
| Hyderbad | 37 | MS428-2 | [88] | |||
| GHBMC V2.0 | C291-T1, C755-T2, C383-T1,T3,T4 | [40] | [50] | |||
| Malaysia | 37 | [89] | ||||
| UCDBTM V2.0 | C755-T2 | C380-T5 | [90] | |||
| Adapt | 37 | C288-T3, C380-T1,T2,T3,T4,T6, C393-T3 | [91] | |||
| Classification | Correlation Score Range |
|---|---|
| Excellent | |
| Good | |
| Fair | |
| Marginal | |
| Unacceptable |
| Peak Accelerations | |||||
|---|---|---|---|---|---|
| Test | Reference | Impact Location | Linear (g) | Rotational (rad/s) | Weight |
| Intracranial Pressure | |||||
| C288-T3 | Hardy et al. [36] | Occipital | 236 | 24,206 | |
| C241-T6 | Hardy et al. [36] | Occipital | 127 | 5792 | |
| C064-T4 | Hardy et al. [36] | Occipital | 122 | 4456 | |
| C380-T4 | Hardy et al. [36] | Temporal | 196 | 14,962 | |
| C380-T5 | Hardy et al. [36] | Parietal | 77 | 6358 | |
| C380-T6 | Hardy et al. [36] | Parietal | 147 | 9797 | |
| C393-T3 | Hardy et al. [36] | Temporal | 159 | 11,584 | |
| C393-T4 | Hardy et al. [36] | Temporal | 180 | 9671 | |
| Relative Brain Displacement | |||||
| C755-T2 | Hardy et al. [35] | Occipital | 22 | 1882 | |
| C755-T5 | Hardy et al. [35] | Frontal | 12 | 843 | |
| C383-T1 | Hardy et al. [35] | Frontal | 62 | 2592 | |
| C383-T4 | Hardy et al. [35] | Occipital | 108 | 10,364 | |
| C288-T3 | Hardy et al. [36] | Occipital | 236 | 24,206 | |
| C241-T5 | Hardy et al. [36] | Occipital | 194 | 8688 | |
| C241-T6 | Hardy et al. [36] | Occipital | 127 | 5792 | |
| C064-T4 | Hardy et al. [36] | Occipital | 122 | 4456 | |
| C380-T4 | Hardy et al. [36] | Temporal | 196 | 14,962 | |
| C380-T5 | Hardy et al. [36] | Parietal | 77 | 6358 | |
| C380-T6 | Hardy et al. [36] | Parietal | 147 | 9797 | |
| C393-T3 | Hardy et al. [36] | Temporal | 159 | 11,584 | |
| C393-T4 | Hardy et al. [36] | Temporal | 180 | 9671 | |
| Brain Deformation | |||||
| C288-T3 | Hardy et al. [36] | Occipital | 236 | 24,206 | |
| C241-T5 | Hardy et al. [36] | Occipital | 194 | 8688 | |
| C241-T6 | Hardy et al. [36] | Occipital | 127 | 5792 | |
| C380-T4 | Hardy et al. [36] | Temporal | 196 | 14,962 | |
| C380-T5 | Hardy et al. [36] | Parietal | 77 | 6358 | |
| C380-T6 | Hardy et al. [36] | Parietal | 147 | 9797 | |
| C393-T3 | Hardy et al. [36] | Temporal | 159 | 11,584 | |
| C393-T4 | Hardy et al. [36] | Temporal | 180 | 9671 | |
| Validation Criteria | |||
|---|---|---|---|
| CORA Parameter | Intracranial Pressure | Relative Brain Displacement | Brain Deformation |
| A_THRES | |||
| B_THRES | |||
| A_VAL | |||
| B_DELTA_END | |||
| T_MIN/MAX | (min) | (min) | |
| D_MIN/MAX | |||
| INT_MIN | |||
| K_V/P/G | |||
| G_V/P/G | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGill, K.; Teixeira-Dias, F.; Callanan, A. A Review of Validation Methods for the Intracranial Response of FEHM to Blunt Impacts. Appl. Sci. 2020, 10, 7227. https://doi.org/10.3390/app10207227
McGill K, Teixeira-Dias F, Callanan A. A Review of Validation Methods for the Intracranial Response of FEHM to Blunt Impacts. Applied Sciences. 2020; 10(20):7227. https://doi.org/10.3390/app10207227
Chicago/Turabian StyleMcGill, K., F. Teixeira-Dias, and A. Callanan. 2020. "A Review of Validation Methods for the Intracranial Response of FEHM to Blunt Impacts" Applied Sciences 10, no. 20: 7227. https://doi.org/10.3390/app10207227
APA StyleMcGill, K., Teixeira-Dias, F., & Callanan, A. (2020). A Review of Validation Methods for the Intracranial Response of FEHM to Blunt Impacts. Applied Sciences, 10(20), 7227. https://doi.org/10.3390/app10207227






