In Vitro Assessment of Antiplasmodial and Antitrypanosomal Activities of Chloroform, Ethyl Acetate and Ethanol Leaf Extracts of Oedera genistifolia
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Plant Material
2.2. GCMS Analysis
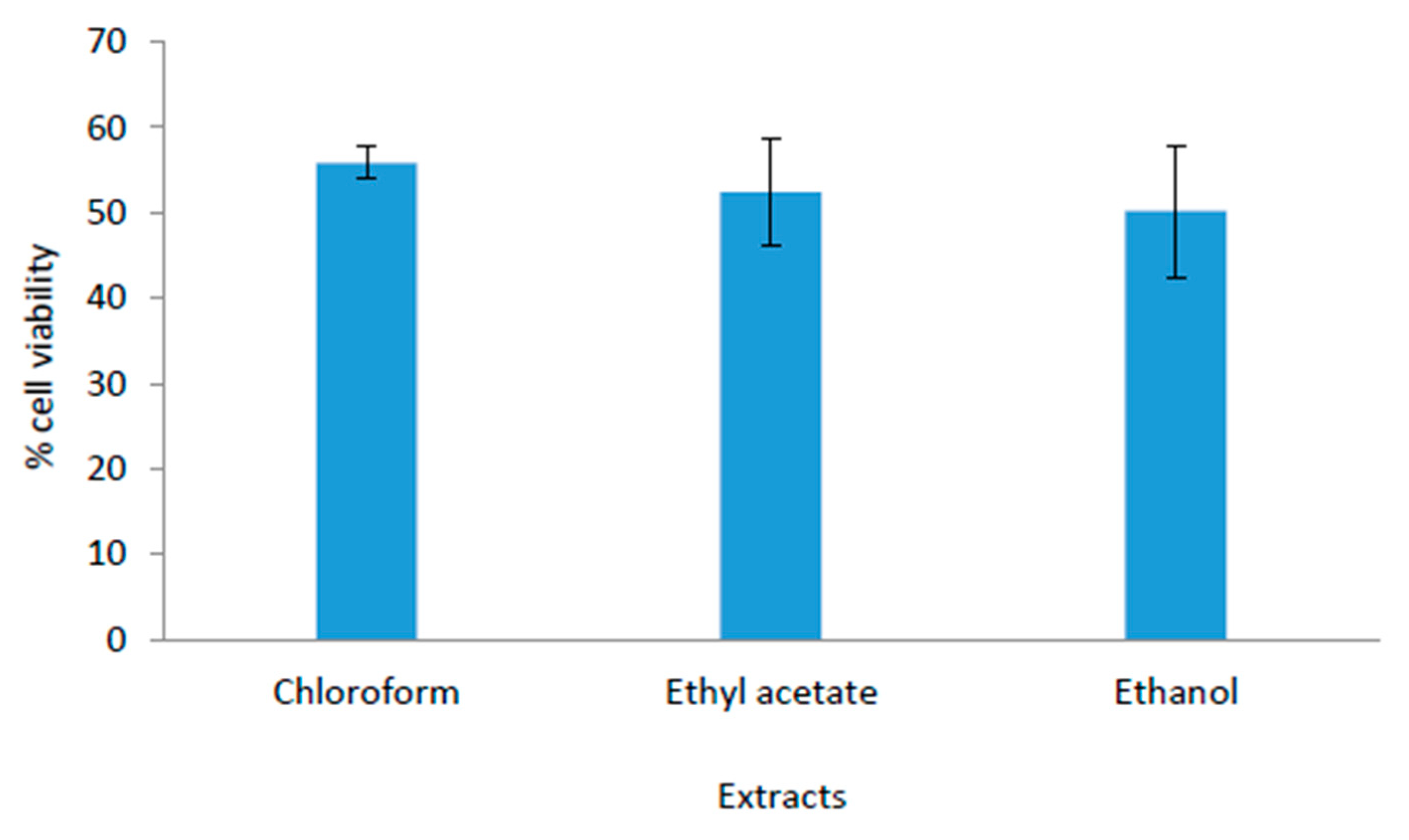
2.3. Biocompatibility Study
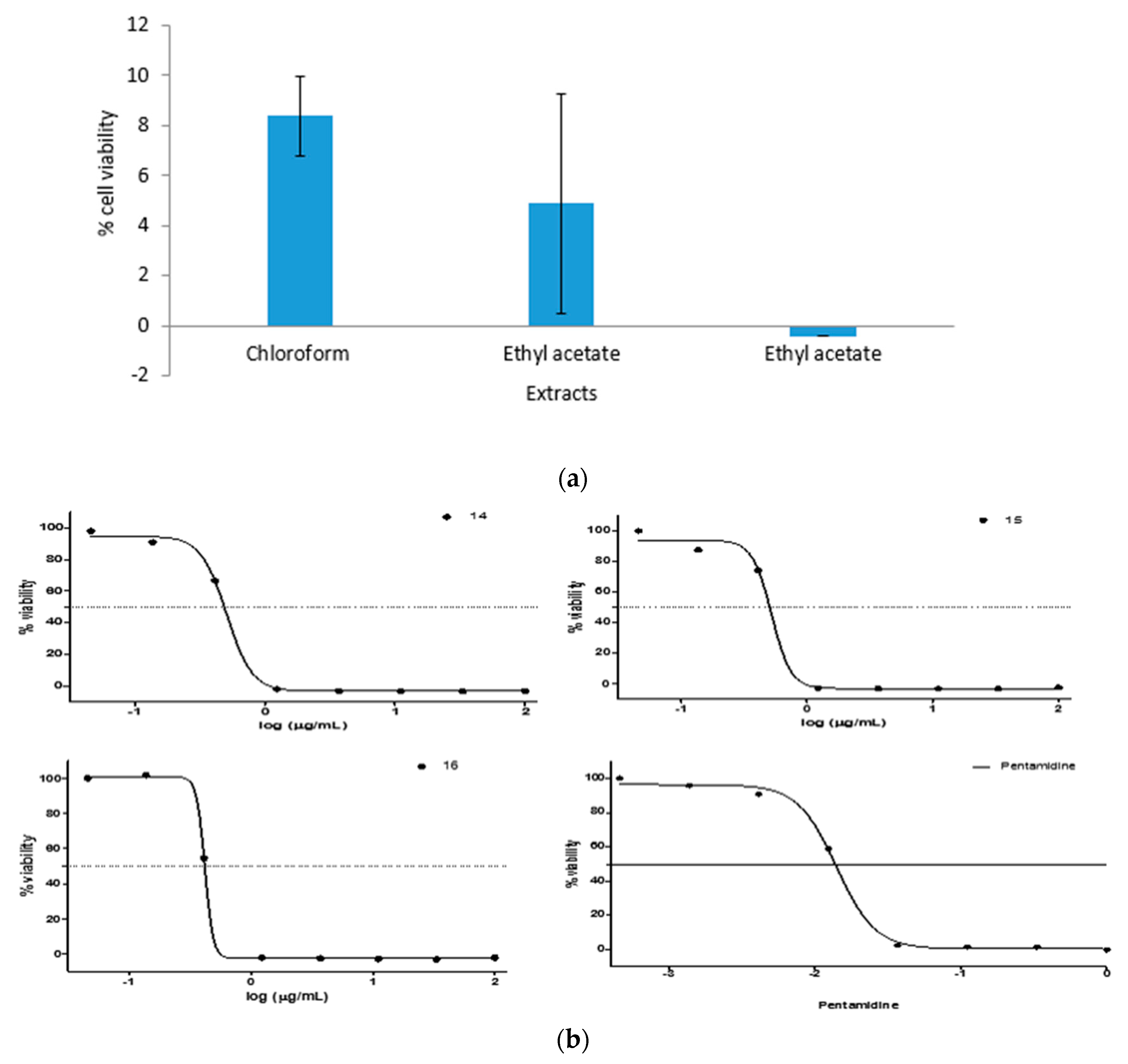
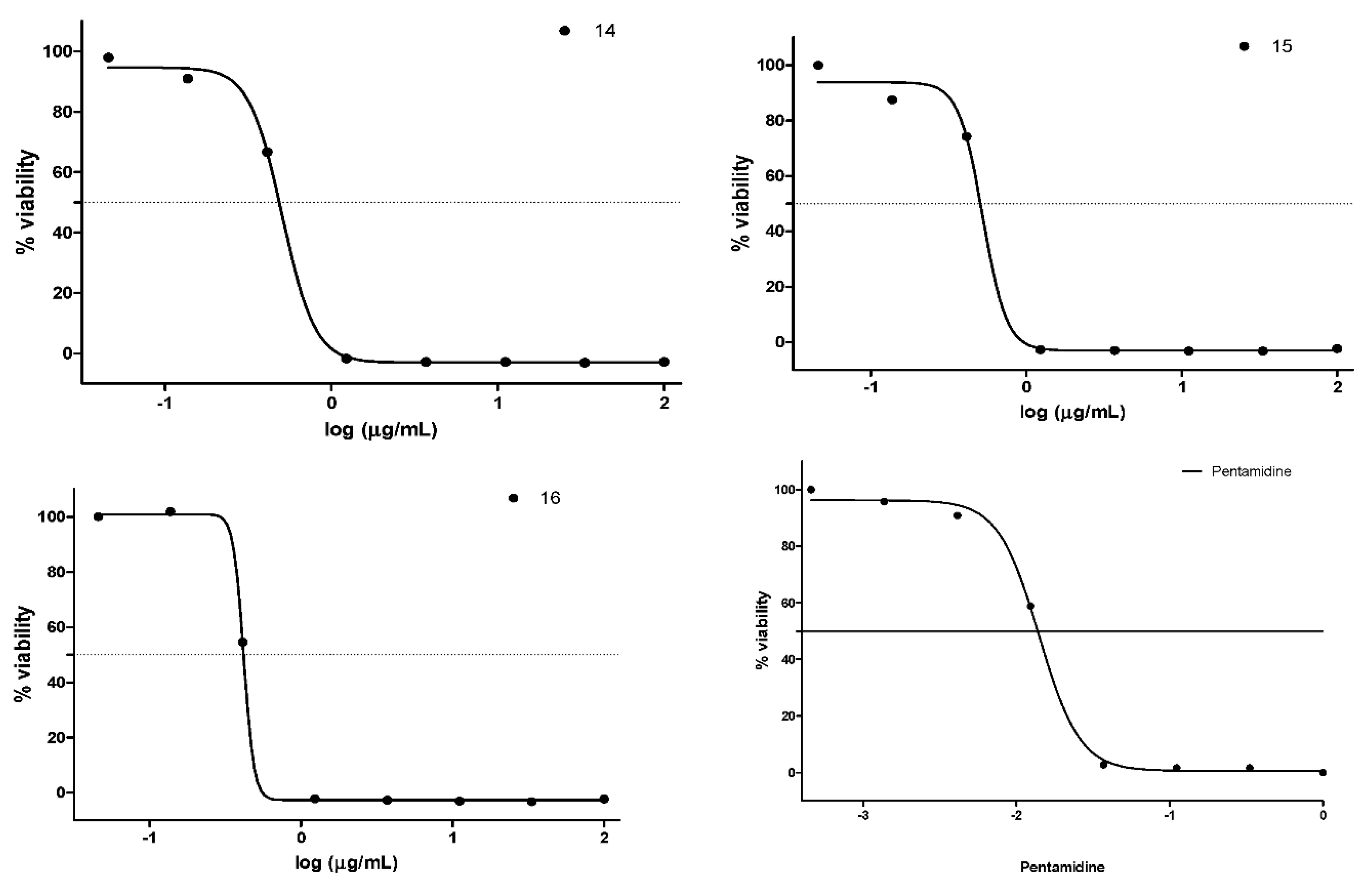
2.4. Antiplasmodial Activity
2.5. Antitrypanosomal Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. GCMS Analysis
3.2. Biocompatibility Study
3.3. Antiplasmodial Study
3.4. Antitrypanosomal Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamaraj, C.; Rahuman, A.A.; Roopan, S.M.; Bagavan, A.; Elango, G.; Zahir, A.A.; Rajakumar, G.; Jayaseelan, C.; Santhoshkumar, T.; Marimuthu, S.; et al. Bioassay-guided isolation and characterization of active antiplasmodial compounds from Murraya koenigii extracts against Plasmodium falciparum and Plasmodium berghei. Parasitol. Res. 2014, 113, 1657–1672. [Google Scholar] [CrossRef] [PubMed]
- Das, N.G.; Rabha, B.; Talukdar, P.K.; Goswami, D.; Dhiman, S. Preliminary in vitro antiplasmodial activity of Aristolochia griffithii and Thalictrum foliolosum DC extracts against malaria parasite Plasmodium falciparum. BMC Res. Notes 2016, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Lawal, B.; Shittu, O.K.; Kabiru, A.Y.; Jigam, A.A.; Umar, M.B.; Berinyuy, E.B.; Alozieuwa, B.U. Potential antimalarials from African natural products: A review. J. Intercult. Ethnopharmacol. 2015, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Mzena, T.; Swai, H.; Chacha, M. Antimalarial activity of Cucumis metuliferus and Lippia kituiensis against Plasmodium berghei infection in mice. Res. Rep. Trop. Med. 2018, 9, 81–88. [Google Scholar] [PubMed]
- Meyer, A.; Holt, H.R.; Selby, R.; Guitian, J. Past and ongoing tsetse and animal Trypanosomiasis control operations in five African countries: A systematic review. PLoS Negl. Trop. Dis. 2016, 10, e0005247. [Google Scholar] [CrossRef] [PubMed]
- Muhanguzi, D.; Mugenyi, A.; Bigirwa, G.; Kamusiime, M.; Kitibwa, A.; Akurut, G.G.; Ochwo, S.; Amanyire, W.; Okech, S.G.; Hattendorf, J.; et al. African animal trypanosomiasis as a constraint to livestock health and production in Karamoja region: A detailed qualitative Past and ongoing tsetse and animal Trypanosomiasis control operations in five African countries: A systematic review and quantitative assessment. BMC Vet. Res. 2017, 13, 355. [Google Scholar] [CrossRef]
- Atouguia, J.; Costa, J. Therapy of human African trypanosomosis: Current situation. Mem Inst. Oswaldo Cruz 1999, 94, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Mohammed, A.; Isah, M.B.; Aliyu, A.B. Anti-trypanosomal activity of African medicinal plants: A review update. J. Ethnopharmacol. 2014, 154, 26–54. [Google Scholar] [CrossRef]
- Ogungbe, I.V.; Setzer, W.N. Comparative Molecular Docking of Antitrypanosomal Natural Products into Multiple Trypanosoma brucei Drug Targets. Molecules 2009, 14, 1513–1536. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.A.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V.G.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases. Part II Curr. Med. Chem. 2012, 19, 2176–2228. [Google Scholar] [CrossRef]
- Deharo, E.; Bourdy, G.; Quenevo, C.; Muñoz, V.; Ruiz, G.; Sauvain, M. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach. Part V. evaluation of the antimalarial activity of plants used by the Tacana Indians. J. Ethnopharmacol. 2001, 77, 91–98. [Google Scholar] [CrossRef]
- Guo, Z.; Vangapandu, S.; Sindelar, R.W.; Walker, L.A.; Sindelar, R.D. Biologically active quassinoids and their chemistry: Potential leads for drug design. Front. Med. Chem. 2009, 4, 1–22. [Google Scholar]
- Krettli, A.U.; Adebayo, J.O.; Krettli, L.G. Testing of natural products and synthetic molecules aiming at new antimalarials. Curr. Drug Targets 2009, 10, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Gurib-Fakim, A.; Subratty, A.H. Screening for alternative antibiotics: An investigation into the antimicrobial activities of medicinal food plants of Mauritius. J. Food Sci. 2010, 75, 173–177. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.J. Natural products as sources of new drugs over the 30 years from 1981 to 2010. Nat. Prod. 2012, 75, 311. [Google Scholar] [CrossRef]
- Ogbole, E.A.; Ogbole, Y.; Peter, J.Y.; Builders, M.I.; Aguiyi, J.C. Phytochemical screening and in vivo antiplasmodial sensitivity study of locally cultivated Artemisia annua leaf extract against Plasmodium berghei. Am. J. Ethnomed. 2014, 1, 42–49. [Google Scholar]
- Nwodo, N.J.; Ibezim, A.; Ntie-Kang, F.; Adikwu, M.U.; Mbah, C.J. Antitrypanosomal activity of Nigerian plants and their constituents. Molecules 2015, 20, 7750–7771. [Google Scholar] [CrossRef]
- Sülsen, V.P.; Cazorla, S.I.; Frank, F.M.; Redko, F.C.; Anesini, C.A.; Coussio, J.D.; Malchiodi, E.L.; Martino, V.S.; Muschietti, L.V. Trypanocidal and leishmanicidal activities of flavonoids from Argentine medicinal plants. Am. J. Trop. Med. Hyg. 2007, 77, 654–659. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Ojemaye, M.O.; Hoppe, H.; Mabinya, L.V.; Okoh, A.I. Phytofabrication of silver/silver chloride nanoparticles using aqueous leaf extract of Oedera genistifolia: Characterization and antibacterial potential. Molecules 2019, 24, 4382. [Google Scholar] [CrossRef]
- Tonisi, S.; Okaiyeto, K.; Mabinya, L.V.; Okoh, A.I. Evaluation of bioactive compounds, free radical scavenging and anticancer activities of bulb extracts of Boophone disticha from Eastern Cape Province, South Africa. Saudi J. Biol. Sci. 2020. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Hoppe, H.; Okoh, A.I. Plant-based synthesis of silver nanoparticles using aqueous leaf extract of Salvia officinalis: Characterization and its antiplasmodial activity. J. Clust. Sci. 2020, 1–9. [Google Scholar] [CrossRef]
- Inbaneson, S.J.; Ravikumar, S.; Suganthi, P. In vitro antiplasmodial effect of ethanolic extracts of traditional medicinal plant Ocimum species against Plasmodium falciparum. Asian Pac. J. Trop. Med. 2012, 5, 103–106. [Google Scholar] [CrossRef]
- Batista, R.; de Jesus Silva, A., Jr.; Braga de Oliveira, A. Plant-derived antimalarial agents: New leads and efficient phytomedicines. Part II. Non-alkaloidal natural products. Molecules 2009, 14, 3037–3072. [Google Scholar] [CrossRef] [PubMed]
- Casuga, F.P.; Castillo, A.L.; Corpuz, M.J.T. GC–MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica (Blanco) (Moraceae) leaves. Asian Pac. J. Trop. Biomed. 2016, 6, 957–961. [Google Scholar] [CrossRef]
- Ajaiyeoba, E.; Falade, M.; Ogbole, O.; kpako, L.; Akinboye, D. In vivo antimalarial and cytotoxicity properties of Annona senegalensis extract. Afr. J. Trad. CAM 2006, 1, 37–41. [Google Scholar]
- Swamy, M.K.; Arumugam, G.; Kaur, R.; Ghasemzadeh, A.; Yusoff, M.M.; Sinniah, U.R. GC-MS based metabolite profiling, antioxidant and antimicrobial properties of different solvent extracts of Malaysian Plectranthus amboinicus leaves. Evid. Based Complementary Altern. Med. 2017, 2017, 1517683. [Google Scholar] [CrossRef]
- Paudel, M.R.; Chand, M.B.; Pant, B.; Pant, B. Assessment of antioxidant and cytotoxic activities of extracts of Dendrobium crepidatum. Biomolecules 2019, 9, 478. [Google Scholar] [CrossRef]
- Sachs, J.; Malaney, P. The economic and social burden of malaria. Nature 2002, 415, 680–685. [Google Scholar] [CrossRef]
- Ogunlesi, M.; Okiei, W.; Ofor, E.; Osibete, A.E. Analysis of the essential oil from the dried leaves of Euphorbia hirta Linn (Euphorbiaceae), a potential medication for asthma. Afr. J. Biotechnol. 2009, 8, 7042–7050. [Google Scholar]
- Santos, C.C.D.M.P.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; de Almeida, A.A.C.; de Oliveira, G.A.L.; Costa, J.P.; de Sousa, D.P.; de Freitas, R.M.; de Almeida, R.N. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef]
- Satyal, P.; Dosoky, N.S.; Poudol, A.; Setzer, W.N. Essential oil constituents and their biologic activities from the leaves of Cassia fistula growing in Nepal. Open Access J. Med. Aromat. Plants 2012, 3, 1–4. [Google Scholar]
- Pejin, B.; Savic, A.; Sokovic, M.; Glamoclija, J.; Ciric, A.; Nikolic, M.; Radotic, K.; Mojovic, M. Further in vitro evaluation of antiradical and antimicrobial activities of phytol. Nat. Prod. Res. 2014, 28, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Sinniah, U.R. A comprehensive review on the phytochemical constituents and pharmacological activities of Pogostemon cablin Benth: An aromatic medicinal plant of industrial importance. Molecules 2015, 20, 8521–8547. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar] [CrossRef] [PubMed]
- Njoya, E.M.; Eloff, J.N.; McGaw, L.J. Croton gratissimus leaf extracts inhibit cancer cell growth by inducing caspase 3/7 activation with additional anti-inflammatory and antioxidant activities. BMC Complementary Altern. Med. 2018, 18, 305. [Google Scholar] [CrossRef]
- Smith, S.M.; Wunder, M.B.; Norris, D.A.; Shellman, Y.G. A simple protocol for using a LDH-based cytotoxicity assay to assess the effects of death and growth inhibition at the same time. PLoS ONE 2011, 6, e26908. [Google Scholar] [CrossRef]
- Pugazhendhia, A.; Prabhub, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B Biol. 2019, 190, 86–97. [Google Scholar] [CrossRef]
- Satish, P.V.V.; Sunita, K. Antimalarial efficacy of Pongamia pinnata (L.) Pierre against Plasmodium falciparum (3D7 strain) and Plasmodium berghei (ANKA). BMC Complementary Altern. Med. 2017, 17, 458. [Google Scholar] [CrossRef]
- Widiyastuti, Y.; Sholikhah, I.Y.M.; Haryanti, S. Cytotoxic activities of ethanolic and dichloromethane extract of leaves, stems, and flowers of Jarong [Stachytarpheta jamaicensis (L.) Vahl.] On HeLa and T47D cancer cell Line. AIP Conf. Proc. 2019, 2202, 020101. [Google Scholar] [CrossRef]
- Al-Sheddi, E.S. Cytotoxic potential of petroleum ether, ethyl acetate, chloroform, and ethanol extracts of Lavandula coronopifolia against human breast carcinoma cell line (MDA-MB-321). Asian Pac. J. Cancer Prev. 2019, 20, 2943–2949. [Google Scholar] [CrossRef]
- Durgawale, P.P.; Patil, M.N.; Joshi, S.A.; Korabu, K.S.; Datkhile, K.D. Studies on phytoconstituents, in vitro antioxidant, antibacterial, antiparasitic, antimicrobial, and anticancer potential of medicinal plant Lasiosiphon eriocephalus decne (Family: Thymelaeaceae). J. Nat. Sci. Biol. Med. 2019, 10, 38–47. [Google Scholar]
- Kalpana, B.; Prakash, M. In vitro cytotoxic activity of leaf extracts of selected medicinal plants. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 3367–3373. [Google Scholar]
- Okoro, I.S.; Tor-Anyiin, T.A. Evaluation of cytotoxicity and phytochemical screening of Pycnanthus angolensis (Welw.) Warb dichloromethane and ethyl acetate stem bark extracts against HeLa cells. Int. J. Cancer Res. 2017, 13, 71–75. [Google Scholar] [CrossRef][Green Version]
- Waiganjo, B.; Moriasi, G.; Onyancha, J.; Elias, N.; Muregi, F. Antiplasmodial and cytotoxic activities of extracts of selected medicinal plants used to treat malaria in Embu County, Kenya. J. Parasitol. Res. 2020, 2020, 8871375. [Google Scholar] [CrossRef]
- Harikrishna, D.; Appa, A.V.; Prabhakar, M.C. Antiplasmodial activity of seven plants used in African folk medicine. Indian J. Pharmacol. 2004, 36, 244–250. [Google Scholar]
- Shimaa, M.A.; Mona, H.H.; Samir, A.R.; Farid, A.B. Antiprotozoal and antimicrobial activity of selected medicinal plants growing in upper Egypt, beni-suef region. World J. Pharm. Pharm. Sci. 2015, 4, 1720–1740. [Google Scholar]
- El-Tahir, A.; Satti, G.M.; Khalid, S.A. Antiplasmodial activity of selected Sudanese medicinal plants with emphasis on Acacia nilotica. Phytother. Res. 1999, 13, 474–478. [Google Scholar] [CrossRef]
- Muganga, R.; Angenot, L.; Tits, M.; Frédérich, M. Antiplasmodial and cytotoxic activities of Rwandan medicinal plants used in the treatment of malaria. J. Ethnopharmacol. 2010, 128, 52–57. [Google Scholar] [CrossRef]
- Beourou, S.; Le Lamer, A.; Maurel-Chevalley, S.; Mutiso, P.; Souard, F.; Moulis, C.; Fabre, N.; Valentin, A. Evaluation of the antiplasmodial activity of extracts of plants used in traditional medicine in Kenya. Int. J. Med. Plants Res. 2013, 2, 219–224. [Google Scholar]
- Shuaibu, M.N.; Wuyep, P.A.; Yanagi, T.; Hirayama, K.; Tanaka, T.; Kouno, I. The use of microfluorometric method for activity-guided isolation of antiplasmodial compound from plant extracts. Parasitol. Res. 2008, 102, 1119–1127. [Google Scholar] [CrossRef]
- Annan, K.; Sarpong, K.; Asare, C.; Dickson, R.; Amponsah, K.I.; Gyan, B.; Ofori, M.; Gbedema, S.Y. In vitro antiplasmodial activity of three herbal remedies for malaria in Ghana: Adenia cissampeloides (Planch.) Harms. Termina liaivorensis A. Chev, and Elaeis guineensis Jacq. Pharmacogn. Res. 2012, 4, 225–229. [Google Scholar]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Zofou, D.; Tene, M.; Ngemenya, M.N.; Tane, P.; Titanji, V.P. In vitro antiplasmodial activity and cytotoxicity of extracts of selected medicinal plants used by traditional healers of Western Cameroon. Malar. Res. Treat. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Bah, S.; Jäger, A.K.; Adsersen, A.; Diallo, D.; Paulsen, B.S. Antiplasmodial and GABA(A)-benzodiazepine receptor binding activities of five plants used in traditional medicine in Mali, West Africa. J. Ethnopharmacol. 2007, 110, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Maikai, V.A.; Nok, J.A.; Adaudi, A.O.; Alawa, C.B.I. In vitro antitrypanosomal activity of aqueous and methanolic crude extracts of stem bark of Ximenia Americana on Trypanosoma congolense. J. Med. Plants Res. 2008, 2, 55–58. [Google Scholar]
- Bala, A.Y.; Adamu, T.; Abubakar, U.; Ladan, M.J. Inhibition of Trypanosoma brucei brucei by Extracts from Waltheria indica L. (Sleepy Morning). Res. J. Parasitol. 2011, 6, 53–59. [Google Scholar] [CrossRef][Green Version]
- Igoli, N.P.; Gray, A.I.; Clements, C.J.; Igoli, J.O.; Nzekwe, U.; Singla, R.K. Scientific investigation of antitrypanosomal activity of Crateva adansonii DC leaves extracts. Indo Glob. J. Pharm. Sci. 2012, 2, 226–229. [Google Scholar]
- Abu, A.H.; Uchendu, C.N.; Ofukwu, R.A. In vitro anti trypanosomal activity of crude extracts of some Nigerian medicinal plants. J. Appl. Biosci. 2009, 21, 1277–1282. [Google Scholar]
- Adeyemi, O.S.; Akanji, M.A.; Oguntoye, S.A. Ethanolic leaf extract of Psidium guajava: Phytochemical and trypanocidal activity in rats infected with Trypanosoma brucei brucei. J. Med. Plant Res. 2009, 3, 420–423. [Google Scholar]
- Agbedahunsi, J.M.; Amao, I.; Adewunmi, C.O.; Croft, S.L. Trypanocidal properties of Terminalia ivorensis A. Chev. (Combretaceae). Afr. J. Tradit. Complementary Altern. Med. 2006, 3, 51–56. [Google Scholar] [CrossRef]
- Ene, A.C.; Atawodi, S.E.; Ameh, D.A.; Nnamani, C.N.; Apeh, Y.E. Antitrypanosomal effects of petroleum ether, chloroform and methanol extracts of Artemisia maciverae Linn. Indian J. Exp. Biol. 2009, 47, 981–986. [Google Scholar] [PubMed]
- Kabiru, Y.A.; Ogbadoyi, E.O.; Okogun, J.I.; Gbodi, T.A.; Makun, H.A. Anti-trypanosomal Potential of Eucalyptus camaldulensis. Br. J. Pharmacol. Toxicol. 2013, 4, 25–32. [Google Scholar] [CrossRef]
- Oluyomi, S.A.; Melissa, L.S.; Musbau, A.A.; Vicky, M.A. Antitrypanosomal and cytotoxic activity of ethanolic extracts of Psidium guajava leaves in Alamar Blue based assays. Vet. Arh. 2011, 81, 623–633. [Google Scholar]
- Shaba, P.; Pandey, N.N.; Sharma, O.P.; Rao, J.R.; Singh, R.K. In vitro trypanocidal activity of methanolic extracts of Quercus borealis leaves and Zingiber officinale roots against Trypanosoma evansi. Greener J. Agric. Sci. 2011, 1, 41–47. [Google Scholar]
| s/n | RT | Area | Qual (%) | Chloroform Extract | Ethyl Acetate Extract | Ethanol Extract |
|---|---|---|---|---|---|---|
| 1 | 8.909 | 1.54 | 96 | (R)-α-Pinene | - | - |
| 2 | 9.455 | 1.53 | 97 | Camphene | - | - |
| 3 | 12.844 | 1.77 | 91 | γ-Terpinene | - | - |
| 4 | 12.875 | 1.37 | 91 | - | Sabinene (β-Thujene) | - |
| 5 | 13.025 | 3.19 | 94 | Eucalyptol | - | - |
| 6 | 13.003 | 2.06 | 94 | - | Eucalyptol | - |
| 7 | 13.284 | 1.43 | 98 | - | - | Eucalyptol |
| 8 | 18.033 | 1.65 | 98 | (+)-2-Bornanone | - | - |
| 9 | 18.037 | 1.51 | 98 | - | (+)-2-Bornanone | - |
| 10 | 18.137 | 1.30 | 98 | - | - | (+)-2-Bornanone |
| 11 | 24.562 | 4.10 | 99 | - | - | Bornyl acetate |
| 12 | 24.583 | 4.42 | 94 | Bornyl acetate | - | - |
| 13 | 24.612 | 4.50 | 94 | - | Bornyl acetate | - |
| 14 | 30.178 | 6.54 | 99 | - | - | Caryophyllene |
| 15 | 30.265 | 7.69 | 99 | Caryophyllene | - | - |
| 16 | 30.311 | 8.58 | 99 | - | Caryophyllene | - |
| 17 | 30.910 | 1.64 | 99 | - | - | Aromandendrene |
| 18 | 30.964 | 2.19 | 99 | Aromandendrene | - | - |
| 19 | 30.997 | 2.42 | - | Aromandendrene | - | |
| 20 | 31.784 | 1.09 | 99 | Alloaromadendrene | - | - |
| 21 | 31.804 | 1.20 | 99 | - | Alloaromadendrene | - |
| 22 | 33.225 | 1.17 | 99 | (+)-Ledene | - | - |
| 23 | 33.258 | 1.35 | 98 | - | Virdiflorene | - |
| 24 | 34.380 | 1.05 | 99 | (+)-δ-Cadinene | - | - |
| 25 | 34.401 | 1.13 | 99 | - | δ-Cadinene, (+)- | - |
| 26 | 36.429 | 1.12 | 99 | - | - | Spatulenol |
| 27 | 36.464 | 1.25 | 99 | Spatulenol | - | - |
| 28 | 36.502 | 1.37 | 99 | - | Spatulenol | - |
| 29 | 39.154 | 1.72 | 99 | - | - | β-Selinenol |
| 30 | 39.215 | 2.01 | 99 | β-Eudesmol | - | - |
| 31 | 39.291 | 2.38 | 96 | - | β-Eudesmol | - |
| 32 | 39.298 | 2.67 | 95 | - | - | γ-Eudesmol |
| 33 | 39.371 | 2.82 | 95 | α-Eudesmol | - | - |
| 34 | 39.427 | 2.92 | 93 | - | 2-(4a,8-Dimethyl-2,3,4,5,6,8a-hexa hydro-1H-naphthalen-2-yl)propan-2- ol | - |
| 35 | 44.148 | 3.02 | 83 | - | - | Acorenone 1 |
| 36 | 44.214 | 3.62 | 83 | Acorenone 1 | - | - |
| 37 | 44.266 | 4.06 | 62 | - | Acorenone 1 | - |
| 38 | 50.332 | 1.05 | 99 | n-Hexadecanoic acid | - | - |
| 39 | 50.495 | 1.37 | 99 | - | n-Hexadecanoic acid | - |
| 40 | 55.541 | 1.03 | 99 | - | - | n-Hexadecanoic acid |
| 41 | 55.739 | 2.19 | 99 | α-Linolenic acid | - | - |
| 42 | 55.843 | 1.90 | 99 | - | α-Linolenic acid | - |
| 43 | 58.879 | 1.72 | 60 | - | - | Estra-1,3,5(10)-trien-17-one, 3-hydroxy-2-methoxy- |
| 44 | 59.930 | 1.38 | 83 | - | - | 2(1H)-Phenanthrenone, 6-(acetyloxy)-3,4,4a,9,10,10a-hexahydro-1,1,4a-trimethyl-7-(1-methylethyl)-, (4aS-trans)- |
| 45 | 60.401 | 1.55 | 99 | - | - | Ferruginol |
| 46 | 60.434 | 1.37 | 99 | Ferruginol | - | - |
| 47 | 60.486 | 1.59 | 99 | - | Ferruginol | - |
| 48 | 61.746 | 4.29 | 95 | - | - | D-Homopregn-17a(20)-ene, (5.alpha., 17aE)- |
| 49 | 61.864 | 6.32 | 94 | D-Homopregn-17a(20)-ene, (5.alpha., 17aE)- | - | - |
| 50 | 61.906 | 6.17 | 56 | - | Benzene, ethenylpentaethyl- | - |
| 51 | 62.537 | 5.40 | 60 | - | 6-Iodo-2-methylquinazolin-4(3H)-on | - |
| 52 | 62.487 | 5.65 | 90 | 2,5-Methano-2H-thieno[3,2-b]thiopy ran-8-ol, hexahydro-, acetate, (2.alpha.,3a.beta.,5.alpha.,7a.beta., 8R*)- | - | - |
| 53 | 67.280 | 1.34 | 70 | - | - | 4-[3-Pyridyl]-3-thiosemicarbazonepiperonal |
| 54 | 67.332 | 1.07 | 99 | (.+/−.)-Demethylsalvicanol | - | - |
| 55 | 67.397 | 1.05 | 95 | - | (.+/−.)-Demethylsalvicanol | - |
| Solvent | Antiplasmodial Activity (µg∙mL−1) | Antitrypanosomal Activity (µg∙mL−1) |
|---|---|---|
| Chloroform extract | 11.6 | 0.5 |
| Ethyl acetate extract | 3.3 | 0.5 |
| Ethanol extract | 3.7 | 0.4 |
| Chloroquine (control) | 0.05 µM | - |
| Pentamidine (control) | - | 0.02 µM |
| Family | Plants | Plant Parts | Solvent | Parasite | IC50 (mg/mL) | Country | Reference |
|---|---|---|---|---|---|---|---|
| Asteraceae | O. genistifolia | Leave | Chloroform Ethyl acetate Ethanol | P. falciparum strain 3D7 | 11.6 (chloroform extract); 3.3 (Ethyl acetate extract) and 3.7 (ethanol extract) | South Africa | Present study |
| Euphorbiaceae | Mellotus appositofolius | Leaves | Chloroform: methanol (1:1) | pf F32 | 40 at 10 | Cameroon | Harikrishna et al. [45] |
| Asteraceae | Artemisia Absinthium | Leaves | Ethanol | pf D6 | 52 | Egypt | Shimaa et al. [46] |
| Balantitiaceae | Balanites aegyptiaca | Stem | Methanol | pfDd2 | 5.2 | Sudan | El-Tahir et al. [47] |
| Fuerstia africana | Fuerstia africana | Leaves | Dichloromethane/MeOH | pf 3D7 | 6.9/40.2 | Rwanda | Muganga et al. [48] |
| Alangiaceae | Alangium chinense | Aerial parts | Hexane/Dichloromethane/Methanol | FcB1 | >50/6.15/2.8 | Kenya | Sylvain et al. [49] |
| Fabaceae | Afzelia africana | Leaves | Methanol | 3D7/K1 | 31.55/39.72 | Nigeria | Shuaibu et al. [50] |
| Passifloraceae | Adenia cissampeloides | Whole plant | Ethanol | pf3D7 | 8.52 | Ghana | Annan et al. [51] |
| Rutaceae | Agathosma puberula | Roots | Dichloromethane | P. f D10 | 33 | South Africa | Clakson et al. [52] |
| Meliaceae | Vismia guinensis | Stem bark | Methanol | pf DD2 | 43.42 | Cameroon | Zofou et al. [53] |
| Fabaceae | Stylosanthes erecta Swartzia | Aerial parts | Dichloromethane/Methanol | Pfk1 | 21.9/23.3 | Mali | Bah et al. [54] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okaiyeto, K.; Okoh, A.I. In Vitro Assessment of Antiplasmodial and Antitrypanosomal Activities of Chloroform, Ethyl Acetate and Ethanol Leaf Extracts of Oedera genistifolia. Appl. Sci. 2020, 10, 6987. https://doi.org/10.3390/app10196987
Okaiyeto K, Okoh AI. In Vitro Assessment of Antiplasmodial and Antitrypanosomal Activities of Chloroform, Ethyl Acetate and Ethanol Leaf Extracts of Oedera genistifolia. Applied Sciences. 2020; 10(19):6987. https://doi.org/10.3390/app10196987
Chicago/Turabian StyleOkaiyeto, Kunle, and Anthony I. Okoh. 2020. "In Vitro Assessment of Antiplasmodial and Antitrypanosomal Activities of Chloroform, Ethyl Acetate and Ethanol Leaf Extracts of Oedera genistifolia" Applied Sciences 10, no. 19: 6987. https://doi.org/10.3390/app10196987
APA StyleOkaiyeto, K., & Okoh, A. I. (2020). In Vitro Assessment of Antiplasmodial and Antitrypanosomal Activities of Chloroform, Ethyl Acetate and Ethanol Leaf Extracts of Oedera genistifolia. Applied Sciences, 10(19), 6987. https://doi.org/10.3390/app10196987





