Bone Healing Evaluation Following Different Osteotomic Techniques in Animal Models: A Suitable Method for Clinical Insights
Abstract
1. Historical Background
2. Osteotomy in Clinical Practice
- End result
- Type
- Approach
- Incision site
3. Osteotomy-Related Tissue Damage
4. How Bone Healing Occurs after Bone Injury
5. Which Animal Model Is Suitable for Bone Investigation of Osteotomic Effects?
- Reduction: the number of animals involved must be the lowest necessary to achieve scientific evidence;
- Refinement: animals’ suffering must be kept to a minimum;
- The type and the aim of the study
- The osteotomy device used.
- The specific bone site and its characteristics (femur, tibia, calvaria, mandible, vertebra)
- Bone macroscopic and microscopic features, including size and biomechanical properties
- Bone composition and density
- Bone turnover rates
- Specific conditions for bone healing, such as the critical size defect
- Costs and aspects regarding the management of the animal (including acquisition costs, housing costs and the need for specifically trained staff).
- The osteotomy instruments that we wanted to test (two different piezosurgical devices and one conventional rotary osteotomy device), which required high dense bone. We consequently excluded mice, dog alveolar bone and rabbit mandible or femur.
- The space that we needed in order to perform several osteotomic lines, which helped us in lowering the number of animals to sacrifice and led us to exclude mouse or rat as potential animal models due to their small size (Reduction in “the three Rs” [70]);
- The European Communities Council Directive, which led us to exclude large animal models (sheep, pork, dog) on an ethical basis [8].
6. Osteotomic Investigation with Rotational Instrument and Piezosurgical Devices: Our Experience
7. What Kind of Information Can We Obtain from In Vivo and Ex Vivo Studies of Osteotomy?
8. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Luvar, M.; Kanthan, S.R.; Roshan, G.; Saw, A. Pattern of Cortical Fracture following Corticotomy for Distraction Osteogenesis. Malays. Orthop. J. 2015, 9, 35–39. [Google Scholar]
- Dabis, J.; Templeton-Ward, O.; Lacey, A.E.; Narayan, B.; Trompeter, A. The history, evolution and basic science of osteotomy techniques. Strateg. Trauma Limb Reconstr. 2017, 12, 169–180. [Google Scholar]
- Green, S.A. Basic Ilizarov Techniques. Tech. Orthop. 1990, 5, 41–52. [Google Scholar]
- Wardak, M.M.; Wardak, E. Percutaneous gigli saw osteotomy. Oper. Orthop. Traumatol. 2010, 22, 414–420. [Google Scholar] [CrossRef]
- Eidelman, M.; Katzman, A.; Zaidman, M.; Keren, Y. Deformity correction using supramalleolar gigli saw osteotomy and Taylor spatial frame: How to perform this osteotomy safely? J. Pediatr. Orthop. Part B 2011, 20, 318–322. [Google Scholar] [CrossRef]
- Makhdoom, A.; Kumar, J.; Siddiqui, A.A. Ilizarov External Fixation: Percutaneous Gigli Saw Versus Multiple Drill-hole Osteotomy Techniques for Distraction Osteogenesis. Cureus 2019, 11, e4973. [Google Scholar] [CrossRef] [PubMed]
- Eralp, L.; Kocaoǧlu, M.; Özkan, K.; Türker, M. A comparison of two osteotomy techniques for tibial lengthening. Arch. Orthop. Trauma Surg. 2004, 124, 298–300. [Google Scholar] [CrossRef]
- Anesi, A.; Ferretti, M.; Cavani, F.; Salvatori, R.; Bianchi, M.; Russo, A.; Chiarini, L.; Palumbo, C. Structural and ultrastructural analyses of bone regeneration in rabbit cranial osteotomy: Piezosurgery versus traditional osteotomes. J. Cranio-Maxillofac. Surg. 2018, 46, 107–118. [Google Scholar] [CrossRef]
- Landes, C.A.; Stübinger, S.; Rieger, J.; Williger, B.; Ha, T.K.L.; Sader, R. Critical Evaluation of Piezoelectric Osteotomy in Orthognathic Surgery: Operative Technique, Blood Loss, Time Requirement, Nerve and Vessel Integrity. J. Oral Maxillofac. Surg. 2008, 66, 657–674. [Google Scholar] [CrossRef]
- Papadaki, M.; Doukas, A.; Farinelli, W.A.; Kaban, L.; Troulis, M. Vertical ramus osteotomy with Er:YAG laser: A feasibility study. Int. J. Oral Maxillofac. Surg. 2007, 36, 1193–1197. [Google Scholar] [CrossRef]
- González-García, A.; Diniz-Freitas, M.; Somoza-Martín, M.; García-García, A. Ultrasonic osteotomy in oral surgery and implantology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.S.; Orenstein, A.; Liaw, L.L.; Berns, M.W. Mid-infrared erbium: YAG laser ablation of bone: The effect of laser osteotomy on bone healing. Lasers Surg. Med. 1989, 9, 362–374. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, R.J.; Deutsch, T.F.; Flotte, T.J.; Lorente, C.A.; Tomford, W.W.; Mankin, H.J.; Schomacker, K.T. Effect of Er:YAG laser holes on osteoinduction in demineralized rat calvarial allografts. J. Orthop. Res. 1996, 14, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Giraud, J.Y.; Villemin, S.; Darmana, R.; Cahuzac, J.P.; Autefage, A.; Morucci, J.P. Bone cutting. Clin. Phys. Physiol. Meas. 1991, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Jain, V.; Gupta, D.; Sharma, A. Parametric effect of vibrational drilling on osteonecrosis and comparative histopathology study with conventional drilling of cortical bone. Proc. Inst. Mech. Eng. H 2018, 232, 975–986. [Google Scholar] [CrossRef]
- Bertollo, N.; Milne, H.R.M.; Ellis, L.P.; Stephens, P.C.; Gillies, R.M.; Walsh, W.R. A comparison of the thermal properties of 2- and 3-fluted drills and the effects on bone cell viability and screw pull-out strength in an ovine model. Clin. Biomech. 2010, 25, 613–617. [Google Scholar] [CrossRef]
- Anitua, E. Biological Bone Drilling in Oral Implantology. J. Dent. Oral Biol. 2017, 2, 2–3. [Google Scholar]
- Buza, J.A.; Einhorn, T. Bone healing in 2016. Clin. Cases Miner. Bone Metab. 2016, 13, 101–105. [Google Scholar] [CrossRef]
- Jegoux, F.; Malard, O.; Goyenvalle, E.; Aguado, E.; Daculsi, G. Radiation effects on bone healing and reconstruction: Interpretation of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2010, 109, 173–184. [Google Scholar] [CrossRef]
- Evans, H.B.; Brown, S.; Hurst, L.N. The Effects of Early Postoperative Radiation on Vascularized Bone Grafts. Ann. Plast. Surg. 1991, 26, 505–510. [Google Scholar] [CrossRef]
- Brasseur, M.; Brogniez, V.; Grégoire, V.; Reychler, H.; Lengelé, B.; D’Hoore, W.; Nyssen-Behets, C. Effects of irradiation on bone remodelling around mandibular implants: An experimental study in dogs. Int. J. Oral Maxillofac. Surg. 2006, 35, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Brogniez, V.; Nyssen-Behets, C.; Reychler, H.; Benoit, L. Implant osseointegration in the irradiated mandible microradiographic and histologic assessment. Clin. Oral Implant. Res. 1998, 234–242. [Google Scholar]
- Schon, R.; Ohno, K.; Kudo, M.; Michi, K. Peri-implant tissue reaction in bone irradiated the fifth day after implantation in rabbits: Histologic and histomorphometric measurements. Int. J. Oral Maxillofac. Implant. 1996, 11, 228–238. [Google Scholar]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2012, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.P.; Chen, M.; Orosco, R.K.; Sirjani, D.; Divi, V.; Hara, W. Association of survival with shorter time to radiation therapy after surgery for US patients with head and neck cancer. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 349–359. [Google Scholar] [CrossRef]
- Davidson, S.R.H.; James, D.F. Drilling in bone: Modeling heat generation and temperature distribution. J. Biomech. Eng. 2003, 125, 305–314. [Google Scholar] [CrossRef]
- Mediouni, M.; Kucklick, T.; Poncet, S.; Madiouni, R.; Abouaomar, A.; Madry, H.; Cucchiarini, M.; Chopko, B.; Vaughan, N.; Arora, M.; et al. An overview of thermal necrosis: Present and future. Curr. Med. Res. Opin. 2019, 35, 1555–1562. [Google Scholar] [CrossRef]
- Dolan, E.B.; Haugh, M.G.; Tallon, D.; Casey, C.; McNamara, L.M. Heat-shock-induced cellular responses to temperature elevations occurring during orthopaedic cutting. J. R. Soc. Interface 2012, 9, 3503–3513. [Google Scholar] [CrossRef]
- Yacker, M.J.; Klein, M. The Effect of Irrigation on Osteotomy Depth and Bur Diameter. Int. J. Oral Maxillofac. Implant. 1996, 11, 634–638. [Google Scholar]
- Matthews, L.S.; Hirsch, C. Temperatures measured in human cortical bone when drilling. J. Bone Jt. Surg. Am. 1972, 54, 297–308. [Google Scholar] [CrossRef]
- Kondo, S.; Okada, Y.; Iseki, H.; Hori, T.; Takakura, K.; Kobayashi, A.; Nagata, H. Thermological study of drilling bone tissue with a high-speed drill. Neurosurgery 2000, 46, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.K.; Panda, S.S. Drilling of bone: A comprehensive review. J. Clin. Orthop. Trauma 2013, 4, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Augustin, G.; Davila, S.; Mihoci, K.; Udiljak, T.; Vedrina, D.S.; Antabak, A. Thermal osteonecrosis and bone drilling parameters revisited. Arch. Orthop. Trauma Surg. 2008, 128, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Iwaniec, U.T.; Wronski, T.J.; Turner, R.T. Histological analysis of bone. Methods Mol. Biol. 2008, 447, 325–341. [Google Scholar] [PubMed]
- Hancox, N.M.; Gejvall, N.G.; Hancox, N.M.; Gejvall, N.G. Biology of Bone, 1st ed.; Press, C.U., Ed.; Cambridge University Press: New York, NY, USA, 1972; Volume 10, ISBN 0521083427. [Google Scholar]
- Bentolila, V.; Boyce, T.M.; Fyhrie, D.P.; Drumb, R.; Skerry, T.M.; Schaffler, M.B. Intracortical remodeling in adult rat long bones after fatigue loading. Bone 1998, 23, 275–281. [Google Scholar] [CrossRef]
- Liao, Z.; Axinte, D.A.; Gao, D. A novel cutting tool design to avoid surface damage in bone machining. Int. J. Mach. Tools Manuf. 2017, 116, 52–59. [Google Scholar] [CrossRef]
- Christen, P.; Ito, K.; Ellouz, R.; Boutroy, S.; Sornay-Rendu, E.; Chapurlat, R.D.; Van Rietbergen, B. Bone remodelling in humans is load-driven but not lazy. Nat. Commun. 2014, 5, 4855. [Google Scholar] [CrossRef]
- Sugita, N.; Osa, T.; Mitsuishi, M. Analysis and estimation of cutting-temperature distribution during end milling in relation to orthopedic surgery. Med. Eng. Phys. 2009, 31, 101–107. [Google Scholar] [CrossRef]
- Lee, J.E.; Gozen, B.A.; Ozdoganlar, O.B. Modeling and experimentation of bone drilling forces. J. Biomech. 2012, 45, 1076–1083. [Google Scholar] [CrossRef]
- Bigham-Sadegh, A.; Oryan, A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int. Wound J. 2015, 12, 238–247. [Google Scholar] [CrossRef]
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol. 2008, 19, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.R.; Daluiski, A.; Einhorn, T.A. The role of growth factors in the repair of bone biology and clinical applications. J. Bone Jt. Surg. Am. 2002, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.; Palumbo, C.; Contri, M.; Marotti, G. Static and dynamic osteogenesis: Two different types of bone formation. Anat. Embryol. (Berl.) 2002, 206, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, C.; Ferretti, M.; Marotti, G. Osteocyte dendrogenesis in static and dynamic bone formation: An ultrastructural study. Anat. Rec. 2004, 278A, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Marotti, G. Static and dynamic osteogenesis in the processes of bone repair. G Ital. di Ortop e Traumatol. 2004, 30, S1–S5. [Google Scholar]
- Marotti, G.; Zaffe, D.; Ferretti, M.; Palumbo, C. Static osteogenesis and dynamic osteogenesis: Their relevance in dental bone implants and biomaterial osseointegration. J. Osteol. Biomater. 2010, 1, 133–139. [Google Scholar]
- Streeten, E.A.; Brandi, M.L. Biology of bone endothelial cells. Bone Miner. 1990, 10, 85–94. [Google Scholar] [CrossRef]
- Villanueva, J.E.; Nimni, M.E. Promotion of calvarial cell osteogenesis by endothelial cells. J. Bone Miner. Res. 2009, 5, 733–739. [Google Scholar] [CrossRef]
- Kasperk, C.H.; Börcsök, I.; Schairer, H.U.; Schneider, U.; Nawroth, P.P.; Niethard, F.U.; Ziegler, R. Endothelin-1 is a potent regulator of human bone cell metabolism in vitro. Calcif. Tissue Int. 1997, 60, 368–374. [Google Scholar] [CrossRef]
- Inoue, A.; Kamiya, A.; Ishiji, A.; Hiruma, Y.; Hirose, S.; Hagiwara, H. Vasoactive peptide-regulated gene expression during osteoblastic differentiation. J. Cardiovasc. Pharmacol. 2000, 36, S286–S289. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.; Palumbo, C.; Bertoni, L.; Cavani, F.; Marotti, G. Does static precede dynamic osteogenesis in endochondral ossification as occurs in intramembranous ossification? Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2006, 288A, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Remedios, A. Bone and Bone Healing. Vet. Clin. N. Am. Small Anim. Pract. 1999, 29, 1029–1044. [Google Scholar] [CrossRef]
- Barré-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Future Sci. OA 2015, 1. [Google Scholar] [CrossRef]
- Peker Tekdal, G.; Bostanci, N.; Belibasakis, G.N.; Gürkan, A. The effect of piezoelectric surgery implant osteotomy on radiological and molecular parameters of peri-implant crestal bone loss: A randomized, controlled, split-mouth trial. Clin. Oral Implant. Res. 2016, 27, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Gülnahar, Y.; Hüseyin Köşger, H.; Tutar, Y. A comparison of piezosurgery and conventional surgery by heat shock protein 70 expression. Int. J. Oral Maxillofac. Surg. 2013, 42, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Aerssens, J.; Boonen, S.; Lowet, G.; Dequeker, J. Interspecies differences in bone composition, density, and quality: Potential implications for in vivo bone research. Endocrinology 1998, 139, 663–670. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal models for bone tissue engineering and modelling disease. Dis. Model. Mech. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cell. Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Reeve-Johnson, L.; Schuetz, M. A Review of Major Animal Models Relevant to Contemporary Orthopaedic Repair of the Appendicular Skeleton in Humans. EC Orthop. 2016, 4, 537–552. [Google Scholar]
- Reichert, J.C.; Saifzadeh, S.; Wullschleger, M.E.; Epari, D.R.; Schütz, M.A.; Duda, G.N.; Schell, H.; van Griensven, M.; Redl, H.; Hutmacher, D.W. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 2009, 30, 2149–2163. [Google Scholar] [CrossRef]
- Schafrum Macedo, A.; Cezaretti Feitosa, C.; Yoiti Kitamura Kawamoto, F.; Vinicius Tertuliano Marinho, P.; dos Santos Dal-Bó, Í.; Fiuza Monteiro, B.; Prado, L.; Bregadioli, T.; Antonio Covino Diamante, G.; Ricardo Auada Ferrigno, C. Animal modeling in bone research—Should we follow the White Rabbit? Anim. Model. Exp. Med. 2019, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Wancket, L.M. Animal Models for Evaluation of Bone Implants and Devices. Vet. Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Cannillo, V.; Anesi, A.; Salvatori, R.; Chiarini, L.; Manfredini, T.; Zaffe, D. Bone Regeneration by Novel Bioactive Glasses Containing Strontium and/or Magnesium: A Preliminary In-Vivo Study. Materials 2018, 11, 2223. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Anesi, A.; Salvatori, R.; Chiarini, L.; Cannillo, V. A comparative in vivo evaluation of bioactive glasses and bioactive glass-based composites for bone tissue repair. Mater. Sci. Eng. C 2017, 79, 286–295. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Human Experimental Technique; Methuen, UFAW: London, UK, 1959; Available online: http://117.239.25.194:7000/jspui/bitstream/123456789/1342/1/PRILIMINERY%20%20AND%20%20CONTENTS.pdf (accessed on 10 April 2020).
- European Commission. Annex to the Communication from the Commission on the European Citizen’s Initiative, ‘Stop Vivisection’. European Commission; European Commission: Brussels, Belgium, 2015; Available online: https://ec.europa.eu/environment/chemicals/lab_animals/pdf/vivisection/en.pdf (accessed on 10 April 2020).
- Strbac, G.D.; Giannis, K.; Unger, E.; Mittlböck, M.; Watzek, G.; Zechner, W. A novel standardized bone model for thermal evaluation of bone osteotomies with various irrigation methods. Clin. Oral Implant. Res. 2014, 25, 622–631. [Google Scholar] [CrossRef]
- Davidson, S.R.H.; James, D.F. Measurement of thermal conductivity of bovine cortical bone. Med. Eng. Phys. 2000, 22, 741–747. [Google Scholar] [CrossRef]
- Repubblica Italiana. Italian Laws for Protection of Animals Used for Scientific Purposes–DECRETO LEGISLATIVO 4 marzo 2014, n. 26–Repubblica Italiana. Available online: http://extwprlegs1.fao.org/docs/pdf/ita153404.pdf (accessed on 10 April 2020).
- Wang, X.; Mabrey, J.D.; Agrawal, C.M. An interspecies comparison of bone fracture properties. Biomed. Mater. Eng. 1998, 8, 1–9. [Google Scholar]
- Stoetzer, M.; Felgenträger, D.; Kampmann, A.; Schumann, P.; Rücker, M.; Gellrich, N.-C.; von See, C. Effects of a new piezoelectric device on periosteal microcirculation after subperiosteal preparation. Microvasc. Res. 2014, 94, 114–118. [Google Scholar] [CrossRef]
- Bellucci, D.; Salvatori, R.; Giannatiempo, J.; Anesi, A.; Bortolini, S.; Cannillo, V. A New Bioactive Glass/Collagen Hybrid Composite for Applications in Dentistry. Materials 2019, 12, 2079. [Google Scholar] [CrossRef]
- Anesi, A.; Generali, L.; Sandoni, L.; Pozzi, S.; Grande, A. From Osteoclast Differentiation to Osteonecrosis of the Jaw: Molecular and Clinical Insights. Int. J. Mol. Sci. 2019, 20, 4925. [Google Scholar] [CrossRef]
- Malavasi, G.; Salvatori, R.; Zambon, A.; Lusvardi, G.; Rigamonti, L.; Chiarini, L.; Anesi, A. Cytocompatibility of Potential Bioactive Cerium-Doped Glasses based on 45S5. Materials 2019, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.-X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, C.; Ferretti, M.; Ardizzoni, A.; Zaffe, D.; Marotti, G. Osteocyte-osteoclast morphological relationships and the putative role of osteocytes in bone remodeling. J. Musculoskelet. Neuronal Interact. 2001, 1, 327–332. [Google Scholar]
- Moss, R.W. Histopathologic reaction of bone to surgical cutting. Oral Surg. Oral Med. Oral Pathol. 1964, 17, 405–414. [Google Scholar] [CrossRef]
- Spatz, S. Early reaction in bone following the use of burs rotating at conventional and ultra speeds; a comparison study. Oral Surg. Oral Med. Oral Pathol. 1965, 19, 808–816. [Google Scholar] [CrossRef]
- Horton, J.E.; Tarpley, T.M.; Wood, L.D. The healing of surgical defects in alveolar bone produced with ultrasonic instrumentation, chisel, and rotary bur. Oral Surg. Oral Med. Oral Pathol. 1975, 39, 536–546. [Google Scholar] [CrossRef]
- Eriksson, A.R.; Albrektsson, T.; Albrektsson, B. Heat caused by drilling cortical bone: Temperature measured in vivo in patients and animals. Acta Orthop. Scand. 1984, 55, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Wächter, R.; Stoll, P. Increase of temperature during osteotomy. In vitro and in vivo investigations. Int. J. Oral Maxillofac. Surg. 1991, 20, 245–249. [Google Scholar] [CrossRef]
- Abouzgia, M.B.; Symington, J.M. Effect of drill speed on bone temperature. Int. J. Oral Maxillofac. Surg. 1996, 25, 394–399. [Google Scholar] [CrossRef]
- Keijser, L.C.; Schreuder, H.W.; Buma, P.; Weinans, H.; Veth, R.P. Cryosurgery in long bones; an experimental study of necrosis and revitalization in rabbits. Arch. Orthop. Trauma Surg. 1999, 119, 440–444. [Google Scholar] [CrossRef]
- Bachus, K.N.; Rondina, M.T.; Hutchinson, D.T. The effects of drilling force on cortical temperatures and their duration: An in vitro study. Med. Eng. Phys. 2000, 22, 685–691. [Google Scholar] [CrossRef]
- Vercellotti, T.; Nevins, M.L.; Kim, D.M.; Nevins, M.; Wada, K.; Schenk, R.K.; Fiorellini, J.P. Osseous response following resective therapy with piezosurgery. Int. J. Periodontics Restor. Dent. 2005, 25, 543–549. [Google Scholar]
- Preti, G.; Martinasso, G.; Peirone, B.; Navone, R.; Manzella, C.; Muzio, G.; Russo, C.; Canuto, R.A.; Schierano, G. Cytokines and growth factors involved in the osseointegration of oral titanium implants positioned using piezoelectric bone surgery versus a drill technique: A pilot study in minipigs. J. Periodontol. 2007, 78, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Maurer, P.; Kriwalsky, M.S.; Block Veras, R.; Vogel, J.; Syrowatka, F.; Heiss, C. Micromorphometrical analysis of conventional osteotomy techniques and ultrasonic osteotomy at the rabbit skull. Clin. Oral Implant. Res. 2008, 19, 570–575. [Google Scholar] [CrossRef]
- Queiroz, T.P.; Souza, F.A.; Okamoto, R.; Margonar, R.; Pereira-Filho, V.A.; Garcia Júnior, I.R.; Vieira, E.H. Evaluation of immediate bone-cell viability and of drill wear after implant osteotomies: Immunohistochemistry and scanning electron microscopy analysis. J. Oral Maxillofac. Surg. 2008, 66, 1233–1240. [Google Scholar] [CrossRef]
- Rashad, A.; Kaiser, A.; Prochnow, N.; Schmitz, I.; Hoffmann, E.; Maurer, P. Heat production during different ultrasonic and conventional osteotomy preparations for dental implants. Clin. Oral Implant. Res. 2011, 22, 1361–1365. [Google Scholar] [CrossRef]
- Augustin, G.; Davila, S.; Udilljak, T.; Staroveski, T.; Brezak, D.; Babic, S. Temperature changes during cortical bone drilling with a newly designed step drill and an internally cooled drill. Int. Orthop. 2012, 36, 1449–1456. [Google Scholar] [CrossRef]
- Heinemann, F.; Hasan, I.; Kunert-Keil, C.; Götz, W.; Gedrange, T.; Spassov, A.; Schweppe, J.; Gredes, T. Experimental and histological investigations of the bone using two different oscillating osteotomy techniques compared with conventional rotary osteotomy. Ann. Anat. 2012, 194, 165–170. [Google Scholar] [CrossRef]
- Hollstein, S.; Hoffmann, E.; Vogel, J.; Heyroth, F.; Prochnow, N.; Maurer, P. Micromorphometrical analyses of five different ultrasonic osteotomy devices at the rabbit skull. Clin. Oral Implant. Res. 2012, 23, 713–718. [Google Scholar] [CrossRef]
- Schütz, S.; Egger, J.; Kühl, S.; Filippi, A.; Lambrecht, J.T. Intraosseous temperature changes during the use of piezosurgical inserts in vitro. Int. J. Oral Maxillofac. Surg. 2012, 41, 1338–1343. [Google Scholar] [CrossRef]
- Claire, S.; Lea, S.C.; Walmsley, A.D. Characterisation of bone following ultrasonic cutting. Clin. Oral Investig. 2013, 17, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Esteves, J.C.; Marcantonio, E.; de Souza Faloni, A.P.; Rocha, F.R.G.; Marcantonio, R.A.; Wilk, K.; Intini, G. Dynamics of bone healing after osteotomy with piezosurgery or conventional drilling–histomorphometrical, immunohistochemical, and molecular analysis. J. Transl. Med. 2013, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Stübinger, S.; Liu, X.L.; Schneider, U.A.; Lang, N.P. Healing of osteotomy sites applying either piezosurgery or two conventional saw blades: A pilot study in rabbits. Int. Orthop. 2013, 37, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Bullon, B.; Bueno, E.F.; Herrero, M.; Fernandez-Palacin, A.; Rios, J.V.; Bullon, P.; Gil, F.J. Effect of irrigation and stainless steel drills on dental implant bed heat generation. J. Mater. Sci. Mater. Med. 2015, 26, 75. [Google Scholar] [CrossRef] [PubMed]
- Lamazza, L.; Laurito, D.; Lollobrigida, M.; Brugnoletti, O.; Garreffa, G.; De Biase, A. Identification of possible factors influencing temperatures elevation during implant site preparation with piezoelectric technique. Ann. Stomatol. (Roma) 2014, 5, 115–122. [Google Scholar] [PubMed]
- Stelzle, F.; Frenkel, C.; Riemann, M.; Knipfer, C.; Stockmann, P.; Nkenke, E. The effect of load on heat production, thermal effects and expenditure of time during implant site preparation–An experimental ex vivo comparison between piezosurgery and conventional drilling. Clin. Oral Implant. Res. 2014, 25, e140–e148. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.E.; Girod, S. Efficacy of bone healing in calvarial defects using piezoelectric surgical instruments. J. Craniofac. Surg. 2014, 25, 149–153. [Google Scholar] [CrossRef]
- Rashad, A.; Sadr-Eshkevari, P.; Heiland, M.; Smeets, R.; Hanken, H.; Gröbe, A.; Assaf, A.T.; Köhnke, R.H.; Mehryar, P.; Riecke, B.; et al. Intraosseous heat generation during sonic, ultrasonic and conventional osteotomy. J. Craniomaxillofac. Surg. 2015, 43, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Boa, K.; Barrak, I.; Varga, E.; Joob-Fancsaly, A.; Varga, E.; Piffko, J. Intraosseous generation of heat during guided surgical drilling: An ex vivo study of the effect of the temperature of the irrigating fluid. Br. J. Oral Maxillofac. Surg. 2016, 54, 904–908. [Google Scholar] [CrossRef]
- Gabrić, D.; Blašković, M.; Gjorgijevska, E.; Mladenov, M.; Tašič, B.; Jurič, I.B.; Ban, T. Evaluation of Bone Healing After Osteotomies Prepared With Er:YAG Laser in Contact and Noncontact Modes and Piezosurgery–An Animal Study. J. Oral Maxillofac. Surg. 2016, 74, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Lamazza, L.; Garreffa, G.; Laurito, D.; Lollobrigida, M.; Palmieri, L.; De Biase, A. Temperature Values Variability in Piezoelectric Implant Site Preparation: Differences between Cortical and Corticocancellous Bovine Bone. BioMed Res. Int. 2016, 2016, 6473680. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sagheb, K.; Kumar, V.V.; Azaripour, A.; Walter, C.; Al-Nawas, B.; Kämmerer, P.W. Comparison of conventional twist drill protocol and piezosurgery for implant insertion: An ex vivo study on different bone types. Clin. Oral Implant. Res. 2016, 28, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Szalma, J.; Vajta, L.; Lempel, E.; Tóth, Á.; Jeges, S.; Olasz, L. Intracanal temperature changes during bone preparations close to and penetrating the inferior alveolar canal: Drills versus piezosurgery. J. Craniomaxillofac. Surg. 2017, 45, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Favero, V.; Sakuma, S.; Apaza Alccayhuaman, K.A.; Benedetto, G.A.; Bengazi, F.; Botticelli, D. Healing at sites prepared using different drilling protocols. An experimental study in the tibiae of sheep. PLoS ONE 2018, 13, e0202957. [Google Scholar] [CrossRef] [PubMed]
- Fugito Junior, K.; Cortes, A.R.; de Carvalho Destro, R.; Yoshimoto, M. Comparative Study on the Cutting Effectiveness and Heat Generation of Rotary Instruments Versus Piezoelectric Surgery Tips Using Scanning Electron Microscopy and Thermal Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 345–350. [Google Scholar] [CrossRef]
- Lajolo, C.; Valente, N.A.; Romandini, W.G.; Petruzzi, M.; Verdugo, F.; D’Addona, A. Bone heat generated using conventional implant drills versus piezosurgery unit during apical cortical plate perforation. J. Periodontol. 2018, 89, 661–668. [Google Scholar] [CrossRef]
- Stacchi, C.; Robiony, M.; Jones, J.M.; Lombardi, T.; Verardi, S.; Lajolo, C.; Valente, N.A.; Romandini, W.G.; Petruzzi, M.; Verdugo, F.; et al. Letter to the editor: Re: Bone heat generated using conventional implant drills versus piezosurgery unit during apical cortical plate perforation. J. Periodontol. 2018, 89, 1161–1162. [Google Scholar] [CrossRef]
- Stocchero, M.; Toia, M.; Jinno, Y.; Cecchinato, F.; Becktor, J.P.; Naito, Y.; Halldin, A.; Jimbo, R. Influence of different drilling preparation on cortical bone: A biomechanical, histological, and micro-CT study on sheep. Clin. Oral Implant. Res. 2018, 29, 707–715. [Google Scholar] [CrossRef]
- Tepedino, M.; Romano, F.; Indolfi, M.; Aimetti, M. Heat Production and Drill Wear Following Osseous Resective Surgery: A Preliminary In Vitro SEM Study Comparing Piezosurgery and Conventional Drilling. Int. J. Periodontics Restor. Dent. 2018, 38, e33–e40. [Google Scholar] [CrossRef]
- Zheng, Q.; Xia, L.; Zhang, X.; Zhang, C.; Hu, Y. Reduction thermal damage to cortical bone using ultrasonically-assisted drilling. Technol. Health Care 2018, 26, 843–856. [Google Scholar] [CrossRef]
- Alam, K.; Al-Ghaithi, A.; Piya, S.; Saleem, A. In-vitro experimental study of histopathology of bone in vibrational drilling. Med. Eng. Phys. 2019, 67, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Pavone, C.; Scardueli, C.R.; de Oliveira, G.J.P.L.; Cerri, P.S.; Marcantonio Junior, E.; Marcantonio, R.A.C. Effects of an Er,Cr:YSGG Laser on Bone Regeneration in Critical-Sized Calvarial Defects of Rats Exposed to Inhalation of Cigarette Smoke. Photobiomodul. Photomed. Laser Surg. 2019, 37, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, L.; Wang, C.; Chen, Z.; Han, S.; Chen, B.; Chen, J. Mechanical and thermal damage in cortical bone drilling in vivo. Proc. Inst. Mech. Eng. H 2019, 233, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Crovace, A.M.; Luzzi, S.; Lacitignola, L.; Fatone, G.; Lucifero, A.G.; Vercellotti, T.; Crovace, A. Minimal invasive piezoelectric osteotomy in neurosurgery: Technic, applications, and clinical outcomes of a retrospective case series. Vet. Sci. 2020, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.C. Effect of Drilling lnto Bone. J. Oral Surg. 1958, 16, 22–30. [Google Scholar]
- Eriksson, A.R.; Albrektsson, T. Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J. Prosthet. Dent. 1983, 50, 101–107. [Google Scholar] [CrossRef]
- Schwieger, K.; Carrero, V.; Rentzsch, R.; Becker, A.; Bishop, N.; Hille, E.; Louis, H.; Morlock, M.; Honl, M. Abrasive water jet cutting as a new procedure for cutting cancellous bone?In vitro testing in comparison with the oscillating saw. J. Biomed. Mater. Res. 2004, 71B, 223–228. [Google Scholar] [CrossRef]
- Sharawy, M.; Misch, C.E.; Weller, N.; Tehemar, S. Heat generation during implant drilling: The significance of motor speed. J. Oral Maxillofac. Surg. 2002, 60, 1160–1169. [Google Scholar] [CrossRef]
- Ark, T.W.; Neal, J.G.; Thacker, J.G.; Edlich, R.F. Influence of irrigation solutions on oscillating bone saw blade performance. J. Biomed. Mater. Res. 1998, 43, 108–112. [Google Scholar] [CrossRef]
- Happe, A. Use of a piezoelectric surgical device to harvest bone grafts from the mandibular ramus: Report of 40 cases. Int. J. Periodontics Restor. Dent. 2007, 3, 241–249. [Google Scholar]
- Sohn, D.-S.; Ahn, M.-R.; Lee, W.H.; Yeo, D.S.; Lim, S.-Y. Piezoelectric osteotomy for intraoral harvesting of bone blocks. Int. J. Periodontics Restor. Dent. 2007, 2, 127–131. [Google Scholar]
- Valente, N.; Cosma, L.; Nocca, G.; D’Addona, A.; Lajolo, C. Piezoelectric Device Versus Conventional Osteotomy Instruments in the Comparison of Three Different Bone Harvesting Methods: An Istomorphometric, Phonometric, and Chronometric Evaluation. Int. J. Oral Maxillofac. Implant. 2019, 34, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Mehmanparast, H.; Petit, Y.; Mac-Thiong, J.-M. Comparison of Pedicle Screw Loosening Mechanisms and the Effect on Fixation Strength. J. Biomech. Eng. 2015, 137, 121003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Yan, Z.; Cai, J.; Kang, F.; Shan, S.; Wang, P.; Zhai, M.; Edward Guo, X.; Luo, E.; et al. Spatiotemporal characterization of microdamage accumulation in rat ulnae in response to uniaxial compressive fatigue loading. Bone 2018, 108, 156–164. [Google Scholar] [CrossRef]
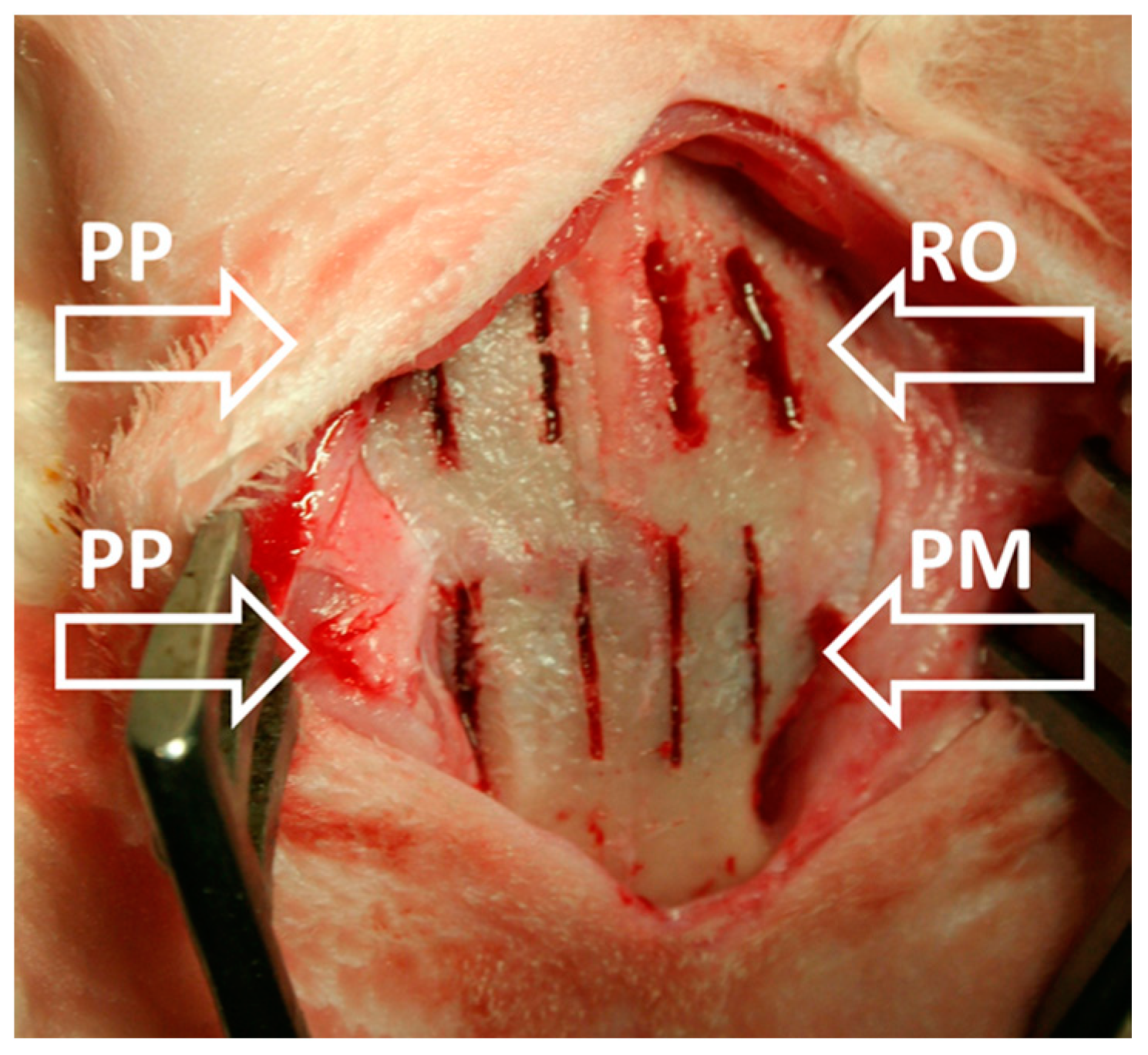
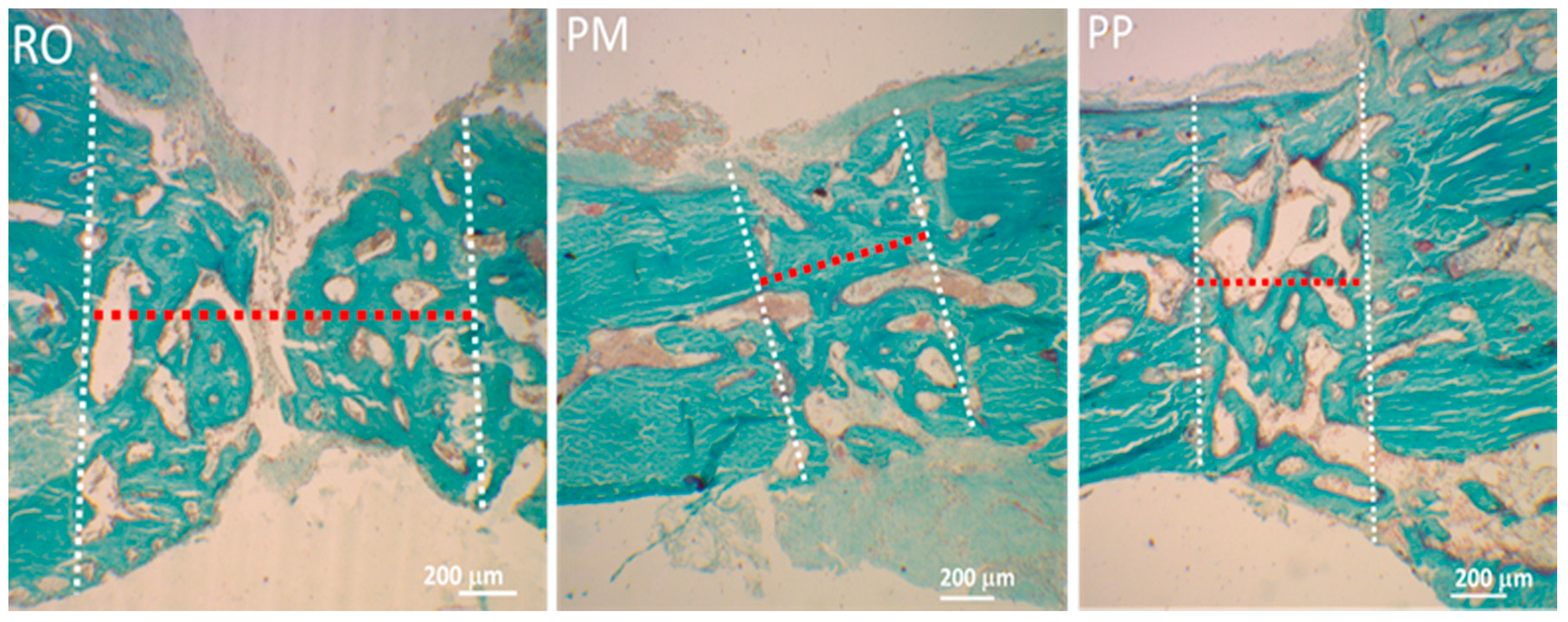



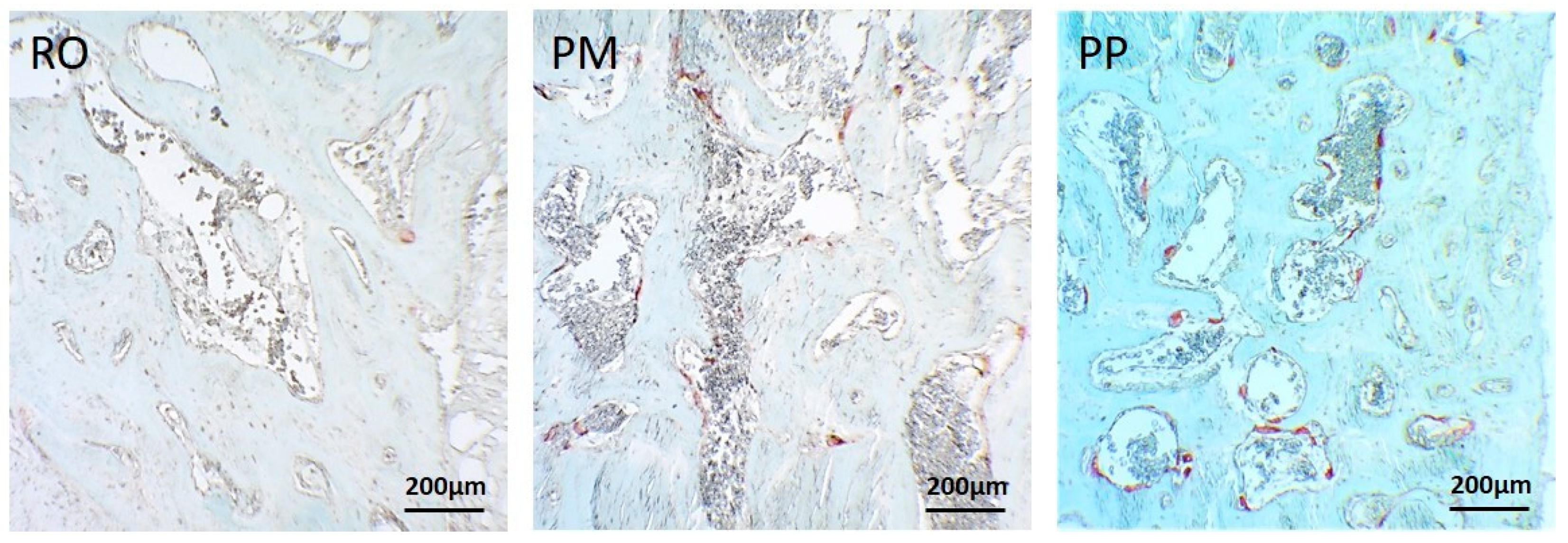
| Cytology and Histology | Molecular Biology and Biochemistry | Physics |
|---|---|---|
Histologic parameters
| PCR BMP, Wnt, osteogenesis, inflammatory cytokines, apoptosis, growth-factors; Hsp70 | Temperature rise
|
| Histomorphometric and Micromorphometric Analysis | ELISA test on the perisulcular fluid to search for RANKL and OPG levels | Micro-CT |
Immunohistochemistry
| CBCT | |
| Scanning electron microscopy (SEM) | Laser 3D scanning | |
| Transmission electron microscopy (TEM) | Laser profilometry | |
| Intravital microscopy |
| Animal | Bone Microscopic and Macroscopic Features | Bone Composition and Remodeling | Animal Management | Best Type of Study |
|---|---|---|---|---|
| Rodents | Mainly primary bone in long bone cortices and minimal cancellous bone. Cortices are thin and fragile. | Limited cortical remodeling and non-Haversian-type remodeling. Limited secondary osteon formation. Higher bone healing capacity in craniofacial bones. | Cheap and easily manageable. Rats are more docile and social than mice, although the latter are cheaper to house and maintain. | Osteoinduction. Cartilage regeneration potential. Bone infection. Extraoral surgical approaches. |
| Rabbit | Cortices are fragile and there is less cancellous bone than in humans. Quick achievement of skeletal maturity. Small size. Lack of biomechanical data. Dense Haversian bone. | Similar bone density to human. Bone metabolism is similar to human, with Haversian-type remodeling, although with a higher rate than humans. | Availability, housing and handling are easy. Cage confinement might worsen their bone healing capability. | Muscolo-skeletal research. Bone implants. Modeling of vertebral fracture repair. Extraoral surgical approaches. |
| Sheep | High trabecular bone density. Good body weight. Different bone microstructure than humans. Big difference between young and mature sheep due to age-dependent changes in bone structure. | Similar bone healing capability to humans. Different remodeling processes. | Although docile, their size requires a lot of space. | Orthopedic research. Bone filler materials in cranial osteotomies. Extraoral surgical approaches. |
| Goats | Good size. Presence of Haversian systems in the tibia, except for the caudal part. | Similar bone healing potential. Similar bone composition. | Docile and tolerant to environmental conditions. A lot of space is needed. | Bone filler materials in cranial osteotomies. Extraoral surgical approaches. Cartilage, ligaments and menisci regeneration. |
| Pig | Plexiform bone, which shifts to dense secondary osteonal bone. Good development of the Haversian system, with medium canals. Similar to humans. | Similar bone density and bone mineral concentration. Similar bone remodeling. | High body weight and aggressive nature. | Extra- and intraoral approaches. Osteonecrosis surgery. Osteogenic regeneration materials in craniofacial bones. Dental implants. |
| Dog | Similar cancellous bone to humans. Presence of secondary osteons with small canals. Thinner articular cartilage. | Variability of trabecular bone remodeling depending on site, age and species. | Docile, easy handling. Good size. | Dental implants and peri-implantitis. |
| Non-human primates | Close to humans. | Comparable to humans. | Difficult to handle and highly trained staff are needed. | Bone implant. |
| Advantages | Disadvantages | |
|---|---|---|
| Ex vivo | Low costs No need for ethical approval Amount of information on bone damage Reproducible conditions Large numbers | Only hypotheses regarding possible clinical correlations Less realistic setting |
| In vivo and immediate sacrifice | Evaluation of bone damage in an in vivo scenario Possibility to obtain baseline results to compare with delayed sacrifice in in vivo results | Impossible to perform bone healing evaluations Intermediate costs |
| In vivo and delayed sacrifice | Bone healing evaluation, with the possibility to obtain both static and dynamic evaluation of osteotomic gap Wide range of parameters to be analyzed Closer to clinical setting in humans | High costs Small numbers Small animal to respect animal testing hierarchy and ethics on animals |
| Author | Animal Model | Type of Study | Material and Methods | Osteotomy Device | Conclusions |
|---|---|---|---|---|---|
| Moss, 1964 [78] | Dog mandible | Ex vivo | Ocular micrometer | Rotary cutting device set at three different speed ranges | Ultra-high-speed cutting did not produce higher bone damage than lower speed mode, especially when used with adequate coolants. Cooling agents reduced bone damage. |
| Spatz, 1965 [79] | Dog jaw | In vivo The animals were sacrificed at 1, 2 and 7 days postoperatively | Photomicrography | Rotating burs at conventional and high speed. | Ultra-high-speed osteotomies showed better and quicker bone healing and smoother cut edge compared to conventional speed burs, particularly evident 1 week after the surgical procedure. |
| Horton et al., 1975. [80] | Dog alveolar bone | In vivo. The animals were sacrificed immediately after the surgical procedure and at 3, 7, 14, 28, 56 and 90 days postoperatively. | Light microscope | Ultrasonic instrument, low-speed rotary cutting bur and surgical chisel | The bur produced the smoothest surface. Healing in later periods appeared histologically to be the best with the use of the surgical chisel. After 90 days, the bone healing was complete. |
| Eriksson et al., 1984 [81] | Rabbit femur, fibula and tibia | In vivo. Animals in the first group were sacrificed immediately after the surgical procedure. Half of the animals of the second group were sacrificed 1 week after the thermic damage, while the other half were followed up for 17 weeks. | Histology, histochemistry and vital microscopy. | Twist driller, thermostatically heated saline solution and thermal chamber | Histochemistry provides a better evaluation of bone viability after heat-induced trauma than histology. Vital microscopy is more sensitive in the evaluation of bone healing following heat trauma than indirect, histological and histochemical methods. |
| Wächter and Stoll, 1991 [82] | Bovine and sheep mandible | Both in vivo and ex vivo The animals were not sacrificed. | Thermocouples | Oscillating saw | Only the combination of intermittent sawing and irrigation allows a safe osteotomy from a thermic point of view. |
| Abouzgia and Symington, 1996 [83] | Bovine femur | Ex vivo | K-type thermocouple (Omega) | Surgical drill (Stryker-100) | Drilling at high speed and large load seems to provide a better combination in terms of temperature rise and duration of temperature elevation. |
| Keijser et al., 1999 [84] | Rabbit tibia and femur | In vivo The animals in the first group were sacrificed after 1 week. The animals in the second group were sacrificed after 1, 3, 5, 7, 9 and 12 weeks. | Histologic examination and temperature measurement. | Cryosurgery | No pathologic fractures were observed in rabbit tibiae. The gap in terms of periosteal bone apposition between human and animal bones was the likely cause of this difference. Rabbit bone, then, is not a suitable model to study this kind of biomechanical dynamics. |
| Bachus et al., 2000 [85] | Human femur | Ex vivo | Thermocouple | Standard surgical drilling | Higher load reduces the risk of thermal necrosis. |
| Vercellotti et al., 2005 [86] | Dog alveolar bone | In vivo The animals were sacrificed immediately after the surgical procedure and at 14, 28 and 56 days postoperatively. Some bone specimens were collected immediately after the surgery as well. | Histomorphometric analysis | Piezosurgery Mectron Dental Technology, carbide bur and diamond bur | By day 56, the surgical sites treated by burs evidenced a loss of bone, versus a bone gain in the piezo-treated sites. |
| Preti et al., 2007 [87] | Mini pig tibia | In vivo The animals were sacrificed at 7, 14, 28 and 56 days postoperatively. Some tibial bone specimens were collected immediately after the surgery as well. | Histomorphology and levels of bone morphogenetic protein (BMP)-4, transforming growth factor (TGF)-β2, tumor necrosis factor-alpha, and interleukin-1β and -10 were evaluated in the peri-implant osseous samples. | Piezosurgery Mectron Dental Technology and drilling according to Branemark protocol | Piezoelectric bone surgery appears to be more efficient in the first phases of bone healing; it induced an earlier increase in BMPs, controlled the inflammatory process better and stimulated bone remodeling as early as 56 days post-treatment. |
| Maurer et al., 2008 [88] | Rabbit skull | Ex vivo | Light microscopy, environmental surface electron microscopy (ESEM) and confocal laser scanning microscopy (CLSM). | Rotating instrument, micro-saw, Piezosurgery—Mectron Dental Technology | Bony structure integrity observed after the ultrasonic technique. |
| Queiroz et al., 2008 [89] | Rabbit tibia | In vivo The animals were sacrificed immediately after the surgical procedure. | Immunoistochemistry, scanning electron microscopy | Conventional drilling | It is preferable to use a less traumatic surgical protocol in order to preserve cell viability. After the thirtieth perforation, it is possible to observe a protein balance alteration. |
| Bertollo et al., 2010 [16] | Pig femur | Ex vivo | Infrared Thermal Imaging Camera (Digicam-IR, Ircon, Niles, IL, USA) | Pneumatic surgical handpiece, 2- and 3-fluted drills—7100 Drill MicroAire Surgical Instruments LLC, Charlottesville, VA, USA | The 3-fluted drills did not grant a clear advantage compared to 2-fluted drills in terms of better bone healing and screw fixation and of reduction of heat rise. |
| Wether tibia | In vivo The animals were sacrificed 2 and 4 weeks after the surgical procedure. | Light microscopy—Olympus, Tokyo, Japan | Said surgical handpiece mounted in a sterilizable mobile drill-press. | ||
| Rashad et al., 2011 [90] | Bovine rib | Ex vivo | Thermocouples | A conventional implant drill system (Straumann, Freiburg, Germany) and two ultrasonic osteotomic devices: Piezosurgery (Mectron Medical Technology) and Variosurg (NSK, Tochigi, Japan). | Implant site preparation with ultrasonic devices with adequate irrigation can provide an equally safe method compared to conventional drilling. |
| Augustin et al., 2012 [91] | Pig femur | Ex vivo | Thermocouple | Combination of internally cooled drill and a two-step drill. | Internally cooled drill causes the lower temperature bone rise. |
| Heinemann et al., 2012 [92] | Pig jaw | Ex vivo | Light microscope. | Piezosurgery—Mectron Medical Technology, SONICflex and the conventional bur method. | The bone matrix adjacent to the defect radius showed intact osteocytes. |
| Hollstein et. al, 2012 [93] | Rabbit skull | Ex vivo | Light microscopy, (ESEM), and confocal laser scanning, microscopy (CLSM). | Piezosurgery 3, Piezon Master Surgery, Piezosurgery—Mectron Medical Technology, VarioSurg, Piezotome 2 | The osseous micro-structure is preserved. Five different piezosurgical devices were evaluated. |
| Schutz et al., 2012 [94] | Pig jaw | Ex vivo | Temperature sensors and digital volume tomography images. | Piezosurgery 3—Mectron Medical Technology | The correct use of the ultrasonic device allowed a safe osteotomy, not causing irreversible thermal damage in the bone. |
| Claire et al., 2013 [95] | Pig femur | Ex vivo | Scanning laser vibrometer, laser profilometer, scanning electron microscope | Piezosurgery 3 with an OP3 style insert tip—Mectron Medical Technology | In the cortical mode, the optimal load was of 150 g. The structure of the bone has to be taken into consideration as well. |
| Esteves et al., 2013 [96] | Rat tibia | In vivo The animals were sacrificed at 3, 7, 14, 30 and 60 days postoperatively. | Histomorphometric analysis, immunohistochemical staining, RT-PCR (reverse transcriptase-polymerase chain reaction). | Piezo Master Surgery and conventional drilling with a 2 mm round diamond coated tip. | Bone healing dynamics after piezosurgery are comparable to those observed with conventional drilling. |
| Gulnahar et al., 2013 [56] | Human mandible | In vivo Bone specimens were collected immediately following the surgical procedure | Heat shock protein 70 (Hsp70) expression. | Conventional bur and piezosurgery. | Conventional burs determined more aggressive procedures, showing significantly higher Hsp70 expression in consequence of the higher stress induced. |
| Ma et al., 2013 [97] | Rabbit skull | In vivo The animals were sacrificed at 1, 2, 3, and 5 weeks postoperatively. | Light microscopy, histomorphometric analysis. | Piezosurgery—Mectron Medical Technology, two types of oscillating steel saw blade. | Advanced bone healing compared to a traditional saw was observed. |
| Bullon et al., 2014 [98] | Bovine rib | Ex vivo | Thermocouple | Drilling with precipitation-hardening stainless steel (K drills) or with martensitic stainless steel (S drills). | Irrigation had a significant impact on heat generation, while drill use and type did not. |
| Lamazza et al., 2014 [99] | Bovine rib | Ex vivo | Load cell and fluoroptic thermometer. | Piezosurgery with different tips (IM1s, IM2s, P2-3, IM3)—Mectron Medical Technology | Load, movements management and bone features play critical roles in temperature rise. Irrigation fluid temperature and the clogging effect also contribute to this phenomenon. |
| Stelzle et al., 2014 [100] | Pig skull | Ex vivo | Thermocouple, histomorphometric analysis. | Piezosurgery—Mectron Medical Technology, spiral burs and trephine burs | Piezosurgery generates more bone damage and higher temperatures than conventional drilling devices when used on high load levels. The maximum load should be 400 g. |
| Stoetzer et al., 2014 [72] | Rat calvaria | In vivo The animals were not sacrificed. The evaluations were performed immediately after the surgery and 3 and 8 days postoperatively. | Intravital microscopy of microcirculatory parameters. | Periosteal elevator and piezoelectric device. | A better periosteal microcirculation was found in piezoelectric device osteotomies. |
| Yang et al., 2014 [101] | Mice skull | In vivo The animals were not sacrificed. The evaluations were performed the day following the surgery and 2, 4 and 8 weeks postoperatively. | Micro-CT (micro–computed tomography). | Surgystar diamond round tip and Piezoelectric System (Synthes), | Piezosurgery provided faster bone healing in comparison with mechanical instrumentation. |
| Rashad et al., 2015 [102] | Bovine rib | Ex vivo | Thermocouple. | Conventional, sonic and ultrasonic osteotomic devices. | Sonic and ultrasonic devices provided the safer osteotomies in terms of heat generation. Irrigation was crucial to prevent temperature rise. |
| Tekdal et al., 2015 [55] | Human maxilla | In vivo The evaluations were performed until 24 weeks postoperatively, with a different timetable for each parameter considered. | Peri-implant sulcular fluid (PISF) analysis, periapical radiographs and cone beam computed tomography (CBCT). | Piezosurgery and conventional drilling. | On the biochemical side, piezosurgery provided a reduced inflammatory response of the bone, while on the radiographic analysis, conventional drilling and piezosurgery had similar crestal bone loss values. |
| Boa et al., 2016 [103] | Bovine rib | Ex vivo | Temperature measurement cavities. | Freehand drilling and surgical-guided drilling, combined with irrigation fluids at different temperatures. | Drilling through a surgical guide allowed the best results, especially in combination with the use of 10 °C pre-cooled irrigation fluid. |
| Gabric et al., 2016 [104] | Rat tibia | In vivo The animals were sacrificed immediately after the surgical procedure and at 1, 2 and 3 weeks postoperatively. | 3D laser scanning technique (i.e., laser triangulation profilometry). | Piezosurgery—Mectron Medical Technology, Er:YAG laser both in contact mode and non-contact mode. | Osteotomies executed with Er:YAG laser in non-contact mode were the fastest to heal. |
| Lamazza et al., 2016 [105] | Bovine rib and femur | Ex vivo | Fiber optic thermometer. | Diamond tip—IM1s, Mectron Medical Technology. | Cortico-cancellous bone samples presented more variability in temperature values compared to cortical bone samples. |
| Sagheb et al. 2017 [106] | Porcine iliac crest and tibia | Ex vivo | Temperature measurement by infrared spectroscopy. Primary implant stability by resonance frequency analysis and extrusion torque. | IM1S; IM2P; IM3P; Piezosurgery—Mectron Medical Technology. | No significant difference in temperature increase between traditional drilling and piezosurgery. |
| Szalma et al., 2017 [107] | Pig mandible | Ex vivo | Thermocouple and infrared thermometer. | Diamond drills, tungsten carbide drills, piezoelectric diamond sphere and saw. | The use of irrigation fluid at 7 °C and pre-drilling is crucial to avoid a potentially nerve-damaging temperature rise in piezosurgical osteotomies. The speed of piezosurgery and of the other devices is similar. |
| Anesi et al., 2018 [8] | Rabbit skull | In vivo The animals were sacrificed 15 days following the surgical procedure. | Histology and enzymatic assay Histomorphometry. Scanning and transmission electron microscope. Nano-mechanical analysis | Piezosurgery Medical® Piezosurgery Plus®, Mectron Medical Technology. Physiodispenser 7000, Nouvag AG. | Piezosurgical group shows more advanced stages of bone healing compared to conventional bur. |
| Favero et al., 2018 [108] | Sheep tibia | In vivo The animals were sacrificed at 1, 2 and 6 weeks postoperatively. | Eclipse Ci microscope (Nikon Corporation, Japan) with a digital video camera (Digital Sight DS-2Mv, Nikon Corporation, Japan). | Conventional drilling, both at high and mixed speed | There was no difference between the two groups. |
| Junior et al., 2018 [109] | Bovine femur | Ex vivo | T-type thermo-couple and Scanning Electron Microscope (SEM) | Piezoelectric tips (Driller) and tri-helicoid dental burs (Dentoflex) | The use of either rotatory burs or piezoelectric tips generates a temperature that does not affect the tissue healing. Burs create a smooth surface, and piezoelectric tips show a rougher and condensed bone surface. The wear of both systems does not cause a relevant increase in temperature after the preparation of 30 surgical beds. |
| Lajolo et al., 2018 [110] | Porcine rib | Ex vivo | Implant site preparation with conventional drill system vs. piezoelectric system. Infrared thermometer was positioned underneath | Premium Surgical Kit Kohno, Sweden & Martina s.p.a. OP4 insert. Piezosurgery—Mectron Medical Technology | “Bone overheating using a piezosurgery unit is a potential risk during implant site preparation”. |
| argued by Stacchi et al., 2018 [111] | Methodological flaws were revealed: OP4 insert is not suitable for implant suite preparation according to manufacturer’s booklet. Excessive pressure load applied on the piezosurgical tip during implant site preparation. No correct movement of the piezosurgical handpiece was applied in the study. | ||||
| Singh et al., 2018 [15] | Bovine bone | Ex vivo | Optical microscope | Vibrational bone drilling | Rotational speed is the major responsibility of heat generation, although all parameters considered affect the result. The best results were obtained with a mid-range rotational speed. |
| Stocchero et al., 2018 [112] | Sheep mandible | In vivo The animals were sacrificed at 5 and 10 weeks postoperatively. | Histomorphometric, μ-CT and biomechanical analysis | Two different drilling protocols | In the long term, no difference was observed between the two groups, although in the early period, there was greater cortical bone remodeling in the undersized preparation group. |
| Tepedino et al., 2018 [113] | Bovine bone | Ex vivo | Thermo-control laser, Scanning Electron Microscopy (SEM) and light microscope | Conventional rotating bur and Piezosurgery 3 with an OP5 insert—Mectron Medical Technology | Piezosurgery caused lower bone damage and temperature rise than the conventional rotating bur. |
| Zheng et al., 2018 [114] | Pig femur | Ex vivo | Infrared camera | Ultrasonically assisted drilling, conventional drilling | Ultrasonically assisted drilling provided less bone damage and generated lower temperatures. |
| Alam et al., 2019 [115] | Bovine femur | Ex vivo | Thermocouples, two-component dynamometer (Type 9271A, Kistler), system microscope (BX53, Olympus), digital microscope (DP22, Olympus) | Vibrational drilling—Orthofix, Italy | The best results were obtained by setting a lower drilling speed, a lower feed rate and a frequency of 20 kHz. |
| Pavone et al., 2019 [116] | Rat calvaria | In vivo The animals were sacrificed immediately after the surgical procedure and at 7, 15, 30 and 60 days postoperatively. | Histometric and histological analysis | Er,Cr:YSGG laser, trephine drill | The Er,Cr:YSSG laser osteotomy allowed the best healing in animals exposed to cigarette smoke. |
| Zhang et al., 2019 [117] | Tibetan pig femur and radius | In vivo | Light microscope | Drilling in five different bits geometries | Chisel edge, drill bit geometry, flute number, edges, steps and direction affect bone damage level and its characteristics. |
| Crovace et al., 2020 [118] | Dogs and cats; calvaria or spine | In vivo. Ostetotomic bone immediate sampling; the animals were not sacrificed. | X-ray or CT scan. Histology | Piezosurgery, Mectron Medical Technology | No signs of bone overheating. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anesi, A.; Di Bartolomeo, M.; Pellacani, A.; Ferretti, M.; Cavani, F.; Salvatori, R.; Nocini, R.; Palumbo, C.; Chiarini, L. Bone Healing Evaluation Following Different Osteotomic Techniques in Animal Models: A Suitable Method for Clinical Insights. Appl. Sci. 2020, 10, 7165. https://doi.org/10.3390/app10207165
Anesi A, Di Bartolomeo M, Pellacani A, Ferretti M, Cavani F, Salvatori R, Nocini R, Palumbo C, Chiarini L. Bone Healing Evaluation Following Different Osteotomic Techniques in Animal Models: A Suitable Method for Clinical Insights. Applied Sciences. 2020; 10(20):7165. https://doi.org/10.3390/app10207165
Chicago/Turabian StyleAnesi, Alexandre, Mattia Di Bartolomeo, Arrigo Pellacani, Marzia Ferretti, Francesco Cavani, Roberta Salvatori, Riccardo Nocini, Carla Palumbo, and Luigi Chiarini. 2020. "Bone Healing Evaluation Following Different Osteotomic Techniques in Animal Models: A Suitable Method for Clinical Insights" Applied Sciences 10, no. 20: 7165. https://doi.org/10.3390/app10207165
APA StyleAnesi, A., Di Bartolomeo, M., Pellacani, A., Ferretti, M., Cavani, F., Salvatori, R., Nocini, R., Palumbo, C., & Chiarini, L. (2020). Bone Healing Evaluation Following Different Osteotomic Techniques in Animal Models: A Suitable Method for Clinical Insights. Applied Sciences, 10(20), 7165. https://doi.org/10.3390/app10207165











