Abstract
When crude oil is extracted out of a subterranean reservoir at high temperature and pressure, it is usually transported via a pipeline, where the crude oil experiences radical changes in its physical and chemical properties, instigating numerous complications. Among the various flow assurance problems, wax deposition and build up are among the most commonly found. However, the accurate mechanism of wax deposition is still unclear and is widely debated among researchers. The mechanism under multiphase conditions is also an ambiguity. This review covers the six wax deposition mechanisms, the challenges in multiphase flow conditions, the latest types of chemical inhibitor, and a summary of factors governing chemical inhibitor performance.
1. Introduction
Paraffin deposition is a major problem, especially during crude oil transportation via pipelines as well as during production in the tubing string, in which the deposition of the unwanted substance causes an increase in the pressure drop (requiring higher pressure to transport the crude oil) [1]. Paraffins are defined as a class of i-alkanes and n-alkanes that comprises a long hydrocarbon chain usually attached via single bonds. High molecular weight paraffins are commonly found in solid wax deposits, where the carbon numbers are in the range of 18 to 75 and where the melting point ranges from 40 °C to 70 °C [2,3]. The lowest-energy chain alkane has a conformational structure of carbon atoms with hydrogen atoms in planes passing perpendicularly through the carbon atoms to the chain axes, as shown in Figure 1. Commonly, the solid phase specific heat ranges between 1.80 and 2.30 kJ/kg·K.
Figure 1.
Structure of the lowest energy chain alkane.
There are two kinds of wax: microcrystalline wax, which contributes to tank-bottom sludges, and macrocrystalline wax, which causes the flow assurance problems in production and transportation operations [4,5]. Before the deposition of paraffin wax occurs, paraffin wax will first crystallize or precipitate out from crude oil. The precipitation of paraffin wax occurs in two stages, which are known as nucleation and crystal growth [6,7,8]. Nucleation occurs when the temperature of the crude oil decreases to the wax appearance temperature (WAT). The wax molecules form clusters, causing a cloudy appearance—prompting the term “cloud point”, which refers to WAT value. Progressively, the paraffin wax molecules attach and detach until they reach a critical size cluster in order to be stable. These clusters are better known as nuclei, and the formation of these nuclei is defined as nucleation. Homogeneous nucleation takes place in the absence of contaminant or nucleating materials [6]. Meanwhile, heterogeneous nucleation occurs in the presence of nucleating material(s) throughout the liquid. The crystal growth process then begins after the nuclei has stabilized as further molecules attach in a plate-like or lamellar structure. In general, the precipitation of wax can cause an increment in crude oil viscosity, although the adhesion of wax to the pipe wall does not occur [9].
The correlation between the crude oil properties and rheology, including the deposition as well as the precipitation of wax during flow, has been extensively studied by a group of researchers [10,11,12,13]. The developed correlation is applied to predict the onset precipitation and deposition of wax in the pipeline. The standard technique used to investigate wax precipitation is by using a cold finger system, flow loop, or viscometer apparatus [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Nonetheless, the precise modelling of deposition along the pipeline walls is challenging. This is because the deposition of wax is a function of thermodynamic variables, hydrodynamic flow, heat and mass transfer, as well as solid–solid and surface–solid interactions. Modelling wax deposition in the presence of multiphase flow is even more complex to derive manually, as numerous variables need to be considered. In addition, a few wax deposition models have been put forward by researchers over the past few years in order to describe wax precipitation using computer simulation software such as OLGATM from Schlumberger, LedaFlow from Kongsberg and FloWax from KBC [30]. A brief explanation of the model utilized by the software is illustrated in Table 1.

Table 1.
Examples of models used in wax deposition studies.
Hence, this review paper aims to discuss the current understanding of the wax deposition mechanism in pipelines. The mechanisms for wax deposition include molecular diffusion, Soret diffusion, Brownian diffusion, gravity settling, shear dispersion, and shear stripping, which will be further discussed in this paper. In addition, the factors affecting wax deposition in multiphase flow, especially in the presence of gas–oil and oil–water flow, respectively, are also discussed. Further, this review covers the latest chemical inhibitors in the literature and the factors that affect the efficiency of chemical inhibitors in developing a sustainable source of knowledge in flow assurance.
2. Wax Deposition Mechanism
Among the six wax deposition mechanisms that have been suggested by researchers, molecular diffusion has been agreed by most researchers to be the dominant deposition mechanism [33]. This is because the pipe walls in deep water pipelines are influenced by cold subsea conditions. This causes the crude oil flowing in the pipeline to experience a radial thermal gradient. This means that the temperature of crude oil situated closer to the pipe wall is lower compared to the temperature of the crude oil at the center of the pipeline [34]. When the temperature of the crude oil reaches the cloud point, the crystallization of wax takes place in the cold region closer to the pipe wall. The crystallization of wax thereby changes the equilibrium of the liquid and solid phases. As the solubility of wax in the crude oil decreases with respect to thermal energy, there will be a concentration gradient present. Cold regions situated closer to the pipe wall will have a lower wax concentration in the liquid phase. This is due to the solid wax depositing out of the bulk liquid crude oil on to the colder pipeline wall. Hence, wax molecular diffusion occurs from the bulk fluid to the pipeline wall [35,36,37].
The wax molecular diffusion or the rate of deposited wax in mass can be predicted by utilizing Fick’s law of diffusion:
where mm is the deposited wax mass, ρd is the solid wax density, Dm is the liquid wax diffusion coefficient, A is the deposition surface area, C is the wax concentration in solution, and r is the radial coordinate. Equation (1) can also be written in terms of the wax solubility coefficient of the crude oil:
where dT/dr is the radial thermal gradient [38]. Expanding the molecular diffusion equation will give the molecular diffusion coefficient [38,39] as follows:
where T is defined as the absolute temperature, is a parameter for association, M is the molecular weight of crude oil, is the dynamic viscosity of crude oil, and V is the molecular volume of wax.
Subsequently, the second deposition mechanism based on thermal gradient is Soret diffusion [40]. Soret diffusion occurs when large molecules and small molecules are dispersed under thermal gradient regions. As yet, the employment of Soret diffusion in the wax deposition model is not fully understood and is recommended as the subject of future studies.
In one case, Soret diffusion was negligible in the wax deposition model [41], while in another case, Soret diffusion was taken into account in the development of the wax deposition model [42]. This notwithstanding, implementing both molecular diffusion and Soret diffusion will develop an accurate wax deposition model. This is because the rate of the deposited wax will be based on the concentration gradient and thermal gradient as well as variables associated with the diffusion coefficient and thermo-diffusion coefficient [40].
The third deposition mechanism due to the movement of particles is Brownian diffusion, which occurs when the surrounding temperature is less than WAT [35,36]. The presence of a solid crystal concentration gradient instigates a net transport of the crystal in the direction of lower concentration. The mass of deposited wax by Brownian motion can be predicted by
where is the deposited wax by Brownian motion, is the Brownian motion wax diffusion coefficient, and C* is defined as the wax concentration of solid wax. Wax deposition caused by Brownian diffusion is dimly understood because, according to its definition, Brownian diffusion flux occurs away from the wall, around the center region of a pipeline [43]. Thus, this dismisses the occurrence of wax deposition onto the pipe wall. On the contrary, it was argued that wax crystals are trapped in the immobile solid layer at the pipe wall—i.e., the concentration of the wax crystals near the wall is ~0—thus permitting Brownian diffusion towards the wall [44].
Not many papers have explained the last three deposition mechanisms, namely the shear dispersion, shear stripping, and gravity settling. Shear dispersion contributes to wax deposition by lateral particle motion immersed in flow-induced conditions, which is also known as shear flow. The shear dispersion mechanism is dominant when the temperatures are lower than the cloud point [45]. Meanwhile, shear stripping is more dominant in the presence of turbulent flow, where the effect of shear forces will be significant compared to laminar flow. Shear forces cause the removal of wax deposits and lead to deposition at regions of lower velocity in the production pipeline as well as reducing the wax deposition thickness [46,47]. Hence, these two former mechanisms are significant in the wax deposition model as well as in wax removal as they can enhance flow improver chemical performance. The later mechanism (gravity settling) is due to the formation of large wax particle sizes. Since the density of crystallized wax is higher than the density of crude oil, the probability for wax crystals to deposit at the bottom of the pipe is high. Depending on the settling velocity coefficient, studies have reported that the mass of deposited wax is found to be identical under vertical and horizontal flows [35,38].
3. Wax Deposition in Multiphase Flow
In comparison to single-phase, literature data on two-phase wax deposition studies (e.g., gas–oil and oil–water) are limited due to the complexity of the experiment. In fact, there is no published study on three-phase gas–oil–water paraffin deposition due to various variables that need to be controlled and considered. Nevertheless, there is a need to extend the study of wax deposition under multiphase flowing conditions since the actual crude oil transportation via subsea pipelines could encounter multiphase flow conditions which lead to different in flow patterns [48,49].
Figure 2i,ii [50] shows two categories of flow pattern which resulted from the inclination or elevation of the crude oil pipeline. For a horizontal pipeline, the flow patterns are bubbly flow, wavy flow, intermittent flow (plug and slug), and annular flow, as shown in Figure 2i. Meanwhile, flow patterns in a vertical pipeline are bubbly flow, intermittent flow (slug and churn), annular flow, and annular mist, as shown in Figure 2ii. In general, wax deposition distribution depends on the position of the pipeline, the flow pattern, and the multiphase system (such as the gas–oil system or the oil–water system).
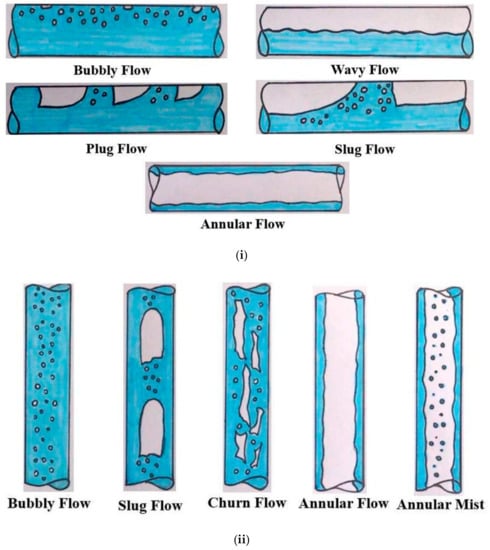
Figure 2.
Flow patterns in pipelines. (i) Flow patterns of near-horizontal pipeline [50]. (ii) Flow patterns of near-vertical flow [50].
A limited database can be found for wax deposition studies in gas–oil two-phase flow conducted by researchers worldwide [48,49,51,52,53]. Figure 3i,ii shows the variation of wax deposit distribution for a gas–oil system, based on the pipeline position and flow pattern. It is reported that wax deposited around the circumference of the pipeline (i.e., the internal pipeline wall) with different thickness and hardness when the pipeline position is vertical. It is notable that the flow pattern has no major effect on the shape of the deposited wax. A similar observation has been reported when the pipeline position is horizontal. For horizontal stratified flow, the deposit was distributed in a crescent shape and was found to be softer and thicker. Meanwhile, for horizontal intermittent flow, a harder deposit was produced than that of stratified flow, while the wax hardness for annular and intermittent flow was similar.
Figure 3.
Wax deposition distribution. (i) Wax deposition distribution (in shaded area) for horizontal flow. (ii) Wax deposition distribution (in shaded area) for vertical flow.
In terms of increasing gas velocity, the wax deposit thickness in gas–oil two-phase flow was reported to be thicker [48]. In contrast, the wax deposit thickness in single-phase flow was reported to be thinner at increasing flow velocity [16]. The equivalent wax deposition in gas–oil two-phase flow depends on of two factors: the deposition-promoting and the deposition-inhibiting factors. The deposition-promoting factor is an increase in heat transfer or thermal gradient due to the increase in the crude oil velocity simultaneously as the gas velocity increases. In contrast, the deposition-hindering factor is attributed to the surface area of the crude oil pipeline which is in-contact with the oil when the liquid holdup decreases as the gas velocity increases. Few studies have found that the deposition-hindering factor is amplified for bubbly, wavy, and intermittent flow patterns [49,54].
4. Oil−Water Wax Deposition
Most of the wax deposition studies in oil–water two-phase flow have used a flow loop or cold finger system [55,56,57,58,59,60]. Like the gas–oil system, the wax deposit distribution for the oil–water system is also dependent on the pipeline position and flow pattern. Remarkably, the correlation of wax deposits (i.e., shape or location of solid deposit at the pipeline circumference) in oil–water two-phase flow is even more complex. The presence of water in the system can greatly enhance gel formation in crude oil and change the wax particle adsorption capability at the liquid–liquid interface.
It was reported that a wax deposit appeared at either the top region, bottom region, or on the entire pipeline circumference, depending on the type of dispersion (incomplete or complete dispersion), oil–water fluid velocity. and water cut. For instance, in the case of incomplete dispersion, the wax deposit would appear either at the top region or bottom region of the pipeline wall. Hypothetically, oil floats in water; thus, the oil film will be at the top region, in the position of the wax deposit. This is valid for both high and low oil-viscosity systems [55,58]. Unless the oil–water fluid velocity is high enough to suppress the oil film towards the bottom region, the wax deposition occurs at the bottom region [58]. In case of complete dispersion, the wax deposit was found to be on the entire pipeline circumference, in which its thickness decreased at an increasing oil–water fluid velocity [55].
Further, it was observed that an increment in water cut could lead to a decrease in wax deposit thickness [56,57,61]. This is because a high water cut means a decrease in the oil volume present in the pipeline. Hence, the amount of wax crystal required for deposition to occur in production feed is reduced. In addition, a high water cut could also lower the heat transfer rate, as water has a higher specific heat value compared to oil. Some researchers have reported that the wax deposit thickness increases and then decreases at higher water cuts [26,62]. As yet, there are no correlation models which are available to preview the impact of water cut on wax deposition distribution. It is important to conduct further investigation in order to manage the risk of wax deposit formation in the crude oil pipelines.
5. Wax Chemical Inhibitors
Between the mechanical method and chemical method, the latter is preferred by the industry in inhibiting the wax deposition issue [5,63,64]. Table 2, Table 3 and Table 4 show the progress of studies involving three types of chemical inhibitor, namely the wax dispersant, pour point depressant (PPD), and wax crystal modifier.

Table 2.
Examples of wax dispersant used in deposition studies.

Table 3.
Examples of pour point depressant used in was deposition study.

Table 4.
Examples of wax crystal modifier used in was deposition study.
5.1. Wax Dispersant
Wax dispersants are a group of surfactants that adsorb onto pipe wall surfaces and decrease the wax adhesion either by altering the wettability of the pipe wall or by forming a thin layer where wax crystals shear off easily. A wax dispersant also adsorbs onto wax crystals and forms a wax crystal lattice structure in the crude oil. The wax crystal structure later reduces the growing crystals’ morphology and delays the formation of a three-dimensional crystal. This altered spherical-like crystal from a large plate-like crystal is expected to increase the ability of the crude oil to flow [81,82]. Note that the wax dispersant has limited effectiveness when not used with a polymeric flow improver. It is reported that a wax dispersant works extremely well with a polymeric flow improver due to its characteristic of hindering wax from settling and depositing at the pipe wall surface.
In the presence of water, surfactants such as alkyl sulfonates and fatty amine ethoxylates can also function as wax dispersants. Interaction between the wax particles and hydrophilic groups of the surfactant disperses the wax particles to smaller sizes. This can lead to the prevention of wax agglomeration and deposition [83,84]. Attributed to the molecular structural factor, these surfactants were reported to perform the best with longer chain esters in reducing the pour point and surface tension, altering the morphology as well as reducing co-crystallization [85,86,87].
5.2. Pour Point Depressant (PPD)
PPDs function by co-crystallizing into the paraffin structure via van der Waals forces. This allows the polar end tail (unattached) to form a steric interference with the alignment of other new wax molecules. PPDs decrease the pour point temperatures of the crude oil and then weaken the wax deposition solid structure, thus allowing for the easy removal of the deposition by shear force. It should be noted that the decrease in the crude oil’s PPD does not reflect the decrease in the WAT of the crude oil, as PPDs do not inhibit the crystallization of the wax crystals but rather inhibit the growth of the wax crystals [33,88]. The difference between PPD and wax dispersant is that the wax dispersant adsorbs into the wax crystal and hinders the further sticking of the wax molecules. Examples of PPD are polyethylene vinyl acetate (EVA), methyl methacrylate (MMA), olefin-maleic anhydride copolymer (MAC), and diethanolamine (DEA) [63,89].
One of the most common PPDs used in the industry is polyethylene vinyl acetate (EVA). EVA is the product of the copolymerization of ethylene and vinyl acetate (VA). There are two processes involved in the wax deposition inhibition mechanism. Firstly, the polar compound, which is VA, comprises methyl and methylene groups that contains two active oxygen atoms—due to this, EVA exhibits strong van der Waals interaction with the long-chain paraffin waxes. As a result, the wax solubility is increased, the gel strength of the wax is reduced, and ultimately there is a reduction in the wax deposition [75,90,91]. Secondly, the non-polar long alkyl moieties of EVA will interact with the long-chain paraffin waxes. This interaction will then alter the wax crystallization process, which leads to a reduction in the pour point value (or a lower WAT) [68]. The shape and growth of the wax crystals will form in different axial directions when different EVA concentrations are utilized [91]. Meanwhile, the concentration of EVA does not directly influence the PPD performance. For example, EVA (with 20 wt % VA content) at 50 ppm and 500 ppm concentrations both reduced the pour point by more than 26 °C [76].
Another type of PPD that is commonly used in the industry is the comb polymer, which can be categorised into two different polymer groups: maleic anhydride copolymer (MAC) and poly-acrylate/ methacrylate (PA or PMA) ester polymers. The length of the pendant chain as well as the length of the chain wax molecule should be similar to obtain optimum deposition inhibition performance. The polyvinyl backbone of the polymer has little or no effect on the performance of the inhibitor; in contrast, amending the backbone regularity significantly affects the structure of the polymer which functions as a pour point as well as the degree of the crystallinity reducer [46,89]. MAC, PA and PMA performed best with longer alkyl chains compared to short alkyl chains by reducing the wax crystal size. This is because the longer the alkyl chain, the higher the solubility of the copolymer in the paraffin wax structure; thus, the pour point is reduced from 27 °C to −3 °C (at 10,000 ppm) [84,92].
Li et al. (2012) also reported that MAC is not only a good pour point reducer agent but also an excellent reducer of the deposition rate of wax and the yield stress of the wax gel [93]. The disadvantage of utilizing comb polymers is that the series of comb polymers must be compatible with the paraffin chain length to prevent the wax crystals from interlocking. This means that the outcome of the wax inhibition effectiveness will depend on the crude oil composition and the paraffin chain length [75]. High molecular weight copolymers are suggested if a crude oil contains a high range of normal paraffins and low carbon number. Meanwhile, for a crude oil that contains a narrow range of normal paraffins and high carbon number, low molecular weight copolymers are recommended [84,94,95].
Due to challenges in dealing with waxy crude oil, these polymeric PPDs showed limited wax inhibition performance. Recent findings showed that nanotechnology had contributed in the development of wax deposition inhibitors. Examples of polymeric nano-hybrids which have showed potential include polyoctadecyl acrylate, EVA co-polymers, and methacrylate [46,89]. The ability of nanoparticles is that they can alter the nucleation site and hence form dispersed nucleation sites, alter the wax crystals growth (co-crystallization) and consequently depress the pour point better than utilizing EVA alone [96,97]. It was reported that the pour point of crude oil was reduced by 25% when EVA was added, while the pour point was further reduced by up to 50% when a nano-hybrid was added together with EVA [96].
Another effective PPD that has been widely used includes the addition of organic solvents prior to polymeric wax inhibitors, which reduces the viscosity of the crude oil and enhances the oil flowability [98]. Examples of effective organic solvents include benzene-, carbon disulphide-, chlorinated hydrocarbons-, xylene- and toluene-based chemicals. For example, when trichloroethylene-xylene (TEX) was added to Nigerian crude oil in a ratio of 0.01 to 0.1 wt %, TEX was able to depress WAT, reduce the total wax deposition from 0.050 g/g to 0.0015 g/g (at 0.1 wt %), and further increase the wax deposition inhibition performance by adding a corrosion inhibitor [99]. However, there are safety concerns around the storage and handling of these organic solvents due to the toxicity of the solvents and their low flash point [100,101].
Non-ionic surfactants have been reported to behave in a similar manner to polymeric PPDs by altering the wax crystals’ morphology, impeding the three-dimensional wax formation [102,103]. Non-ionic surfactants are generally viscous sticky liquids, which have extra surface-active properties and can act as a durable emulsifier. Non-ionic surfactants do not ionize in aqueous solution due to the presence of hydrophilic groups (alcohols, phenol, ester, and ether). In addition, non-ionic surfactants are better than anionic surfactants, as they are more reactive at higher temperature, hence performing better at reservoir temperature. Also, the low viscous emulsions make the non-ionic surfactants easier to recover at the refinery.
Presently, natural surfactants are also being investigated under the category of PPDs and have been found to be much more promising than conventional surfactants. This is mainly due to their lower toxicity, ability to biodegrade, wide structural variety, and the fact that they can be obtained from cheap renewable materials as well as being stable under wide range of pH values [104,105]. The natural surfactants that show promising results include sapindus mukorossi sp. and halomonas xianhensis sp. nov. bacteria. For example, halomonas xianhensis sp. nov. bacteria were able to reduce the pour point of crude oil by up to 24 °C and reduce the crude oil viscosity by up to 70% [66,72]. Recently, polyamine amide from canola oil showed potential as an excellent pour point reducer with small concentration [106]. Akinyemi et al. (2018) also reported that Jatropha seeds and castor seed oil were able to reduce the pour point (from 24 °C to 8 °C) and the viscosity of waxy crude oils when a low dosage was used (0.1–0.3 vol %) [18].
5.3. Wax Crystal Modifier
Wax crystal modifier can incorporate into wax crystals during the nucleation process and change the growth and surface characteristics of the crystals, causing them to reassemble into micelle-like aggregates. This forms more subcritical nuclei and reduces the supersaturation properties of the crude oil. Subsequently, smaller wax crystals form and remain stable in the oil phase [68,107,108]. Wax crystal modifier will also aid in reducing the tendency of the wax crystals to form a three-dimensional network, hence reducing the pour point and the oil viscosity.
There are reports in which the term wax crystal modifier is interchangeable with pour point depressant. However, the exact mechanism of how the crystal modifier functions is still ambiguous and requires further investigation. Some researchers have instigated a theory that wax crystal modifier reduces the pour point by forming hindering needle star-like crystals (spherulites) [109]. Examples of wax crystal modifiers are polyalkyl methacrylate, polymeric fatty ester, methacrylic acid ester, and crystalline-amorphous copolymers such as polyethylene–polyethylene propylene (PE-PEP) and polyethylene butene (PEB). It has been reported that PE-PEP and PEB could aid in regulating the rheological properties and the size of the wax crystals in the middle distillate crude oils and fuels [110,111].
6. Factors Affecting Wax Inhibition Performance
6.1. Flow Regimes
The variance in flow regimes affects the thermal gradients and plays a vital role in deposition behavior as well as wax inhibition performance. The two main flow regimes that are involved in crude oil fluid flow behavior are laminar and turbulent flow. Deposited wax under laminar flow conditions would result in the deposition of a low paraffin content wax deposit solid. In a turbulent flow, the deposit solid would comprise a high paraffin content wax deposit [112]. Further, the wax deposition mass under laminar flow conditions is higher compared to the wax deposited under turbulent flow conditions. This is expected as, in turbulent flow, the shear force acting on the deposited wax deposit causes softer low paraffin content wax deposit to be sheared off the pipe wall, leaving behind the harder high paraffin content wax deposit [14,15,112]. This hard-and-high content paraffin wax deposit makes pigging operations difficult. In general, the flow regime in the crude oil pipeline must be considered when selecting chemical inhibitors.
6.2. Temperature
The performance of a chemical inhibitor is temperature-dependent. When the temperature increases, the deposited wax mass reduces while the critical carbon number increases. The increment in the critical carbon number is due to the longer paraffin inhibitor chain used, which hinders the lower n-paraffin components but leads to the increase in the deposition of higher n-paraffin components [113]. The critical carbon number determines the hardness of the wax deposition [114]. The harder the wax deposited, the higher the concentration of chemical inhibitor required. Others have described that chemical inhibitors are efficient at lower bulk oil temperatures but highly inefficent at higher temperatures [5,15,38,115,116]. Hence, a suitable operating temperature condition during the injection of chemical inhibitors must be considered to reduce the deposition of wax as well as the critical carbon number.
6.3. Wax Content
The wax content present in the crude oil determines the amount as well as the thickness of the wax that will be deposited. Various research has shown that approximately 2 wt % of paraffin wax is sufficient to induce the gelling of a virgin waxy crude oil [117]. The higher the wax content in the crude oil, the higher the pour point of the crude oil. This does not indicate that a higher concentration of chemical inhibitor is required to reduce the pour point; however, some authors have concluded from their research that the interaction of the molecular structure of the chemical inhibitor and the wax molecules is a more prominent factor which dictates the amount of chemical inhibitor required to inhibit wax deposition [26,38,118,119,120,121].
6.4. Chemical Inhibitor’s Molecular Structure
Most chemical inhibitors are polymers that have a backbone and a hydrocarbon pendant chain which interacts with the wax molecule in the crude oil and inhibits the agglomeration of large wax crystal structures. This contributes to the list of factors that influence the performance of the chemical inhibitors: the polymer backbone, the length of the hydrocarbon pendant chain, and the molecular weight. Despite earlier findings that the polymer backbone factor was not significant in affecting the pour point reduction [117], the polymer backbone of ethylene vinyl acetate co-polymer was reported to affect the polymer’s inhibition performance. The length of the hydrocarbon pendant chain length is expected to match the length of wax molecules in the crude oil to obtain the inhibitor optimum performance [118]. A short, low molecular weight polymer may not have the molecular volume to interrupt the co-crystallization of the wax crystals, while a long, high molecular weight polymer may be insoluble or could interact with itself and not with the wax molecule in the crude oil. Also, the solubility of the polymer in the crude oil could be limited, thus inducing wax crystallization and increasing the pour point of the crude oil [118].
6.5. Effect of Solvent and Dilution
Undiluted chemical inhibitors are often solid under room temperature. For the transportation and distribution of the chemical inhibitors, the use of a solvent is therefore necessary for dilution. However, the solvent can influence the effective hydrodynamic specific volume of the chemical inhibitor, and thus the inhibition performance. The effective hydrodynamic specific volume is known as the degree of the chemical inhibitor which reacts with the solvent as well as the measure of the degree of the coiled and uncoiled chemical inhibitor in the solvent [15]. A chemical inhibitor or polymer which has a strong interaction with a good solvent as well as the polymer is uncoiled, and a high radius of gyration is said to have a high effective hydrodynamic specific volume. This means that the chemical inhibitor exhibits high performance. A good polymer solvent portrays a higher package viscosity and more fully expanded compound than a poor polymer solvent. The expansion polymer structure leads to a complex polymer/solvent formation which appears as an enormous molecule with an increase in viscosity [119]. On the contrary, a poor polymer solvent has a weak polymer–solvent interaction, causing the polymer to be coiled. The coiled polymer–solvent exhibits a low radius of gyration and is said to have a low effective hydrodynamic specific volume, which means the chemical inhibitor exhibits low performance.
The dilution or net concentration of a polymer in a solvent has a large influence on the polymer’s physical properties. Theoretically, at a low dilution (high concentration), the polymer could interact with other polymer molecules which causes coiling effects, hence limiting the accessibility of the polymer to the wax molecules in the crude oil. This means that a concentrated chemical inhibitor could have low performance. At a high dilution (low concentration), the polymer is completely solvated and completely accessible to interact with the wax molecule in the crude oil [15,119,120]. The difference in the chemical inhibitor performance can be altered by introducing a mixing procedure while adding the solvent into the chemical inhibitor [118].
6.6. Polar Crude Fractions
Polar extracts from crude and distillate oils such as asphaltenes, aromatics, and resins have potential as low-cost flow improvers [116,121]. It was reported that the presence of asphaltenes reduced the gelation temperature of wax–crude oil solutions. Further addition of the asphaltenes resulted in a macroscopic phase separation of the wax–crude oil solutions caused by gravity settling. It is expected that the flocculation of the asphaltene strongly affects the wax crystallization mechanism on crude oils. Unfortunately, the mechanisms by which polar asphaltenes form a molecular interaction (intra- or inter-) with wax molecules and how asphaltenes affect the size of the wax crystals are not fully understood.
7. Conclusions
Wax deposition is among the most common flow assurance problem endured by petroleum industries, especially during the production and transportation of crude oils. Continuous research works have led to a comprehensive understanding of how to mitigate the occurrence of pipeline blockages caused by wax deposition. This review describes the differences between six types of wax deposition mechanism, issues related to deposition mechanism, challenges that are attributed to multiphase flow condition, and the latest chemical inhibitors used by researchers, as well as a list of factors that affect the inhibitor performance.
Author Contributions
Conceptualization, T.R.; H.H. and C.D.W.; Resources, T.R.; H.H. and C.D.W.; Writing—Original Draft Preparation, T.R.; Writing—Review & Editing, H.H.; C.D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This postgraduate work conducted by the first author was funded by YUTP-FRG grant (015LC0-064).
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| DEA | Diethanolamine |
| EVA | Ethylene vinyl acetate |
| MAC | Olefin-maleic anhydride copolymer |
| MMA | Methyl methacrylate |
| PA | Poly-acrylate |
| PEB | Polyethylene butene |
| PE-PEP | Polyethylene–polyethylene propylene |
| PMA | Poly-methacrylate |
| PPDs | Pour point depressants |
| TEX | Trichloroethylene-xylene |
| VA | Vinyl acetate |
| WAT | Wax appearance temperature |
| HTGC | High temperature gas chromatography |
| Superficial gas velocity | |
| Superficial liquid velocity |
References
- Arnold, K.; Stewart, M. Surface Production Operations; Gulf Professional Publishing: Oxford, UK, 2008. [Google Scholar]
- Pedersen, K.S.; Fredenslund, A.; Thomassen, P. Properties of Oils and Natural Gases 5; Gulf Pub Co: Pittsburgh, PA, USA, 1989. [Google Scholar]
- Srivastava, S.; Handoo, J.; Agrawal, K.; Joshi, G. Phase-transition studies in n-alkanes and petroleum-related waxes—A review. J. Phys. Chem. Solids 1993, 54, 639–670. [Google Scholar] [CrossRef]
- Ferworn, K.A.; Fluid, D.B.R.; Hammami, A.; Robinson, D.B.; Ellis, H. Control of Wax Deposition: An Experimental Investigation of Crystal Morphology and an Evaluation of Various Chemical Solvents. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 18–21 February 1997; pp. 1–20. [Google Scholar]
- Aiyejina, A.; Chakrabarti, D.P.; Pilgrim, A.; Sastry, M. Wax formation in oil pipelines: A critical review. Int. J. Multiph. Flow 2011, 37, 671–694. [Google Scholar] [CrossRef]
- Behbahani, T.J.; Beigi, A.A.M.; Taheri, Z.; Ghanbari, B. Investigation of wax precipitation in crude oil: Experimental and modeling. Petroleum 2015, 1, 223–230. [Google Scholar] [CrossRef][Green Version]
- Lira-Galeana, C.; Hammami, A. Chapter 21 Wax precipitation from petroleum fluids: A Review. Dev. Pet. Sci. 2000, 40, 557–608. [Google Scholar]
- Weingarten, J.S.; Euchner, J.A. Methods for predicting wax precipitation and deposition. SPE Prod. Eng. 1988, 3, 121–126. [Google Scholar] [CrossRef]
- Struchkov, I.A.; Rogachev, M.K. Wax precipitation in multicomponent hydrocarbon system. J. Pet. Explor. Prod. Technol. 2017, 7, 543–553. [Google Scholar] [CrossRef]
- Garcia, M.D.C.; Urbina, A. Effect of crude oil composition and blending on flowing properties. Pet. Sci. Technol. 2003, 21, 863–878. [Google Scholar] [CrossRef]
- García, M.D.C.; Orea, M.; Carbognani, L.; Urbina, A. The effect of paraffinic fractions on crude oil wax crystallization. Pet. Sci. Technol. 2001, 19, 189–196. [Google Scholar] [CrossRef]
- Hammami, A.; Ratulowski, J.; Coutinho, J.A.P. Cloud points: Can we measure or model them? SPE Repr. Ser. 2004, 21, 144–154. [Google Scholar] [CrossRef]
- Daraboina, N.; Soedarmo, A.; Sarica, C. Microscopic Study of Wax Inhibition Mechanism. In Proceedings of the Offshore Technology Conference, Society of Petroleum Engineers, Houston, TX, USA, 2–5 May 2016; pp. 1–10. [Google Scholar]
- Chi, Y.; Daraboina, N.; Sarica, C. Investigation of inhibitors efficacy in wax deposition mitigation using a laboratory scale flow loop. AIChE J. 2016, 62, 4131–4139. [Google Scholar] [CrossRef]
- Perez, P.; Boden, E.; Chichak, K.; Gurnon, A.K.; Hu, L.; Lee, J.; McDermott, J.; Osaheni, J.; Peng, W.; Richards, W.; et al. Evaluation of Paraffin Wax Inhibitors: An Experimental Comparison of Bench-Top Test Results and Small-Scale Deposition Rigs for Model Waxy Oils. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4–7 May 2015; pp. 4–7. [Google Scholar]
- Singh, P.; Venkatesan, R.; Fogler, H.S.; Nagarajan, N. Formation and aging of incipient thin film wax-oil gels. AIChE J. 2000, 46, 1059–1074. [Google Scholar] [CrossRef]
- Soedarmo, A.A.; Daraboina, N.; Sarica, C. Validation of wax deposition models with recent laboratory scale flow loop experimental data. J. Pet. Sci. Eng. 2017, 149, 351–366. [Google Scholar] [CrossRef]
- Akinyemi, O.; Udonne, J.; Oyedeko, K. Study of effects of blend of plant seed oils on wax deposition tendencies of Nigerian waxy crude oil. J. Pet. Sci. Eng. 2018, 161, 551–558. [Google Scholar] [CrossRef]
- Correra, S.; Fasano, A.; Fusi, L.; Primicerio, M. Modelling wax diffusion in crude oils: The cold finger device. Appl. Math. Model. 2007, 31, 2286–2298. [Google Scholar] [CrossRef]
- Deshmukh, S.; Bharambe, D.P. Wax dispersant additives for improving the low temperature flow behavior of waxy crude oil. Energy Sources Part A Recover. Util. Environ. Eff. 2012, 34, 1121–1129. [Google Scholar] [CrossRef]
- Santos, A.D.; Fernandes, A.; Giulietti, M. Study of the paraffin deposit formation using the cold finger metholodogy for Brazilian crude oils. J. Pet. Sci. Eng. 2004, 45, 47–60. [Google Scholar] [CrossRef]
- Fan, K.; Huang, Q.; Li, S.; Zhao, D. Wax Deposition Study in a Cold-finger System with Model Oil. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Bali, Indonesia, 20–22 October 2015; pp. 20–22. [Google Scholar]
- Hunt, E.B., Jr. Laboratory study of paraffin deposition. J. Pet. Technol. 1962, 14, 1259–1269. [Google Scholar] [CrossRef]
- Ridzuan, N.; Adam, F.; Yaacob, Z. Effects of shear rate and inhibitors on wax deposition of Malaysian crude oil. Orient. J. Chem. 2015, 31, 1999–2004. [Google Scholar] [CrossRef]
- Wei, F.; Acosta, E.; Gawas, K.; Krishnamurthy, P. Targeting High Molecular Weight Wax. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 13–15 April 2015; pp. 13–15. [Google Scholar]
- Zhang, Y.; Gong, J.; Wu, H. An experimental study on wax deposition of water in waxy crude oil emulsions. Pet. Sci. Technol. 2010, 28, 1653–1664. [Google Scholar] [CrossRef]
- Akinyemi, O.P.; Udonne, J.D.; Efeovbokhan, V.E.; Ayoola, A.A. A study on the use of plant seed oils, triethanolamine and xylene as flow improvers of Nigerian waxy crude oil. J. Appl. Res. Technol. 2016, 14, 195–205. [Google Scholar] [CrossRef]
- Patel, M.R.; Chitte, P.S.; Bharambe, D. Oleic acid based polymeric flow improvers for Langhnaj (North Gujarat, India) crude oil. Egypt. J. Pet. 2017, 26, 895–903. [Google Scholar] [CrossRef]
- Hoffmann, R.; Amundsen, L. Single-phase wax deposition experiments. Energy Fuels 2010, 24, 1069–1080. [Google Scholar] [CrossRef]
- Leporini, M.; Terenzi, A.; Marchetti, B.; Giacchetta, G.; Corvaro, F. Experiences in numerical simulation of wax deposition in oil and multiphase pipelines: Theory versus reality. J. Pet. Sci. Eng. 2019, 174, 997–1008. [Google Scholar] [CrossRef]
- Rosvold, K. Wax Deposition Models; Department of Petroleum Engineering, NTNU: Taipei, Taiwan, 2008. [Google Scholar]
- Coutinho, J.A.P.; Edmonds, B.; Moorwood, T.; Szczepanski, R.; Zhang, X. Reliable wax predictions for flow assurance. Energy Fuels 2006, 20, 1081–1088. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, S.; Fogler, H.S. Wax Deposition: Experimental Characterizations, Theoretical Modeling, and Field Practices, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Chi, Y.; Yang, J.; Sarica, C.; Daraboina, N. A critical review of controlling paraffin deposition in production lines using chemicals. Energy Fuels 2019, 33, 2797–2809. [Google Scholar] [CrossRef]
- Harun, A.; Ab Lah, N.K.I.N.; Husin, H.; Hassan, Z. An overview of wax crystallization, deposition mechanism and effect of temperature & shear. In Proceedings of the ICIMSA 2016 3rd International Conference on Industrial Engineering, Management Science and Applications, Jeju, Korea, 23–26 May 2016; pp. 1–5. [Google Scholar]
- Leiroz, A.; Azevedo, L. Studies on the Mechanisms Of Wax Deposition In Pipelines. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 2–5 May 2005. [Google Scholar]
- Li, M.; Su, J.; Wu, Z.; Yang, Y.; Ji, S. Study of the mechanisms of wax prevention in a pipeline with glass inner layer. Colloids Surf. A Phys. Eng. Asp. 1997, 123, 635–649. [Google Scholar] [CrossRef]
- Burger, E.; Perkins, T.; Striegler, J. Studies of Wax Deposition in the Trans Alaska Pipeline. J. Pet. Technol. 1981, 33, 1075–1086. [Google Scholar] [CrossRef]
- Wilke, C.R.; Chang, P. Correlation of diffusion coefficients in dilute solutions. AIChE J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Ekweribe, C.K.; Civan, F.; Lee, H.S.; Singh, P. Interim report on pressure effect on waxy-crude pipeline-restart conditions investigated by a model system. SPE Proj. Facil. Constr. 2009, 4, 61–74. [Google Scholar] [CrossRef]
- Merino-Garcia, D.; Correra, S. Cold Flow: A review of a technology to avoid wax deposition. Pet. Sci. Technol. 2008, 26, 446–459. [Google Scholar] [CrossRef]
- Banki, R.; Hoteit, H.; Firoozabadi, A. Mathematical formulation and numerical modeling of wax deposition in pipelines from enthalpy–porosity approach and irreversible thermodynamics. Int. J. Heat Mass Transf. 2008, 51, 3387–3398. [Google Scholar] [CrossRef]
- Majeed, A.; Bringeal, B.; Overa, S. Model calculates wax deposition for North sea. J. Oil Gas 1990, 88, 63–69. [Google Scholar]
- Azevedo, L.F.A.; Teixeira, A.M. A critical review of the modeling of wax deposition mechanisms. Pet. Sci. Technol. 2003, 21, 393–408. [Google Scholar] [CrossRef]
- Fasano, A.; Fusi, L.; Correra, S. Mathematical models for waxy crude oils. Meccanica 2004, 39, 441–482. [Google Scholar] [CrossRef]
- Hao, L.Z.; Al-Salim, H.S.; Ridzuan, N. A review of the mechanism and role of wax inhibitors in the wax deposition and precipitation. Pertanika J. Sci. Technol. 2019, 27, 499–526. [Google Scholar]
- Hernandez, O.; Hensley, H.; Sarica, C.; Brill, J.; Volk, M.; Dellecase, E. Improvements in Single-Phase Paraffin Deposition Modeling. In Proceedings of the SPE Annual Technical Conference and Exhibition, Society of Petroleum Engineers, Denver, CO, USA, 5–8 October 2003. [Google Scholar]
- Gong, J.; Zhang, Y.; Liao, L.; Duan, J.; Wang, P.; Zhou, J. Wax deposition in the oil/gas two-phase flow for a horizontal pipe. Energy Fuels 2011, 25, 1624–1632. [Google Scholar] [CrossRef]
- Matzain, A.; Apte, M.S.; Zhang, H.-Q.; Volk, M.; Brill, J.P.; Creek, J.L. Investigation of Paraffin Deposition During Multiphase Flow in Pipelines and Wellbores—Part 1: Experiments. J. Energy Resour. Technol. 2002, 124, 180–186. [Google Scholar] [CrossRef]
- Sultan, R.; Leulmi, H. CFD Simulation Investigation of Natural Gas Components through a Drilling Pipe Faculty of Engineering and Applied Sciences; University of Newfoundland: St. John’s, NL, Canada, 2016. [Google Scholar]
- Matzain, A. Multiphase Flow Paraffin Deposition Modeling. 1999. Available online: https://www.osti.gov/servlets/purl/834175 (accessed on 22 September 2019).
- Taitel, Y.; Dukler, A. A model for predicting flow regime transitions in horizontal and near horizontal gas-liquid ow. AIChE J. 1976, 22, 47–55. [Google Scholar] [CrossRef]
- Kilincer, N. Multiphase Paraffin Deposition Behavior of a Garden Banks Condensate, M.E. Project. 2003. Available online: https://www.osti.gov/servlets/purl/834175 (accessed on 21 September 2019).
- Rittirong, A.; Panacharoensawad, E.; Sarica, C. Experimental Study of Paraffin Deposition under Two-Phase Gas/Oil Slug Flow in Horizontal Pipes. SPE Prod. Oper. 2016, 32, 99–117. [Google Scholar]
- Anosike, C.F. Effect of Flow Patterns on Oil−Water Flow Paraffin Deposition in Horizontal Pipes; University of Tulsa: Tulsa, OK, USA, 2007. [Google Scholar]
- Bruno, A. Paraffin Deposition of Crude Oil and Water Dispersions under Flowing Conditions; Tulsa University: Tulsa, OK, USA, 2006. [Google Scholar]
- Couto, G.H.; Chen, H.; Delle-Case, E.; Sarica, C.; Volk, M. An Investigation of Two-Phase Oil/Water Paraffin Deposition. SPE Prod. Oper. 2008, 23, 49–55. [Google Scholar] [CrossRef]
- Vuong, D.H.; Zhang, H.-Q.; Sarica, C.; Li, M. Experimental Study on High Viscosity Oil/Water Flow in Horizontal and Vertical Pipes. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, Louisiana, USA, 4–7 October 2009; pp. 1–10. [Google Scholar]
- Huang, Z.; Lu, Y.; Hoffmann, R.; Amundsen, L.; Fogler, H.S. The effect of operating temperatures on wax deposition. Energy Fuels 2011, 25, 5180–5188. [Google Scholar] [CrossRef]
- Robustillo, M.D.; Coto, B.; Martos, C.; Espada, J.J. Assessment of different methods to determine the total wax content of crude oils. Energy Fuels 2012, 26, 6352–6357. [Google Scholar] [CrossRef]
- Phan, T.; Faust, M.; Balsamo, V.; Champion, N. A More Effective Solution to Treat Paraffinic Crude Oil Wells. In Proceedings of the SPE Production & Operations, Galveston, TX, USA, 8–9 April 2019; pp. 8–9. [Google Scholar]
- Kasumu, A.S.; Mehrotra, A.K. Solids deposition from two-phase wax–solvent–water “Waxy” mixtures under turbulent flow. Energy Fuels 2013, 27, 1914–1925. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Abang, S.; Bono, A.; Krishnaiah, D.; Karali, R.; Safuan, M.K. Wax inhibitor based on ethylene vinyl acetate with methyl methacrylate and diethanolamine for crude oil pipeline. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 12074. [Google Scholar] [CrossRef]
- Patton, C.C. Paraffin deposition from refined wax-solvent systems. Soc. Petrol. Eng. 1970, 10, 17–24. [Google Scholar] [CrossRef]
- Subramanie, P.A.; Padhi, A.; Ridzuan, N.; Adam, F. Experimental study on the effect of wax inhibitor and nanoparticles on rheology of Malaysian crude oil. J. King Saud Univ.-Eng. Sci. 2019, in press. [Google Scholar] [CrossRef]
- Kumar, R.; Banerjee, S.; Mandal, A.; Naiya, T.K. Flow improvement of heavy crude oil through pipelines using surfactant extracted from soapnuts. J. Pet. Sci. Eng. 2017, 152, 353–360. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Ghazawy, R.A.; Morsy, F.A.; Hebishy, A.M.S.; Elmorsy, A. Adsorption of polymeric additives based on Vinyl Acetate copolymers as wax dispersant and its relevance to polymer crystallization mechanisms. J. Chem. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Yang, F.; Paso, K.; Norrman, J.; Li, C.; Oschmann, H.; Sjöblom, J. Hydrophilic nanoparticles facilitate wax inhibition. Energy Fuels 2015, 29, 1368–1374. [Google Scholar] [CrossRef]
- Tung, N.P.; Phong, N.T.P.; Long, B.Q.K.; Thuc, P.D.; Son, T.C. Studying the Mechanisms of Crude Oil Pour Point and Viscosity Reductions When Developing Chemical Additives With the Use of Advanced Analytical Tools. In Proceedings of the Society of Petroleum Engineers, Houston, TX, USA, 13–16 February 2001; pp. 1–12. [Google Scholar]
- Ruwoldt, J.; Sørland, G.H.; Simon, S.; Oschmann, H.-J.; Sjöblom, J. Inhibitor-wax interactions and PPD effect on wax crystallization: New approaches for GC/MS and NMR, and comparison with DSC, CPM, and rheometry. J. Pet. Sci. Eng. 2019, 177, 53–68. [Google Scholar] [CrossRef]
- Adeyanju, O.A.; Oyekunle, L.O. Experimental study of water-in-oil emulsion flow on wax deposition in subsea pipelines. J. Pet. Sci. Eng. 2019, 182, 106294. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; Khidr, T.T. Some biosurfactants used as pour point depressant for waxy egyptian crude oil. Pet. Sci. Technol. 2016, 34, 1475–1482. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; El-Din, M.R.N.; Morsi, R.E.; Elsabee, M.Z.; El-Din, M.R.N. Styrene-Maleic Anhydride copolymer esters as flow improvers of waxy crude oil. J. Dispers. Sci. Technol. 2009, 30, 420–426. [Google Scholar] [CrossRef]
- Hafiz, A.; Khidr, T. Hexa-triethanolamine oleate esters as pour point depressant for waxy crude oils. J. Pet. Sci. Eng. 2007, 56, 296–302. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; El-Kafrawy, A.F.; Khidr, T.T.; El-Ghazawy, R.A.; Mishrif, M.R. Synthesis and evaluation of some novel polymeric surfactants ased on aromatic amines used as wax dispersant for waxy gas oil. J. Dispers. Sci. Technol. 2007, 28, 976–983. [Google Scholar] [CrossRef]
- Machado, A.L.C.; Lucas, E.F.; González, G. Poly(ethylene-co-vinyl acetate) (EVA) as wax inhibitor of a Brazilian crude oil: Oil viscosity, pour point and phase behavior of organic solutions. J. Pet. Sci. Eng. 2001, 32, 159–165. [Google Scholar] [CrossRef]
- Lim, Z.H.; Al Salim, H.S.; Ridzuan, N.; Nguele, R.; Sasaki, K. Effect of surfactants and their blend with silica nanoparticles on wax deposition in a Malaysian crude oil. Pet. Sci. 2018, 15, 577–590. [Google Scholar] [CrossRef]
- He, C.; Ding, Y.; Chen, J.; Wang, F.; Gao, C.; Zhang, S.; Yang, M. Influence of the nano-hybrid pour point depressant on flow properties of waxy crude oil. Fuel 2016, 167, 40–48. [Google Scholar] [CrossRef]
- Farag, R.K. Poly (Cinnamoyloxy Ethyl Methacrylate-Co-Octadecyl Acrylate) as flow improver for Egyptian waxy crude oils. Int. J. Polym. Mater. 2008, 57, 189–202. [Google Scholar] [CrossRef]
- Taraneh, J.B.; Rahmatollah, G.; Hassan, A.; Alireza, D. Effect of wax inhibitors on pour point and rheological properties of Iranian waxy crude oil. Fuel Process. Technol. 2008, 89, 973–977. [Google Scholar] [CrossRef]
- Ansaroudi, H.R.J.; Vafaie-Sefti, M.; Masoudi, S.; Behbahani, T.J.; Jafari, H. Study of the morphology of wax crystals in the presence of Ethylene-co-vinyl Acetate copolymer. Pet. Sci. Technol. 2013, 31, 643–651. [Google Scholar] [CrossRef]
- Soni, H.P.; Kiranbala, K.S.; Agrawal, A.; Bharambe, D.P. Designing maleic anhydride-α-olifin copolymeric combs as wax crystal growth nucleators. Fuel Process. Technol. 2010, 91, 997–1004. [Google Scholar] [CrossRef]
- Al-Yaari, M. Paraffin Wax Deposition: Mitigation and Removal Techniques. In Proceedings of the SPE Saudi Arabia, Dhahran, Saudi Arabia, 14–16 March 2011. [Google Scholar]
- Zhang, C.; Gao, C.; Gao, F.; Wang, J.; Zhang, D.; Wang, Y.; Xu, D. Synthesis of comb bipolymers and their pour point depressing properties. Pet. Sci. 2014, 11, 155–160. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Khidr, T.T.; Ismail, D.A. Effect of gemini surfactant additives on pour point depressant of crude oil. J. Dispers. Sci. Technol. 2018, 39, 1160–1164. [Google Scholar] [CrossRef]
- Maithufi, M.N.; Joubert, D.J.; Klumperman, B. Application of Gemini Surfactants as diesel fuel wax dispersants. Energy Fuels 2011, 25, 162–171. [Google Scholar] [CrossRef]
- Sahai, M.; Singh, R.K.; Kukrety, A.; Kumar, A.; Ray, S.S.; Chhibber, V.K.; Kumar, S. Application of Triazine-Based Gemini surfactants as viscosity reducing agents of Tar Sand derived bituminous crude. Energy Fuels 2018, 32, 3031–3038. [Google Scholar] [CrossRef]
- Coto, B.; Martos, C.; Espada, J.J.; Robustillo, M.D.; Peña, J.L. Experimental study of the effect of inhibitors in wax precipitation by different techniques. Energy Sci. Eng. 2014, 2, 196–203. [Google Scholar] [CrossRef]
- Sivakumar, P.; Sircar, A.; Deka, B.; Anumegalai, A.S.; Moorthi, P.S.; Yasvanthrajan, N. Flow improvers for assured flow of crude oil in midstream pipeline—A review. J. Pet. Sci. Eng. 2018, 164, 24–30. [Google Scholar] [CrossRef]
- Taiwo, E.; Otolorin, J.; Afolabi, T. Crude Oil Transportation: Nigerian Niger Delta Waxy Crude, Crude Oil Exploration in the World 2010. 2012. Available online: https://www.intechopen.com/books/crude-oil-exploration-in-the-world/crude-oil-transportation-nigerian-niger-delta-waxy-crude-oil (accessed on 15 September 2019).
- Ridzuan, N.; Adam, F.; Yaacob, Z.; Ump, P. Molecular Recognition of Wax Inhibitor through Pour Point Depressant. Spetember 2014, pp. 1–9. Available online: http://www.pertanika.upm.edu.my/Pertanika%20PAPERS/JST%20Vol.%2027%20(1)%20Jan.%202019/29%20JST-1110-2018.pdf (accessed on 15 September 2019).
- Xu, J.; Zhang, X.; Sun, J.; Li, L.; Guo, X. How comb-type poly (maleic acid alkylamide-co-a-olefin) assemble in waxy oils and improve flowing ability. Asia-Pac. J. Chem. Eng. 2018, 58, 2254–2261. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Tinsley, J.; Adamson, D.; Pethica, B.; Huang, J.; Prud’homme, R.; Guo, X. Improvement of oil flowability by assembly of comb-type copolymers with paraffin and asphaltene. AIChE J. 2012, 58, 2254–2261. [Google Scholar] [CrossRef]
- Borthakur, A.; Chanda, D.; Choudhury, S.R.D.; Rao, K.V.; Subrahmanyam, B. Alkyl Fumarate−Vinyl Acetate copolymer as flow improver for high waxy Indian crude oils. Energy Fuels 1996, 10, 844–848. [Google Scholar] [CrossRef]
- Yang, F.; Li, C.; Lin, M.; Li, Z.; Yu, T. Depressive effect of polyacrylate (PA) pour point depressant on waxy crude oils. J. Petrochem. Univ. 2009, 22, 20–25. [Google Scholar]
- Wang, F.; Zhang, D.; Ding, Y.; Zhang, L.; Yang, M.; Jiang, B.; Huo, L. The effect of nanohybrid materials on the pour-point and viscosity depressing of waxy crude oil. Chin. Sci. Bull. 2011, 56, 14–17. [Google Scholar] [CrossRef]
- Yang, F.; Yao, B.; Li, C.; Shi, X.; Sun, G.; Ma, X. Performance improvement of the ethylene-vinyl acetate copolymer (EVA) pour point depressant by small dosages of the polymethylsilsesquioxane (PMSQ) microsphere: An experimental study. Fuel 2017, 207, 204–213. [Google Scholar] [CrossRef]
- Gateau, P.; Henaut, I.; Barre, L.; Argillier, J. Heavy oil dilution. Oil Gas Sci. Technol. 2004, 59, 503–509. [Google Scholar] [CrossRef]
- Bello, O.; Fasesan, S.; Teodoriu, C.; Reinicke, K. An Evaluation of the performance of selected wax inhibitors on paraffin deposition of Nigerian crude oils. Pet. Sci. Technol. 2006, 24, 195–206. [Google Scholar] [CrossRef]
- Straub, T.; Autry, S.; King, G. An Investigation into Practical Removal of Downhole Paraffin by Thermal Methods and Chemical Solvents. In Proceedings of the SPE Production & Operations, Olahoma City, OK, USA, 13–14 March 1989. [Google Scholar]
- Woo, G.; Garbis, S.; Gray, T. Long-Term Control of Paraffin Deposition. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 16–19 September 1984. [Google Scholar]
- Ahmed, N.S.; Nassar, A.M.; Zaki, N.N.; Gharieb, H.K. Stability and rheology of heavy crude oil-in-water emulsion stabilized by an anionic- nonionic surfactant mixture. Pet. Sci. Technol. 1999, 17, 553–576. [Google Scholar] [CrossRef]
- Khidr, T.T.; Doheim, M.M.; El-Shamy, O.A.A. Pour point depressant of fuel oil using non-ionic surfactants. Pet. Sci. Technol. 2015, 33, 1619–1626. [Google Scholar] [CrossRef]
- Okoliegbe, L.; Agarry, O. Application of microbial surfactant (A review). Sch. J. Biotechnol. 2012, 1, 15–23. [Google Scholar]
- Oguntimein, G.; Erdmann, H.; Schmid, R. Lipase catalysed synthesis of sugar eser in organic solvents. Biotechnol. Lett. 1993, 15, 175–180. [Google Scholar] [CrossRef]
- Chen, G.; Bai, Y.; Zhang, J.; Yuan, W.; Song, H.; Jeje, A. Synthesis of new flow improvers from canola oil and application to waxy crude oil. Pet. Sci. Technol. 2016, 34, 1285–1290. [Google Scholar] [CrossRef]
- Marie, E.; Chevalier, Y.; Eydoux, F.; Germanaud, L.; Flores, P. Control of n-alkanes crystallization by ethylene–vinyl acetate copolymers. J. Colloid Interface Sci. 2005, 290, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Naiya, T.K.; Banerjee, S.; Kumar, R.; Mandal, A. Heavy Crude Oil Rheology Improvement Using Naturally Extracted Surfactant. In Proceedings of the SPE Oil & Gas India Conference and Exhibition, Society of Petroleum Engineers, Mumbai, India, 24–26 November 2015. [Google Scholar]
- Wei, B. Recent advances on mitigating wax problem using polymeric wax crystal modifier. J. Pet. Explor. Prod. Technol. 2015, 5, 391–401. [Google Scholar] [CrossRef]
- Ashbaugh, H.S.; Fetters, L.J.; Adamson, D.; Prud’homme, R.K. Flow improvement of waxy oils mediated by self-aggregating partially cystallizable diblock copolymers. J. Rheol. 2002, 46, 763–776. [Google Scholar] [CrossRef]
- Leube, W.; Monkenbusch, M.; Schneiders, D.; Richter, D.; Adamson, D.; Fetters, L.; Dounis, P.; Lovegrove, R. Wax-Crystal modification for fuel oils by self-aggregating partially crystallizable hydrocarbon block copolymers. Energy Fuels 2000, 14, 419–430. [Google Scholar] [CrossRef]
- Chi, Y.; Daraboina, N.; Sarica, C. Effect of the Flow Field on the Wax Deposition and Performance of Wax Inhibitors: Cold Finger and Flow Loop Testing. Energy Fuels 2017, 31, 4915–4924. [Google Scholar] [CrossRef]
- Dubey, A.; Chi, Y.; Daraboina, N. Investigating the Performance of Paraffin Inhibitors under Different Operating Conditions. In Proceedings of the SPE Annual Technical Conference and Exhibitionn, Society of Petroleum Engineers, San Antonio, Texas, USA, 9–11 October 2017; pp. 1–20. [Google Scholar]
- Quan, Q.; Gong, J.; Wang, W.; Gao, G. Study on the aging and critical carbon number of wax deposition with temperature for crude oils. J. Pet. Sci. Eng. 2015, 130, 1–5. [Google Scholar] [CrossRef]
- Jennings, D.W.; Breitigam, J. Paraffin inhibitor formulations for different application environments: From heated injection in the desert to extreme cold arctic temperatures. Energy Fuels 2010, 24, 2337–2349. [Google Scholar] [CrossRef]
- Kelland, M. Production Chemicals for the Oil and Gas Industry. CRC Press. 2014. Available online: https://books.google.com.my/books/about/Production_Chemicals_for_the_Oil_and_Gas.html?id=Aw6xjq2cBaQC&redir_esc=y (accessed on 1 October 2019).
- Beiny, D.H.M.; Mullins, J.W.; Lewtas, K. Crystallization of N-Dotriacontaine from hydrocarbon solution with polymeric additives. J. Cryst. Growth 1990, 102, 801–806. [Google Scholar] [CrossRef]
- Manka, J.S.; Ziegler, K.L. Factors affecting the performance of crude oil wax-control additives. Soc. Pet. Eng. SPE 2001, 67326, 1–7. [Google Scholar]
- Qian, J.W.; Qi, G.R.; Xu, Y.L.; Yang, S.L. Solvent effect on the action of ethylene-vinyl acetate copolymer pour point depressant in waxy solutions. J. Appl. Polym. Sci. 1996, 60, 1575–1578. [Google Scholar] [CrossRef]
- Xiong, C.X. The structure and activity of polyalphaolefins as pour point depressants. Lub. Eng. 1993, 49, 196–200. [Google Scholar]
- Venkatesan, R.; Östlund, J.-A.; Chawla, H.; Wattana, P.; Nydén, M.; Fogler, H.S. The effect of asphaltenes on the gelation of waxy oils. Energy Fuels 2003, 17, 1630–1640. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).