Geopolymer-Bonded Laminated Veneer Lumber as Environmentally Friendly and Formaldehyde-Free Product: Effect of Various Additives on Geopolymer Binder Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Geopolymer Binder
2.3. Experimental Design
2.3.1. Part 1: Effect of Hot-Pressing Conditions
2.3.2. Part 1: Effect of Additives Content
2.4. Geopolymer Binder Characterization
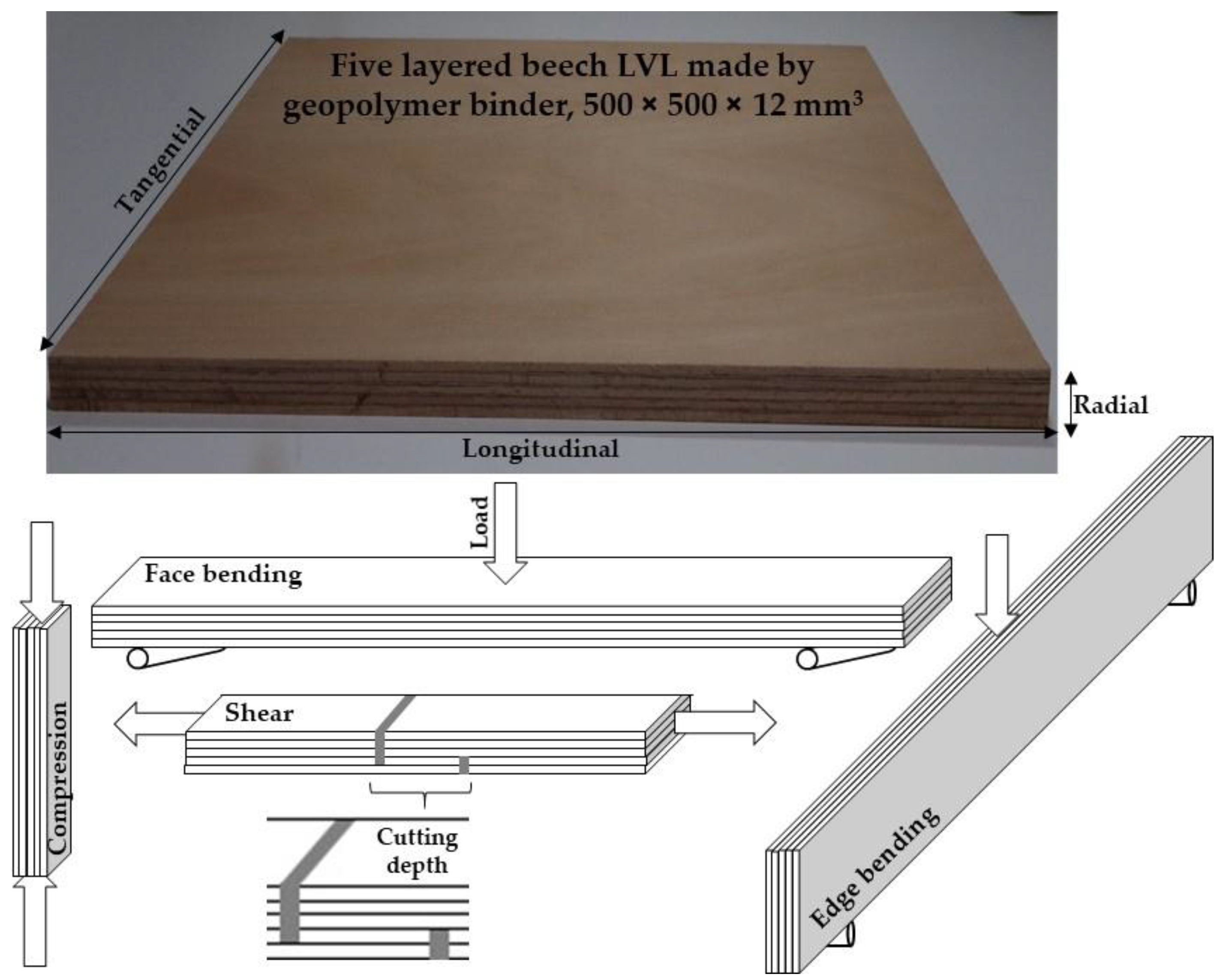
2.5. Manufacturing of LVL Panels
2.6. Characterization of LVL Panels
3. Results and Discussion
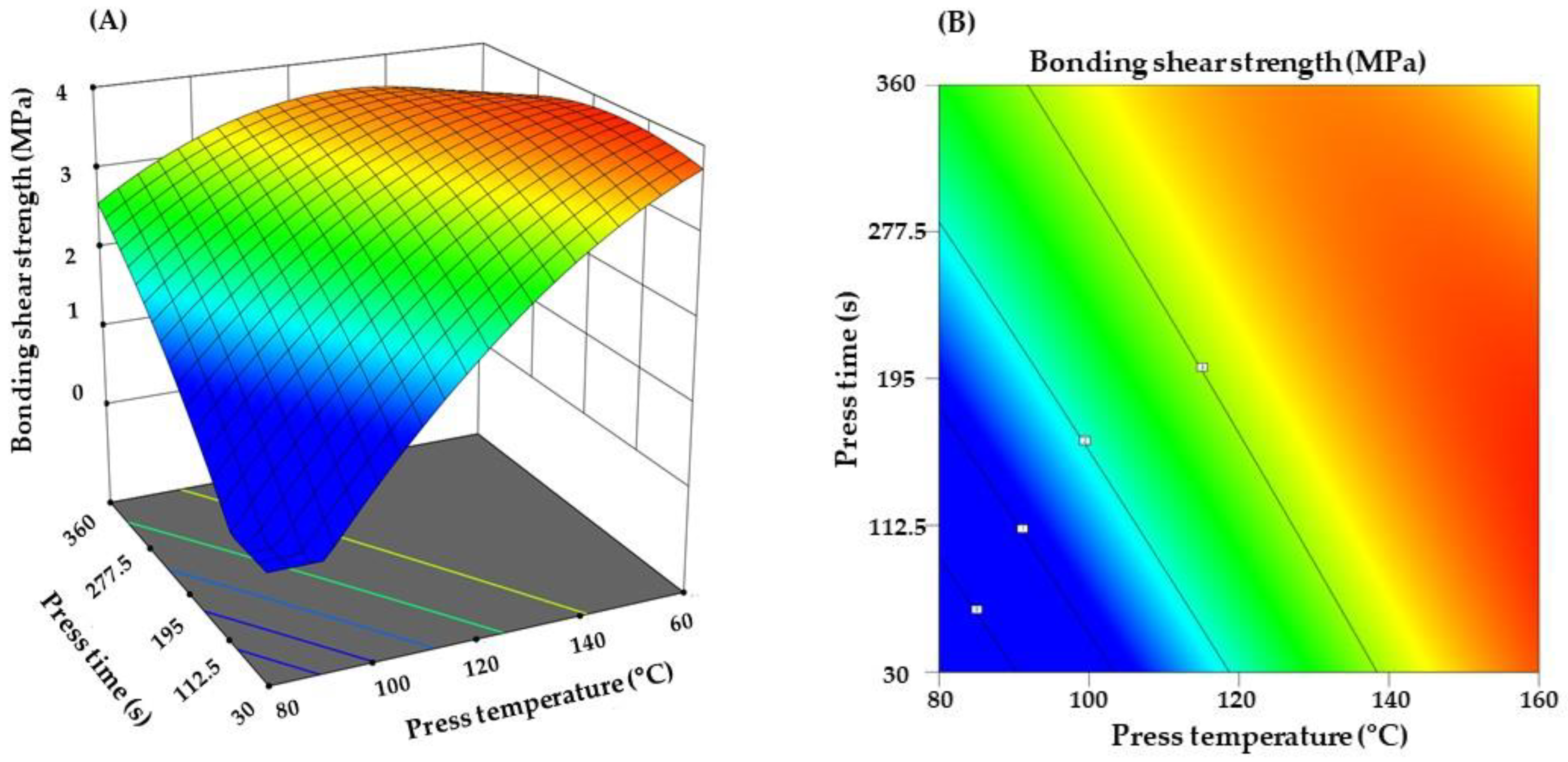
3.1. Effect of Processing Parameters on Binder Performance
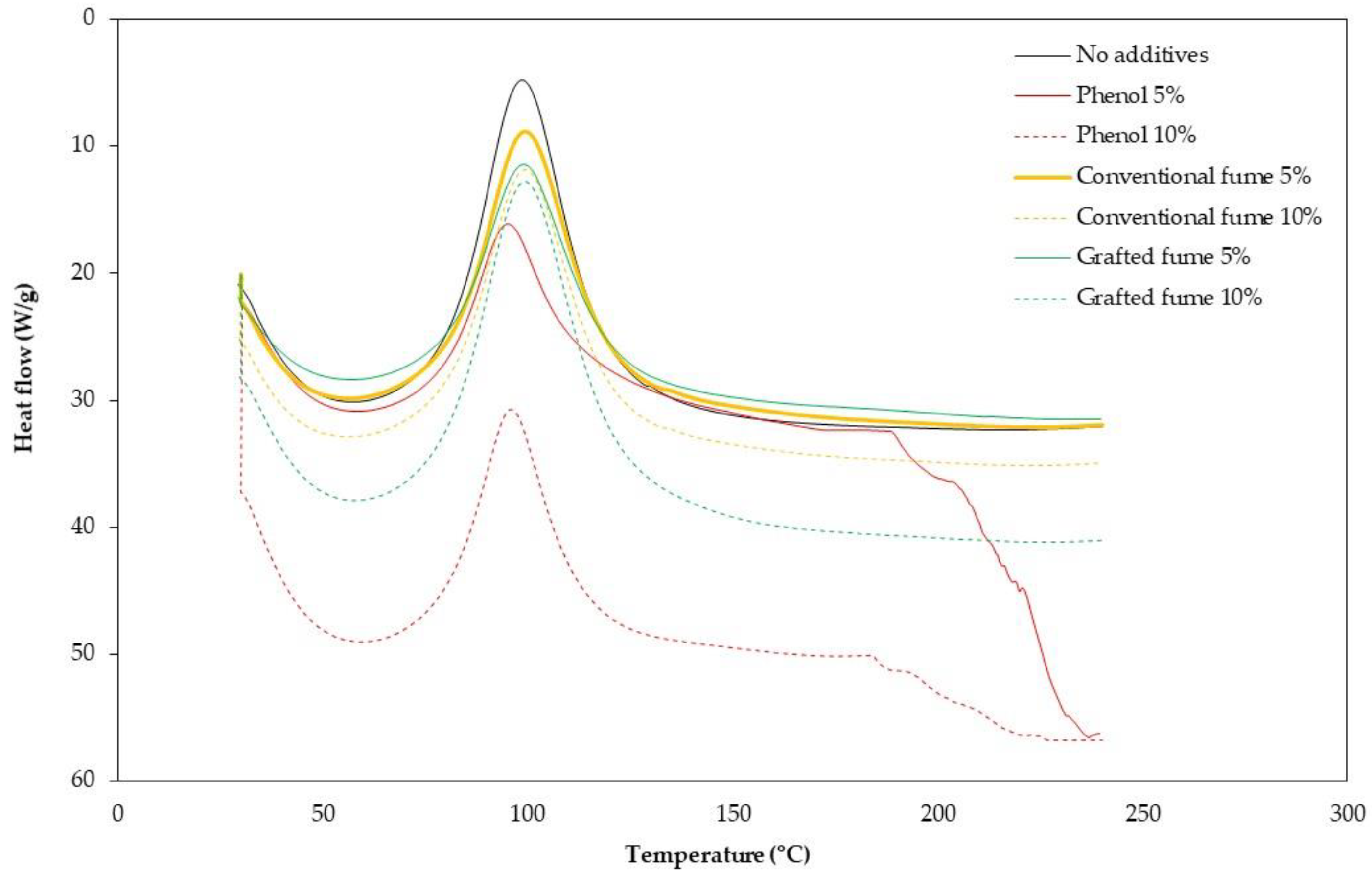
3.2. Binder Characterisation
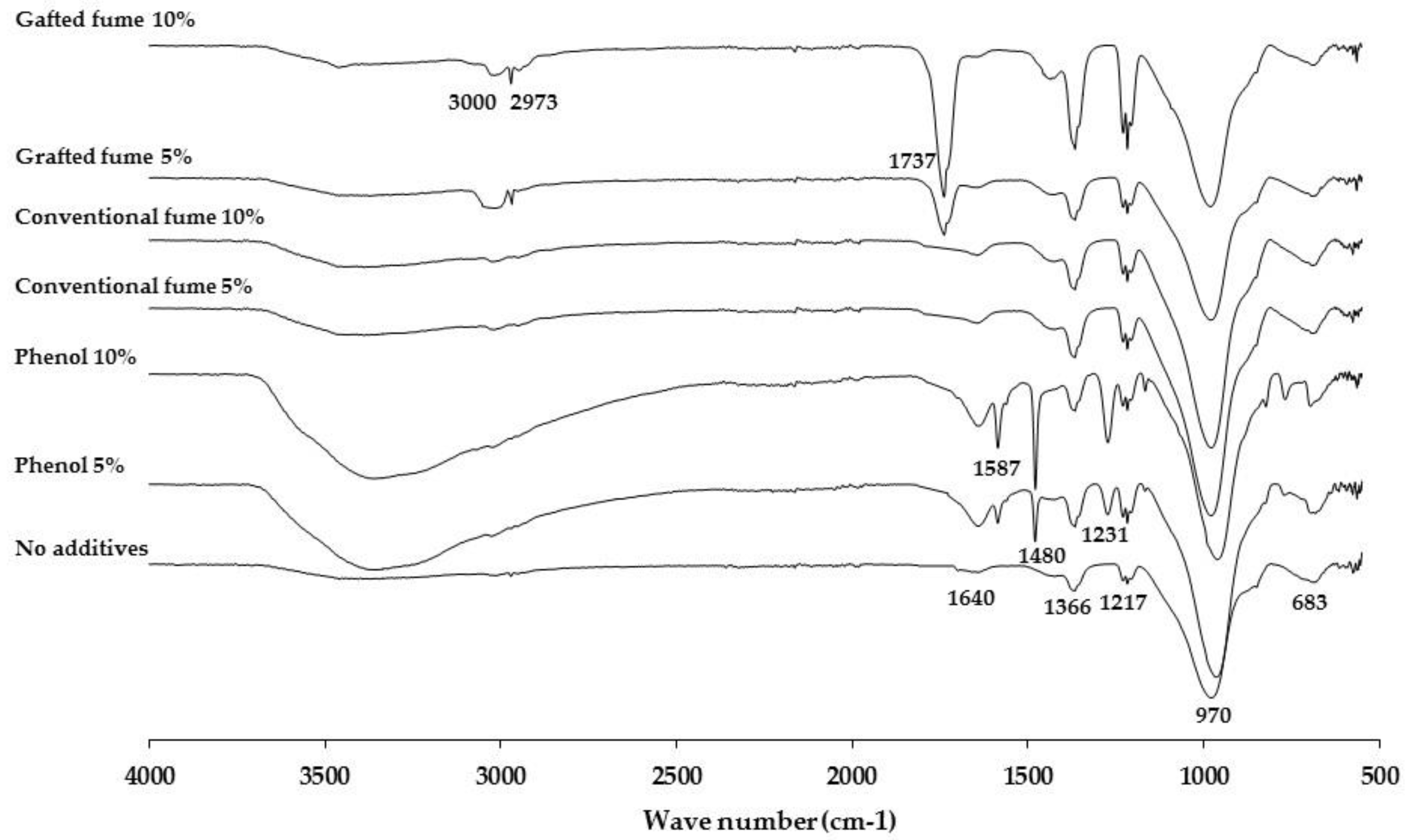
3.3. ATR-FTIR Characterization
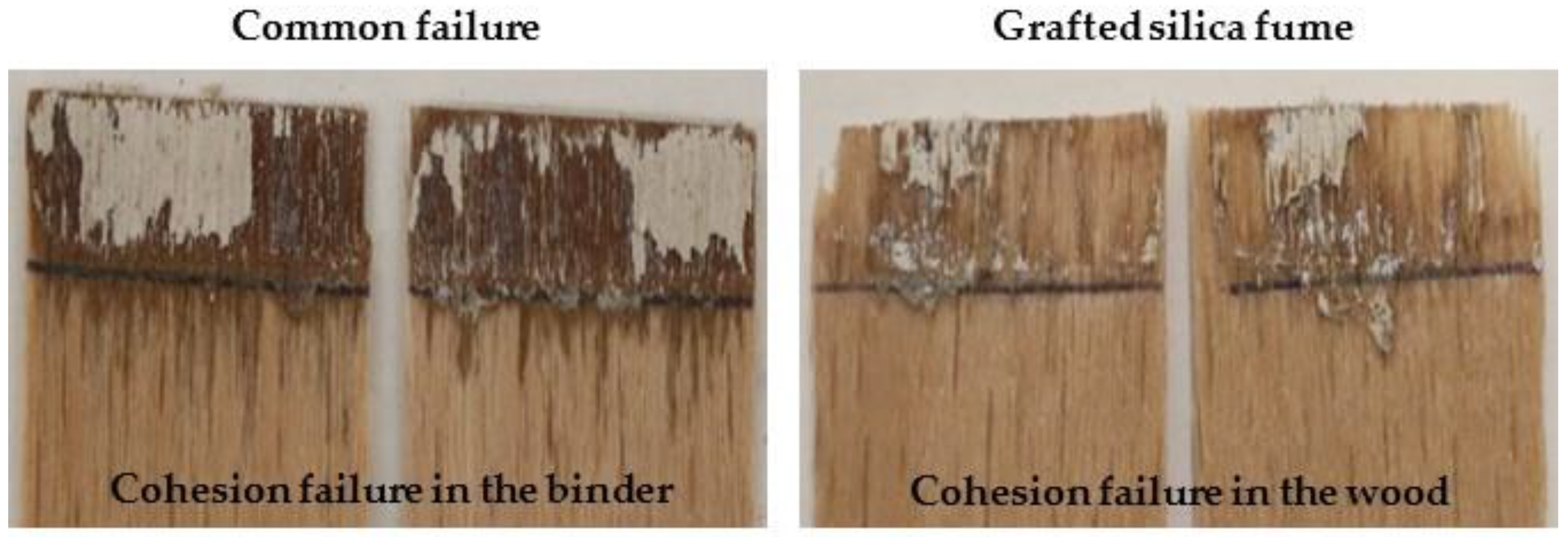
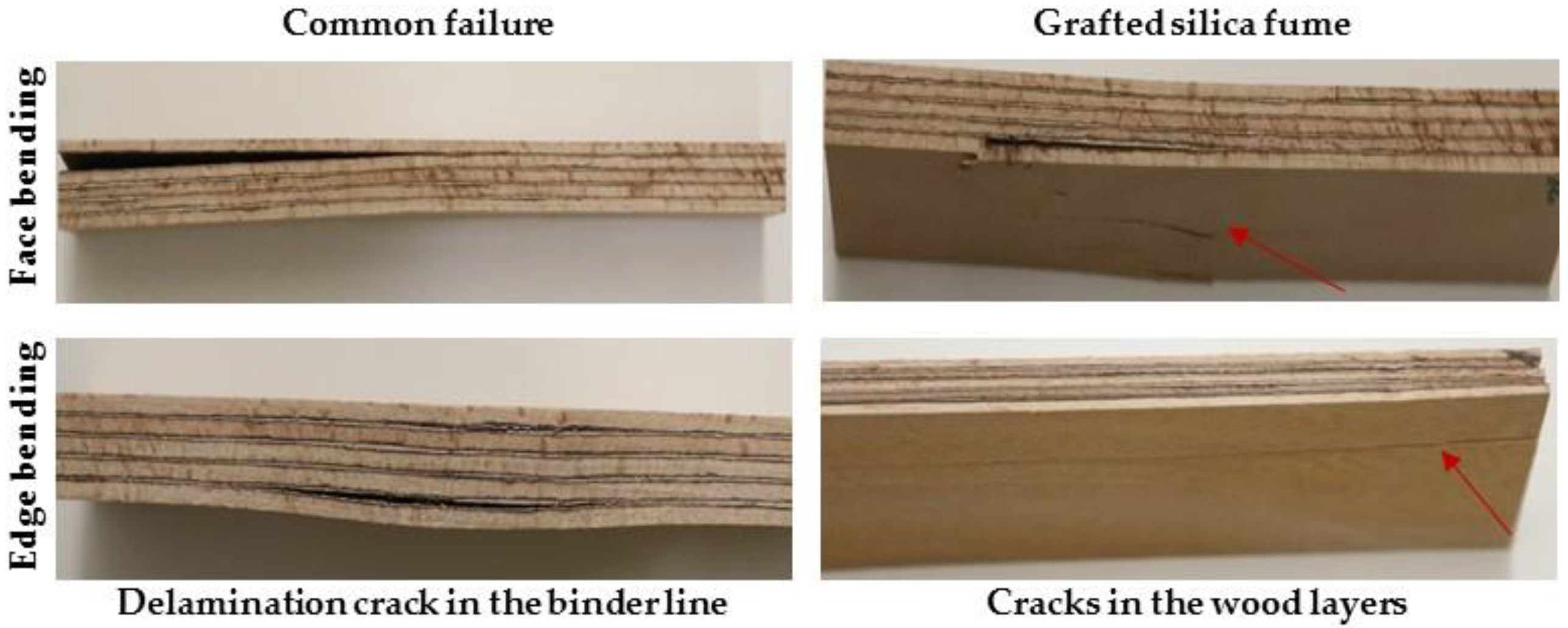
3.4. Shear Strength Analysis
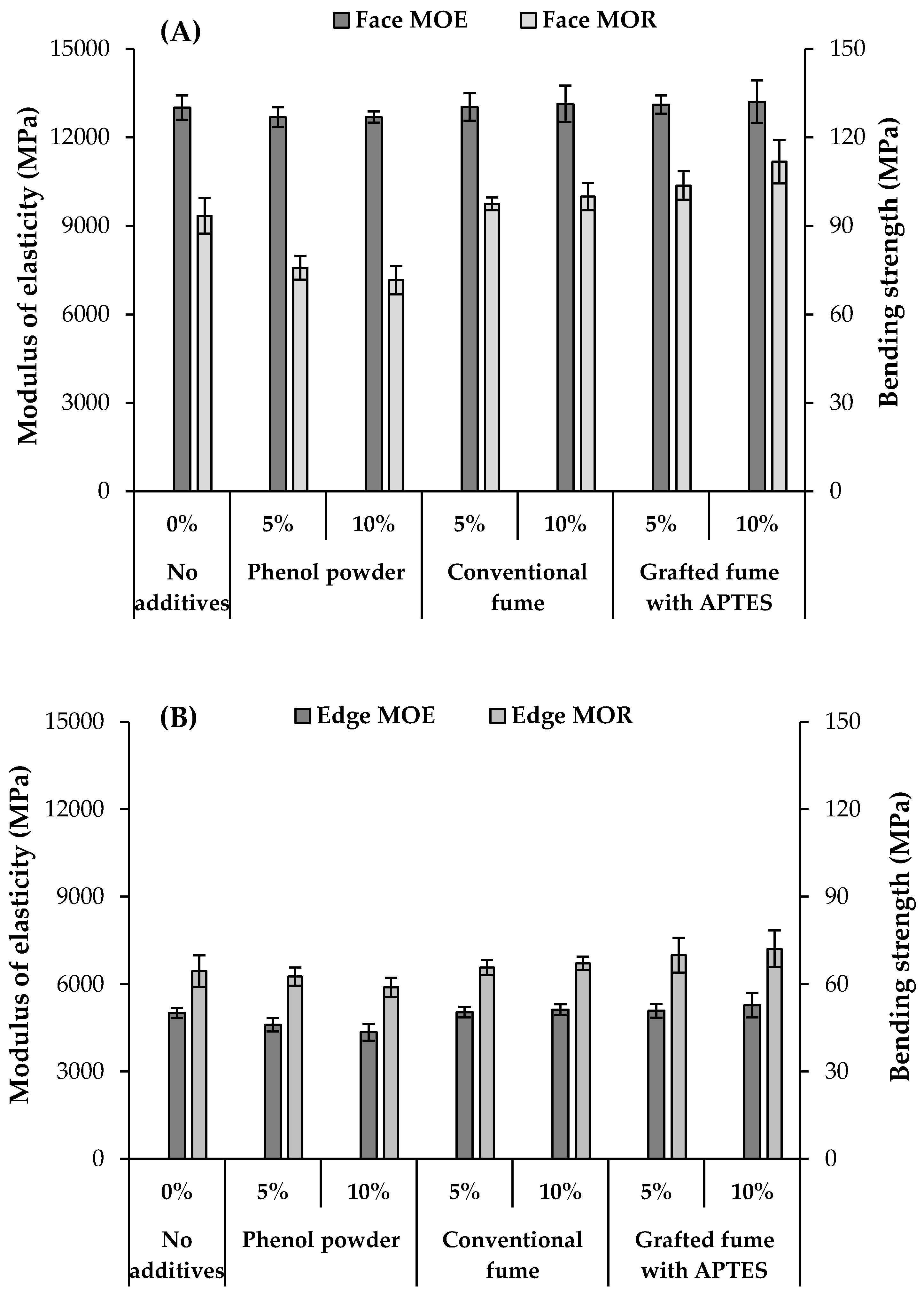
3.5. Bending Properties
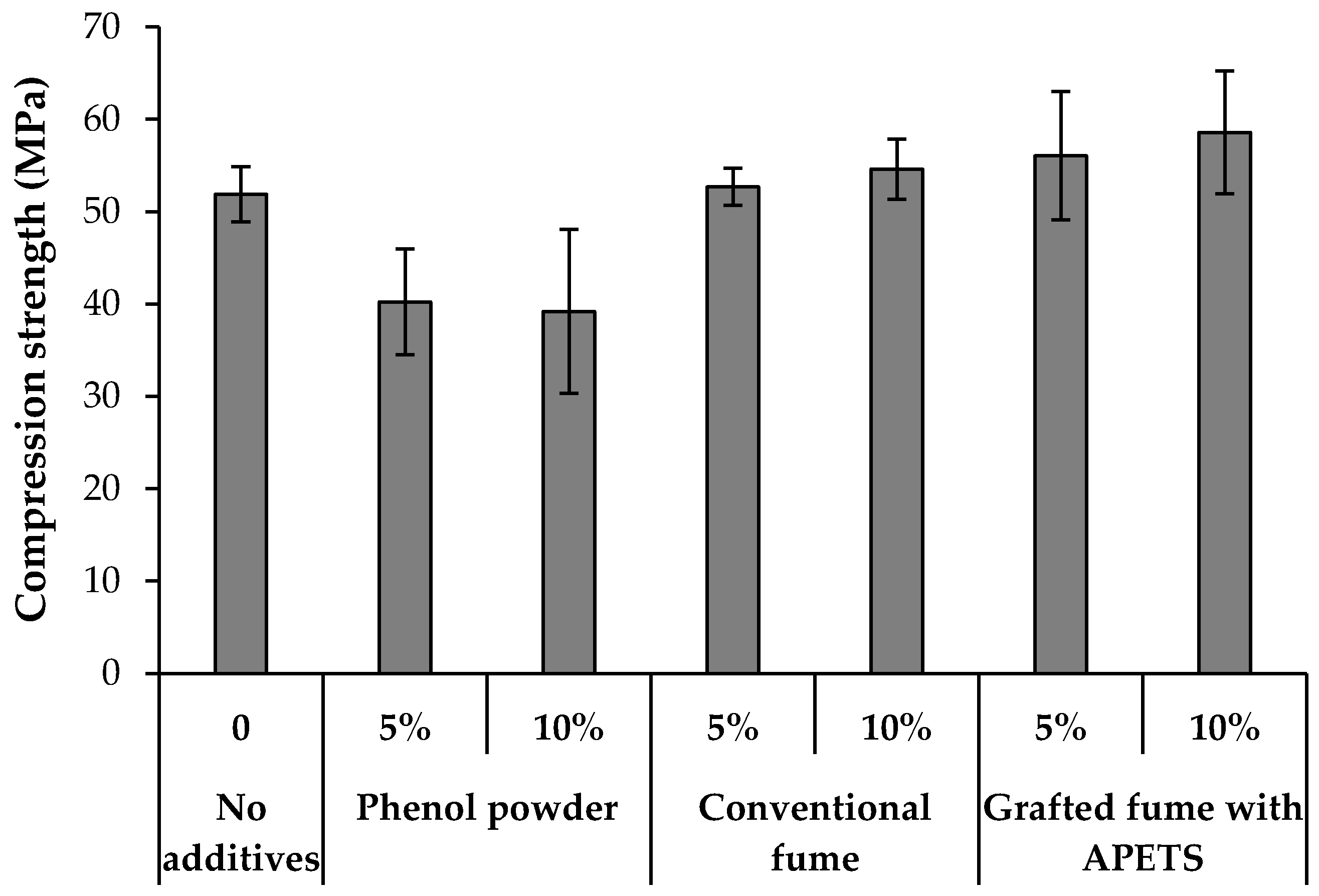
3.6. Compression Strength
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ozarska, B. A review of the utilisation of hardwoods for LVL. Wood Sci. Technol. 1999, 33, 341–351. [Google Scholar] [CrossRef]
- Eckelman, C.A. Potential uses of laminated veneer lumber in furniture. For. Prod. J. 1993, 43, 19–24. [Google Scholar]
- Ferdosian, F.; Pan, Z.; Gao, G.; Zhao, B. Bio-based adhesives and evaluation for wood composites application. Polymers 2017, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Ayrilmis, N.; Lee, Y.-K.; Kwon, J.H.; Han, T.-H.; Kim, H.-J. Formaldehyde emission and VOCs from LVLs produced with three grades of urea-formaldehyde resin modified with nanocellulose. Build. Environ. 2016, 97, 82–87. [Google Scholar] [CrossRef]
- Hassannejad, H.; Shalbafan, A.; Rahmaninia, M. Reduction of formaldehyde emission from medium density fiberboard by chitosan as scavenger. J. Adhes. 2018, 8464, 1–17. [Google Scholar] [CrossRef]
- Pizzi, A.; Mittal, K.L. Handbook of Adhesive Technology, 3rd ed.; Taylor & Francis: Boca Raton, FL, USA, 2018; Volume 66, ISBN 9781498736442. [Google Scholar]
- Chen, Z.X.; Lei, Q.; He, R.L.; Zhang, Z.F.; Chowdhury, A.J.K. Review on antibacterial biocomposites of structural laminated veneer lumber. Saudi J. Biol. Sci. 2016, 23, S142–S147. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Dundar, T.; Candan, Z.; Akbulut, T. Wettability of fire retardant treated laminated veneer lumber (LVL) manufactured from veneers dried at different temperatures. BioResources 2009, 4, 1536–1544. [Google Scholar]
- Bahrami, M.; Shalbafan, A.; Welling, J. Development of plywood using geopolymer as binder: Effect of silica fume on the plywood and binder characteristics. Eur. J. Wood Wood Prod. 2019, 77, 981–994. [Google Scholar] [CrossRef]
- Sarmin, S.N.; Welling, J.; Krause, A.; Shalbafan, A. Investigating the possibility of geopolymer to produce inorganic-bonded wood composites for multifunctional construction material—A review. BioResources 2014, 9, 7941–7950. [Google Scholar]
- Jin, S.; Li, K.; Li, J.; Chen, H. A low-cost, formaldehyde-free and high flame retardancywood adhesive from inorganic adhesives: Properties and performance. Polymers 2017, 9, 513. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 2nd ed.; Institute Geopolymere: Saint-Quentin, France, 2008; ISBN 9782951482012. [Google Scholar]
- Steinerova, M.; Matulova, L.; Vermach, P.; Kotas, J. The brittleness and chemical stability of optimized geopolymer composites. Materials 2017, 10, 396. [Google Scholar] [CrossRef] [PubMed]
- Bing-hui, M.; Zhu, H.; Xue-min, C.; Yan, H.; Si-yu, G. Effect of curing temperature on geopolymerization of metakaolin-based geopolymers. Appl. Clay Sci. 2014, 99, 144–148. [Google Scholar]
- Khater, H.M. Effect of silica fume on the characterization of the geopolymer materials. Int. J. Adv. Struct. Eng. 2013, 5, 1–10. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Wang, Y.; Feng, J. Preparation and properties of alkali activated metakaolin-based geopolymer. Materials 2016, 9, 767. [Google Scholar] [CrossRef]
- Singh, N. Fly ash-based geopolymer binder: A future construction material. Minerals 2018, 8, 299. [Google Scholar] [CrossRef]
- Lee, W.K.W.; Van Deventer, J.S.J. Use of infrared spectroscopy to study geopolymerization of heterogeneous amorphous aluminosilicates. Langmuir 2003, 19, 8726–8734. [Google Scholar] [CrossRef]
- Petrič, M. Influence of silicon-containing compounds on adhesives for and andhesion to wood and lignocellulosic materials: A critical review. Rev. Adhes. Adhes. 2018, 6, 26–81. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, Y.; Yu, Z.; Mu, J. Effects of cellulose, hemicellulose, and lignin on the morphology and mechanical properties of metakaolin-based geopolymer. Constr. Build. Mater. 2018, 173, 10–16. [Google Scholar] [CrossRef]
- Prud’homme, E.; Michaud, P.; Peyratout, C.; Smith, A.; Rossignol, S.; Joussein, E.; Sauvat, N. Geomaterial foam to reinforce wood. Strateg. Mater. Comput. Des. Ceram. Eng. Sci. Proc. 2010, 31, 3–10. [Google Scholar]
- Gouny, F.; Fouchal, F.; Maillard, P.; Rossignol, S. Study of the effect of siliceous species in the formation of a geopolymer binder: Understanding the reaction mechanisms among the binder, wood, and earth brick. Ind. Eng. Chem. Res. 2014, 53, 3559–3569. [Google Scholar] [CrossRef]
- Berzins, A.; Morozovs, A.; Gross, U.; Iejavs, J. Mechanical properties of wood-geopolymer composite. Eng. Rural Dev. 2017, 16, 1167–1173. [Google Scholar]
- Chen, T.; Niu, M.; Xie, Y.; Wu, Z.; Liu, X.; Cai, L.; Zhuang, B. Modification of ultra-low density fiberboards by an inorganic film formed by Si-Al deposition and their mechanical properties. BioResources 2015, 10, 538–547. [Google Scholar] [CrossRef]
- Chen, T.; Wu, Z.; Niu, M.; Xie, Y.; Wang, X. Effect of Si–Al molar ratio on microstructure and mechanical properties of ultra-low density fiberboard. Eur. J. Wood Wood Prod. 2016, 74, 151–160. [Google Scholar] [CrossRef]
- Shalbafan, A.; Welling, J.; Hasch, J. Geopolymers as potential new binder class for the wood based composite industry. Holzforschung 2016, 70, 755–761. [Google Scholar] [CrossRef]
- Bekhta, P.; Sedliačik, J. Environmentally-friendly high-density polyethylene-bonded plywood panels. Polymers 2019, 11, 1166. [Google Scholar] [CrossRef]
- Berzins, A.; Morozovs, A.; Van Den Bulcke, J.; Acker, J. Van Softwood surface compatibility with inorganic geopolymer. Adv. Mater. Proc. 2017, 2, 793–798. [Google Scholar] [CrossRef]
- Shalbafan, A.; Welling, J.; Hasch, J. Effect of aluminosilicate powders on the applicability of innovative geopolymer binders for wood-based composites. Eur. J. Wood Wood Prod. 2017, 75, 893–902. [Google Scholar] [CrossRef]
- Tavor, D.; Wolfson, A.; Shamaev, A.; Shvarzman, A. Recycling of industrial wastewater by its immobilization in geopolymer cement. Ind. Eng. Chem. Res. 2007, 46, 6801–6805. [Google Scholar] [CrossRef]
- Prud’homme, E.; Michaud, P.; Joussein, E.; Peyratout, C.; Smith, A.; Arrii-Clacens, S.; Clacens, J.M.; Rossignol, S. Silica fume as porogent agent in geo-materials at low temperature. J. Eur. Ceram. Soc. 2010, 30, 1641–1648. [Google Scholar] [CrossRef]
- Song, W.; Wei, W.; Wang, D.; Zhang, S. Preparation and properties of new plywood composites made from surface modified veneers and polyvinyl chloride films. BioResources 2017, 12, 8320–8339. [Google Scholar]
- European Committee for Standardisation. Laminated Veneer Lumber (LVL)—Defenitions, Classification and Specifications (Includes Amendment A1:2009); European Norm EN 14279:2009; European Committee for Standardization: Brussels, Belgium, 2009. [Google Scholar]
- European Committee for Standardisation. Wood-Based Panels—Determination of Modulus of Elasticicty in Bending and of Bending Strength; European Norm EN 310:1993; European Committee for Standardization: Brussels, Belgium, 1993. [Google Scholar]
- European Committee for Standardisation. Plywood—Bonding Qulaity—Part 1: Test Methods; European Norm EN 314-1:2005; European Committee for Standardization: Brussels, Belgium, 2005. [Google Scholar]
- European Committee for Standardisation. Plywood—Bonding Quality—Part 2: Requirements; European Norm EN 314-2:1993; European Committee for Standardization: Brussels, Belgium, 1993. [Google Scholar]
- European Committee for Standardisation. Timber Structures—Structural Timber and Glued Laminated Timber—Determination of Some Physical and Mechanical Properties; European Norm EN 408:1995; European Committee for Standardization: Brussels, Belgium, 1995. [Google Scholar]
- Asadi, M.; Nemati, A.; Naghizadeh, R.; Arzani, K.; Fahim, J. Effect of temperature and activator molar of Na2O to SiO2 in the process of synthesis and microstructure of cement geopolymer. J. Adv. Mater. Process. 2013, 1, 3–10. [Google Scholar]
- Kotorlenko, L.A.; Aleksandrova, V.S. Spectral manifestations of change in electronic structure in phenol-phenolate anion-phenoxy radical series. Theor. Exp. Chem. 1982, 18, 97–99. [Google Scholar] [CrossRef]
- Xing, C.; Deng, J.; Zhang, S.Y.; Riedl, B.; Cloutier, A. Differential scanning calorimetry characterization of urea-formaldehyde resin curing behavior as affected by less desirable wood material and catalyst content. J. Appl. Polym. Sci. 2005, 98, 2027–2032. [Google Scholar] [CrossRef]
- Pizarro, J.; Castillo, X.; Jara, S.; Ortiz, C.; Navarro, P.; Cid, H.; Rioseco, H.; Barros, D.; Belzile, N. Adsorption of Cu2+ on coal fly ash modified with functionalized mesoporous silica. Fuel 2015, 156, 96–102. [Google Scholar] [CrossRef]
- Erdem, A.; Shahwan, T.; Çaĝir, A.; Eroĝlu, A.E. Synthesis of aminopropyl triethoxysilane-functionalized silica and its application in speciation studies of vanadium (IV) and vanadium (V). Chem. Eng. J. 2011, 174, 76–85. [Google Scholar] [CrossRef]
- European Committee for Standardisation. Solid Wood Panels (SWP)—Requirements; European Norm EN 13353: 2003; European Committee for Standardization: Brussels, Belgium, 2003. [Google Scholar]
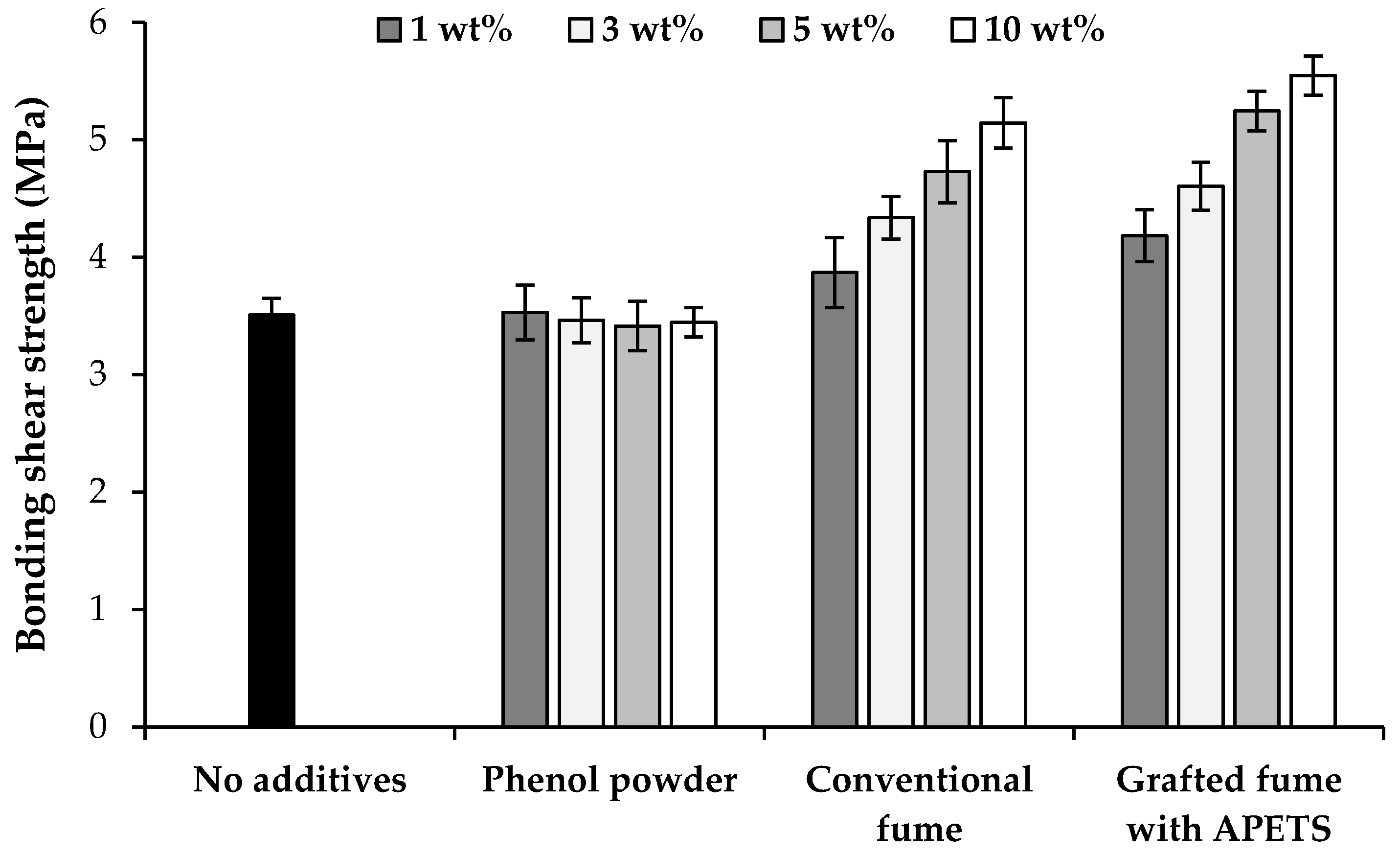


| Sample Code | Types of Additives | Content of Additives (wt %) 1 |
|---|---|---|
| N | Geopolymer with no additives | - |
| P | Phenol flakes | 1, 3, 5, 10 |
| CF | Conventional silica fume | 1, 3, 5, 10 |
| GF | Grafted silica fume with 3-aminopropyltriethoxysilane (APTES) | 1, 3, 5, 10 |
| Experiment | Independent Variables | Dependent Variables ** | ||
|---|---|---|---|---|
| No. | A1 (°C) | A2 (s) | SSM (MPa) | SSP (MPa) |
| 1 | 100 | 112.5 | 1.58 1.46 1.57 | 1.61 |
| 2 | 140 | 112.5 | 3.40 3.60 3.69 | 3.43 |
| 3 | 100 | 277.5 | 2.64 2.96 2.68 | 2.85 |
| 4 | 140 | 277.5 | 3.62 3.84 3.96 | 3.68 |
| 5 | 80 | 195 | 1.37 1.22 1.23 | 1.15 |
| 6 | 160 | 195 | 3.50 3.90 3.77 | 3.82 |
| 7 | 120 | 30 | 2.04 2.23 1.92 | 2.07 |
| 8 | 120 | 360 | 3.62 3.48 3.61 | 3.55 |
| 9 | 120 | 195 | 3.13 3.06 3.37 2.94 3.20 | 3.14 |
| 10 * | 140 | 112.5 | 3.66 3.52 3.34 | 3.44 |
| Model 1 | Coefficients of Variation (%) | Coefficient of Determination (R2) | Adjusted R2 | Adequate Precison |
|---|---|---|---|---|
| Y = −11.49 + 0.16X1 + 0.027X2 − 0.00015 X1X2 − 0.00040X12 − 0.000012X22 | 6.15 | 0.970 | 0.967 | 35.52 |
| Additives in Geopolymer | Amount (wt %) | Viscosity (cP) | Gel Time (s) | DSC Parameters | ||
|---|---|---|---|---|---|---|
| Onset Temperature (°C) | Peak Temperature (°C) | Delta H (J/g) | ||||
| No additives | 0 | 1040 (48) * | 83.7 (1.7) * | 79 | 98.8 | 160.4 |
| Phenol flakes | 5 | 6282 (387) | 89.7 (1.8) | 78 | 96.0 | 118.1 |
| 10 | 10,740 (417) | 91.3 (2.4) | 79 | 95.2 | 85.4 | |
| Conventional silica fume | 5 | 1564 (27) | 80.3 (1.9) | 80 | 99.4 | 168.2 |
| 10 | 2237 (13) | 79.7 (1.1) | 81 | 99.6 | 174.3 | |
| Grafted silica fume with APTES | 5 | 1194 (9) | 79.0 (0.7) | 80 | 99.2 | 166.8 |
| 10 | 1773 (6) | 74.3 (1.8) | 79 | 99.5 | 169.1 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalbafan, A.; Thoemen, H. Geopolymer-Bonded Laminated Veneer Lumber as Environmentally Friendly and Formaldehyde-Free Product: Effect of Various Additives on Geopolymer Binder Features. Appl. Sci. 2020, 10, 593. https://doi.org/10.3390/app10020593
Shalbafan A, Thoemen H. Geopolymer-Bonded Laminated Veneer Lumber as Environmentally Friendly and Formaldehyde-Free Product: Effect of Various Additives on Geopolymer Binder Features. Applied Sciences. 2020; 10(2):593. https://doi.org/10.3390/app10020593
Chicago/Turabian StyleShalbafan, Ali, and Heiko Thoemen. 2020. "Geopolymer-Bonded Laminated Veneer Lumber as Environmentally Friendly and Formaldehyde-Free Product: Effect of Various Additives on Geopolymer Binder Features" Applied Sciences 10, no. 2: 593. https://doi.org/10.3390/app10020593
APA StyleShalbafan, A., & Thoemen, H. (2020). Geopolymer-Bonded Laminated Veneer Lumber as Environmentally Friendly and Formaldehyde-Free Product: Effect of Various Additives on Geopolymer Binder Features. Applied Sciences, 10(2), 593. https://doi.org/10.3390/app10020593






