Abstract
Around 200 cytokines with roles in cell signaling have been identified and studied, with the vast majority belonging to the four-α-helix bundle family. These proteins exert their function by binding to specific receptors and are implicated in many diseases. The use of several cytokines as therapeutic targets has been approved by the FDA, however their rapid clearance in vivo still greatly limits their efficacy. Nano-based drug delivery systems have been widely applied in nanomedicine to develop safe, specific and controlled delivery techniques. Nevertheless, each nanomaterial has its own specifications and their suitability towards the biochemical and biophysical properties of the selected drug needs to be determined, weighing in the final choice of the ideal nano drug delivery system. Nanoparticles remain the most used vehicle for cytokine delivery, where polymeric carriers represent the vast majority of the studied systems. Liposomes and gold or silica nanoparticles are also explored and discussed in this review. Additionally, surface functionalization is of great importance to facilitate the attachment of a wide variety of molecules and modify features such as bioavailability. Since the monitoring of cytokine levels has an important role in early clinical diagnosis and for assessing therapeutic efficacy, nanotechnological advances are also valuable for nanosensor development.
1. Introduction
Cytokines are a group of small proteins with a molecular weight of less than 30 kDa, used by cells as mediators for cell–cell interactions, with an important role in cell signaling. They regulate a wide range of biological functions for the maintenance of normal host physiology, such as inflammation, repair, proliferation and differentiation, among several others [1,2]. In addition, the multifunctional activity of these proteins depends on the context of the specific tissue/organ and cytokine environment; it is concentration-dependent, being often designated as pleiotropic, as one cytokine can display different functions [2]. For instance, Oncostatin M (OSM), which belongs to the IL-6 family of proteins, can be involved in impaired healing in chronically inflamed wounds, but is proved to stimulate mitogenic responses of dermal fibroblasts and restore the extracellular matrix, important features for skin repair [3].
Nowadays, around 200 cytokines have been identified and studied, with the vast majority belonging to the four-α-helix bundle family [4]. They can be classified into pro- or anti-inflammatory cytokines, growth factors, lymphokines or chemokines [5]. In addition to the cytokines being grouped into different families according to their structure and specificity, their respective receptor complex composition is also important for classification [6]. These small proteins exert their function through binding to specific receptors present on the membrane of target cells. They are usually composed of one polypeptide chain (the cytokine specific α subunit) and a signal-transducing β subunit [4]. Cytokines are involved in many diseases and redundancy is another well-known hallmark, as multiple cytokines can mediate similar functions [7]. For instance, the inflammatory cytokines TNF-α, IL-1 or IL-6 modulate myocardial function and promote the progression of heart failure [8]. Furthermore, IL-4 and IL-10 play an important role in pregnancy and a deficiency in these cytokines contributes to infertility or hypertensive disorders [7]. TNF-α and IL-4, -5, -13 are involved in the inflammatory process of bronchial asthma disease [9]. In chronic kidney disease, the up-regulation of pro-inflammatory cytokines, such as IL-1, -6 and IFN-γ, is responsible for the activation of leucocytes resulting in apoptosis of nephrons [10]. Cytokines are also biomarkers of HIV infection, since the production of IL-1, -6, -4, -10, or TNF-α is increased in infected patients, stimulating HIV replication [11]. The presence of an abnormal production of cytokines (IL-1α and IL-1β) was also observed in Alzheimer’s disease, the most common form of dementia [12]. Even in cancer, the presence and concentration of cytokines influence cancer cell proliferation. IL-1β and IL-8 promote angiogenesis and growth of different cancer cells [13].
The use of several cytokines as therapeutic targets is currently approved by FDA [4]. Although cytokines may be suitable biomarkers for health and disease, and their exploitation as therapeutic agents has been greatly expanded, the rapid clearance by proteases and excretion mechanisms once in vivo still limit their application [14,15]. In fact, it is necessary to improve half-life and protein stability. The relatively large size of cytokines or growth factors as well as their potential toxicity at high doses suggest that safe, specific and controlled delivery techniques are required in clinical administration [16]. Nanotechnology, currently employed in therapeutics, diagnostics and imaging [17], has been widely used to overcome many of these limitations. Nowadays, there are many nano-based drug delivery systems applied in nanomedicine, from nanoparticles to nanogels, nanotubes, or nanochips; however, the first remain the most widely used [16,18]. They have the advantage of encapsulating or incorporating bioactive compounds and delivering them to target tissues with a controlled/sustained release and minimal dosage [16]. In addition, their size facilitates cell uptake of the drug and reduces side effects [18]. Nevertheless, each nanomaterial has its own limitations and the suitability of the biochemical and biophysical properties of the selected drug need to be determined, weighing in the final choice of the ideal nano drug delivery system [18]. Moreover, the monitoring of cytokine levels has an important role in early clinical diagnosis and for assessing therapeutic efficacy. The nanotechnology advances are also of great value in the development of nanosensors that are capable of displaying higher sensitivity, stability and limit detection and other key sensor parameters, compared to conventional cytokine detection assays.
In this review, we discuss and update recent advances in the use of nanomaterials, especially nanoparticles, to mediate cytokine delivery. Furthermore, we approach both structure and binding affinity of IL-6 cytokine family members as a model. This review explores the increasing use of polymeric nanoparticles, as one of the best studied vehicles for cytokine delivery. Additionally, the use of liposomes and gold or silica nanoparticles have also been used for the same purpose, and are mentioned. In this review, we give particular attention to the functionalization of these nanoparticles, which is essential for a successful delivery, as well as to their clinical potential as therapeutic agents, with the main focus on the IL-6 cytokine family. Some essential features of nanomaterial-based sensors are addressed, since they have attracted increasing attention in molecular diagnosis, allowing the monitoring of cytokine levels.
2. Il-6 Cytokine Family
Composed of interleukins (IL)-6, -11, -27, -31, cardiotrophin-1 (CT-1), cardiotrophin-like cytokine factor 1, ciliary neurotrophic protein factor (CNTF), leukemia inhibitory factor (LIF) and oncostatin M (OSM), the IL-6 cytokine family comprises multifaceted cytokines [19]. The members of this family exert effects at the level of different organs such as skin, pancreas, liver, lung, reproductive system, brain, bone metabolism and inflammation, with impact on cancer and other disorders, playing both pro- or anti-inflammatory roles (reviewed in [20,21]).
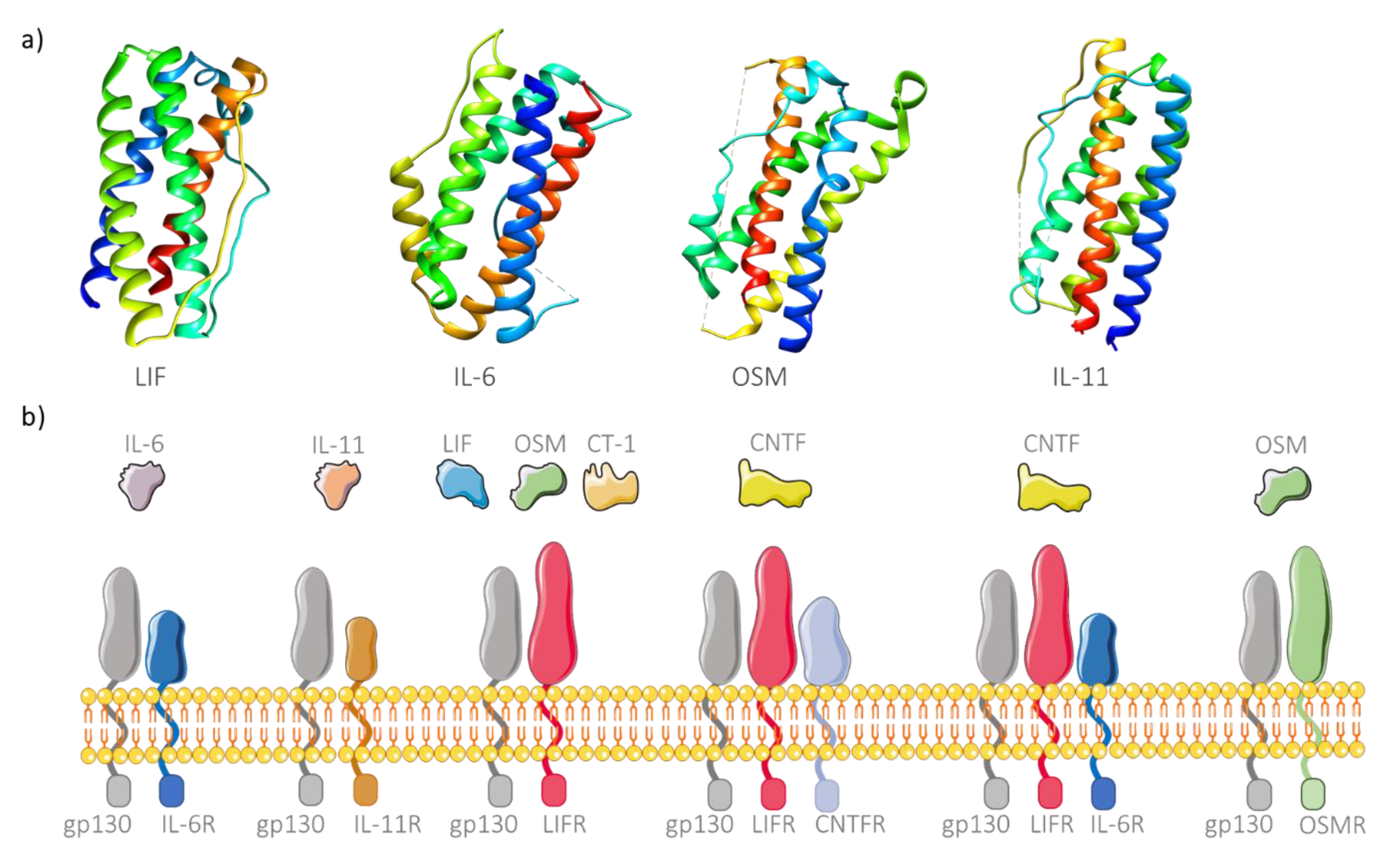
These cytokines comprise four long α-helices arranged to form an up-up-down-down topology (Figure 1a), thus forming a subfamily of the helix bundle cytokines [22]. The main characteristic responsible for grouping these cytokines in the same family is the existence of a common signaling receptor subunit glycoprotein 130 kDa (gp130), present in the membrane of many cell types [23]. Recently, two other interleukins have been added to this family, IL-35 and IL-39, due to this shared common feature [24,25]. The cytokine receptor is also composed of another polypeptide chain, the cytokine specific subunit, which is also important for intracellular signaling [26]. Different cytokines from this family can share the same receptors or use more than one receptor to perform signaling (Figure 1b). However, there are also α subunits in the free soluble form, able to form cytokine/receptor complexes and posteriorly bind to the membrane-bound gp130, a process designated as trans-signaling. Additionally, free soluble gp130 is also considered an inhibitor of IL-6 trans-signaling since it can bind to cytokine/receptor complexes outside the membrane, thus preventing intracellular signaling [27]. Subsequently to the binding event, the cell surface receptor complex activates different pathways such as the JAK/STAT, RAS/MAPK or PI3K signaling pathways [28], leading to various pathological and physiological functions which depend on the biochemical pathways. While highly attractive for biomedical use due to their pleiotropic activity, these cytokines, when administered in vivo, are rapidly degraded [14,15]. The use of nanomaterials to deliver these molecules provides a valid solution, acting as a protective vehicle from degradation. Although encapsulation of cytokines allows controlled and specific delivery to target cells, the nanomaterials must be able to release the molecules, as in most cases, cytokines needs to bind the membrane receptor for its activity to occur. An alternative approach is to link the cytokine to the surface of the nanomaterial. However, the potential ligation to free soluble gp130 is problematic and can hinder effective cytokine delivery. Similarly, nanogram levels of delivered TNF α can bind to its soluble receptor molecules, resulting in the lower binding to the membrane receptors and inhibition of posterior intracellular signaling [29]. This drawback can be overcome by functionalizing the surface of the produced nanomaterials with protective molecules (e.g., PEG) in order to mask the cytokines until they reach the target cells.
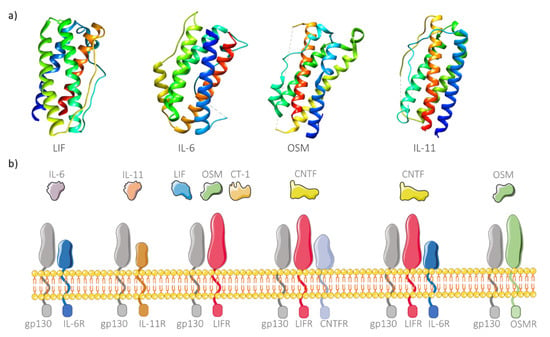
Figure 1.
Structure and binding affinity of IL-6 family members. (a) Three-dimensional structure of four members of IL-6 cytokine family: LIF (leukemia inhibitory factor; PDB ID 1emr), IL-6 (interleukin-6; PDB ID 1alu), OSM (oncostatin M; PDB ID 1evs) and IL-11 (PDB ID 4mhl), showing the characteristic top (helix A, blue), top (helix B, light blue to green), down (helix C, green to yellow), down (helix D, orange to red) helix arrangement. (b) Receptor complexes of IL-6 cytokine family. The signaling receptor subunit gp130 is shared by all family members. IL-6 and IL11 have their specific subunits (IL6R and IL-11R, respectively). OSM can bind to OSMR and gp130 but also to LIFR. In turn, LIF and CT-1 (cardiotrophin-1) transduce intracellular signaling through binding to gp130 and LIFR. A CNTF (ciliary neurotrophic protein factor) receptor is normally composed of three complexes, namely CNTFR, LIFR and gp130, but can also signal through binding to IL-6R, LIFR and gp130.
Although several applications of cytokines in treatments or even in diagnosis are recognized, their delivery to the sites of interest, using nanocarriers, is insufficiently explored, mainly with regard to the IL-6 family. In fact, IL-6 is still the most studied protein of this family, and it is important to transfer this knowledge to the remaining cytokines so to improve or complement the type of treatment adjusted to the disease.
3. Nanoparticles as Cytokine Delivery Systems
The use of cytokines for disease treatments has a large range of applications by modulating the immune system. However, systemic administration generally results in harmful side effects due to the toxicity associated with high concentrations of cytokines required to achieve a therapeutic outcome [30]. Nowadays, there is a wide choice of possible nanomaterials to be used to reduce these and other side effects, though not all vehicles may be suitable for the delivery of cytokines. Solvent, pH, temperature, charge, size and other parameters used in the nanoformulations will influence the encapsulation efficiency, nanomaterial toxicity or even cytokine stability [31]. The latter is one of the major reasons why the delivery of cytokines is yet to be fully explored. Regardless, nanoparticles remain the most used vehicle for this purpose and many types have been studied that include sources such as polymeric [32,33], chitosan [14], gold [34], silica [35], magnetic [36] to liposomal [37,38] nanoparticles (Table 1).

Table 1.
Nanoparticle-based systems for the delivery of cytokines and applications in biomedicine.
The cytokines can be encapsulated, adsorbed or conjugated into the nanocarrier system, and the nanometric size of these delivery systems increases the absorption of the drug by the cells, when compared to larger particles. One has to, however, take into account that these immunostimulatory agents need to be delivered in sufficient amounts to attain the desired effect. In addition, the nanocarriers are frequently detected as foreign by the immune system, which can lead to unwanted toxicity or even immune clearance. In this way, the immunotoxicity of the nanocarrier should be evaluated by measuring pro-inflammatory cytokine levels, which can be associated with adverse reactions and low therapeutic efficacy of nanoparticles, depending on the applied treatment [43]. To overcome this pitfall, nanoparticles can be functionalized to alter their surface properties and facilitate the attachment of a wide variety of molecules, such as receptor ligands, thereby increasing the bioactivity of these nanomaterials [44].
3.1. Types of Nanoparticles for Cytokine Delivery
3.1.1. Polymeric Nanoparticles
Polymeric carriers represent one of the best studied delivery vehicles, ranging from gels to nanoparticles, and have been used to improve circulation times, stability, and the capacity to encapsulate and supply therapeutic molecules compared to lipid formulations [45]. This type of particle often exhibits excellent biocompatibility, low toxicity and biodegradability profiles. The most commonly used polymer is polylactic-coglycolic acid (PLGA), which is a US FDA-approved polymer synthesized using the oil-in-water emulsion method [16,45]. However, other methods such as spray-drying, salting out and nanoprecipitation can be employed to produce these nanoparticles and it is important to consider that the chosen method will influence their properties, such as size and surface [44]. Other important characteristics of these nanocarriers include: high stability, easy encapsulation or adsorption of both hydrophobic and hydrophilic molecules [46], facile surface and structure modification allowing the design of multifunctional engineered delivery systems [44]. Kamaly and co-workers reported the development of PLGA polyethylene glycol (PEG) nanoparticles encapsulating IL-10 to prevent plaque formation in advanced atherosclerosis lesions, and argued that the encapsulated cytokine may have a longer circulation life compared to its adsorption to the nanoparticle [39]. The PLGA polymer was charged at terminal portions for more effective electrostatic interactions with IL-10, and PEG was used to functionalize the nanoparticle, allowing a greater nanoparticle stability and prolonged systemic circulation by immune shielding [39,47]. In addition, the researchers proved that neither the IL-10-containing nanoparticles nor the empty ones caused any in vitro or in vivo toxicity [39]. Kim and co-workers developed nanoparticles containing chitosan, a well-studied polysaccharide polymer commonly used due to its biocompatibility and biodegradability properties [15], encapsulating IL-10 conjugated with iron oxide nanoparticles for magnetic resonance imaging (MRI) ability, without affecting the mechanical and targeting properties of the nanoparticles. The authors demonstrated that these nanocarriers had easy and efficient loading, and controlled release, effectively targeting plaques in atherosclerotic mice [41,48]. The arginylglycylaspartic acid (RGD) peptide was used as a targeting ligand to functionalize the nanocarriers. Therefore, the delivery of IL-10 allowed for the inhibition of pro-inflammatory cytokines, when compared to the empty nanoparticles. An important parameter that was evaluated in this study was the half-life of the cytokine, which extended to 8.59 ± 0.21 h, much longer than free IL-10 (0.58 ± 0.06 h) [41]. Depending on the application, this parameter is essential for the clinical pharmacokinetics of the therapeutic route, yet it is not always assessed. The use of cytokines has also been extensively studied in tissue regeneration, since they are essential for proliferation and differentiation of cells in this process [49]. However, their low local concentration and short half-lives limit their application. Wang and co-workers developed self-assembled chitosan/Heparine (CSO/H) nanoparticles that could load vascular endothelial growth factor (VEGF) to stimulate proliferation in vitro and vascularization in vivo [15]. Despite the aforementioned advantages of chitosan, the in vivo application of this biopolymer is sometimes limited, due to poor pH stability at values above 6.5 and considering that many cytokines need to be at pH 7.4 to be active. However, by using a low molecular weight chitosan soluble at physiological pH, the authors demonstrated high cytokine encapsulation efficiency at around 90% (tested also for stromal cell derived factor-1α), able to preserve cytokine’s bioactivity for tissue regeneration [15]. This indicates that chitosan-based NPs could be an appropriate system for loading and delivery of other cytokines [15]. Furthermore, the CSO/H nanoparticles demonstrated to be uniform in size and within the optimal range (50–150 nm) for the design of nanoparticles for drug delivery purposes, a feature not always obtained with particles constituted by other molecules [15].
3.1.2. Liposomes
Liposomes are well-studied carriers exploited for the delivery of numerous types of biomolecules, including cytokines [50,51,52,53]. Vesicles composed of one or more lipid membranes are able to easily cross lipid bilayers and cell membranes [44], but can be rapidly eliminated in vivo; therefore, stabilization, for instance with PEG, is essential in these nanocarriers [54]. In addition, the conditions used to prepare liposomal formulations are of great importance: solvents such as chloroform are often used [55] as well as high temperatures or low pH that decrease nanoparticles’ biosafety or induce cytokine denaturation [31]. Van Slooten and collaborators demonstrated that liposomes containing IFN-γ can be useful for tumor vaccination, providing a reservoir for cytokines at the site of antigen presentation. However, low but detectable cytokine/liposome amounts were found in blood, liver, spleen, kidneys and lymph nodes [38], probably due to the non-functionalization of these nanoparticles. Although the nanoparticles were completely cleared in 168 h, 70% of the injected dose was eliminated from the injection site in 8 h, thus exhibiting a release profile which does not seem to be fully suited for the application [38].
In another study, Zhang and co-workers demonstrated that combined treatment with IL-2 and anti-CD137 caused anti-tumor immunity, but was accompanied by severe systemic toxicity. Thus, the authors developed PEGylated liposomes (with a lipid mixture of DOPC, cholesterol, DSPE-PEG, and DSPE-PEG-maleimide) containing IL-2 and anti-CD137 conjugated to the particles’ surface. The produced liposomes provoked stronger anti-tumor activity and showed an absence of toxicity when compared to systemic delivery of free drug combination, by the evaluation of pro-inflammatory cytokine levels in the serum [42]. This is a useful approach for the delivery of cytokines, as these are available to bind to the respective receptors at the target cells’ surface. Moreover, it allows the increment of cytokine activity and the introduction of an additional drug to be delivered, potentiating the desired effect, usually termed as co-delivery. As an example, Postma and co-workers confirmed that TNF-α coupled to the outer surface of the liposome improved protection against Plasmodium berghei-induced experimental cerebral malaria in mice compared to liposomes encapsulating TNF-α, or even the free cytokine [56].
3.1.3. Gold Nanoparticles
The use of gold nanoparticles (AuNPs) in biological applications is increasing, partly due to their chemical inertness and unique optical and physical properties, suitable for use as imaging agents [57]. These nanoparticles are easy to functionalize and to synthetize with controlled dispersion, and are characterized by a high surface area-to-volume ratio. AuNPs proved to be great therapeutic agents as they can easily travel to target cells and support high drug load [58]. Curnis and coworkers demonstrated that gold nanoparticles can be used as nanocarriers for cytokine delivery to tumors in mice models with fibrosarcoma [34]. The researchers proved that TNF-loaded gold nanoparticles could deliver active cytokine to tumors without associated toxicity (as judged by animal weight loss), when compared to the administration of the free cytokine that showed no activity for the same dosage. The nanocarriers were functionalized with a novel tumor-homing peptide, Asn-Gly-Arg (NGR), which is a CD13 ligand expressed in the tumor neovasculature [34]. This example demonstrates that AuNPs can be used as a platform for the delivery of cytokines for cancer therapy. Although generally described as biocompatible, gold nanoparticles can, in some cases, elicit an immune reaction and it is thus essential to evaluate cellular responses such as cytokine or reactive oxygen species (ROS) production, an overlooked aspect in many reports. For instance, Srijampa and co-workers showed that the surface charge of gold nanoparticles determined whether pro-inflammatory cytokine production is increased or decreased [59,60]. However, other physical and chemical properties of the nanoformulations, such as size and hydrophobicity of the particle coating, may also be responsible for causing cytotoxicity, influencing compatibility with cellular immune responses such as transport processes or cellular damage [60]. In this way, the design of nanoparticles for biomedical applications should respect these key aspects as well as the final objective and type of application. The modification and functionalization of the surface remains one of the most relevant parameters when the main objective is to escape interactions with cells of the immune system.
3.1.4. Silica Nanoparticles
Silica nanoparticles have also been used as a cytokine delivery system, showing high colloidal stability, extensive surface functionalization and possibility to control both structure and pore size [61]. Silica nanoparticles present limitations for the delivery of cytokines, due to the low internalization efficiency for larger biomolecules. Nonetheless, Kwon and collaborators solved this limitation by developing mesoporous silica nanoparticles with extra-large pores that can be applied to cytokine delivery in vivo [35]. These nanoparticles were tested for the delivery of IL-4 to modulate immune responses in mice by inducing M2 macrophage polarization, with clinical applications in anti-inflammatory or tissue homeostasis therapies. The cytokine half-life was improved, while showing minimal toxicity, which was evaluated through ROS and pro-inflammatory cytokine production [35]. Moreover, in addition to the efficient loading and delivery of IL-4, a cytokine with proven ability to stimulate M2 macrophage polarization [62], the nanoparticles were taken up by phagocytic cells, such as macrophages, demonstrating the potential application for in vivo anti-inflammatory M2 macrophage polarization.
3.2. The Specific Case of the IL-6 Cytokine Family
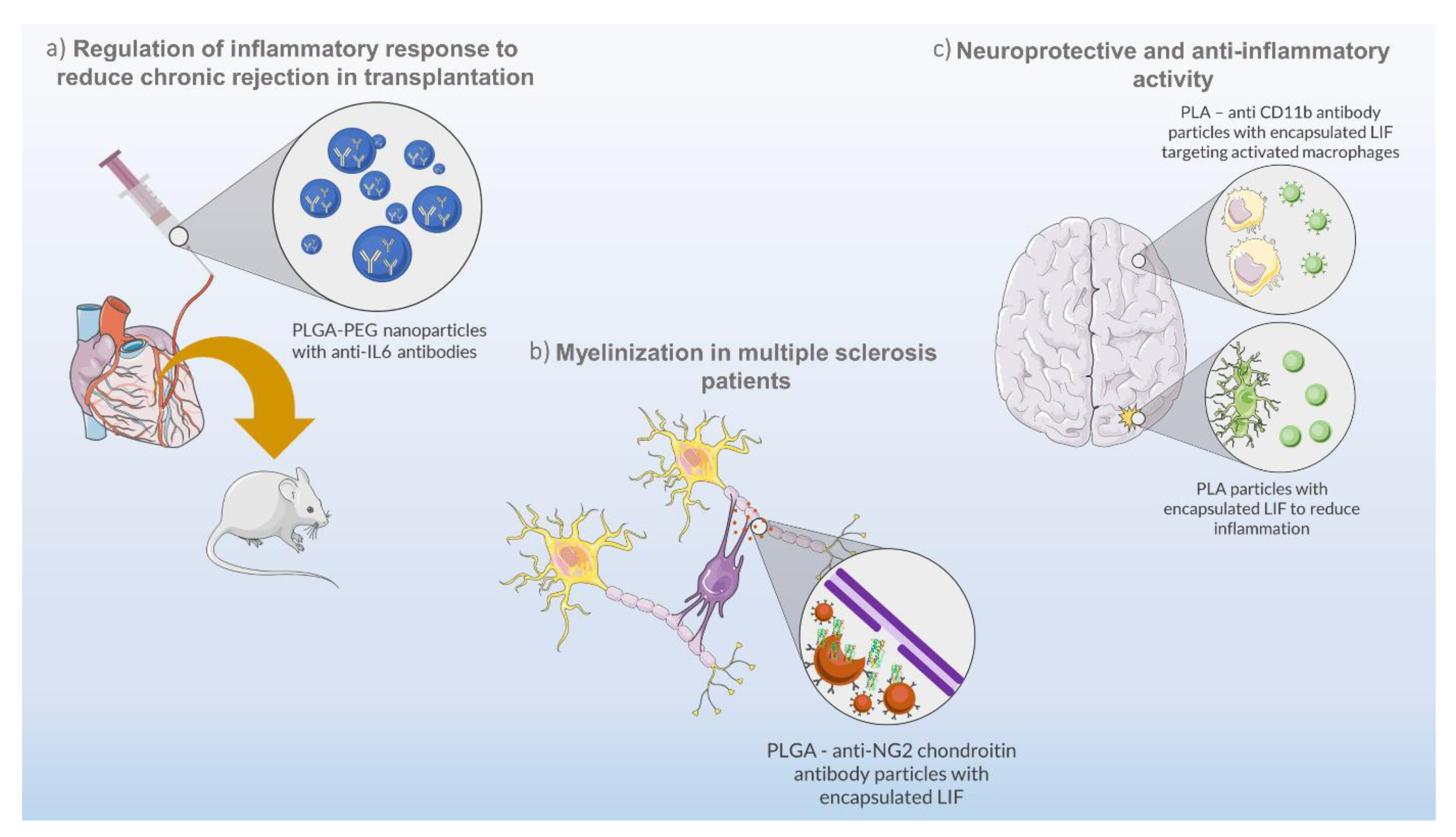
Occasionally, unregulated cytokine production can result in unwanted conditions of inflammation, a common situation in organ transplants. In such cases, immunosuppressive therapies using immunoregulatory molecules are a viable option. Solhjou and co-workers developed a controlled formulation composed of nanoparticles made of PLGA-PEG and loaded with anti-IL-6 antibody for the targeting of IL-6-mediated inflammatory responses (Figure 2a) [40]. For that, the authors perfused a heart with nanoparticles to deliver anti-IL-6 antibody prior to transplantation, in order to suppress chronic rejection. In addition, the direct delivery of these nanoparticles prevented systemic immunosuppression and allowed the use of reduced anti-IL-6 doses (more than 200 times when compared to the systemic administration) with positive results and minimal side effects [40].
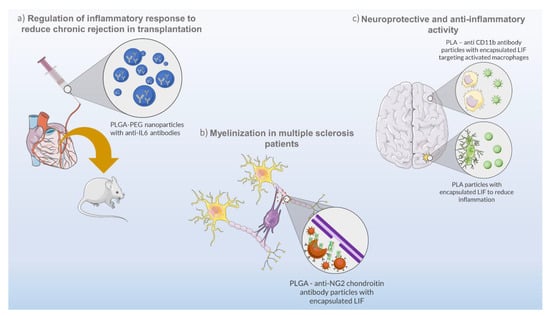
Figure 2.
Biotechnological approaches using nanoparticles and cytokines from IL-6 family. (a) PLGA (polylactic-coglycolic acid)–PEG (polyethylene glycol) nanoparticles encapsulating anti-IL-6 antibodies for regulation of the inflammatory response in heart transplantation in mice. (b) PLGA–anti-NG2 chondroitin antibody particles encapsulating LIF for remyelination of the central nervous system as a therapeutic strategy for multiple sclerosis disease. (c) PLA (poly(lactic acid))–anti CD11b antibody particles encapsulating LIF and providing neuroprotective and anti-inflammatory effects to damaged brain sites.
Another study using LIF-loaded PLGA nanoparticles was carried out by Sonja Rittchen and co-workers [33]. LIF is a pleiotropic cytokine of the IL-6 family which can act in diverse organs and play several functions in health and disease, depending on the cell type and environment [63]. The objective of the study was to develop a biodegradable and biocompatible nanocarrier capable of maintaining load stability and a controlled cytokine release for a prolonged therapeutic effect, verified over several days. The produced nanoparticles were tested for the delivery of pro-remyelinating factors (LIF) to the central nervous system as a therapeutic strategy for multiple sclerosis disease (Figure 2b). LIF has been proven to promote remyelination and tissue repair [64,65], avoiding off-target effects by nanoparticle functionalization with anti-NG2 chondroitin sulphate proteoglycan antibodies. This functionalization demonstrated to be effective for the targeted delivery of LIF to oligodendrocyte precursor cells, promoting their differentiation to repair myelin, and avoiding a systemic exposure to the cytokine. Moreover, the potency of the targeted drug delivery system was high, requiring minimal doses of LIF (picomolar range) to increase remyelination, in vivo, using a mouse model [33].
The pleiotropic activity of LIF was shown in a study by Davis and co-workers, in which the authors demonstrated that LIF decreases neural cell degeneration in vitro and in vivo (Figure 2c) [66]. However, LIF-dependent neuroprotection is influenced by the short half-life and biostability of the cytokine in blood [32]. Thus, the authors developed LIF-loaded nanoparticles made of PEG-poly(lactic acid) (PLA) to deliver and prolong the stability and activity of the cytokine for in vivo applications [32]. The PEG-PLA LIF-loaded nanoparticles, termed as NanoLIF, demonstrated a gradual release of LIF in in vitro tests, which enhances the therapeutic efficacy for future in vivo applications, and an increase in LIF half-life (30 h) in comparison to other nanomaterials (2 h) [67]. The NanoLIF system also demonstrated high loading capacity (up to 331 ng/mL LIF) and the ability to protect the cytokine from thermal degradation. Additionally, the authors also devised a strategy for the target delivery of LIF to inflammatory macrophages through surface functionalization of NanoLIF with CD11b antibody (CD11b-NanoLIF), that can bind to the CD11b membrane protein overexpressed on macrophages [32]. In this way, a combined approach would improve the therapeutic efficacy of LIF by providing neuroprotective and anti-inflammatory effects. While NanoLIF slowly releases LIF in the vicinities of damaged brain sites for a prolonged time, suppressing initial brain damage, CD11b-NanoLIF targets activated macrophages to inhibit secondary inflammation in the brain.
4. More Than Meets the Eye: Cytokines and Nanocarriers as Novel Biosensors
The cytokines present in body fluids and tissues play an important role as potentially useful biomarkers for several diseases. The monitoring of their levels is of great importance for early clinical diagnosis and for assessing therapeutic efficacy. However, quantification of cytokine secretion in the respective microenvironment still remains a challenge due to the use of laborious and time-consuming detection platforms, such as enzyme-linked immunosorbent assay (ELISA). Although well-established and effective, ELISA includes a long assay time (3–8 h), a large amount of sample volume and low sensitivity [68,69,70] when compared to some recently developed biosensors based on nanotechnology. Overall, a biosensor is composed of a biorecognition element, which binds to or interacts with the analyte, a signal transducer, which transduces the biorecognition event into a signal, and a detector, which detects the signal via several modalities [71]. A biosensor must follow some general criteria, which include sensitivity in detecting the concentration of analyte molecules and selectivity for the discrimination between different analytes. Other important features are the dynamic range (concentration range within the linear range of response), response time (time required to indicate a defined percentage of final response), accuracy (agreement between the measured result and the true value), precision (reproducibility of measurements), detection limit (the lowest concentration that produces a measurable signal response), and lifetime (the time length over which the sensor can be operated without significant deterioration in performance) [69]. Nanomaterial-based sensors have attracted increasing attention in molecular diagnosis, since they offer excellent sensitivity, stability and their reduced size is useful to improve their limit detection and analyte–sensor interactions [68].
Zhuang Hao and co-workers developed a graphene-based field effect transistor nanosensor capable of sampling and quantifying cytokine biomarkers in human body fluids, such as sweat [70]. The nanosensor was functionalized with a short DNA aptamer consisting of 25 nucleotides that acted as a cytokine-specific receptor in order to achieve affinity recognition of the biomarker and enhanced sensitivity of cytokine detection. The researchers proved that the nanosensor was capable of responding to changes in TNF-α concentration within 5 min with a limit of detection as low as 26 pM [70]. In turn, Guozhen Liu and co-workers reported the use of graphene quantum dot nanosensors for in vitro and ex vivo detection of very small amounts of INF-γ [72]. By conjugating graphene quantum dots (GQD) with aptamers of INF-γ (Ap-GQD conjugate) and with epitopes of INF γ (Ep-GQD conjugate), the authors developed a “switch-on” cytokine nanosensor based on the aggregation and disaggregation of GQDs: in the absence of INF-γ, Ap-GQD and Ep-GQD specifically interact, forming aggregates with no resulting fluorescence. In the presence of the cytokine, the competitive affinity binding between INF-γ and Ap-GQD results in the disaggregation of the Ep-GQD and Ap-GQD complex, turning on fluorescence, owing to the strong fluorescent signal of each individual conjugate [72]. The nanosensor was efficiently delivered into living cells, presenting great solubility, stability, biocompatibility and high sensitivity, capable of detecting concentrations of intracellular INF-γ as low as 2 pg/mL [72].
With the increasing knowledge on biosensors, a translation from in vitro to in vivo is required, as the first is not able to comply with the complexity of the latter. Consequently, in vivo biosensing has been the focus of research aiming at the continuous long-term monitoring of biological processes. Still, in vivo biosensing presents several other challenges such as long-term stability in biological fluids, biocompatibility, tissue response, cellular uptake, biodistribution and clearance, among others. Even though extensive progress has been made in the development of in vitro biosensors, these often fail to be translated into in vivo systems giving indications of reduced performance. Some of these biosensors are reviewed by Mark A. Eckert and co-workers [71] and Rong and co-workers [73], however, to the best of our knowledge, there are a lack of developed in vivo sensors for the detection of cytokines or for the expression of their altered receptors, which can be very useful in certain pathologies.
5. Conclusions
Cytokines, proteins secreted by immune cells, play important roles in a variety of biological processes and are increasingly used as therapeutic agents. However, their reduced half-life and related systemic toxicity limit their use, barriers that can be overcome with the use of nanotechnology. A large set of nano-based drug delivery systems are currently available, with most resorting to the use of nanoparticles. The choice of the nanomaterial to be used depends on several features, as mentioned, that influence encapsulation efficiency, nanomaterial toxicity or even cytokine stability. For cytokine delivery, nanocarriers display better results compared to the use of free cytokines, in particular nanoparticles made of polymers such as PLGA, chitosan or other polymers, including for delivery of IL-6 family members. As there are few incursions on the therapeutic delivery of these specific cytokines, it would be pertinent to test nanocarriers previously validated with other cytokines, since the general properties of these proteins are very similar. The use of nanotechnology for nanosensor development is also useful due to their reduced size, which improves the limit detection and analyte–sensor interactions. Although the use of nanosensors to detect cytokine levels or the expression of their altered receptors is still underexplored, we strongly believe these devices will revolutionize the future of healthcare.
Author Contributions
Conceptualization, R.M., A.C.G. and A.d.C.; writing—original draft preparation, A.G.; writing—review and editing, R.M., A.C.G. and A.d.C.; supervision, R.M., A.C.G. and A.d.C.; project administration, A.C.G.; funding acquisition, A.C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the “Contrato-Programa” UIDB/04050/2020 funded by national funds through the FCT I.P. and project FUN2CYT: Harnessing the potential for biomedical applications of pleiotropic cytokines LIF and oncostatin M (POCI-01-0145-FEDER-030568) supported by Programa Operacional Competitividade e Internacionalização (FEDER) and FCT, IP. Anabela Gonçalves acknowledges her PhD scholarship from FCT (SFRH/BD/146807/2019).
Acknowledgments
The authors credit Servier Medical Art for providing vector images for figure creation under a Creative Commons Attribution 3.0 Unported License.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, J.-M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Historical insights into cytokines. Eur. J. Immunol. 2007, 37, S34–S45. [Google Scholar] [CrossRef] [PubMed]
- Goren, I.; Kämpfer, H.; Müller, E.; Schiefelbein, D.; Pfeilschifter, J.; Frank, S. Oncostatin M expression is functionally connected to neutrophils in the early inflammatory phase of skin repair: Implications for normal and diabetes-impaired wounds. J. Investig. Dermatol. 2006, 126, 628–637. [Google Scholar] [CrossRef]
- Ray, A.; Gulati, S.G.K.; Joshi, N.R.J. Cytokines and their role in health and disease: A brief overview. MOJ Immunol. 2016, 4, 1–9. [Google Scholar] [CrossRef]
- Barnes, P.J. The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Investig. 2008, 118, 3546–3556. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 family cytokines. Cold Spring Harb. Perspect. Biol. 2017, 10, a028415. [Google Scholar] [CrossRef] [PubMed]
- Kakar, S. Cytokines evolution: Role in various diseases. Curr. Med. Res. Pr. 2015, 5, 176–182. [Google Scholar] [CrossRef]
- Aukrust, P.; Ueland, T.; Lien, E.; Bendtzen, K.; Müller, F.; Andreassen, A.K.; Nordøy, I.; Aass, H.; Espevik, T.; Simonsen, S.; et al. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1999, 83, 376–382. [Google Scholar] [CrossRef]
- Kips, J.C. Cytokines in asthma. Eur. Respir. J. 2001, 18, 24–33. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Molfino, A.; Bollea, M.R.; Fanelli, F.R. Malnutrition and wasting in renal disease. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 378–383. [Google Scholar] [CrossRef]
- Kedzierska, K.; Crowe, S.M. Cytokines and HIV-1: Interactions and clinical implications. Antivir. Chem. Chemother. 2001, 12, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L. Nuanced roles of cytokines in three major human brain disorders. J. Clin. Investig. 2008, 118, 3557–3563. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Lampaki, S.; Yarmus, L.; Kioumis, I.; Pitsiou, G.; Katsikogiannis, N.; Hohenforst-Schmidt, W.; Li, Q.; Huang, H.; Sakkas, A.; et al. Interleukin-7 and Interleukin-15 for cancer. J. Cancer 2014, 5, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, D.H.; Basser, R.; Davis, I.D.; Cebon, J.S.; Mitchell, P.; Underhill, C.; Kilpatrick, T.; Reardon, K.; Green, M.D.; Bardy, P.; et al. A phase I study of recombinant human leukemia inhibitory factor in patients with advanced cancer. Clin. Cancer Res. 2003, 9, 2056–2065. [Google Scholar] [PubMed]
- Tang, H.; Wang, B.; Tan, L.; Deng, D.; Lü, T.; Zhou, C.; Li, Z.; Tang, Z.; Wu, Z. Novel stable cytokine delivery system in physiological pH solution: Chitosan oligosaccharide/heparin nanoparticles. Int. J. Nanomed. 2015, 10, 3417–3427. [Google Scholar] [CrossRef]
- Zhuang, J.; Holay, M.; Park, J.H.; Fang, R.H.; Zhang, J.; Zhang, L. Nanoparticle delivery of immunostimulatory agents for cancer immunotherapy. Theranostics 2019, 9, 7826–7848. [Google Scholar] [CrossRef]
- Langer, R.; Weissleder, R. Nanotechnology. JAMA 2015, 313, 135–136. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.-S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Richards, C.D. The enigmatic cytokine oncostatin M and roles in disease. ISRN Inflamm. 2013, 2013, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. The soluble interleukin-6 receptor and related proteins. Best Pr. Res. Clin. Endocrinol. Metab. 2015, 29, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Collison, L.W.; Delgoffe, G.M.; Guy, C.S.; Vignali, K.M.; Chaturvedi, V.; Fairweather, D.; Satoskar, A.R.; Garcia, K.C.; Hunter, C.A.; Drake, C.G.; et al. The composition and signaling of the IL-35 receptor are unconventional. Nat. Immunol. 2012, 13, 290–299. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Y.; Xiao, H.; Liu, X.; Zhang, Y.; Han, G.; Chen, G.; Hou, C.; Ma, N.; Shen, B.; et al. A novel IL-23p19/Ebi3 (IL-39) cytokine mediates inflammation in Lupus-like mice. Eur. J. Immunol. 2016, 46, 1343–1350. [Google Scholar] [CrossRef]
- Taga, T.; Hibi, M.; Hirata, Y.; Yamasaki, K.; Yasukawa, K.; Matsuda, T.; Hirano, T.; Kishimoto, T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell 1989, 58, 573–581. [Google Scholar] [CrossRef]
- Wolf, J.; Rose-John, S.; Garbers, C. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine 2014, 70, 11–20. [Google Scholar] [CrossRef]
- Kishimoto, T.; Akira, S.; Narazaki, M.; Taga, T. Interleukin-6 family of cytokines and gp130. Blood 1995, 86, 1243–1254. [Google Scholar] [CrossRef]
- Curnis, F.; Sacchi, A.; Corti, A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J. Clin. Investig. 2002, 110, 475–482. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M.; Chang, A.E.; Avis, F.P.; Leitman, S.; Linehan, W.M.; Robertson, C.N.; Lee, R.E.; Rubin, J.T.; et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and Interleukin-2 or high-dose Interleukin-2 alone. N. Engl. J. Med. 1987, 316, 889–897. [Google Scholar] [CrossRef]
- Yu, M.; Wu, J.; Shi, J.; Farokhzad, O.C. Nanotechnology for protein delivery: Overview and perspectives. J. Control. Release 2016, 240, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.M.; Reichel, D.; Bae, Y.; Pennypacker, K. Leukemia inhibitory factor-loaded nanoparticles with enhanced cytokine metabolic stability and anti-inflammatory activity. Pharm. Res. 2018, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rittchen, S.; Boyd, A.; Burns, A.; Park, J.; Fahmy, T.M.; Metcalfe, S.M.; Williams, A. Myelin repair In Vivo is increased by targeting oligodendrocyte precursor cells with nanoparticles encapsulating leukaemia inhibitory factor (LIF). Biomaterials 2015, 56, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Curnis, F.; Fiocchi, M.; Sacchi, A.; Gori, A.; Gasparri, A.; Corti, A. NGR-tagged nano-gold: A new CD13-selective carrier for cytokine delivery to tumors. Nano Res. 2016, 9, 1393–1408. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Cha, B.G.; Cho, Y.; Min, J.; Park, E.-B.; Kang, S.-J.; Kim, J. Extra-large pore mesoporous silica nanoparticles for directing In Vivo M2 macrophage polarization by delivering IL-4. Nano Lett. 2017, 17, 2747–2756. [Google Scholar] [CrossRef]
- Mejías, R.; Pérez-Yagüe, S.; Gutiérrez, L.; Cabrera, L.I.; Spada, R.; Acedo, P.; Serna, C.; Lázaro, F.J.; Villanueva, A.; Morales, M.D.P.; et al. Dimercaptosuccinic acid-coated magnetite nanoparticles for magnetically guided in vivo delivery of interferon gamma for cancer immunotherapy. Biomaterials 2011, 32, 2938–2952. [Google Scholar] [CrossRef]
- Duits, A.J.; Van Puijenbroek, A.; Vermeulen, H.; Hofhuis, F.M.; Van De Winkel, J.G.; Capel, P.J. Immunoadjuvant activity of a liposomal IL-6 formulation. Vaccine 1993, 11, 777–781. [Google Scholar] [CrossRef]
- Van Slooten, M.L.; Storm, G.; Zoephel, A.; Küpcü, Z.; Boerman, O.; Crommelin, D.J.A.; Wagner, E.; Kircheis, R. Liposomes containing interferon-gamma as adjuvant in tumor cell vaccines. Pharm. Res. 2000, 17, 42–48. [Google Scholar] [CrossRef]
- Kamaly, N.; Fredman, G.; Fojas, J.J.R.; Subramanian, M.; Choi, W.I.; Zepeda, K.; Vilos, C.; Yu, M.; Gadde, S.; Wu, J.; et al. Targeted Interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS Nano 2016, 10, 5280–5292. [Google Scholar] [CrossRef]
- Solhjou, Z.; Uehara, M.; Bahmani, B.; Maarouf, O.H.; Ichimura, T.; Brooks, C.R.; Xu, W.; Yilmaz, M.; Elkhal, A.; Tullius, S.G.; et al. Novel application of localized nanodelivery of anti-interleukin-6 protects organ transplant from ischemia-reperfusion injuries. Arab. Archaeol. Epigr. 2017, 17, 2326–2337. [Google Scholar] [CrossRef]
- Kim, M.; Sahu, A.; Hwang, Y.; Kim, G.B.; Nam, G.H.; Kim, I.S.; Chan Kwon, I.; Tae, G. Targeted delivery of anti-inflammatory cytokine by nanocarrier reduces atherosclerosis in Apo E(-/-) mice. Biomaterials 2020, 226, 119550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, N.; Suh, H.; Irvine, D.J. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Wooley, K.L. Cytokines as biomarkers of nanoparticle immunotoxicity. Chem. Soc. Rev. 2013, 42, 5552–5576. [Google Scholar] [CrossRef] [PubMed]
- Conniot, J.; Silva, J.M.; Fernandes, J.G.; Silva, L.C.; Gaspar, R.; Ebrocchini, S.; Florindo, H.F.; Barata, T.S. Cancer immunotherapy: Nanodelivery approaches for immune cell targeting and tracking. Front. Chem. 2014, 2, 105. [Google Scholar] [CrossRef]
- Christian, D.A.; Hunter, C.A. Particle-mediated delivery of cytokines for immunotherapy. Immunotherapy 2012, 4, 425–441. [Google Scholar] [CrossRef]
- Gelperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef]
- Gu, F.; Zhang, L.; Teply, B.A.; Mann, N.; Wang, A.Z.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl. Acad. Sci. USA 2008, 105, 2586–2591. [Google Scholar] [CrossRef]
- Kim, M.; Sahu, A.; Kim, G.B.; Nam, G.H.; Um, W.; Shin, S.J.; Jeong, Y.Y.; Kim, I.-S.; Kim, K.; Kwon, I.C.; et al. Comparison of In Vivo targeting ability between cRGD and collagen-targeting peptide conjugated nano-carriers for atherosclerosis. J. Control. Release 2018, 269, 337–346. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Perspective article: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Almer, G.; Frascione, D.; Pali-Schöll, I.; Vonach, C.; Lukschal, A.; Stremnitzer, C.; Diesner, S.C.; Jensen-Jarolim, E.; Prassl, R.; Mangge, H. Interleukin-10: An anti-inflammatory marker to target atherosclerotic lesions via PEGylated liposomes. Mol. Pharm. 2012, 10, 175–186. [Google Scholar] [CrossRef]
- Van Slooten, M.; Boerman, O.; Romøren, K.; Kedar, E.; Crommelin, D.; Storm, G. Liposomes as sustained release system for human interferon-γ: Biopharmaceutical aspects. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2001, 1530, 134–145. [Google Scholar] [CrossRef]
- Nii, A.; Fan, D.; Fidler, I.J. Cytotoxic potential of liposomes containing tumor necrosis factor-alpha against sensitive and resistant target cells. J. Immunother. 1991, 10, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Neville, M.E.; Robb, R.J.; Popescu, M.C. In Situ vaccination against a non-immunogenic tumour using intratumoural injections of liposomal Interleukin 2. Cytokine 2001, 16, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Mugabe, C.; Azghani, A.O.; Omri, A. Preparation and characterization of dehydration–rehydration vesicles loaded with aminoglycoside and macrolide antibiotics. Int. J. Pharm. 2006, 307, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Postma, N.S.; Crommelin, D.J.; Eling, W.M.; Zuidema, J. Treatment with liposome-bound recombinant human tumor necrosis factor-alpha suppresses parasitemia and protects against Plasmodium berghei k173-induced experimental cerebral malaria in mice. J. Pharmacol. Exp. Ther. 1999, 288, 114–120. [Google Scholar] [PubMed]
- Meir, R.; Shamalov, K.; Sadan, T.; Motiei, M.; Yaari, G.; Cohen, C.J.; Popovtzer, R. Fast image-guided stratification using anti-programmed death ligand 1 gold nanoparticles for cancer immunotherapy. ACS Nano 2017, 11, 11127–11134. [Google Scholar] [CrossRef]
- Chithrani, D.B.; Dunne, M.; Stewart, J.; Allen, C.; Jaffray, D.A. Cellular uptake and transport of gold nanoparticles incorporated in a liposomal carrier. Nanomedicine 2010, 6, 161–169. [Google Scholar] [CrossRef]
- Srijampa, S.; Buddhisa, S.; Ngernpimai, S.; Sangiamdee, D.; Chompoosor, A.; Tippayawat, P. Effects of gold nanoparticles with different surface charges on cellular internalization and cytokine responses in monocytes. BioNanoScience 2019, 9, 580–586. [Google Scholar] [CrossRef]
- Srijampa, S.; Buddhisa, S.; Ngernpimai, S.; Leelayuwat, C.; Proungvitaya, S.; Chompoosor, A.; Tippayawat, P. Influence of gold nanoparticles with different surface charges on localization and monocyte behavior. Bioconjug. Chem. 2020, 31, 1133–1143. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Pinho, V.; Fernandes, M.; da Costa, A.; Machado, R.; Gomes, A.C. Leukemia inhibitory factor: Recent advances and implications in biotechnology. Cytokine Growth Factor Rev. 2019, 52, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Deverman, B.E.; Patterson, P.H. Exogenous leukemia inhibitory factor stimulates oligodendrocyte progenitor cell proliferation and enhances hippocampal remyelination. J. Neurosci. 2012, 32, 2100–2109. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, S.M.; Strom, T.B.; Williams, A.; Fahmy, T.M. Multiple sclerosis and the LIF/IL-6 axis: Use of nanotechnology to harness the tolerogenic and reparative properties of LIF. Nanobiomedicine 2015, 2, 5. [Google Scholar] [CrossRef]
- Davis, S.M.; Collier, L.A.; Leonardo, C.C.; Seifert, H.A.; Ajmo, C.T.; Pennypacker, K. Leukemia inhibitory factor protects neurons from ischemic damage via upregulation of superoxide dismutase 3. Mol. Neurobiol. 2016, 54, 608–622. [Google Scholar] [CrossRef]
- Park, J.; Gao, W.; Whiston, R.; Strom, T.B.; Metcalfe, S.; Fahmy, T.M. Modulation of CD4+ T Lymphocyte lineage outcomes with targeted, nanoparticle-mediated cytokine delivery. Mol. Pharm. 2010, 8, 143–152. [Google Scholar] [CrossRef]
- Chen, P.; Huang, N.-T.; Chung, M.-T.; Cornell, T.T.; Kurabayashi, K. Label-free cytokine micro and nano-biosensing towards personalized medicine of systemic inflammatory disorders. Adv. Drug Deliv. Rev. 2015, 95, 90–103. [Google Scholar] [CrossRef]
- Singh, M.; Truong, J.; Reeves, W.B.; Hahm, J.-I. Emerging cytokine biosensors with optical detection modalities and nanomaterial-enabled signal enhancement. Sensors 2017, 17, 428. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, Z.; Li, Y.; Zhu, Y.; Wang, X.; De Moraes, C.G.; Pan, Y.; Zhao, X.; Lin, Q. Measurement of cytokine biomarkers using an aptamer-based affinity graphene nanosensor on a flexible substrate toward wearable applications. Nanoscale 2018, 10, 21681–21688. [Google Scholar] [CrossRef]
- Eckert, M.A.; Vu, P.Q.; Zhang, K.; Kang, D.; Ali, M.M.; Xu, C.; Zhao, W. Novel molecular and nanosensors for In Vivo sensing. Theranostics 2013, 3, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, K.; Ma, K.; Care, A.; Hutchinson, M.R.; Goldys, E.M. Graphene quantum dot based “switch-on” nanosensors for intracellular cytokine monitoring. Nanoscale 2017, 9, 4934–4943. [Google Scholar] [CrossRef] [PubMed]
- Rong, G.; Corrie, S.R.; Clark, H.A. In Vivo biosensing: Progress and perspectives. ACS Sens. 2017, 2, 327–338. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).