Endoscopic Optical Imaging Technologies and Devices for Medical Purposes: State of the Art
Abstract
1. Introduction
2. Medical Imaging Applications
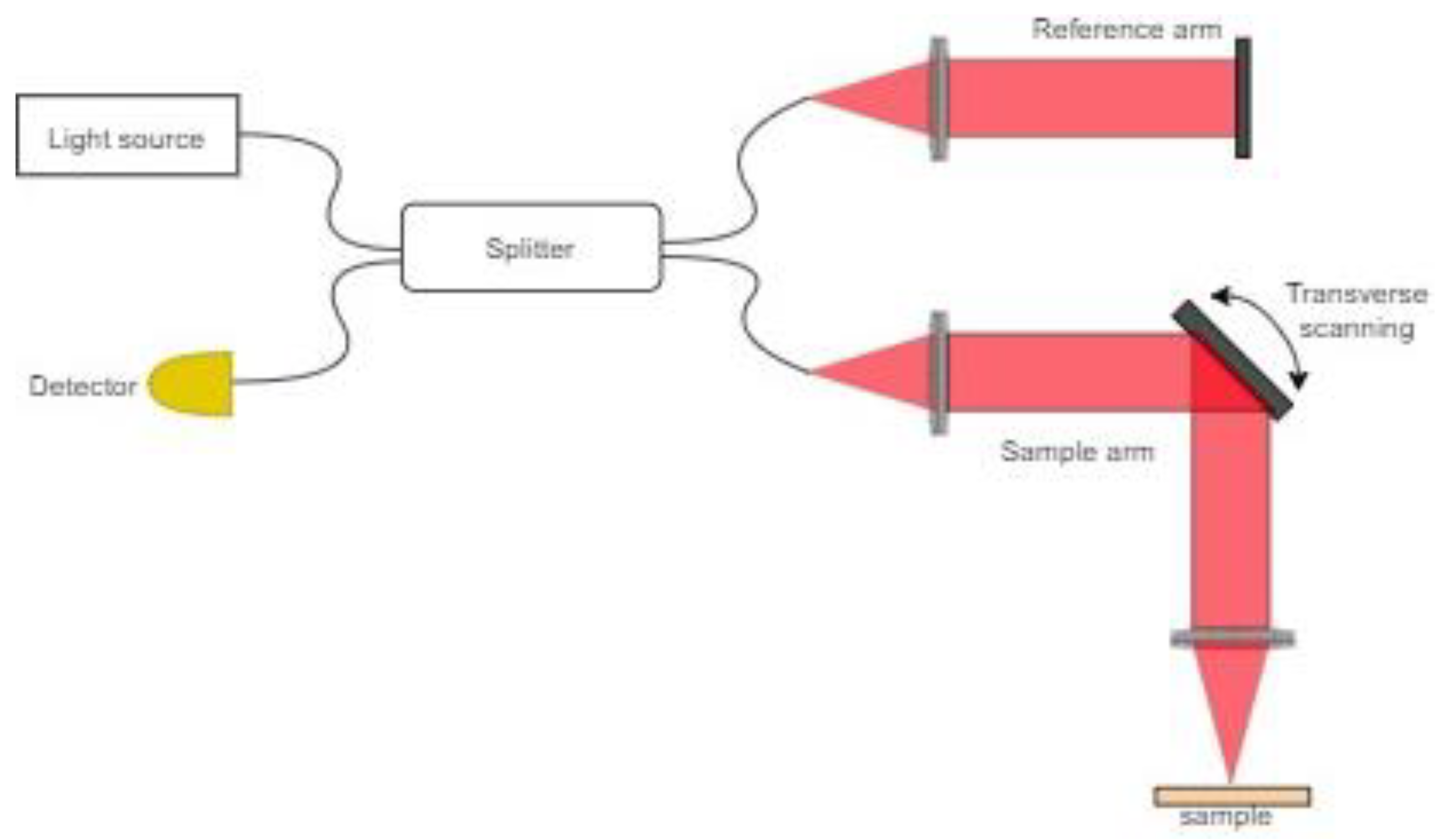
2.1. Optical Coherence Tomography
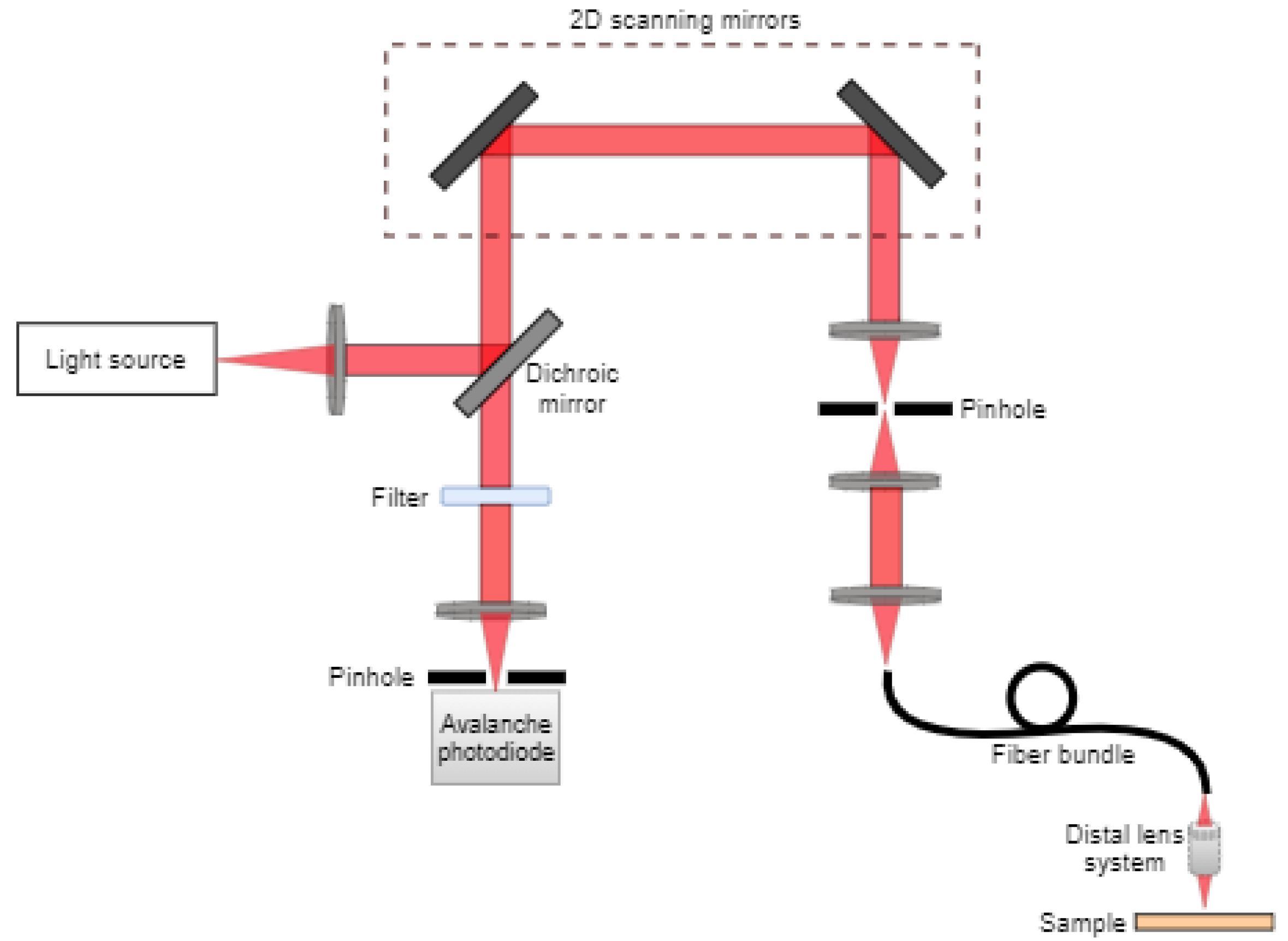
2.2. Confocal Microscopy
2.3. Nonlinear Microscopy
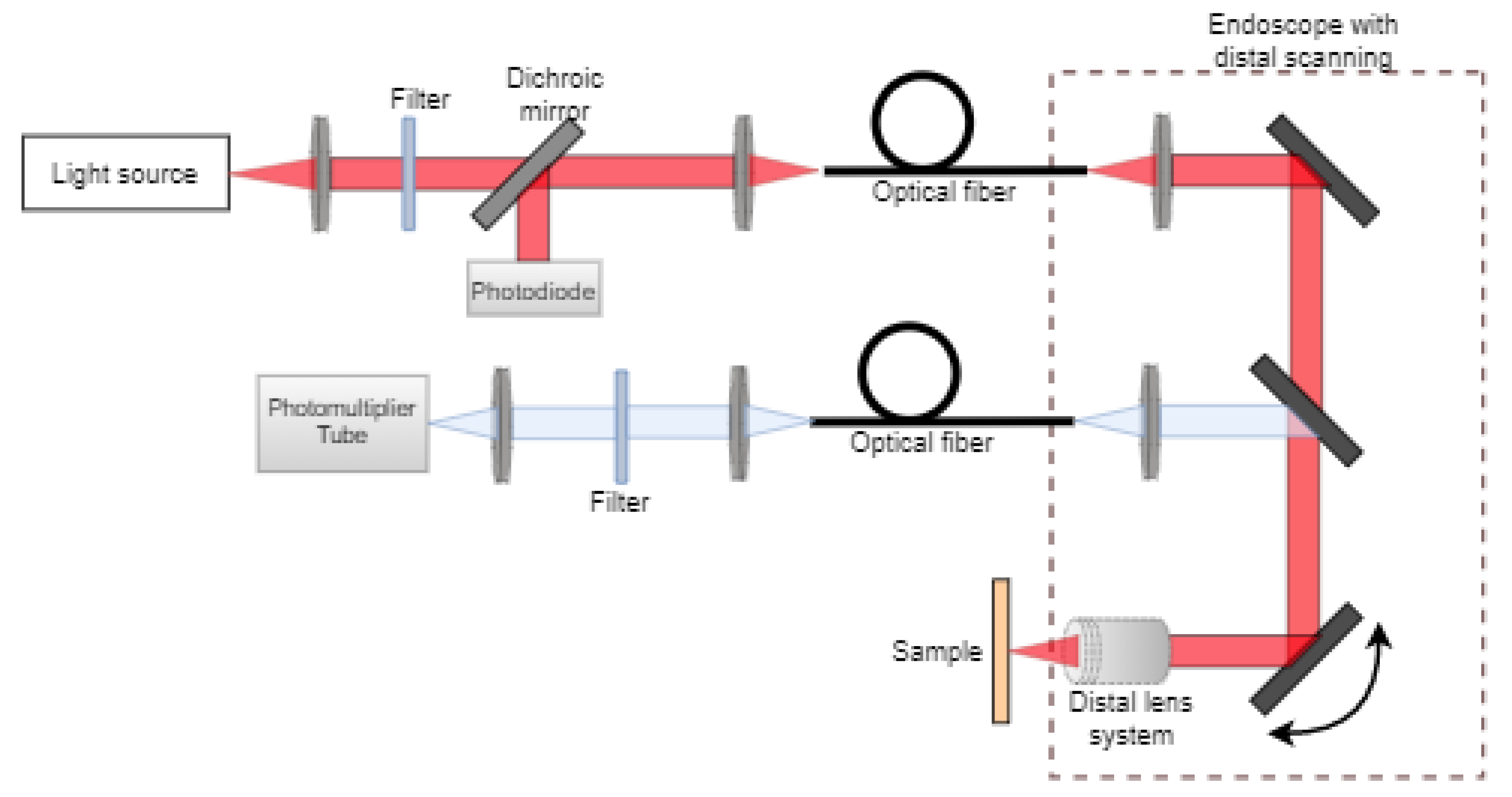
2.3.1. Multiphoton Microscopy
2.3.2. Higher Harmonic Generation Microscopy
2.3.3. Raman Scattering Microscopy
2.4. Photoacoustic Imaging
3. Scanning Directions
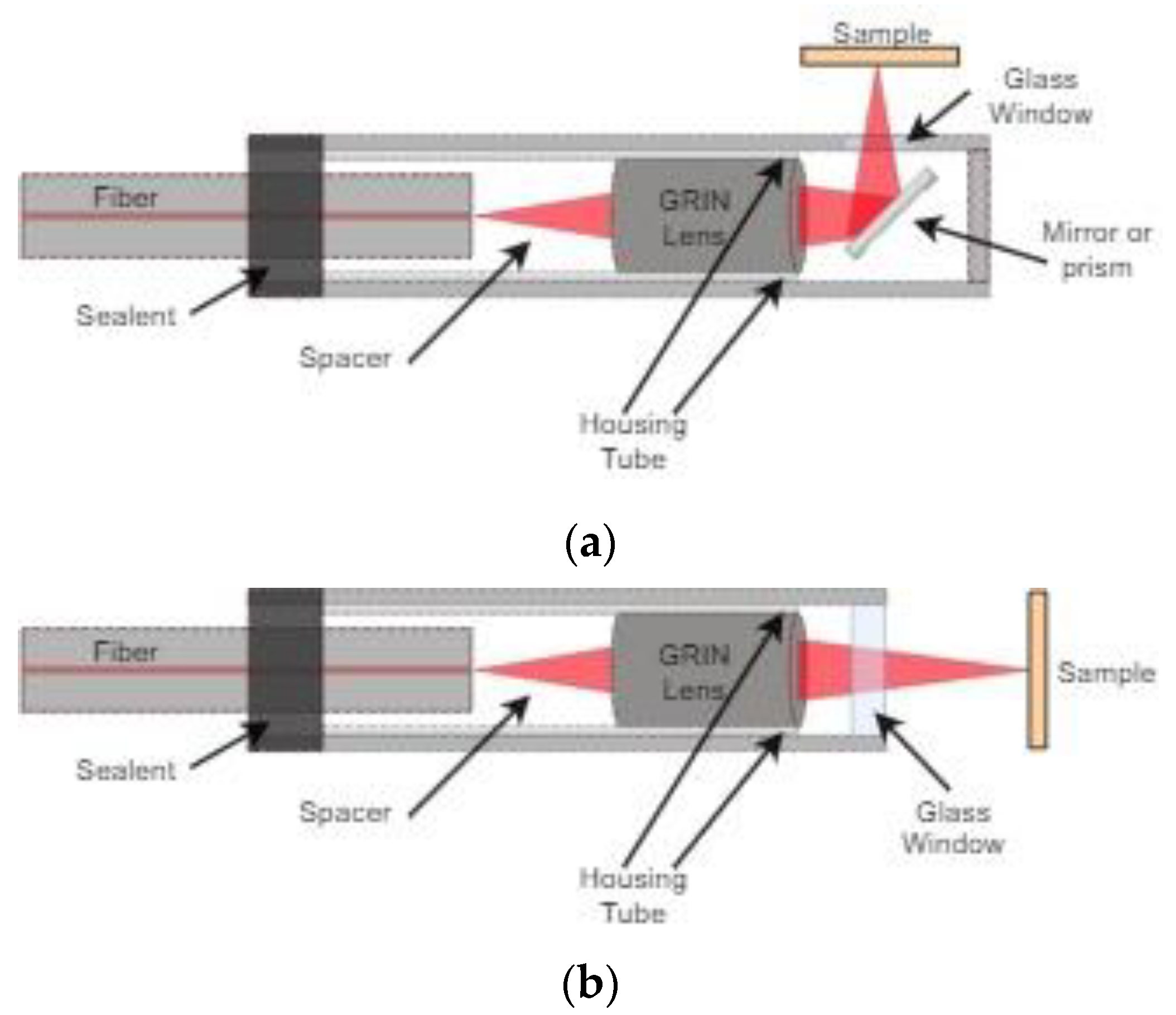
3.1. Side View Imaging
3.2. Forward View Imaging
4. Scanning Principle
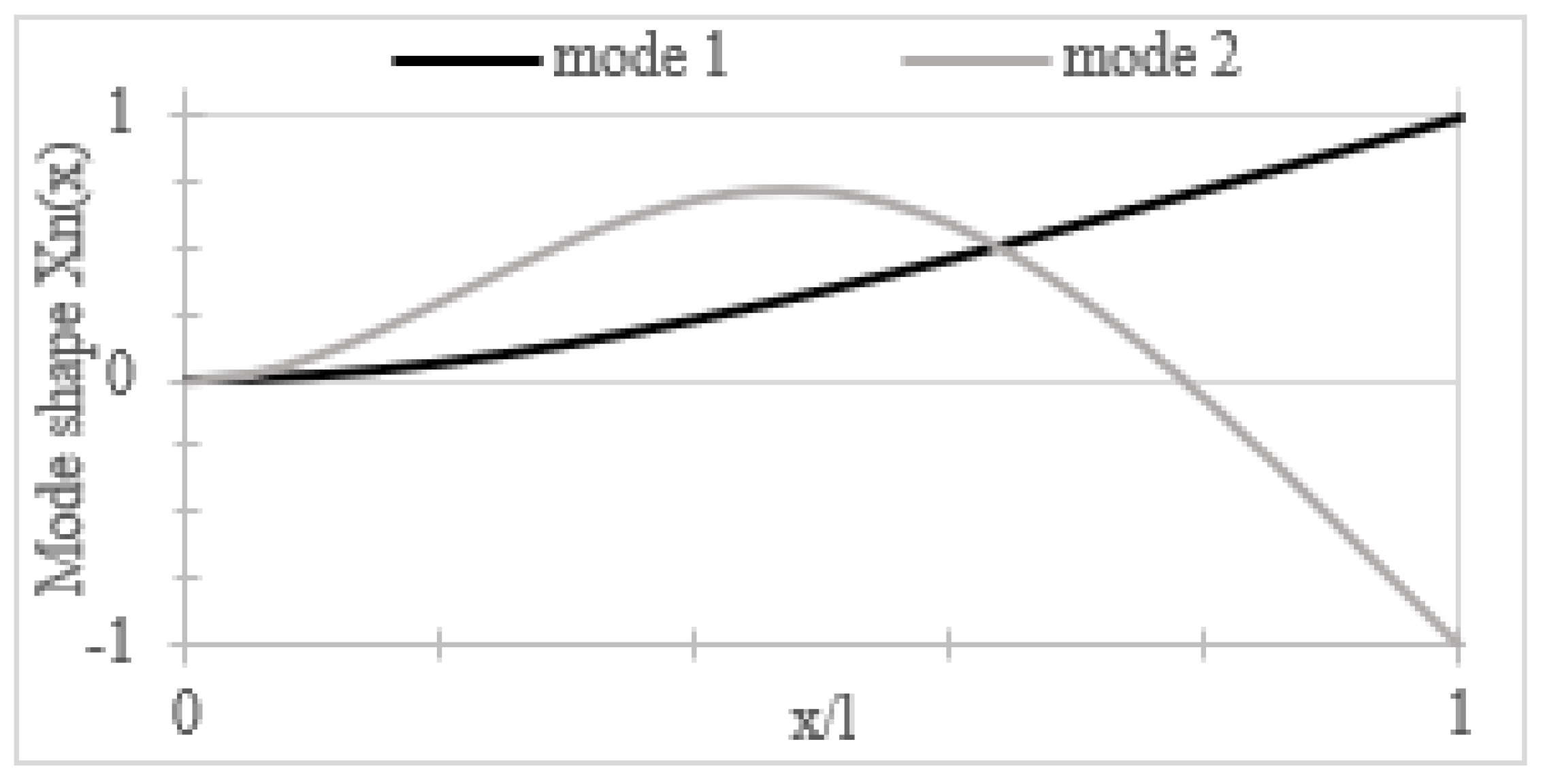
4.1. Resonant Scanner
4.2. Non-Resonant Scanner
4.3. Semi-Resonant Scanner
5. Actuation Methods
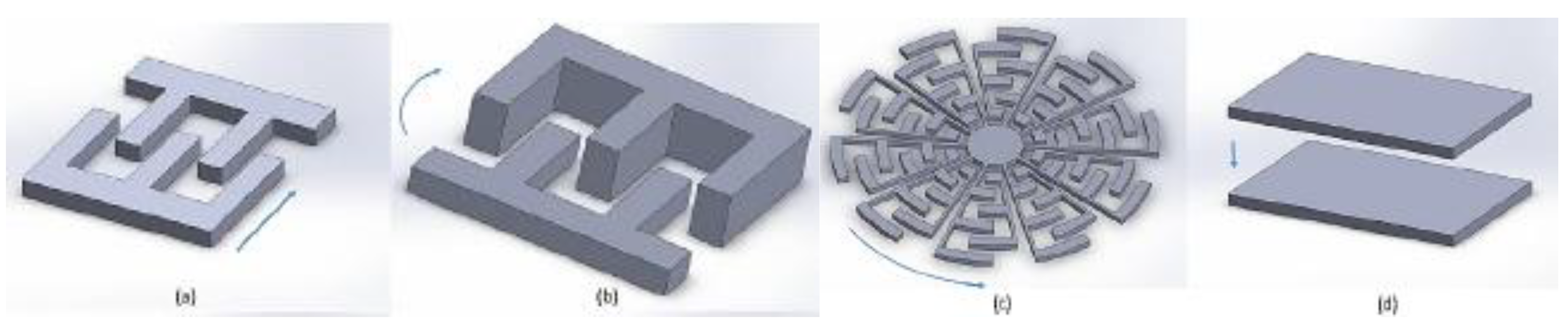
5.1. Electrostatic Actuators
5.2. Electrothermal Actuators
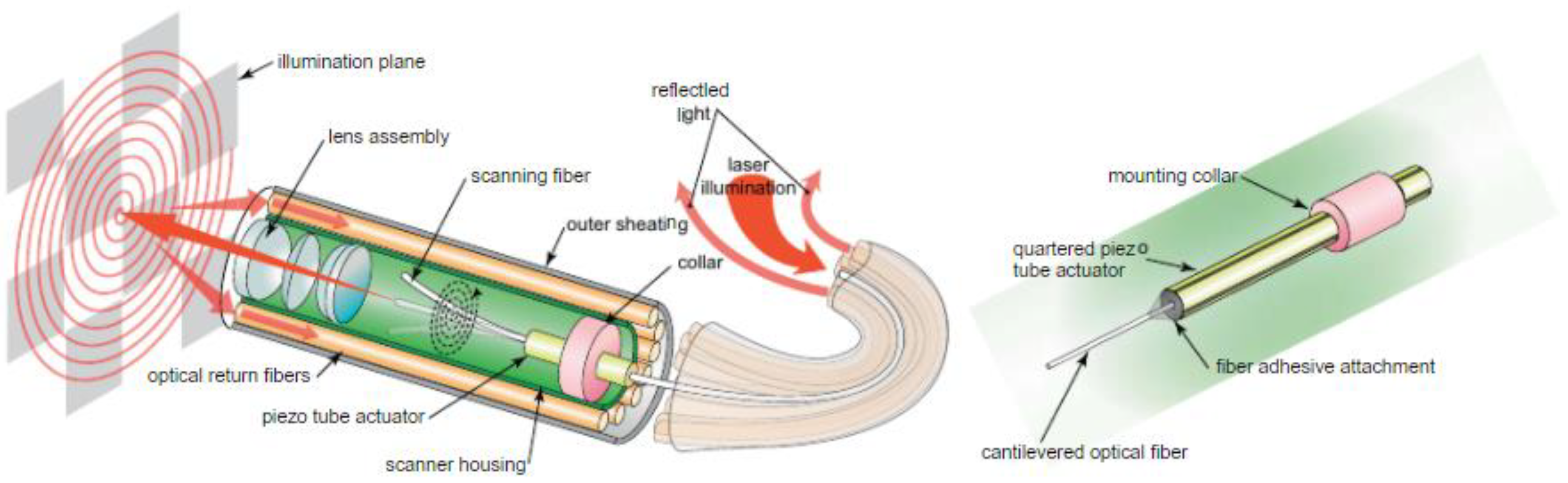
5.3. Piezoelectric Actuators
5.4. Electromagnetic Actuators
5.5. Shape Memory Alloy Actuators
6. Scanning Patterns
6.1. Raster Scanning
6.2. Spiral Scanning
6.3. Lissajous Scanning
6.4. Circular Scanning
6.5. Propeller Scanning
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kiesslich, R.; Goetz, M.; Hoffman, A.; Galle, P.R. New imahing techniques and opportunities in endoscopy. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 547–553. [Google Scholar] [CrossRef]
- Qiu, Z.; Piyawattanametha, W. MEMS Actuators for Optical Microendoscopy. Micromachines 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Engelbrecht, C.J.; Soper, T.D.; Helmchen, F.; Seibel, E.J. Scanning fiber endoscopy with highly flexible, 1-mm catheterscopes for wide-field, full-color imaging. J. Biophotonics 2010, 3, 385–407. [Google Scholar] [CrossRef] [PubMed]
- Udovich, J.A.; Kirkpatrick, N.D.; Kano, A.; Tanbakuchi, A.; Utzinger, U.; Gmitro, A.F. Spectral background and transmission characteristics of fiber optic imaging bundles. Appl. Opt. 2008, 47, 4560–4568. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, D. Endoscopes Use CMOS Image Sensors. Vision Systems Design. Available online: https://www.vision-systems.com/home/article/16750278/endoscopes-use-cmos-image-sensors (accessed on 28 July 2020).
- Kaur, M.; Brown, M.; Lane, P.M.; Menon, C. An Electro-Thermally Actuated Micro-Cantilever-Based Fiber Optic Scanner. IEEE Sens. J. 2020, 20, 9877–9885. [Google Scholar] [CrossRef]
- Wang, L.V.; Wu, H.-I. Biomed. Opt.: Principles and Imaging; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Fujimoto, J.G.; Pitris, C.; Boppart, S.A.; Brezinski, M.E. Optical Coherence Tomography: An Emerging Technology for Biomedical Imaging and Optical Biopsy. Neoplasia 2000, 2, 9–25. [Google Scholar] [CrossRef]
- Zysk, A.M. Boppart, Optical Coherence Tomography. In Optical Imaging and Microscopy: Techniques and Advanced Systems, 2nd ed.; Török, P., Kao, F.-J., Eds.; Springer: Heidelberg/Berlin, Germany, 2007; pp. 401–436. [Google Scholar]
- Boudoux, C. Fundamentals of Biomedical Optics: From Light Interactions with Cells to Complex Imaging Systems; Pollux: Montreal, QC, Canada, 2017. [Google Scholar]
- Popescu, D.P.; Choo-Smith, L.-P.; Flueraru, C.; Mao, Y.; Chang, S.; Disano, J.; Sherif, S.; Sowa, M.G. Optical coherence tomography: Fundamental principles, instrumental designs and biomedical applications. Biophys. Rev. 2011, 3, 155–169. [Google Scholar] [CrossRef]
- Kim, B.J.; Irwin, D.J.; Song, D.D.; Daniel, E.R.; Leveque, J.S.; Raquib, J.L.; Pan, W.L.; Ying, G.-S.L.; Aleman, T.L.; Dunaief, J.L.; et al. Optical coherence tomography identifies outer retina thinning in frontotemporal degeneration. Neurology 2017, 89, 1604–1611. [Google Scholar] [CrossRef]
- Lumbroso, B.; Huang, D.; Chen, C.J.; Jia, Y.; Rispoli, M.; Romano, A.; Waheed, N.K. Clinical OCT Angiography Atlas; Jaypee Brothers Medical Publishers: New Delhi, India, 2015. [Google Scholar]
- Cunha, J.P.; Proença, R.; Dias-Santos, A.; Almeida, R.; Águas, H.; Alves, M.; Papoila, A.L.; Louro, C.; Castanheira-Dinis, A. OCT in Alzheimer’s disease: Thinning of the RNFL and superior hemiretina. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1827–1835. [Google Scholar] [CrossRef]
- Tearney, G.J.; Boppart, S.A.; Bouna, B.E.; Brezinski, M.E.; Weissman, N.J.; Southern, J.F.; Fujimoto, J.G. Scanning single-mode fiber optic catheter-endoscope for optical coherence tomography. Opt. Lett. 1996, 21, 543–545. [Google Scholar] [CrossRef]
- Boppart, S.A.; Bouma, B.E.; Pitris, C.; Tearney, G.J.; Fujimoto, J.G.; Brezinski, M.E. Forward-imaging instruments for optical coherence tomography. Opt. Lett. 1997, 22, 1618–1620. [Google Scholar] [CrossRef]
- Paddock, S.W.; Fellers, T.J.; Davidson, M.W. Introductory Confocal Concepts. Available online: https://www.microscopyu.com/techniques/confocal/introductory-confocal-concepts (accessed on 23 June 2020).
- Liu, L.; Wang, E.; Zhang, X.; Liang, W.; Li, X.; Xie, H. MEMS-based 3D confocal scanning microendoscope using MEMS scanners for both lateral and axial scan. Sens. Actuators A Phys. 2014, 215, 89–95. [Google Scholar] [CrossRef]
- Dickensheets, D.L.; Kino, G.S. Micromachined scanning confocal optical microscope. Opt. Lett. 1996, 21, 764–766. [Google Scholar] [CrossRef]
- Maitland, K.C.; Shin, H.J.; Ra, H.; Lee, D.; Solgaard, O.; Richards-Kortum, R. Single fiber confocal microscope with a two-axis gimbaled MEMS scanner for cellular imaging. Opt. Express 2006, 14, 8604–8612. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.W.; Lopez, D.; Zhen, Q.; Piyawattanametha, W. 2-D MEMS Scanner for Handheld Multispectral Dual-Axis Confocal Microscopes. J. Microelectromechanical Syst. 2018, 27, 605–612. [Google Scholar] [CrossRef]
- Cellvizio Targeted Biopsies. Mauna Kea Technolgies. Available online: https://www.maunakeatech.com/en/cellvizio/10-cellvizio-targeted-biopsies (accessed on 25 August 2020).
- Laemmel, E.; Genet, M.; Le Goualher, G.; Perchant, A.; Le Gargasson, J.F.; Vicaut, E. Fibered Confocal Fluorescence Microscopy (Cell-ViZioTM) Facilitates Extended Imaging in the Field of Microcirculation. J. Vasc. Res. 2004, 41, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Duan, X.; Lee, M.; Birla, M.; Chen, J.; Oldham, K.R.; Wang, T.D.; Li, H. Ultra-Compact Microsystems-Based Confocal Endomicroscope. IEEE Trans. Med. Imaging 2020, 39, 2406–2414. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Zhou, Y.; Zong, H.; Chen, M.; Sun, M. Advances in nonlinear optical microscopy for biophotonics. J. Nanophotonics 2018, 12, 033007. [Google Scholar] [CrossRef]
- Piston, D.W.; Fellers, T.J.; Davidson, M.W. Multiphoton Microscopy. Available online: https://www.microscopyu.com/techniques/multi-photon/multiphoton-microscopy (accessed on 23 June 2020).
- Huang, J.; Li, N.; Wang, Q.; Gu, Y.; Wang, P. A lysosome-targetable and two-photon fluorescent probe for imaging endogenous β-galactosidase in living ovarian cancer cells. Sens. Actuators B Chem. 2017, 246, 833–839. [Google Scholar] [CrossRef]
- Liu, Z.-R.; Tang, Y.; Xu, A.; Lin, W. A new fluorescent probe with a large turn-on signal for imaging nitroreductase in tumor cells and tissues by two-photon microscopy. Biosens. Bioelectron. 2017, 89, 853–858. [Google Scholar] [CrossRef]
- Kawakami, R.; Sawada, K.; Kusama, Y.; Fang, Y.-C.; Kanazawa, S.; Kozawa, Y.; Sato, S.; Yokoyama, H.; Nemoto, T. In vivo two-photon imaging of mouse hippocampal neurons in dentate gyrus using a light source based on a high-peak power gain-switched laser diode. Biomed. Opt. Express 2015, 6, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, T.S. Endomicroscopy. In Biomedical Optical Imaging Technologies: Design and Applications; Liang, R., Ed.; Springer: Heidelberg/Berlin, Germany, 2013; pp. 255–296. [Google Scholar]
- Campagnola, P.J.; Wei, M.-D.; Lewis, A.; Loew, L.M. High-Resolution Nonlinear Optical Imaging of Live Cells by second Harmonic Generation. Biophys. J. 1999, 77, 3341–3349. [Google Scholar] [CrossRef]
- Liu, H.; Qin, W.; Saho, Y.; Ma, Z.; Ye, T.; Borg, T.; Gao, B.Z. Myofibrillogenesis in live neonatal cardiomyocytes observed with hybrid two-photon excitation fluorescence-second harmonic generation microscopy. J. Biomed. Opt. 2011, 16, 126012. [Google Scholar] [CrossRef] [PubMed]
- Romeike, B.F.; Meyer, T.; Reichart, R.; Kalff, R.; Petersen, I.; Dietzek, B.; Popp, J. Coherent anti-Stokes Raman scattering and two photon excited fluorescence for neurosurgery. Clin. Neurol. Neurosurg. 2015, 131, 42–46. [Google Scholar] [CrossRef]
- Le, T.T.; Huff, T.B.; Cheng, J.-X. Coherent anti-Stokes Raman scattering imaging of lipids in cancer metastasis. BMC Cancer 2009, 9, 42. [Google Scholar] [CrossRef]
- Wei, L.; Yu, Y.; Shen, Y.; Wang, M.C.; Min, W. Vibrational imaging of newly synthesized proteins in live cells by stimulated Raman scattering microscopy. Proc. Natl. Acad. Sci. USA 2013, 110, 11226–11231. [Google Scholar] [CrossRef]
- Haasterecht, L.V.; Zada, L.; Schmidt, R.W.; de Bakker, E.; Barbe’, E.; Leslie, H.A.; Vethaak, A.D.; Gibbs, S.; de Boer, J.F.; Niessen, F.B.; et al. Label-Free stimulated Raman scattering imaging reveals silicone breast implant material in tissue. J. Biophotonics 2020, 13, e201960197. [Google Scholar] [CrossRef]
- Hu, S.; Maslov, K.; Wang, L.V. Three-Dimensional Optical-Resolution Photoacoustic Microscopy. In Biomedical Optical Imaging Technologies: Design and Applications; Liang, R., Ed.; Springer: Heidelberg/Berlin, Germany, 2013; pp. 55–77. [Google Scholar]
- Yao, J.; Wang, L.V. Photoacoustic Microscopy. Laser Photonics Rev. 2013, 7, 758–778. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.V. Photoacoustic imaging and characterization of the microvasculature. J. Biomed. Opt. 2010, 15, 011101. [Google Scholar] [CrossRef]
- Stein, E.W.; Maslov, K.; Wang, L.V. Noninvasive mapping of the electrically stimulated mouse brain using photoacoustic microscopy. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2008: The Ninth Conference on Biomedical Thermoacoustics, Optoacoustics, and Acousto-optics, San Jose, CA, USA, 28 February 2008; International Society for Optics and Photonics: Bellingham, WA, USA, 2008; Volume 6856, p. 68561J. [Google Scholar] [CrossRef]
- Xie, Z.; Roberts, W.; Carson, P.; Liu, X.; Tao, C.; Wang, X. Evaluation of bladder microvasculature with high resolution photoacuostic imaging. Opt. Lett. 2011, 36, 4815–4817. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.V. Neurovascular Photoacoustic Tomography. Front. Neuroeng. 2010, 2, 10. [Google Scholar] [CrossRef]
- Ermilov, S.A.; Khamapirad, T.; Conjusteau, A.; Leonard, M.H.; Lacewell, R.; Mehta, K.; Miller, T.; Oraevsky, A.A. Laser optoacoustic imaging system for detection of breast cancer. J. Biomed. Opt. 2009, 14, 024007. [Google Scholar] [CrossRef] [PubMed]
- Maslov, K.; Ku, G.; Wang, L.V. Photoacoustic microscopy with submicron resolution. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2010, San Francisco, CA, USA, 23 February 2010; International Society for Optics and Photonics: Bellingham, WA, USA, 2010; Volume 7564, p. 75640W. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef]
- Chan, J.; Zheng, Z.; Bell, K.; Le, M.; Reza, P.H.; Yeow, J.T.W. Photoacoustic Imaging with Capacitive Micromachined Ultrasound Transducers: Principles and Developments. Sensors 2019, 19, 3617. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zipfel, W.; Shear, J.B.; Williams, R.M.; Webb, W.W. Multiphoton fluorescence excitation: New spectral windows for biological nonlinear microscopy. Proc. Natl. Acad. Sci. USA 1996, 93, 10763–10768. [Google Scholar] [CrossRef]
- Hoffmann, D.M.; Kuhnke, K.; Kern, L. Sum-frequency generation microscope for opaque and reflecting samples. Rev. Sci. Instrum. 2002, 73, 3221–3226. [Google Scholar] [CrossRef]
- Zumbusch, A.; Holtom, G.R.; Xie, S.X. Three-Dimensional Vibrational Imaging by Coherent Anti-Stokes Raman Scattering. Phys. Rev. Lett. 1999, 82, 4142–4145. [Google Scholar] [CrossRef]
- Campagnola, P. Second Harmonic Generation Imaging Microscopy: Applications to Diseases Diagnostics. Anal. Chem. 2011, 83, 3224–3231. [Google Scholar] [CrossRef]
- Ganikhanov, F.; Carrasco, S.; Xie, S.X.; Katz, M.; Seitz, W.; Kopf, D. Broadly tunable dual-wavelength light source for coherent anti-Stokes Raman scattering microscopy. Opt. Lett. 2006, 31, 1292–1294. [Google Scholar] [CrossRef]
- Baik, J.W.; Kim, J.Y.; Cho, S.; Choi, S.; Kim, J.; Kim, C. Super Wide-Field Photoacoustic Microscopy of Animals and Humans In Vivo. IEEE Trans. Med. Imaging 2020, 39, 975–984. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, J.W.; Yun, S.H. 350-um side-view optical probe for imaging the murine brain in vivo from the cortex to the hypothalamus. J. Biomed. Opt. 2013, 18, 050502. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.; Mukai, D.S.; Brenner, M.; Chen, Z. In vivo endoscopic optical coherence tomography by use of a rotational microelectromechanical system probe. Opt. Lett. 2004, 29, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Chandler, J.E.; Seibel, E.J. Wide Field Fluorescence Imaging in Narrow Passageways Using Scanning Fiber Endoscope Technology. In Proceedings of the Endoscopic Microscopy V, Proc. of SPIE 7558, San Francisco, CA, USA, 18 February 2010. [Google Scholar] [CrossRef]
- Zhang, E.Z.; Beard, P.C. A miniature all-optical photoacoustic imaging probe. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2011, Proc. SPIE 7899, San Francisco, CA, USA, 10 February 2011. [Google Scholar] [CrossRef]
- Tearney, G.J.; Brezinski, M.E.; Bouma, B.E.; Boppart, S.A.; Pitris, C.; Southern, J.F.; Fujimoto, J.G. In Vivo Endoscopic Optical Biopsy with Optical Coherence Tomography. Science 1997, 276, 2037–2039. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chudoba, C.; Ko, T.; Pitris, C.; Fujimoto, J.G. Imaging needle for optical coherence tomography. Opt. Lett. 2000, 25, 1520–1522. [Google Scholar] [CrossRef] [PubMed]
- Lorenser, D.; Yang, X.; Kirk, R.W.; Quirk, B.C.; McLaughlin, R.A.; Sampson, D.D. Ultrathin side-viewing needle probe for optical coherence tomography. Opt. Lett. 2011, 36, 3894–3896. [Google Scholar] [CrossRef] [PubMed]
- Yang, V.X.; Mao, Y.X.; Munce, N.; Standish, B.; Kucharczyk, W.; Marcon, N.E.; Wilson, B.C.; Vitkin, I.A. Interstitial Doppler optical coherence tomography. Opt. Lett. 2005, 30, 1791–1793. [Google Scholar] [CrossRef]
- Herz, P.R.; Chen, Y.; Aguirre, A.D.; Schneider, K.; Hsiung, P.; Fujimoto, J.G.; Madden, K.; Schmitt, J.; Goodnow, J.; Petersen, C. Micromotor endoscope catheter for in vivo, ultrahigh-resolution optical coherence tomography. Opt. Lett. 2004, 29, 2261–2263. [Google Scholar] [CrossRef]
- Paulson, B.; Lee, S.; Kim, Y.; Moon, Y.; Kim, J.K. Miniaturized omnidirectional flexible side-view endoscope for rapid monitoring of thin tubular biostructures. Biomed. Opt. Express 2019, 10, 2264–2274. [Google Scholar] [CrossRef]
- Park, H.-C.; Seo, Y.-H.; Jeong, K.-H. Lissajous fiber scanning for forward viewing optical endomicroscopy using asymmetric modulation. Opt. Express 2014, 22, 5818–5825. [Google Scholar] [CrossRef]
- Seibel, E.J.; Johnston, R.S.; Melville, C.D. A full-color scanning fiber endoscope. Opt. Fibers Sens. Med. Diag. Treat. Appl. 2006, 6083. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, B.H.; Maguluri, G.N.; Lee, T.W.; Rogomentich, F.J.; Bancu, M.G.; Bouma, B.E.; de Boer, J.F.; Bernstein, J.J. Two-axis magnetically-driven MEMS scanning catheter for endoscopic high-speed optical coherence tomography. Opt. Express 2007, 15, 18130–18140. [Google Scholar] [CrossRef]
- Yao, J.; Peng, T.; Sun, B.; Zhang, H.; Zhao, M.; Dai, B.; Liu, H.; Ding, G.; Swada, R.; Yang, Z. A Single-Fiber Endoscope Scanner Probe Utilizing Two-Degrees-of-Freedom (2DOF) High-Order Resonance to Realize Larger Scanning Angle. IEEE Trans. Compon. Packag. Manuf. Technol. 2019, 9, 2332–2340. [Google Scholar] [CrossRef]
- Acemoglu, A.; Mattos, L.S. Magnetic Laser Scanner for Endoscopic Microsurgery. In Proceedings of the IEEE International Conference on Robotics and Automation, Singapore, 29 May–3 June 2017; pp. 4215–4220. [Google Scholar] [CrossRef]
- Seo, Y.-H.; Hwang, K.; Park, H.-C.; Jeong, K.-H. Electrothermal mems fiber scanner with lissajous patterns for endomicroscopic applications. Opt. Express 2016, 24, 3903–3909. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.-H.; Hwang, K.; Jeong, K.-H. 1.65 mm diameter forward-viewing confocal endomicroscopic catheter using a glip-chip bonded electrothermal MEMS fiber scanner. Opt. Express 2018, 26, 4780–4785. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Matsunaga, T.; Haga, Y. Active Bending Electric Endoscope Using Shape Memory Alloy wires. In New Trends in Medical and Service Robots: Assistive, Surgical and Educational Robotics; Bleuler, H., Bouri, M., Mondada, F., Pisla, D., Rodic, A., Helmer, P., Eds.; Springer: Cham, Switzerland, 2016; pp. 131–139. [Google Scholar]
- Wang, Y.; Bachman, M.; Li, G.P.; Guo, S.; Wong, B.J.; Chen, Z. Low-voltage polymer-based scanning cantilever for in vivo optical coherence tomography. Opt. Lett. 2005, 30, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Zara, J.M.; Yazdanfar, S.; Rao, K.D.; Izatt, J.A.; Smith, S.W. Electrostatic micromachine scanning mirror for optical coherence tomography. Opt. Lett. 2003, 28, 628–630. [Google Scholar] [CrossRef]
- Pan, Y.; Xie, H.; Fedder, G.K. Endoscopic optical coherence tomography based on a microelectromechanical mirror. Opt. Lett. 2001, 26, 1966–1968. [Google Scholar] [CrossRef]
- Gilchrist, K.H.; McNabb, R.P.; Izatt, J.P.; Grego, S. Piezoelectric scanning mirrors for endoscopic optical coherence tomography. J. Micromech. Microeng. 2009, 19, 095012. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Z.; Xie, T.; Chu, C.R. Hand-held arthroscopic optical coherence tomography for in vivo high-resolution imaging of articular cartilage. J. Biomed. Opt. 2003, 8, 648–654. [Google Scholar] [CrossRef]
- Göbel, W.; Kerr, J.N.; Nimmerjahn, A.; Helmchen, F. Miniaturized two-photon microscope based on a flexible coherent fiber bundle and a gradient-index lens objective. Opt. Lett. 2004, 29, 2521–2523. [Google Scholar] [CrossRef]
- Wu, J.; Conry, M.; Gu, C.; Wang, F.; Yaqoob, Z.; Yang, C. Paired-angle-rotation scanning optical coherence tomography forward-imaging probe. Opt. Lett. 2006, 31, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Sarunic, M.V.; Han, S.; Wu, J.; Yaqoob, Z.; Humayun, M.; Yang, C. Endoscopic Optical Coherence Tomography of the Retina at 1310 nm Using Paired-Angle-Rotating Scanning. In Proceedings of the Coherence Domain Optical Methods and Optical Coherence Tomography in Biomedicine XI, Proc. of SPIE 6429, San Jose, CA, USA, 22–24 January 2007. [Google Scholar] [CrossRef]
- Wu, T.; Pan, R.; Cao, K.; Zhang, L.; Wang, J.; Lu, Y.; He, C.; Liu, Y. Side-viewing endoscopic imaging probe for swept source optical coherence. Opt. Commun. 2020, 467, 125596. [Google Scholar] [CrossRef]
- Inman, D.J. Engineering Vibrations; Pearson: Hoboken, NJ, USA, 2014. [Google Scholar]
- Holmstrom, S.T.S.; Baran, U.; Urey, H. Mems Laser Scanners: A Review. J. Microelectromech. Syst. 2014, 23, 259–275. [Google Scholar] [CrossRef]
- Haight, E.C.; King, W.W. stability of Nonlinear Oscillations of an elastic Rod. J. Acoust. Soc. Am. 1972, 52, 899–911. [Google Scholar] [CrossRef]
- Hyer, M.W. Whirling of a base-excited cantilever beam. J. Acoust. Soc. Am. 1979, 65, 931–939. [Google Scholar] [CrossRef]
- Kundrat, M.J.; Reinhall, P.G.; Lee, C.M.; Seibel, E.J. High Performance Open Loop Control of Scanning with a Small Cylindrical Cantilever Beam. J. Sound Vib. 2011, 330, 1762–1771. [Google Scholar] [CrossRef]
- Wu, L.; Ding, Z.; Huang, G. Realization of 2D scanning pattern of a fiber cantilever by nonlinear coupling. In Proceedings of the Fifth International Conference on Photonics and Imaging in Biology and Medicine, Proc. of SPIE 6534, Bellingham, WA, USA, 1 April 2007. [Google Scholar] [CrossRef]
- Rivera, D.R.; Brown, C.M.; Ouzounov, D.G.; Pavlova, I.; Kobat, D.; Webb, W.W.; Xu, C. Compact and flexible raster scanning multiphoton endoscope capable of imaging unstained tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 17598–17603. [Google Scholar] [CrossRef]
- Hendriks, B.H.W.; Bierhoff, W.C.J.; Horikx, J.J.L.; Desjardins, A.E.; Hezemans, C.A.; Hooft, G.W.; Lucassen, G.W.; Mihajlovic, N. High-resolution resonant and nonresonant fiber-scanning confocal microscope. J. Biomed. Opt. 2011, 16, 026007. [Google Scholar] [CrossRef]
- Park, H.-C.; Zhang, X.; Yuan, W.; Zhou, L.; Xie, H.; Li, X. Ultralow-voltage electrothermal MEMS based fiber-optic scanning probe for forward-viewing endoscopic OCT. Opt. Lett. 2019, 44, 2232–2235. [Google Scholar] [CrossRef]
- Naono, T.; Fujii, T.; Esashi, M.; Tanaka, S. Non-resonant 2-D piezoelectric MEMS optical scanner actuated by Nb doped PZT thin film. Sens. Actuators A Phys. 2015, 233, 147–157. [Google Scholar] [CrossRef]
- Sawinski, J.; Denk, W. Miniature random-access fiber scanner for in vivo multiphoton imaging. J. Appl. Phys. 2007, 102, 034701. [Google Scholar] [CrossRef]
- Moon, S.; Lee, S.-W.; Rubinstein, M.; Wong, B.J.F.; Chen, Z. Semi-resonant operation of a fiber-cantilever piezotube scanner for stable optical coherence tomography endoscope imaging. Opt. Express 2010, 18, 21183–21197. [Google Scholar] [CrossRef] [PubMed]
- Bourouina, T.; Fujita, H.; Reyne, G.; Motamedi, M.E. Optical scanning. In MOEMS: Micro-Opto-Electro-Mechanical Systems; Motamedi, M.E., Ed.; SPIE: Bellingham, WA, USA, 2005; pp. 323–367. [Google Scholar]
- Pengwang, E.; Rabenorosoa, K.; Rakotondrabe, M.; Andreff, N. Scanning Micromirror Platform Based on MEMS Technology for Medical Application. Micromachines 2016, 7, 7020024. [Google Scholar] [CrossRef] [PubMed]
- Henri, C.; Franck, L. Fabrication, Simulation and experiment of a rotating electrostatic silicon mirror with large angular deflection. In Proceedings of the IEEE Thirteen Annual International Conference on Micro Electro Mechanical Systems, Miyazaki, Japan, 23–27 January 2000. [Google Scholar]
- Schenk, H.; Durr, P.; Kunze, D.; Lakner, H.; Kuck, H. A resonantly excited 2D-micro-scanning-mirror with large deflection. Sens. Actuators A Phys. 2001, 89, 104–111. [Google Scholar] [CrossRef]
- Aguirre, A.D.; Herz, P.R.; Chen, Y.; Fujimoto, J.G.; Piyawattanametha, W.; Fan, L.; Wu, M.C. Two-axis MEMS Scanning Catheter for Ultrahigh Resolution Three-dimensional and En Face Imaging. Opt. Express 2007, 15, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Piyawattanametha, W.; Barretto, R.P.J.; Ko, T.H.; Flusberg, B.A.; Cocker, E.D.; Ra, H.; Lee, D.; Solgaard, O.; Schnitzer, M.J. Fast-scanning two-photon fluorescence imaging based on a microelectromechanical systems two-dimensional scanning mirror. Opt. Lett. 2006, 31, 2018–2020. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Q. A review on actuation and sensing techniques for MEMS-based microgrippers. J. Micro Bio Robot. 2017, 13, 1–14. [Google Scholar] [CrossRef]
- Rahafrooz, A.; Hajjam, A.; Pourkamali, S. Thermal Actuation of High Frequency Micromechanical Resonators. In Proceedings of the 2009 IEEE International SOI Conference, Foster City, CA, USA, 5–8 October 2009. [Google Scholar] [CrossRef]
- Potekhina, A.; Wang, C. Review of Electrothermal Actuators and Applications. Actuators 2019, 8, 69. [Google Scholar] [CrossRef]
- Buser, R.A.; de Rooij, N.F.; Tischhauser, H.; Dommann, A.; Staufert, G. Biaxial scanning mirror activated by bimorph structures for medical applications. Sens. Actuators A Phys. 1992, 31, 29–34. [Google Scholar] [CrossRef]
- Schweizer, S.; Calmes, S.; Laudon, M.; Renaud, P. Thermally actuated optical microscanner with large angle and low consumption. Sens. Actuators A Phys. 1999, 76, 470–477. [Google Scholar] [CrossRef]
- Evstafyev, S.S.; Timoshenkov, S.P.; Britkov, I.M.; Samoilykov, V.K.; Tereshhenko, A.M. A bimorph electrothermal actuator for micromirror devices. In Proceedings of the Micro- and Nanotechnology Sensors, Systems, and Applications VII, Proc. of SPIE 9467, Baltimore, MD, USA, 22 April 2015. [Google Scholar] [CrossRef]
- Tanguy, Q.A.; Gaiffe, O.; Passilly, N.; Cote, J.-M.; Cabodevila, G.; Bargiel, S.; Lutz, P.; Xie, H.; Gorecki, C. Real-time Lissajous imaging with a low-voltage 2-axis MEMS scanner based on electro-thermal actuation. Opt. Express 2020, 28, 8512–8527. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Tanguy, Q.; Pozzi, A.; Xie, H. Optical coherence tomography endoscopic probe based on a tilted MEMS mirror. Biomed. Opt. Express 2016, 7, 3345–3354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Advanced Industrial Control Technology; Elsevier: Oxford, UK, 2010; pp. 73–116. [Google Scholar]
- Mansoor, M.B.; Koble, S.; Wong, W.T.; Woias, P.; Goldschmidtboing, F. Design, Characterization and Sensitivity Analysis of a Piezoelectric Ceramic/Metal Composite Transducer. Micromachines 2017, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Myaing, M.T.; MacDonald, D.J.; Li, X. Fiber-optic scanning two-photon fluorescence endoscope. Opt. Lett. 2006, 31, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cobb, M.J.; Chen, Y.; Kimmey, M.B.; Li, X. Rapid-scanning forward-imaging miniature endoscope for real-time optical coherence tomography. Opt. Lett. 2004, 29, 1763–1765. [Google Scholar] [CrossRef]
- Kretschmer, S.; Jager, J.; Vilches, S.; Ataman, C.; Zapper, H. A bimodal endoscopic imager in a glass package. J. Micromech. Microeng. 2018, 28, 105009. [Google Scholar] [CrossRef]
- Wu, T.; Ding, Z.; Wang, K.; Chen, M.; Wang, C. Two-dimensional scanning realized by an asymmetry fiber cantilever driven by single piezo bender actuator for optical coherence tomography. Opt. Lett. 2009, 17, 13819–13829. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, L.; Gao, F.; Zhang, X. Design and implementation of 2-D endoscopic optical fiber scanner. In Proceedings of the Seventh International Conference on Photonics and Imaging in Biology and Medicine, Proc. of SPIE 7280, Wuhan, China, 6 March 2009. [Google Scholar] [CrossRef]
- Joos, K.M.; Shen, J.-H. Miniature real-time intraoperative forward imaging optical coherence tomography probe. Biomed. Opt. Express 2013, 4, 1342–1350. [Google Scholar] [CrossRef]
- Sun, B.; Sawada, R.; Yang, Z.; Zhang, Y.; Itoh, T.; Maeda, R. Design and Fabrication of Driving Microcoil with Large Tilt-Angle for Medical Scanner Application. In Proceedings of the 2014 Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS (DTIP), Cote D’Azur, France, 1–4 April 2014; pp. 1–6. [Google Scholar]
- Sun, B.; Nogami, H.; Pen, Y.; Sawada, R. Microelectromagnetic actuator based on a 3D printing process for fiber scanner application. J. Micromech. Microengi. 2015, 25, 075014. [Google Scholar] [CrossRef]
- Barbaroto, P.R.; Ferreira, L.O.S.; Doi, I. Micromachined scanner actuated by electromagnetic induction. In Proceedings of the Optomechatronic Systems III, Proc. of SPIE 4902, Stuttgart, Germany, 18 October 2002. [Google Scholar] [CrossRef]
- Miyajima, H.; Asaoka, N.; Isokawa, T.; Ogata, M.; Aoki, Y.; Imai, M.; Fujimori, O.; Katashiro, M.; Matsumoto, K. A MEMS Electromagnetic Optical Scanner for a Commercial Confocal Laser Scanning Microscope. J. Microelectromech. Syst. 2003, 12, 243–251. [Google Scholar] [CrossRef]
- Miyajima, H. MEMS electromagnetic optical scanner for a commercial laser scanning microscope. In Proceedings of the MOEMS Display and Imaging Systems, Proc. of SPIE 4985, San Jose, CA, USA, 20 January 2003. [Google Scholar] [CrossRef]
- Mitsui, T.; Takahashi, Y.; Watanabe, Y. A 2-axis optical scanner driven nonresonantly by electromagnetic force for OCT imaging. J. Micromech. Microeng. 2006, 16, 2482–2487. [Google Scholar] [CrossRef]
- Yuan, S.; Wan, Y.; Mao, Y.; Song, S.; Meng, M.Q.-H. Design of a Novel Electromagnetic Actuation System for Actuating Magnetic Capsule Robot. In Proceedings of the 2019 IEEE International Conference on Robotics and Biomimetics (ROBIO), Dali, China, 6–8 December 2019; pp. 1513–1519. [Google Scholar] [CrossRef]
- Hoang, M.C.; Le, V.H.; Nguyen, K.T.; Nguyen, V.D.; Kim, J.; Choi, E.; Bang, S.; Kang, B.; Park, J.-O.; Kim, C.-S. A Robotic Biopsy Endoscope with Magnetic 5-DOF Locomotion and a Retractable Biopsy Punch. Micromachines 2020, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; Wiley: Hoboken, NJ, USA, 2009; pp. 241–273. [Google Scholar]
- Bourouina, T.; Lebrasseur, E.; Reyne, G.; Debray, A.; Fujita, H.; Ludwig, A.; Quandt, E.; Muro, H.; Oki, T.; Asaoka, A. Integration of Two Degree-of-Freedom Magnetostrictive Actuation and Piezoresistive Detection: Application to a Two-Dimensional Optical scanner. J. Microelectromech. Syst. 2002, 11, 355–361. [Google Scholar] [CrossRef]
- Maeda, S.; Abe, K.; Yamamoto, K.; Tohyama, O.; Ito, H. Active endoscope with SMA (Shape Memory Alloy) coil springs. In Proceedings of the Ninth International Workshop on Micro Electromechanical Systems, San Diego, CA, USA, 11–15 February 1996; pp. 290–295. [Google Scholar] [CrossRef]
- Haga, Y.; Tanahashi, Y.; Esashi, M. Small diameter active catheter using shape memory alloy. In Proceedings of the IEEE. Eleventh Annual International Workshop on Micro Electro Mechanical Systems. An Investigation of Micro Structures, Sensors, Actuators, Machines and Systems. Proceedings MEMS 98, Heidelberg, Germany, 25–29 January 1998. [Google Scholar] [CrossRef]
- Makishi, W.; Matunaga, T.; Haga, Y.; Esashi, M. Active Bending Electric Endoscope Using Shape Memory Alloy Coil Actuators. In Proceedings of the First IEEE/RAS-EMBS International Conference on Biomedical Robotics and Biomechatronics, Pisa, Italy, 20–22 February 2006; pp. 217–219. [Google Scholar]
- Hofmann, U.; Janes, J.; Quenzer, H.J. High-Q MEMS Resonators for Laser Beam Scanning Displays. Micromachines 2012, 3, 509–528. [Google Scholar] [CrossRef]
- Vinegoni, C.; Lee, S.; Feruglio, P.F.; Weissleder, R. Advanced Motion Compensation Methods for Intravital Optical Microscopy. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 83–91. [Google Scholar] [CrossRef]
- Duma, V.-F.; Lee, K.-S.; Meemon, P.; Rolland, J.P. Experimental investigations of the scanning functions of galvanometer-based scanners with applications in OCT. Appl. Opt. 2011, 50, 5735–5749. [Google Scholar] [CrossRef]
- Hwang, K.; Seo, Y.-H.; Ahn, J.; Kim, P.; Jeong, K.-H. Frequency selection rule for high definition and high frame rate Lissajous scanning. Sci. Rep. 2017, 7, 14075. [Google Scholar] [CrossRef]
- Kovács, A. Scanning strategies for imaging arrays. In Proceedings of the Millimeter and Submillimeter Detectors and Instrumentations for Astronomy IV, Proc. of SPIE 7020, Marseille, France, 18 July 2008. [Google Scholar] [CrossRef]
- Huang, G.; Ding, Z. Rapid two-dimensional transversal scanning fiber probe for optical coherence tomography. In Proceedings of the Coherence Domain Optical Methods and Optical Coherence Tomography in biomedicine XI, Proc. of SPIE 6429, California, CA, USA, 2 July 2007. [Google Scholar] [CrossRef]
- Tekpinar, M.; Khayatzadeh, R.; Ferhanoglu, O. Mutliple-pattern generating piezoelectric fiber scanner toward endoscopic applications. Opt. Eng. 2019, 58, 023101. [Google Scholar] [CrossRef]
- Ahrabi, A.A.; Kaur, M.; Li, Y.; Lane, P.; Menon, C. An Electro-Thermal Actuation Method for Resonance vibration of a Minaturized Optical-Fiber Scanner for Future Scanning Fiber Endoscope Design. Actuators 2019, 8, 21. [Google Scholar] [CrossRef]
- Bouma, B.E.; Tearney, G.J. Clinical Imaging with Optical Coherence Tomography. Acad. Radiol. 2002, 9, 942–953. [Google Scholar] [CrossRef]
- Glakova, N.D.; Petrova, G.A.; Nikulin, N.K.; Radenska-Lopovok, S.G.; Snopova, L.B.; Chumakov, Y.P.; Nasonova, V.A.; Gelikonov, V.M.; Gelikonov, G.V.; Kuranov, R.V.; et al. In vivo optical coherence tomography imaging of human skin: Norm and pathology. Skin Res. Technol. 2000, 6, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Fried, D.; Xie, J.; Shafi, S.; Featherstone, J.D.B.; Breunig, T.M.; Le, C. Imaging caries lesions and lesion progression with polarization sensitive optical coherence tomography. J. Biomed. Opt. 2002, 7, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M. Confocal Laser Endomicroscopy: Applications in Clinical and Translational Science—A Comprehensive Review. Int. Sch. Res. Netw. ISRN Pathol. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Adur, J.; Carvalho, H.F.; Cesar, C.L.; Casco, V.H. Nonlinear Microscopy Techniques: Principles and Biomedical Applications. In Microscopy and Analysis; Stanciu, S.G., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Sun, J.; Guo, S.; Wu, L.; Liu, L.; Choe, S.-W.; Sorg, B.S.; Xie, H. 3D In Vivo optical coherence tomography based on a low-voltage, large-scan-range 2D MEMS mirror. Opt. Express 2010, 18, 12065–12075. [Google Scholar] [CrossRef]
- Akhoundi, F.; Qin, Y.; Peyghambarian, N.; Barton, J.K.; Kieu, K. Compact fiber-based multi-photon endoscope working at 1700nm. Biomed. Opt. Express 2018, 9, 2326–2335. [Google Scholar] [CrossRef]
- OmniVision. OVM6948. Available online: https://www.ovt.com/sensors/OVM6948 (accessed on 27 May 2020).
- Dickensheets, D.L.; Kino, G.S. Silicon-Micromachined Scanning Confocal Optical Microscope. J. Microelectromech. Syst. 1998, 7, 38–47. [Google Scholar] [CrossRef]
- Piyawattanametha, W.; Cocker, E.D.; Burns, L.D.; Barretto, R.P.J.; Jung, J.C.; Ra, H.; Solgaard, O.; Schnitzer, M.J. In vivo brain imaging using a portable 2.9 g two-photon microscope based on a microelectromechanical systems scanning mirror. Opt. Lett. 2009, 34, 2309–2311. [Google Scholar] [CrossRef]











| OCT | CM | Nonlinear Microscopy | Photoacoustic Imaging | |||||
|---|---|---|---|---|---|---|---|---|
| Multiphoton | Harmonic Generation | Raman Scattering | OR-PAM | AR-PAM | PAT | |||
| Lateral resolution | 2–25 µm [2,10] | 1 µm [10,18] | ≈0.2 µm [47] | 4.9 µm [48] | 300 nm [49] | ~0.5–10 µm [44,45] | 45 µm [41,45] | 70–720 µm [41,45] |
| Axial resolution | 1–15 µm [2,10] | 7 µm [18] | ≈0.6 µm [47] | 3.1 µm [48] | 1.6 µm [49] | 10 µm [44] | 15 µm [41,45] | 25–640 µm [41,45] |
| Penetration depth | 1–3 mm [2,3,10] | >400 µm [18] | >200 µm [47] | 100–300 µm [50] | ~130 µm [51] | ~1 mm [45] | ~3–5 mm [45] | 70 mm [45] |
| FOV | 2–5 mm [2] | 0.25–1 mm [2] | 200–500 µm [2] | 170 µm [50] | 205 µm × 205 µm [36] | 1–2 mm [2] | upto 36 mm × 80 mm [52] | ~40 mm [52] |
| Orientation | Cross-section [10] | en face [10] | en face [10] | Cross-section [41] | ||||
| wavelength | Near IR | Visible or Near IR | Near IR | Near IR or IR | ||||
| Source | Low coherence [10] | Continuous wave or pulsed [10] | Pulsed [10] | Pulsed [41] | ||||
| Frame rate | >60 Hz [2] | >15 Hz [2] | >5 Hz [2] | ~10 Hz (depends on scanning area) [2] | ||||
| Advantages | High sensitivity, high imaging speed, deep tissue penetration, independent of source spectrum | High contrast due to rejection of out-of-focus scattered photons, isotropic and fine resolution, ability to control depth of field, ability to change magnification by changing the scanned area | Label free technology, High spatial resolution, less phototoxicity, and photobleaching due to exciting event taking place at focus point | High spatial resolution, High contrast, High imaging speed, and deep tissue penetration | ||||
| Disadvantages | Expensive detector, depth resolution dependent on NA, small dynamic range | Two or more nearby fluorescence signals can overlap | Expensive laser source, needs dispersion compensation | Expensive transducers required to detect the poor acoustic signal, Signal to noise ratio decreases with the tissue penetration | ||||
| Forward View | Side View | References. | |
|---|---|---|---|
| Probe diameter | >250 µm (proximal scanning) >1.65 mm (distal scanning) | >250 µm (proximal scanning) >2.4 mm (distal scanning) | [54,56,77] |
| Rigid length | >9 mm | >11 mm | [64,79] |
| FOV | 50–400 µm | ~3–4 mm | [23,53,75] |
| Image orientation | en face | Peripherical surface | |
| Advantages | Can be used for image guidance to relocate and control the position of the medical devices, can directly image the extent of the malignancy and cancerous surface | Can image the finer cavities of the body, gives information about the wall/section of tissue layer involved in the malignancy, higher field of view, less expensive | |
| Disadvantages | Limited field of view, limitation of miniaturization limits the ability to image the narrower sections | Difficult to guide the probe in the body due to lack of guidance |
| Resonant Scanner | Semi-Resonant Scanner | Non-Resonant Scanner | |
|---|---|---|---|
| Scan area | ✓✓✓ | ✓✓ | ✓ |
| Power consumption | ✓ | ✓✓ | ✓✓✓ |
| Operating frequency | High | Intermediate | Low |
| Advantages | Large displacement amplitude, low power consumption | Large scanning amplitude than non-resonant scanners, variable imaging field, stable working conditions | Operable at very low frequencies, stable to small variations of operating conditions, image field is variable |
| Disadvantages | Offsetting the image field requires complex systems, Instability can lead to whirling motion | Performance highly depending on the working frequency | Small scanning amplitude, high power consumption |
| Electrostatic | Electro-Thermal | Piezoelectric | Electromagnetic | Shape Memory Alloy | |
|---|---|---|---|---|---|
| Force | ✓ | ✓ | ✓✓✓ | ✓✓ | ✓✓✓ |
| Displacement amplitude | ✓✓ | ✓✓✓ | ✓ | ✓✓✓ | ✓✓ |
| Compactness | ✓✓✓ | ✓✓✓ | ✓✓ | ✓ | ✓✓ |
| Working principle | Electrostatic force | Thermal expansion | Piezoelectric effect | Magnetization effect | Material deformation |
| Advantages | Fast response, low voltage required, easy fabrication, and no hysteresis | Large displacement, low operating voltage, small dimensions | Large force generated, wide operating frequency range, low power consumption | Large displacement obtained, quick and linear response, easy to control | Flexibility, large frequency response |
| Disadvantages | Large device dimensions, pull-in problem, complicated circuit | High working temperature, not operable at very high frequencies | Limited displacement | Large device dimensions, difficult to manufacture | Low displacement |
| Raster | Spiral | Lissajous | Circular | Propeller | |
|---|---|---|---|---|---|
| Scanning pattern |  |  |  |  |  |
| Actuation pattern |  |  |  |  |  Y(t) constant rotation |
| Advantages | Uniform light intensity | Easy to get, area is swept by changing the driving voltage | Uniform light intensity, most used | Possible to get circular pattern with 1D actuation, area is swept by changing the driving voltage | Easy to generate |
| Disadvantages | Points are scanned at different times can lead to motion artifacts | Light intensity is higher in center | Fill factor highly depends on the frequency ratio, quasi-random pattern | Light intensity is higher in center | Non uniform light intensity, the rotation of miniaturized structure requires complex and expensive devices |
| Working Principle | Frequency | FOV | Drive Voltage | Scanner Dimensions | Scanning Pattern | References | |
|---|---|---|---|---|---|---|---|
| OCT using rotational MEMS probe | Micromotor | 1 kHz | - | >1 V | 2.4 mm (in diameter) | Radial | [54] |
| MEMS fiber scanner for confocal microscopy | Electrothermal actuation | 239 Hz (x-axis) 207 Hz (y-axis) | 378 µm × 439 µm | 16 Vpp (duty cycle 13%) | Diameter (1.65 mm) Rigid length (28 mm) | Lissajous scanning | [69] |
| Fiber scanner for forward viewing endoscope | Piezoelectric tube | 86 Hz (x-axis) 97 Hz (y-axis) | 732 µm × 591.7 µm | 40 Vac | Diameter (3.2 mm) Rigid length (50 mm) | Lissajous scanning | [63] |
| OCT based on 2D MEMS mirror | Electrothermal actuation | 1.25 Hz (fast scan actuator pair) 0.0125 Hz (longitudinal) | 2.3 mm × 2.3 mm | 0–4 V ramp (fast scan) 0.5–3.5 V ramp (slow axis) | Diameter (5.8 mm) Rigid length (12 mm) | Lissajous scanning | [140] |
| Scanning fiber endoscope | Piezoelectric tube | 5 kHz | 200 µm (in diameter) | <20 Vac | Diameter (1.2 mm -1.7 mm) Rigid length (9 mm) | Spiral scan pattern | [55,64] |
| Multi-Photon Endoscope | Piezoelectric tube | 35 Hz | 900 µm (in diameter) | 40 Vac | Diameter (5 mm) Rigid length (4 cm) | Circular pattern | [141] |
| OmniVision camera | Chip on tip camera | - | 364 µm × 364 µm | 3.3 Vac | 650 µm × 650 µm × 1158 µm | - | [142] |
| FCFM using Cellvizio | Scanning mirrors at proximal end | 4 kHz | 160 µm × 120 µm ÷ 400 µm × 280 µm | - | 350 µm ÷ 1.8 mm diameter 20 mm Rigid length | - | [23] |
| Scanning confocal microscope | Electrostatic torsional mirrors | 4.3 kHz (fast scan) 1.07 kHz (slow scan) | 100 µm (in diameter) | 20 V | 1.2 mm × 2.5 mm × 6.5 mm | Lissajous scanning | [19,143] |
| Two-photon microscope | Electrostatically actuated mirrors | 1.08 kHz (fast scan) 0.65 kHz (slow scan) | 295 µm × 100 µm | 60 V | 2.0 cm × 1.9 cm × 1.1 cm | Raster scanning | [144] |
| Side view endomicroscope | Electrostatically driven mirror | 12 kHz (fast scan) 3 kHz (slow scan) | 350 µm × 350 µm | 60 Vpp | 2.4 mm (in diameter) | Lissajous scanning | [24] |
| Non-resonant MEMS scanner for OCT | Thin film piezoelectric | 50 Hz | 1 mm × 0.7 mm | 40 Vpp | 2.2 mm × 2.7 mm | Lissajous scanning | [89] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, M.; Lane, P.M.; Menon, C. Endoscopic Optical Imaging Technologies and Devices for Medical Purposes: State of the Art. Appl. Sci. 2020, 10, 6865. https://doi.org/10.3390/app10196865
Kaur M, Lane PM, Menon C. Endoscopic Optical Imaging Technologies and Devices for Medical Purposes: State of the Art. Applied Sciences. 2020; 10(19):6865. https://doi.org/10.3390/app10196865
Chicago/Turabian StyleKaur, Mandeep, Pierre M. Lane, and Carlo Menon. 2020. "Endoscopic Optical Imaging Technologies and Devices for Medical Purposes: State of the Art" Applied Sciences 10, no. 19: 6865. https://doi.org/10.3390/app10196865
APA StyleKaur, M., Lane, P. M., & Menon, C. (2020). Endoscopic Optical Imaging Technologies and Devices for Medical Purposes: State of the Art. Applied Sciences, 10(19), 6865. https://doi.org/10.3390/app10196865







