3-D Modeling of Gas–Solid Two-Phase Flow in a π-Shaped Centripetal Radial Flow Adsorber
Abstract
Featured Application
Abstract
1. Introduction
2. Experiments
2.1. Materials
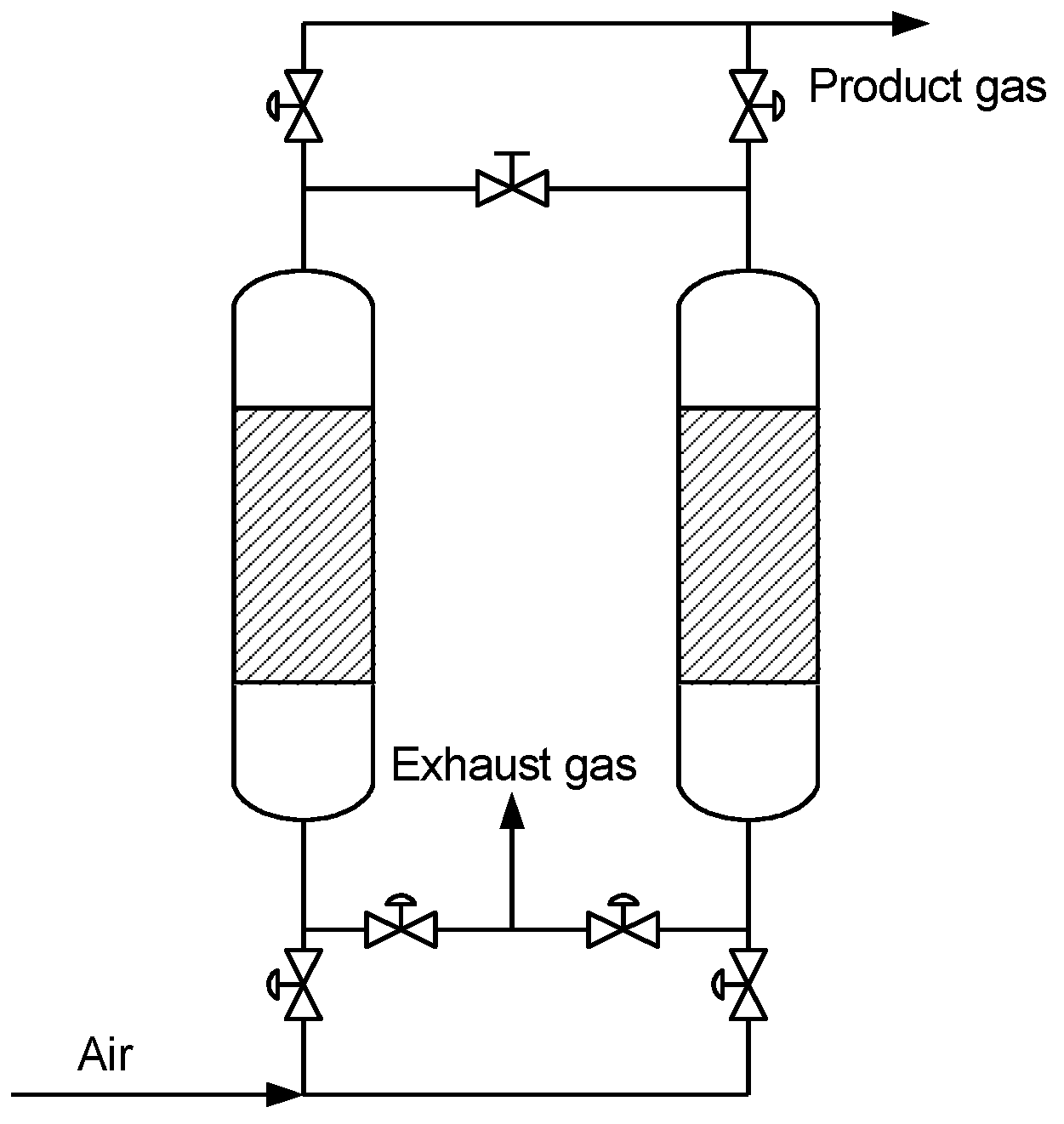
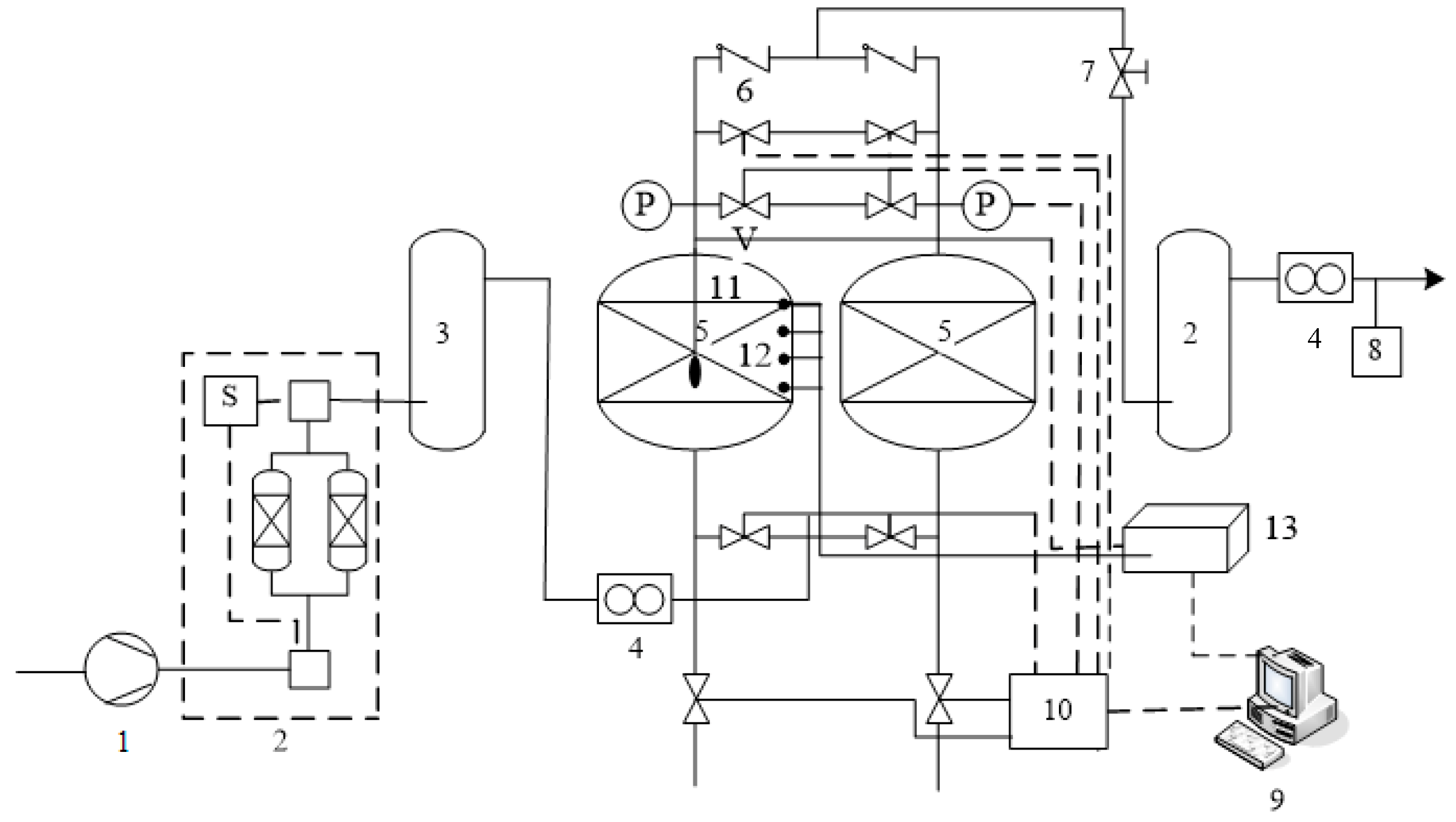
2.2. Experiment Process
3. Numerical Model
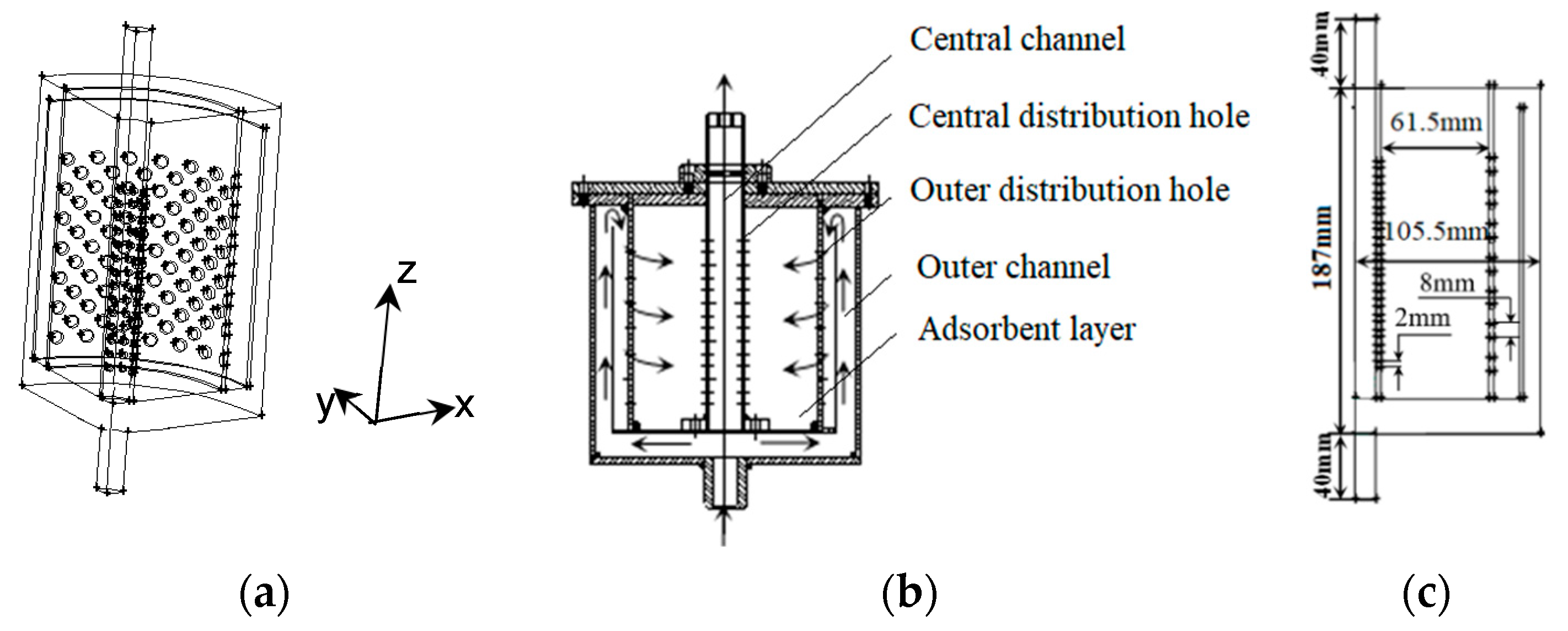
3.1. Physical Model
3.2. Mathematical Model
3.3. Initial and Boundary Conditions and Model Parameters
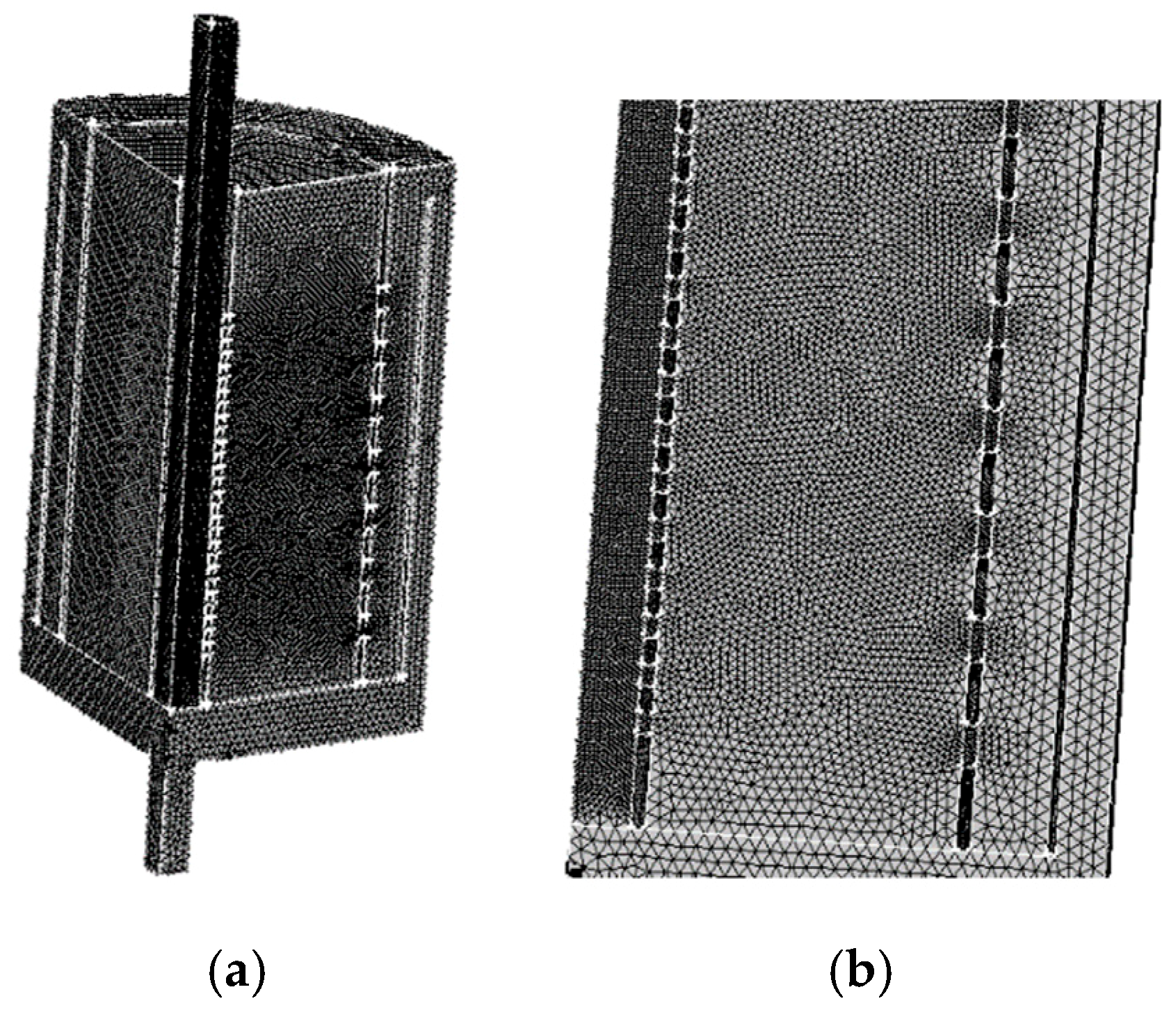
3.4. Meshing and Method of Solution
4. Results and Discussion
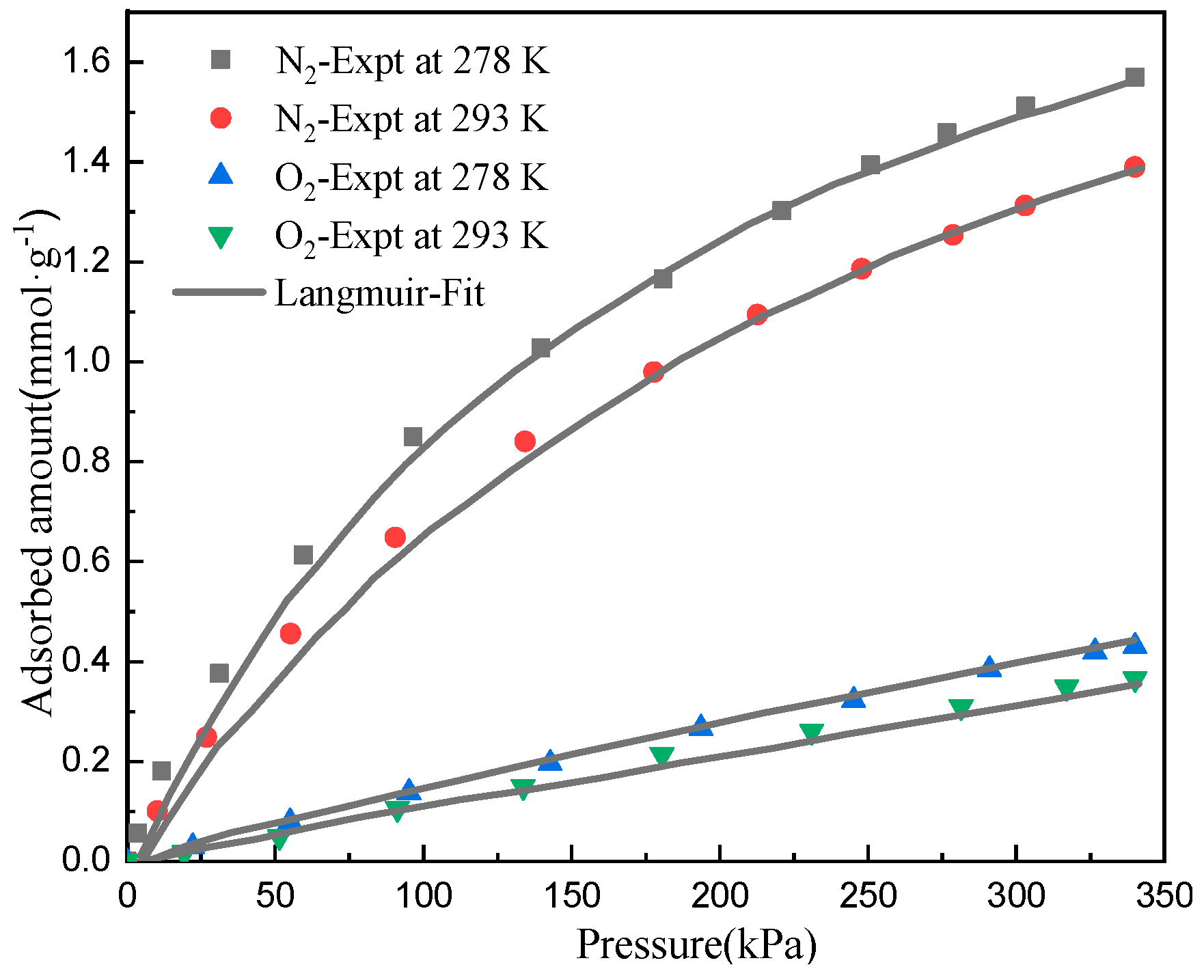
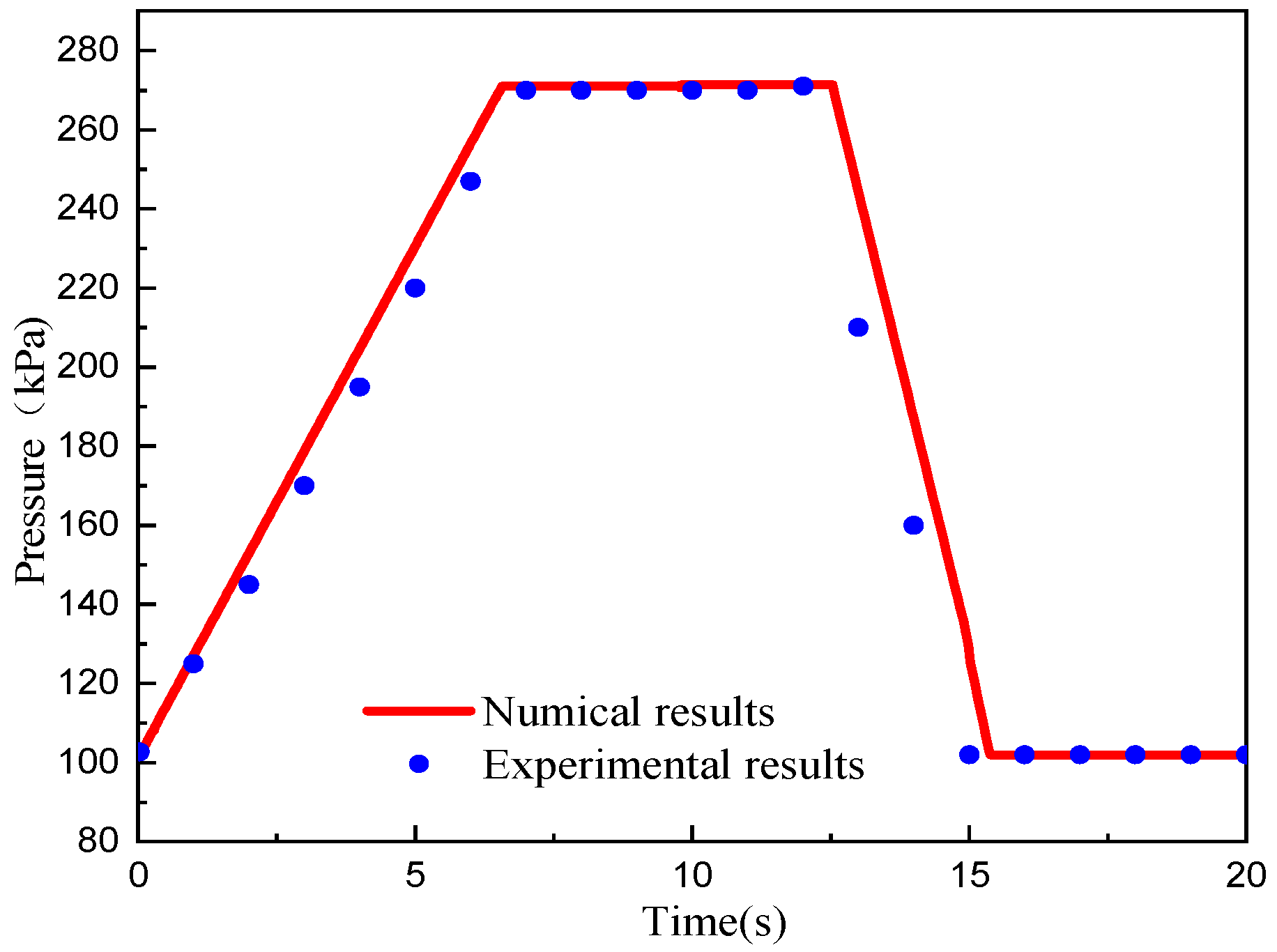
4.1. Model Validation
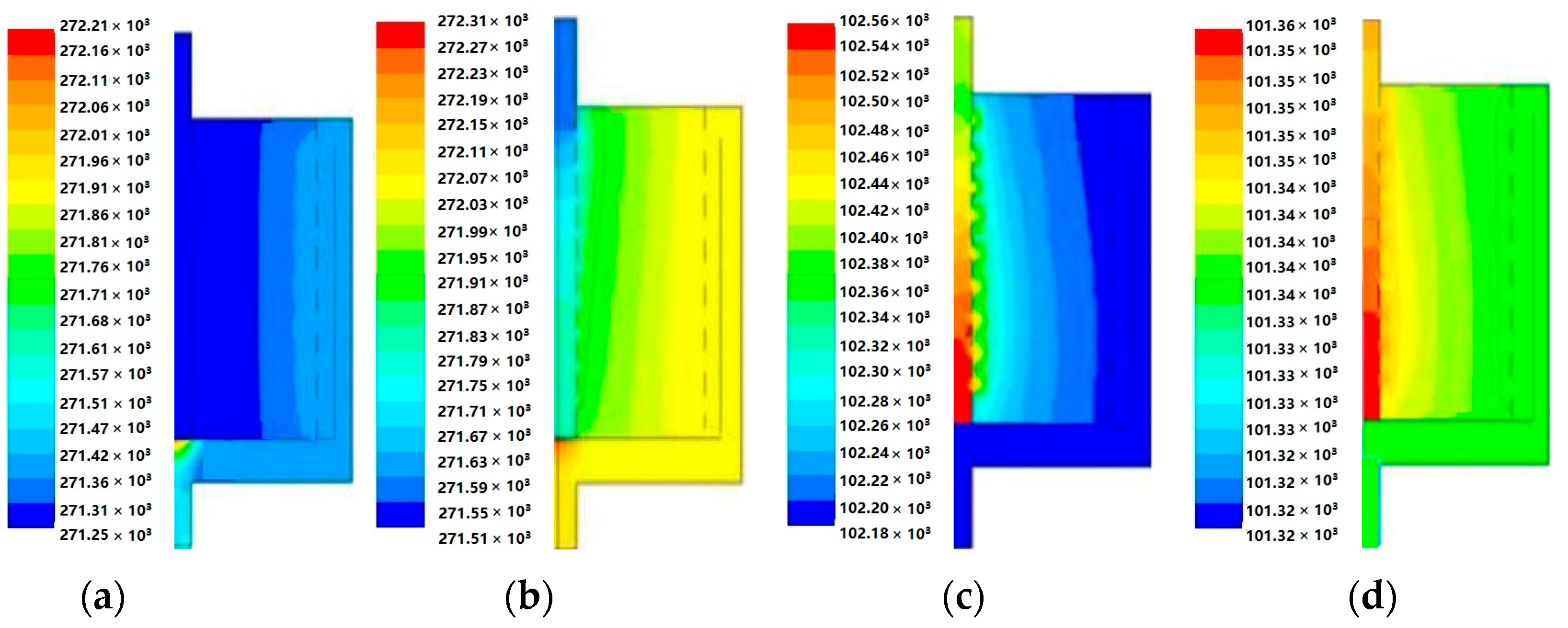
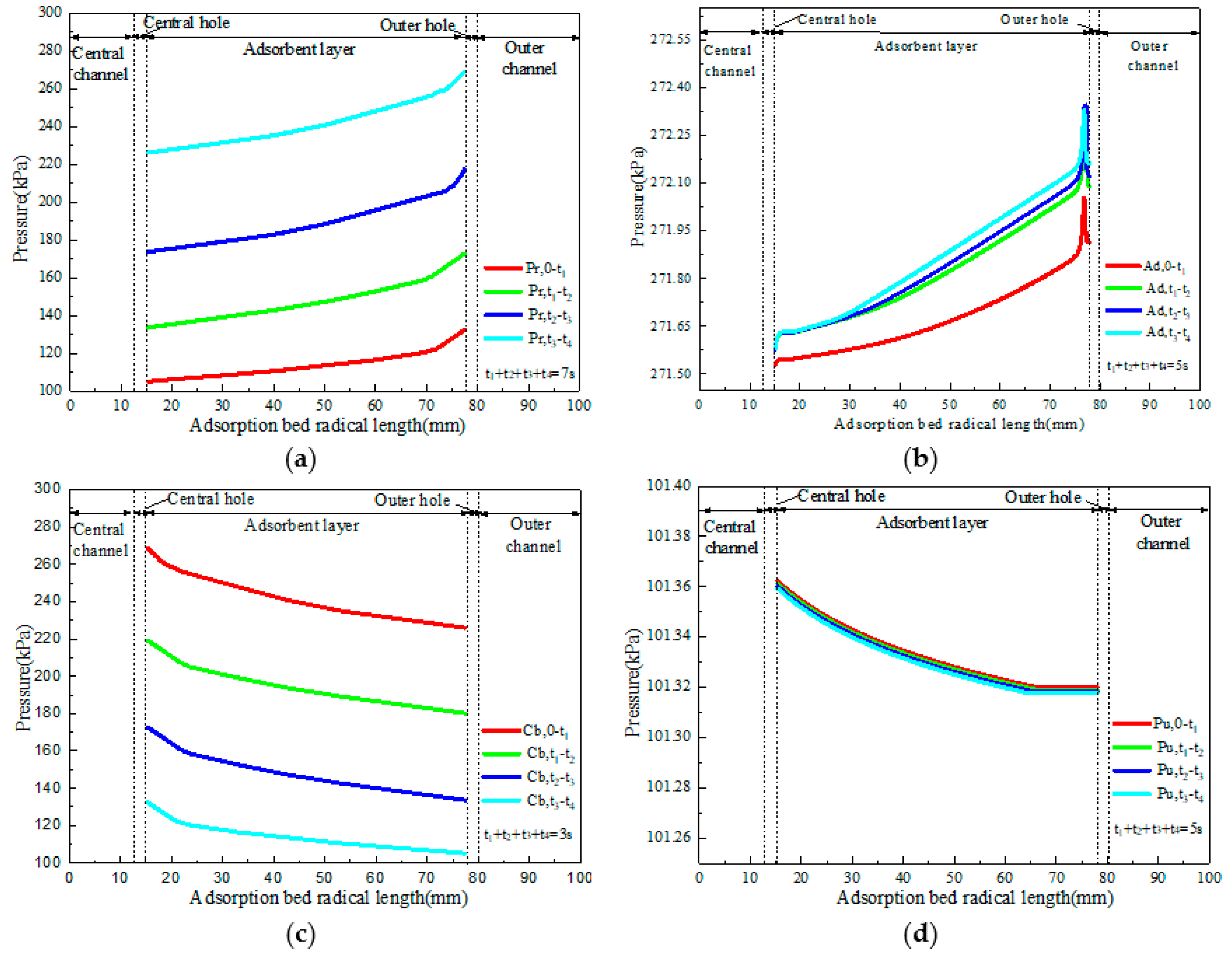
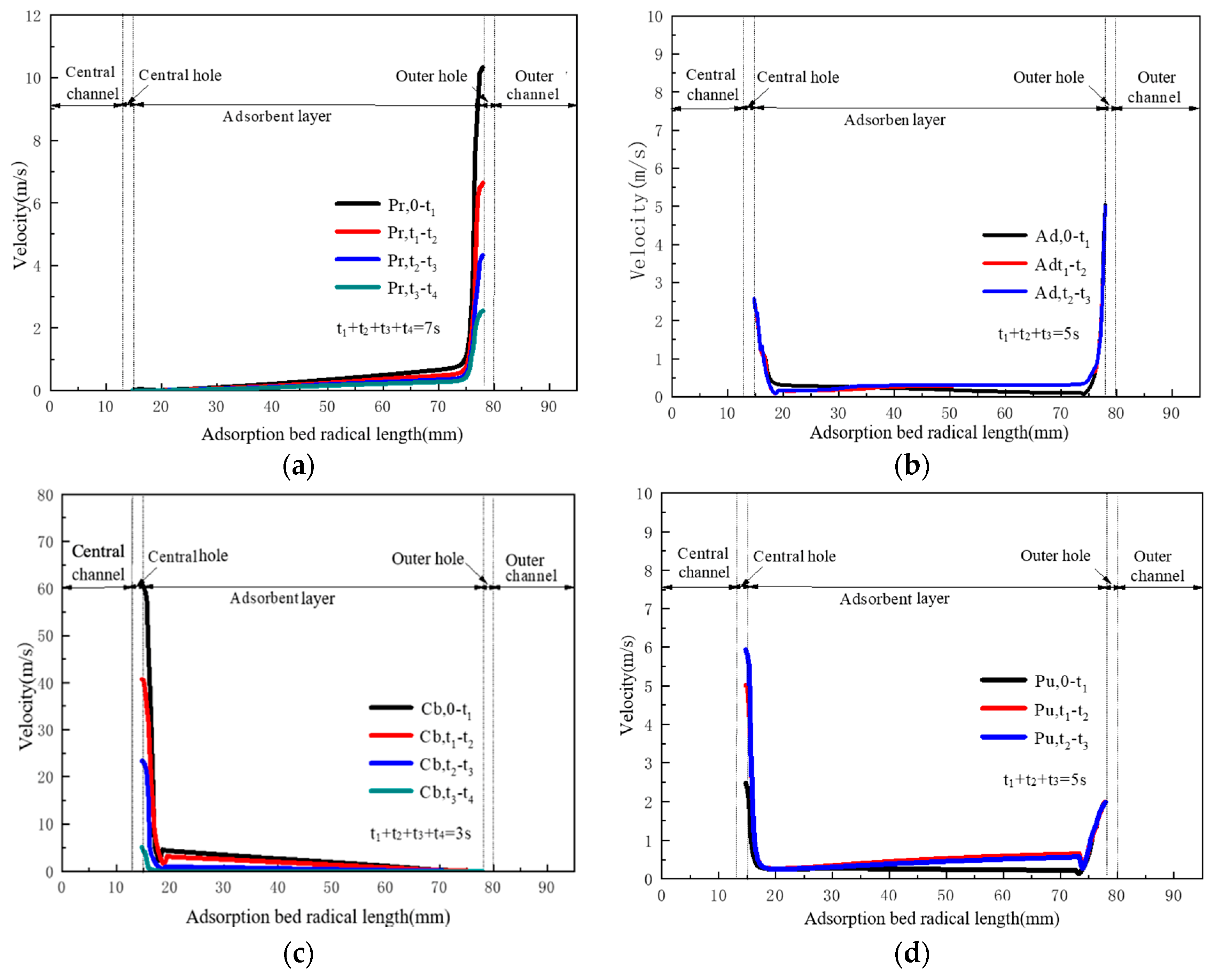
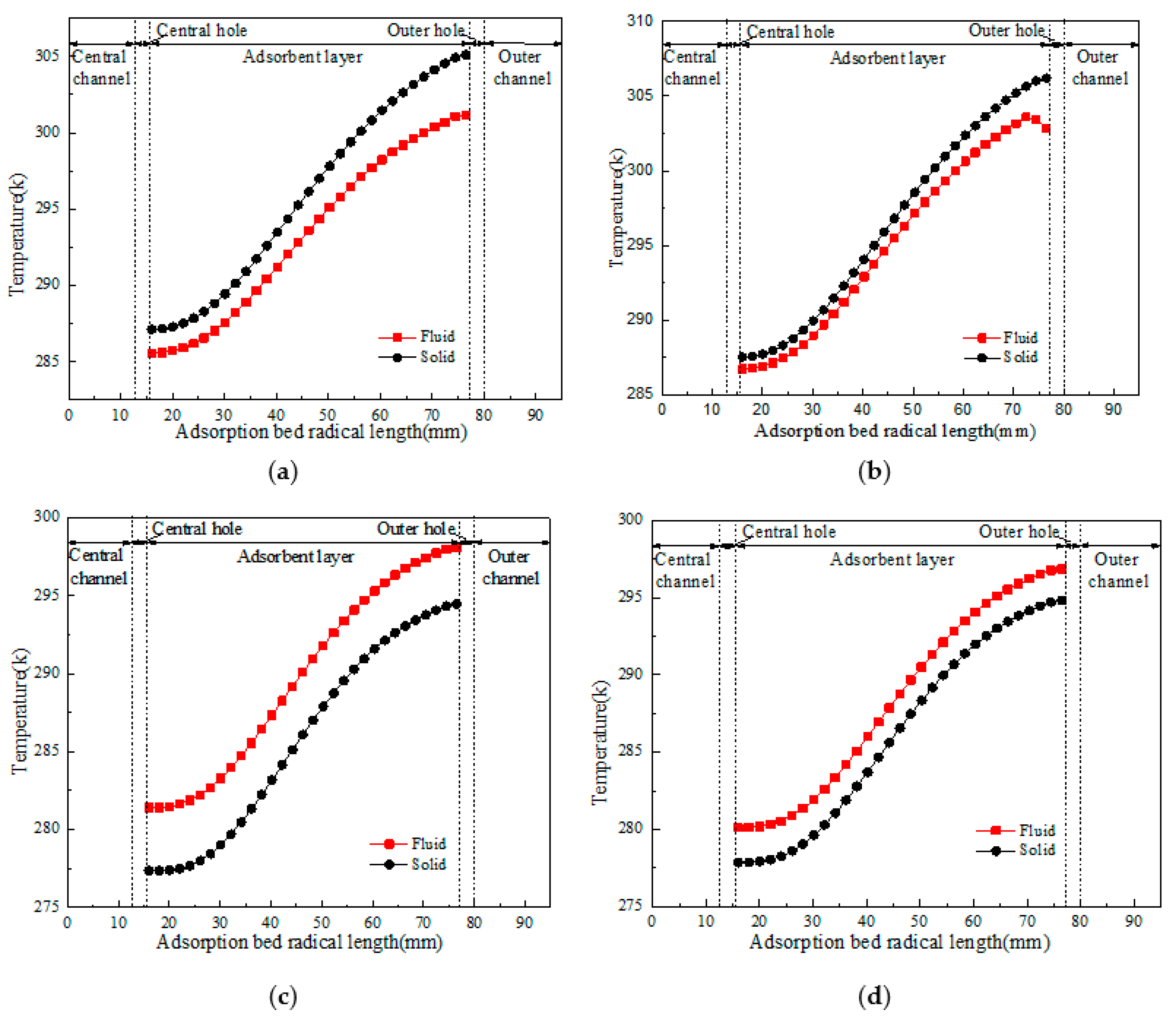
4.2. Flow and Heat Transfer Characteristics of CP-π RFA
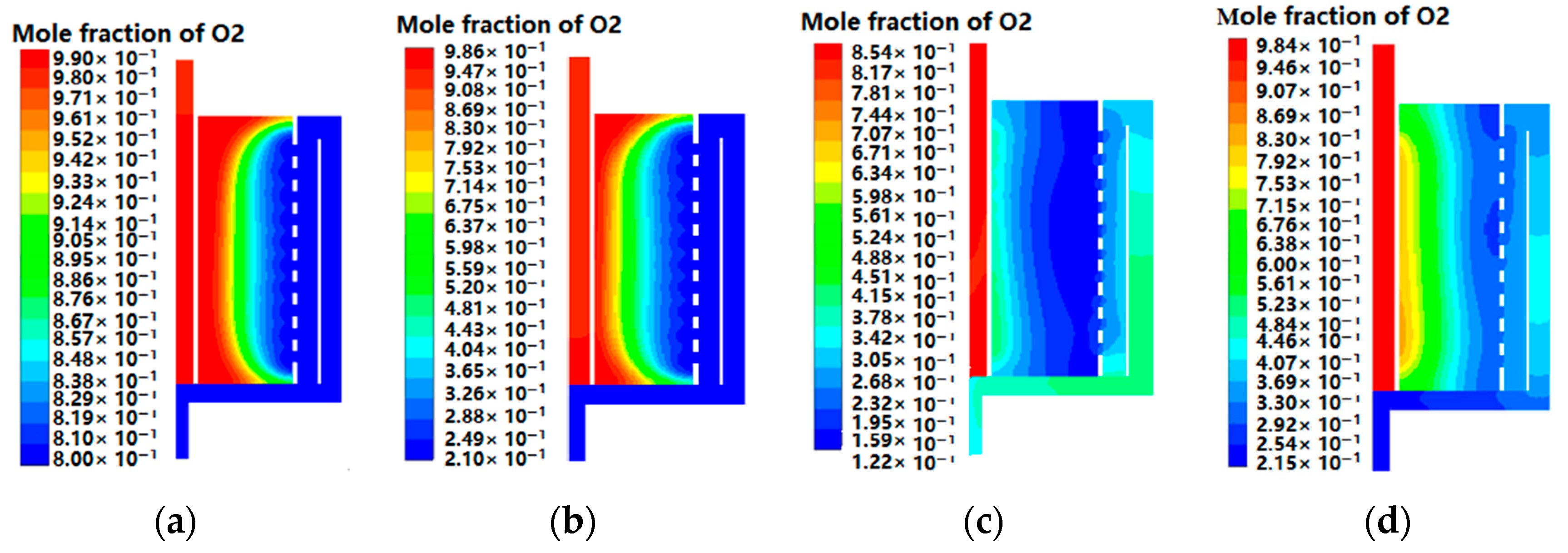
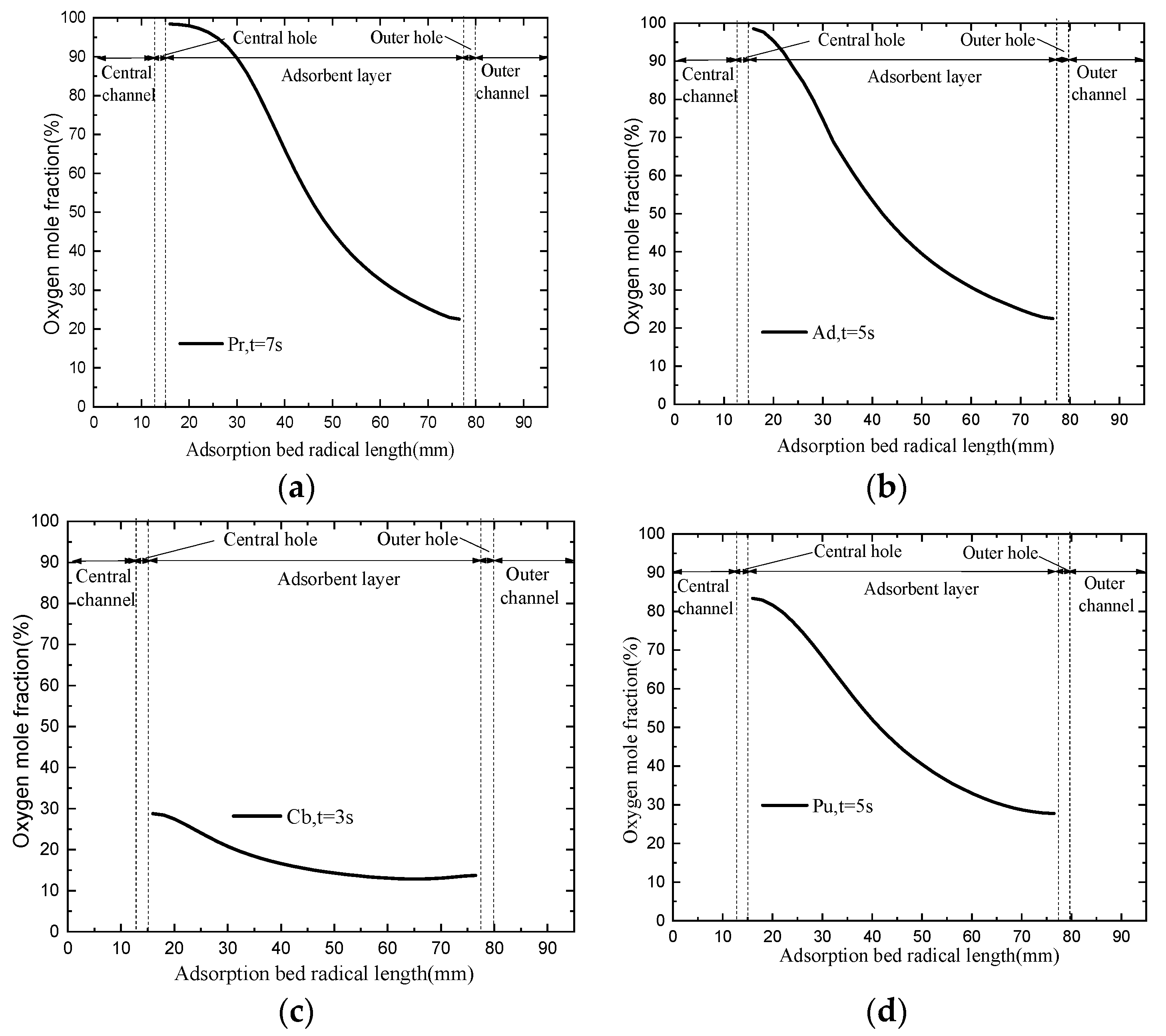
4.3. Oxygen Distributions of CP-π RFA
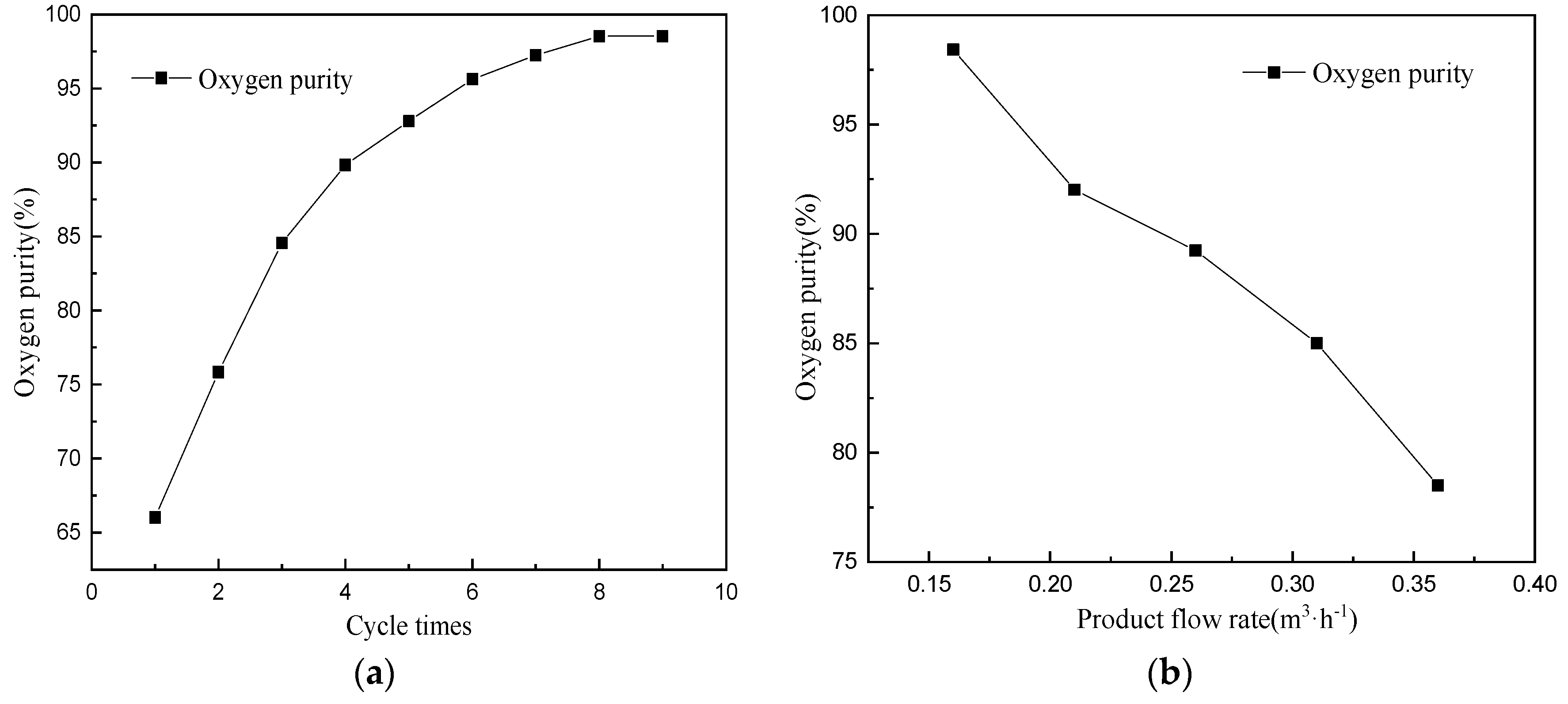
4.4. Effect of Product Flow Rate and Cycle Times on Oxygen Production Performance of CP-π RFA
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| dp | Diameter of adsorbent particle, m |
| Di | Mass dispersion rate, m2·s−1 |
| ef | Total fluid energy, J·kg−1 |
| ep | Total solid medium energy, J·kg−1 |
| Heat of adsorption, kJ·mol−1 | |
| Ki | Langmuir parameter, mol·kg−1·kPa−1 |
| k1 | Langmuir parameter, mol·kg−1·kPa−1 |
| k2,k4 | Langmuir parameter, K |
| k3 | Langmuir parameter, kPa−1 |
| keff | Effective bed thermal conductivity, W·m−2·K |
| ki | Mass transfer constant, s−1 |
| Mi | Molar weight of component i, kg·mol−1 |
| p | Gas pressure, Pa |
| pi | Partial pressure of component i, Pa |
| qi | Solid-phase adsorbate concentration, mol·kg−1 |
| qi* | Adsorbate concentration in equilibrium with gas phase, mol·kg−1 |
| Q | Volume flow rate, m3·s−1 |
| Si | Mass source term of the component i, kg·m−3·s−1 |
| Sm | Total mass source term, kg·m−3·s−1 |
| Sv | Momentum source term, N·m−3 |
| t | Adsorption time, s |
| T | Temperature, K |
| u | Velocity vector, m s−1 |
| Yi | Mass fraction of component i |
| ε | Porosity of the fixed bed |
| μ | Dynamic viscosity of the fluid, Pa·s |
| ρp | Density of adsorbent particle, kg·m−3 |
| ρf | Fluid density, kg·m−3 |
References
- Skarstrom, C.W. Method and Apparatus for Fractionating Gaseous Mixtures by Adsorption. U.S. Patent No. 3,082,166, 12 July 1960. [Google Scholar]
- Zeng, R.; Guan, J. Progress in pressure swing adsorption models during the recent 30 years. Chin. J. Chem. Eng. 2002, 10, 228–235. [Google Scholar]
- Daszkowski, T.; Eigenberger, G. A re-evaluation of fluid flow, heat transfer and chemical reaction in catalyst filled tubes. Chem. Eng. Sci. 1992, 4, 2245–2250. [Google Scholar] [CrossRef]
- Ponzi, P.R.; Kaye, L.A. Effects of flow maldistribution on conversion and selectivity in radial flow fixed-bed reactors. AIChE J. 1979, 25, 100–108. [Google Scholar] [CrossRef]
- Dai, Z.; Yu, M.; Rui, D.; Xue, J.; Yang, Z. Investigation on a vertical radial flow adsorber designed by a novel parallel connection method. Chin. J. Chem. Eng. 2018, 26, 484–493. [Google Scholar] [CrossRef]
- Tian, Q.; He, G.; Wang, Z.; Cai, D.; Chen, L. A novel radial adsorber with parallel layered beds for prepurification of large-scale air separation units. Ind. Eng. Chem. Res. 2015, 54, 7502–7515. [Google Scholar] [CrossRef]
- Ruthven, D.M.; Farooq, S.; Knaebel, K.S. Pressure Swing Adsorption; VCH Publishers: New York, NY, USA, 1994. [Google Scholar]
- Smolarek, J.; Leavitt, F.W.; Nowobilski, J.J.; Bergsten, V.E.; Fassbaugh, J.H. Radial Bed Vaccum/Pressure Swing Adsorber Vessel. U.S. Patent No. 5,759,242, 2 June 1998. [Google Scholar]
- LaCava, A.I.; Shirley, A.I.; Ramachandran, R. How to specify pressure-swing adsorption units. Chem. Eng. 1998, 105, 110–118. [Google Scholar]
- Kareeri, A.A.; Zughbi, H.D.; Al-Ali, H.H. Simulation of flow distribution in radial flow reactors. Ind. Eng. Chem. Res. 2006, 45, 2862–2874. [Google Scholar] [CrossRef]
- Celik, C.E.; Smolarek, J. Radial Bed Flow Distributor for Radial Pressure Adsorber Vessel. U.S. Patent No. 7,128,775, 12 April 2004. [Google Scholar]
- Zhang, X.; Lu, J.; Qiu, L.; Zhang, X.; Wang, X. A mathematical model for designing optimal shape for the cone used in Z-flow type radial flow adsorbers. Chin. J. Chem. Eng. 2013, 21, 494–499. [Google Scholar] [CrossRef]
- Farsi, M. DME production in multi-stage radial flow spherical membrane reactors: Reactor design and modeling. J. Nat. Gas Sci. Eng. 2014, 20, 366–372. [Google Scholar] [CrossRef]
- Li, R.; Zhu, Z. Investigations on hydrodynamics of multilayer Π-type radial flow reactors. Asia-Pac. J. Chem. Eng. 2012, 7, 517–527. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Li, Y.; Zhang, H. Velocity distribution in axial adsorber with consideration of mass variation. J. Univ. Sci. Technol. 2011, 33, 1412–1418. [Google Scholar]
- Wang, H.; Liu, Y.; Meng, Y. Effect of the gas distribution system structure of a radial flow adsorber on gas distribution. Chin. J. Eng. 2015, 37, 91–96. [Google Scholar]
- Hamedi, N.; Tohidian, T.; Rahimpour, M.R.; Iranshahi, D.; Raeissi, S. Conversion enhancement of heavy reformates into xylenes by optimal design of a novel radial flow packed bed reactor, applying a detailed kinetic model. Chem. Eng. Res. Des. 2015, 95, 317–336. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Chen, J.; Li, Z.; Liu, Y.; Zhang, C.; Xing, Y. Two-dimensional modeling of pressure swing adsorption (PSA) oxygen gGeneration with radial-flow adsorber. Appl. Sci. 2019, 9, 1153. [Google Scholar] [CrossRef]
- Chiang, A.S.T.; Hong, M.C. Radial flow rapid pressure swing adsorption. Adsorption 1995, 1, 153–164. [Google Scholar] [CrossRef]
- Huang, W.; Chou, C. Comparison of radial-and axial-flow rapid pressure swing adsorption processes. Ind. Eng. Chem. Res. 2003, 42, 1998–2006. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Shi, S. Influence of the structure of radial flow adsorbers on oxygen production with pressure swing adsorption. Chin. J. Eng. 2015, 37, 238–242. [Google Scholar]
- Yang, R.T. Gas Separation by Adsorption Processes; Imperial College Press: London, UK, 1997. [Google Scholar]
- Yang, X.; Epiepang, F.E.; Li, J.; Wei, Y.; Liu, Y.; Yang, R.T. Sr-LSX zeolite for air separation. Chem. Eng. J. 2019, 362, 482–486. [Google Scholar] [CrossRef]
- Epiepang, F.E.; Yang, X.; Li, J.; Liu, Y.; Yang, R.T. Mixed-cation LiCa-LSX zeolite with minimum lithium for air separation. AIChE J. 2018, 64, 406–415. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Yang, X.; Liu, W. Study of a novel rapid vacuum pressure swing adsorption process with intermediate gas pressurization for producing oxygen. Adsorption 2017, 23, 175–184. [Google Scholar] [CrossRef]
- Ding, Z.; Han, Z.; Fu, Q.; Shen, Y.; Tian, C.; Zhang, D. Optimization and analysis of the VPSA process for industrial-scale oxygen production. Adsorption 2018, 24, 1–18. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, X.; Dai, R. Numerical study of flow maldistribution and depressurization strategies in a small-scale axial adsorber. Adsorption 2014, 20, 757–768. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Liu, W. Two-dimensional modeling of the transport phenomena in the adsorber during pressure swing adsorption process. Ind. Eng. Chem. Res. 2010, 49, 11814–11824. [Google Scholar] [CrossRef]
- Fan, M.H.; Panezai, H.; Sun, J.H. Thermal and kinetic performance of water desorption for N2 adsorption in Li-LSX zeolite. J. Phys. Chem. C 2014, 118, 23761–23767. [Google Scholar] [CrossRef]
- Sun, L.M.; Amar, N.B.; Meunier, F. Numerical study on coupled heat and mass transfers in an adsorber with external fluid heating. Heat Recov. Syst. CHP 1995, 15, 19–29. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Wang, H.; Tsai, C.; Yang, X.; Xing, Y.; Zhang, C.; Xiao, P.; Webley, P.A. A numerical modelling study of SO2 adsorption on activated carbons with new rate equations. Chem. Eng. J. 2018, 353, 858–866. [Google Scholar] [CrossRef]
- Li, G.; Xiao, P.; Zhang, J.; Webley, P.A.; Xu, D. The role of water on postcombustion CO2 capture by vacuum swing adsorption: Bed layering and purge to feed ratio. AIChE J. 2014, 60, 673–689. [Google Scholar] [CrossRef]
- Blaczek, J. Computational Fluid Dynamics: Principles and Applications; Butterworth-Heinemann Press: Washington, DC, USA, 2015. [Google Scholar]
- FLENT. ANSYS Fluent User’s Guide; Fluent Inc.: Lebanon, NH, USA, 2012. [Google Scholar]
- FLENT. Fluent 6.3 UDF Manual; Fluent Inc.: Lebanon, NH, USA, 2006. [Google Scholar]
- Wang, H.; Liu, Y.; Zhang, C.; Yang, X.; Chen, J. Study on variable mass flow laws in π-shaped centripetal radial flow adsorber. CIESC J. 2019, 70, 3385–3395. [Google Scholar]
- Wang, H.; Liu, Y.; Zhang, C.; Chen, F.; Ma, X.; Li, C. Heat and mass transfer characteristics of the gas-solid two-phase model in a π-shaped centripetal radial flow adsorber. Chin. J. Eng. 2019, 41, 1473–1483. [Google Scholar]
- Fang, L.; Xiao, J.; Benard, P. Thermal effects on pressure swing adsorption cycles for hydrogen purification. J. Eng. Thermophys. 2018, 39, 1104–1111. [Google Scholar]
- Zhu, X.; Liu, Y.; Yang, X.; Liu, W. Progress of modified adsorbent and pressure swing adsorption for oxygen production in China. Chem. Ind. Eng. Prog. 2015, 34, 19–25. [Google Scholar]
- Budner, Z.; Dula, J.; Podstawa, W.; Gawdzik, A. Study and modelling of the vacuum swing adsorption (VSA) process employed in the production of oxygen. Chem. Eng. Res. Des. 1999, 77, 405–412. [Google Scholar] [CrossRef]
| Adsorbent | Values | Adsorbent Column | Values |
|---|---|---|---|
| Adsorbent | LiX(CECA-G500) | Bed external porosity | 0.4 |
| Type | Sphere | Dispersion coefficient (m2·s−1) | 5 × 10−5 [28] |
| Particle radius (mm) | 0.8 | LDF constant for oxygen, kO2 (s−1) | 62.0 [29] |
| Particle density (kg·m3) | 1035 | LDF constant for nitrogen, kN2 (s−1) | 19.7 [29] |
| Heat capacity (J·kg−1·K−1) | 1100 | Ambient temperature (K) | 298 |
| Thermal conductivity (W·m2·K) | 0.2 |
| Parameter | Values | Parameter | Values |
|---|---|---|---|
| Molar feed composition | O2-N2(21–79% by volume) | Inner diameter of adsorber (mm) | 211 |
| Diameter of adsorber (mm) | 219 | Adsorption bed width (mm) | 61.5 |
| Sorbent filling height (mm) | 187 | Outer channel width (mm) | 25 |
| Entrance radius (mm) | 12 | Central channel width (mm) | 13 |
| Entrance length (mm) | 40 | Flow distributor width (mm) | 3 |
| Opening ratio of outer channel (%) | 27% | Diameter of central channel hole (mm) | 2 |
| Opening ratio of central channel (%) | 17% | Diameter of outer channel hole (mm) | 8 |
| Vent length(mm) | 40 | Vent radius (mm) | 12 |
| Adsorbate | k1 (mol·kg−1·Pa−1) | k2 (K) | k3 (Pa−1) | k4 (K) | ΔH (kJ·mol−1) |
|---|---|---|---|---|---|
| O2 | 7.87 × 10−9 | 1541.211 | 6.79 × 10−10 | 1968.24 | 12 |
| N2 | 9.86 × 10−9 | 2010.908 | 1.67 × 10−9 | 2250 | 18 |
| Parameter | Values |
|---|---|
| Pressure (Pa) | 101,325 |
| Air-temperature (K) | 298 |
| Solid-temperature (K) | 298 |
| Mass fraction of O2 in the gas-phase | 0.233 |
| Amount of O2 adsorbed per unit mass of sorbent (mol·kg−1) | 0.0262832 |
| Amount of N2 adsorbed per unit mass of sorbent (mol·kg−1) | 0.6328067 |
| Step | Schematic Diagram | Duration(s) | Boundary Conditions for Adsorber 1 | |||
|---|---|---|---|---|---|---|
| Entrance | Vent | Adsorber Wall | Adsorber Axis | |||
| Pr Step |  | 7 | mass flow inlet | wall | wall | axis |
| Ad Step |  | 5 | mass flow inlet | pressure outlet | wall | axis |
| Cb Step |  | 3 | pressure outlet | wall | wall | axis |
| Pu Step |  | 5 | pressure outlet | mass flow inlet | wall | axis |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yang, X.; Li, Z.; Liu, Y.; Zhang, C.; Ma, X.; Li, C. 3-D Modeling of Gas–Solid Two-Phase Flow in a π-Shaped Centripetal Radial Flow Adsorber. Appl. Sci. 2020, 10, 614. https://doi.org/10.3390/app10020614
Wang H, Yang X, Li Z, Liu Y, Zhang C, Ma X, Li C. 3-D Modeling of Gas–Solid Two-Phase Flow in a π-Shaped Centripetal Radial Flow Adsorber. Applied Sciences. 2020; 10(2):614. https://doi.org/10.3390/app10020614
Chicago/Turabian StyleWang, Haoyu, Xiong Yang, Ziyi Li, Yingshu Liu, Chuanzhao Zhang, Xiaojun Ma, and Chunwang Li. 2020. "3-D Modeling of Gas–Solid Two-Phase Flow in a π-Shaped Centripetal Radial Flow Adsorber" Applied Sciences 10, no. 2: 614. https://doi.org/10.3390/app10020614
APA StyleWang, H., Yang, X., Li, Z., Liu, Y., Zhang, C., Ma, X., & Li, C. (2020). 3-D Modeling of Gas–Solid Two-Phase Flow in a π-Shaped Centripetal Radial Flow Adsorber. Applied Sciences, 10(2), 614. https://doi.org/10.3390/app10020614






