Increasing Hypopnea in Sleep Breathing Disturbance Improves Postoperative Oxygen Saturation in Patients with Very Severe Obstructive Sleep Apnea
Abstract
1. Introduction
2. Materials and Methods
- Age ≥ 20 years
- Unsuccessful or refusal of CPAP
- AHI ≥ 60 events/h
- Received a one-stage multi-level sleep surgery with the modified ZPP performed with one-layer closure and open partial tongue-base glossectomy
- Available preoperative and postoperative PSGs for required recordings, including desaturation index and SpO2.
3. Ethical Statements
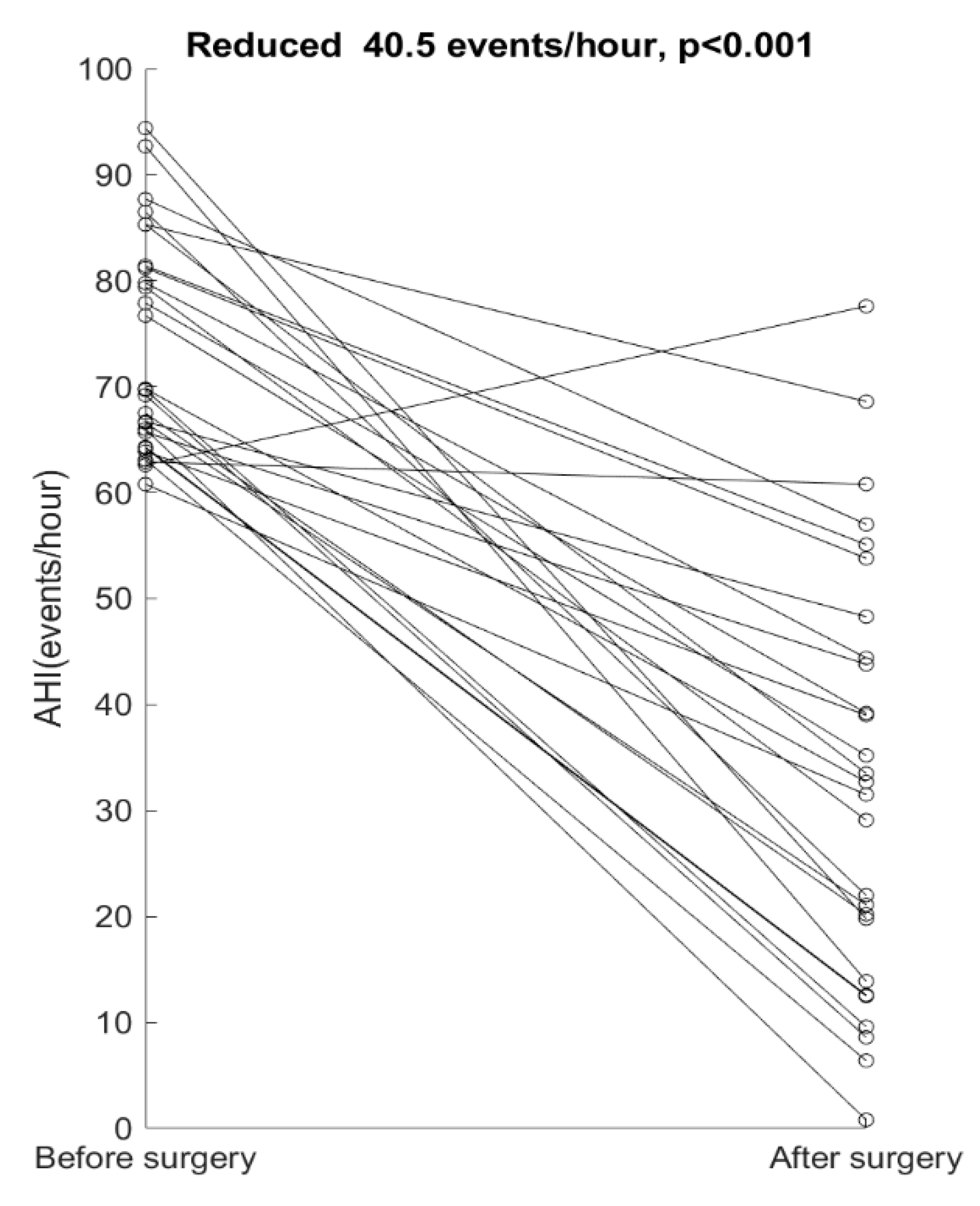
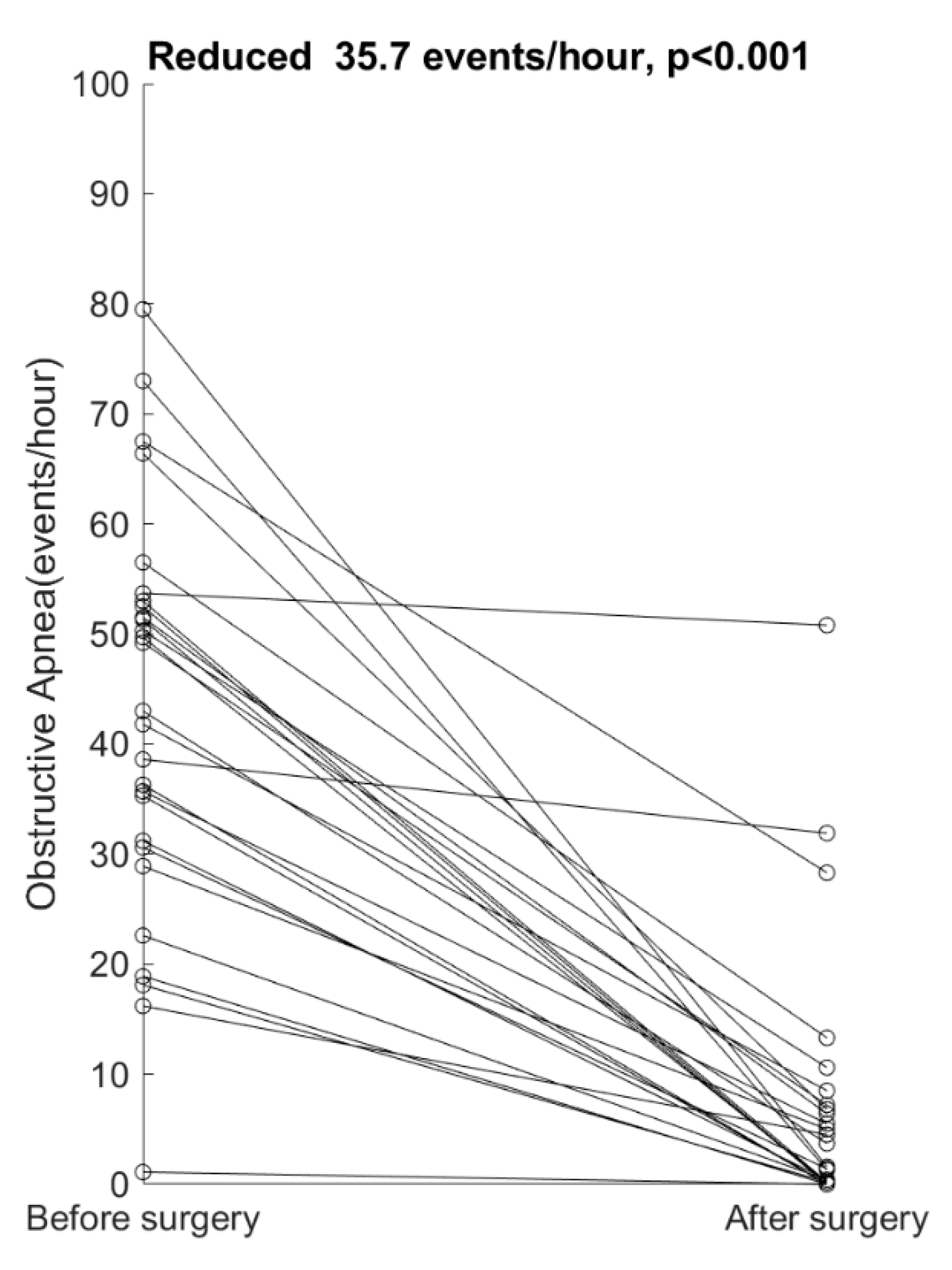
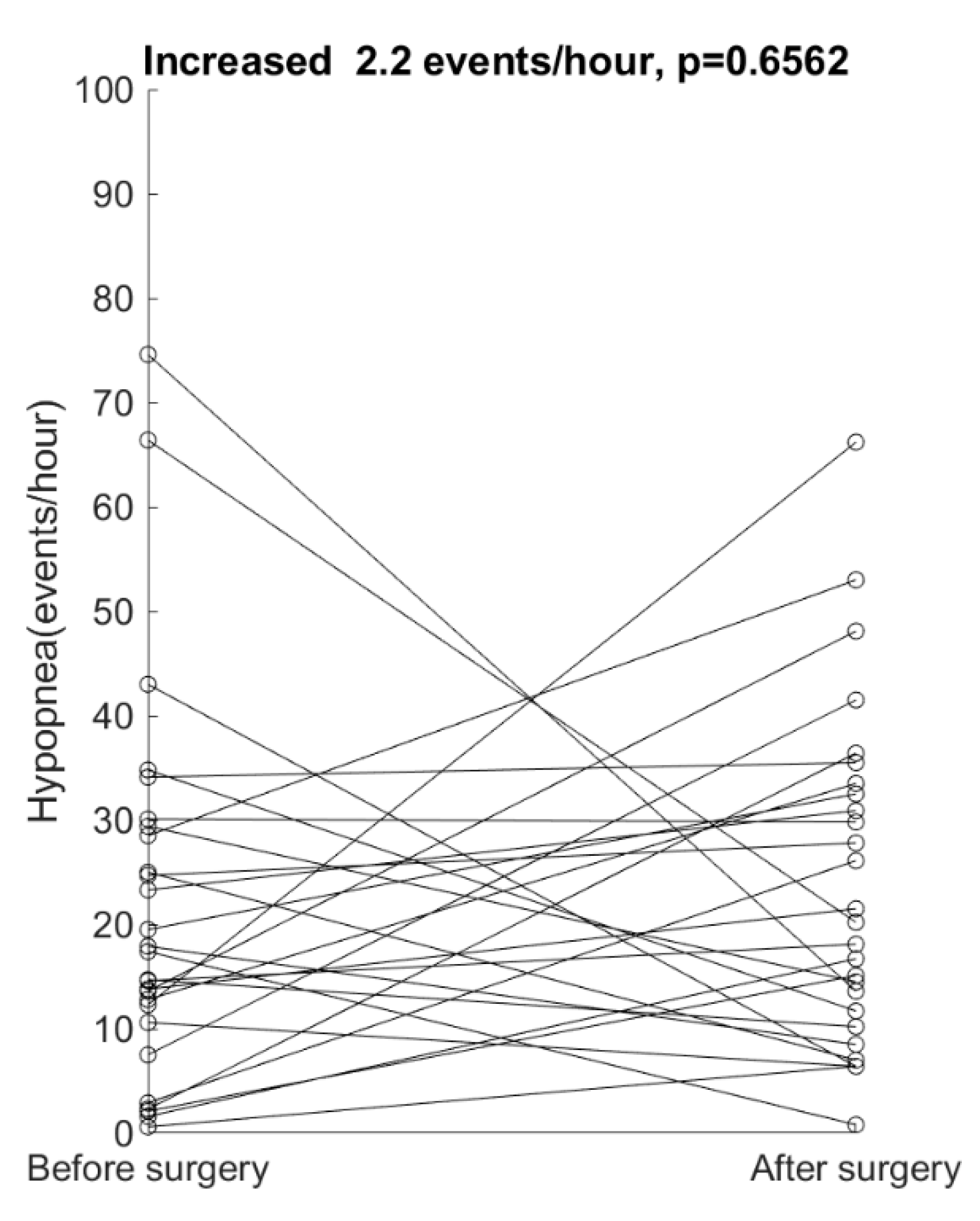
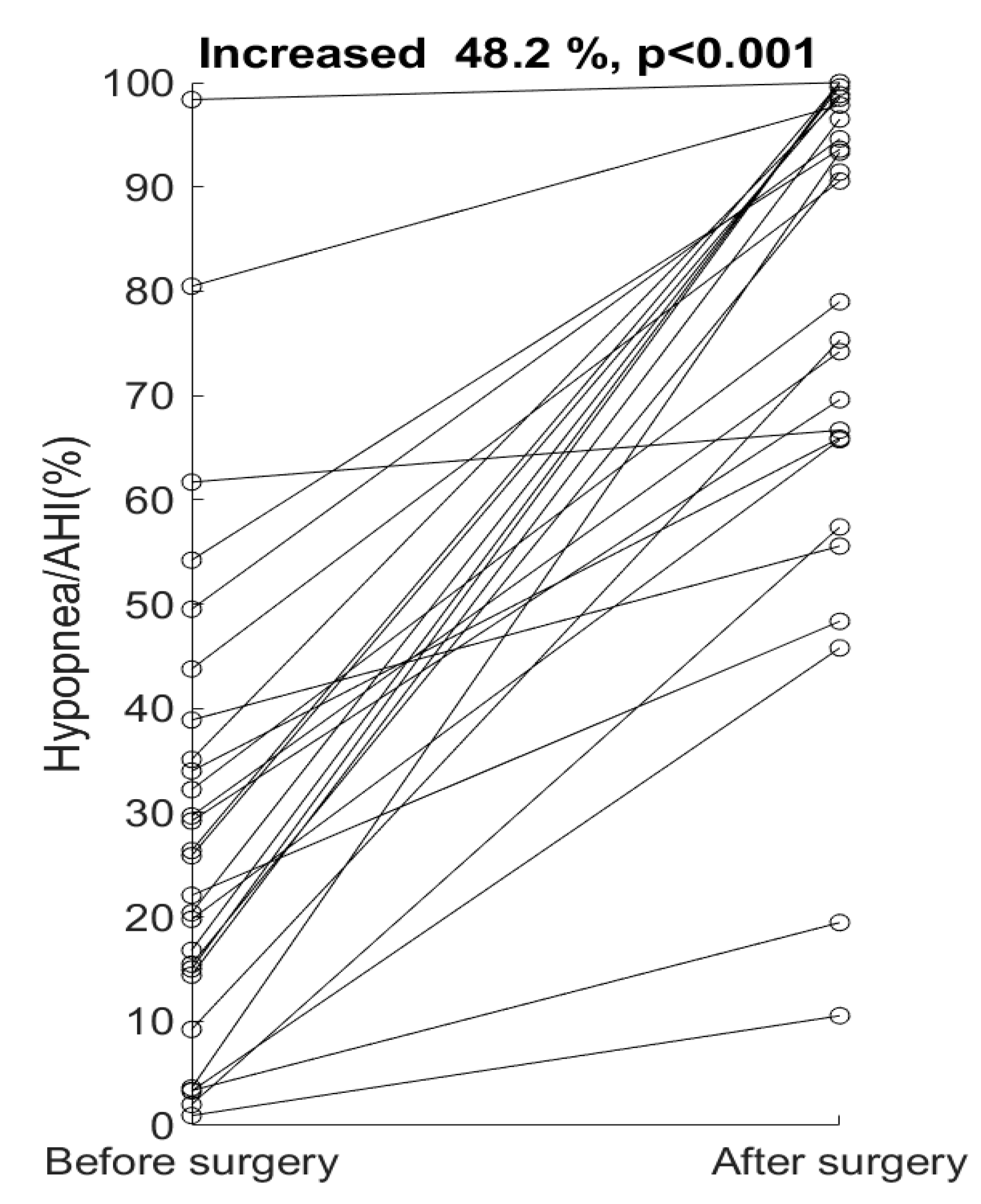
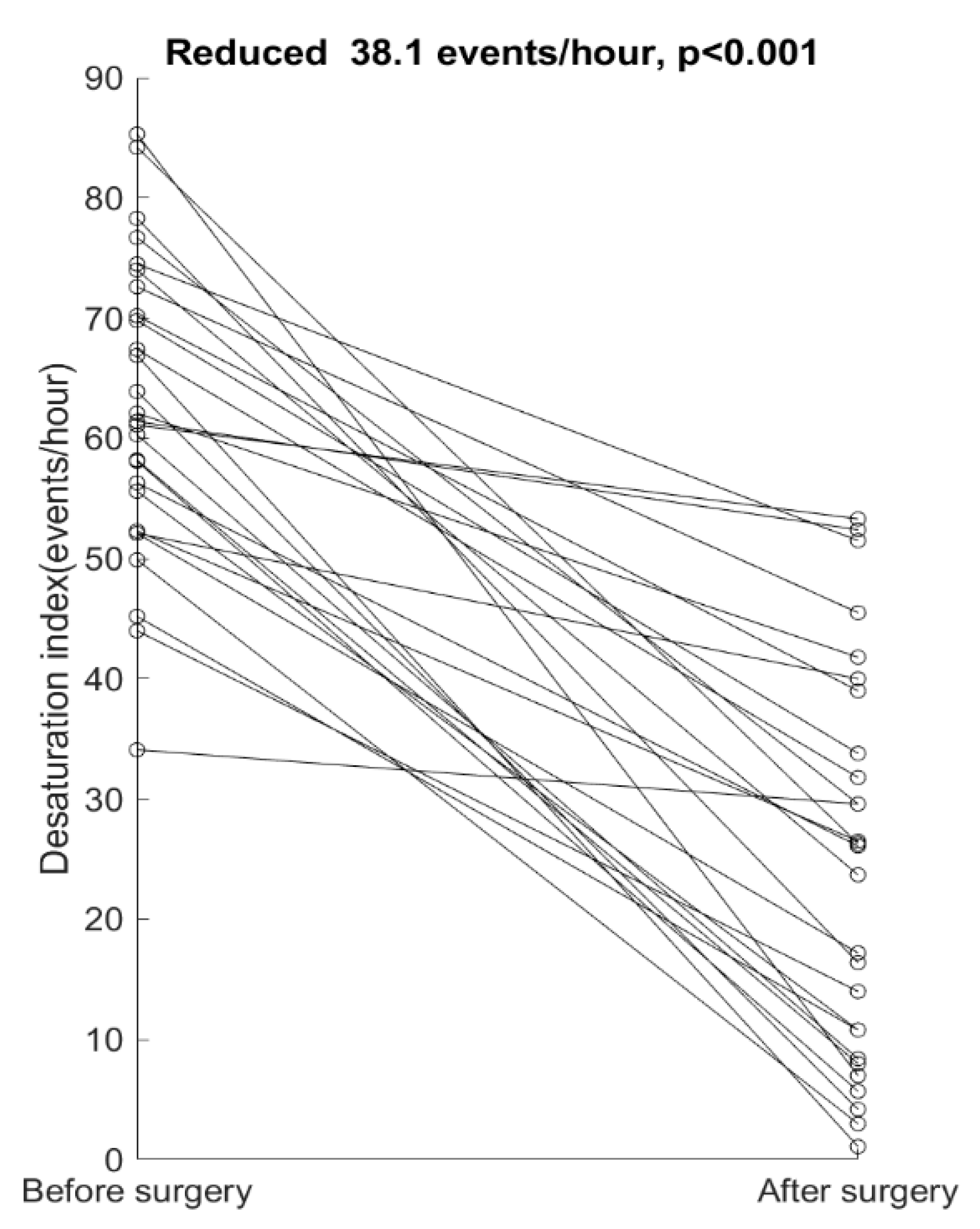
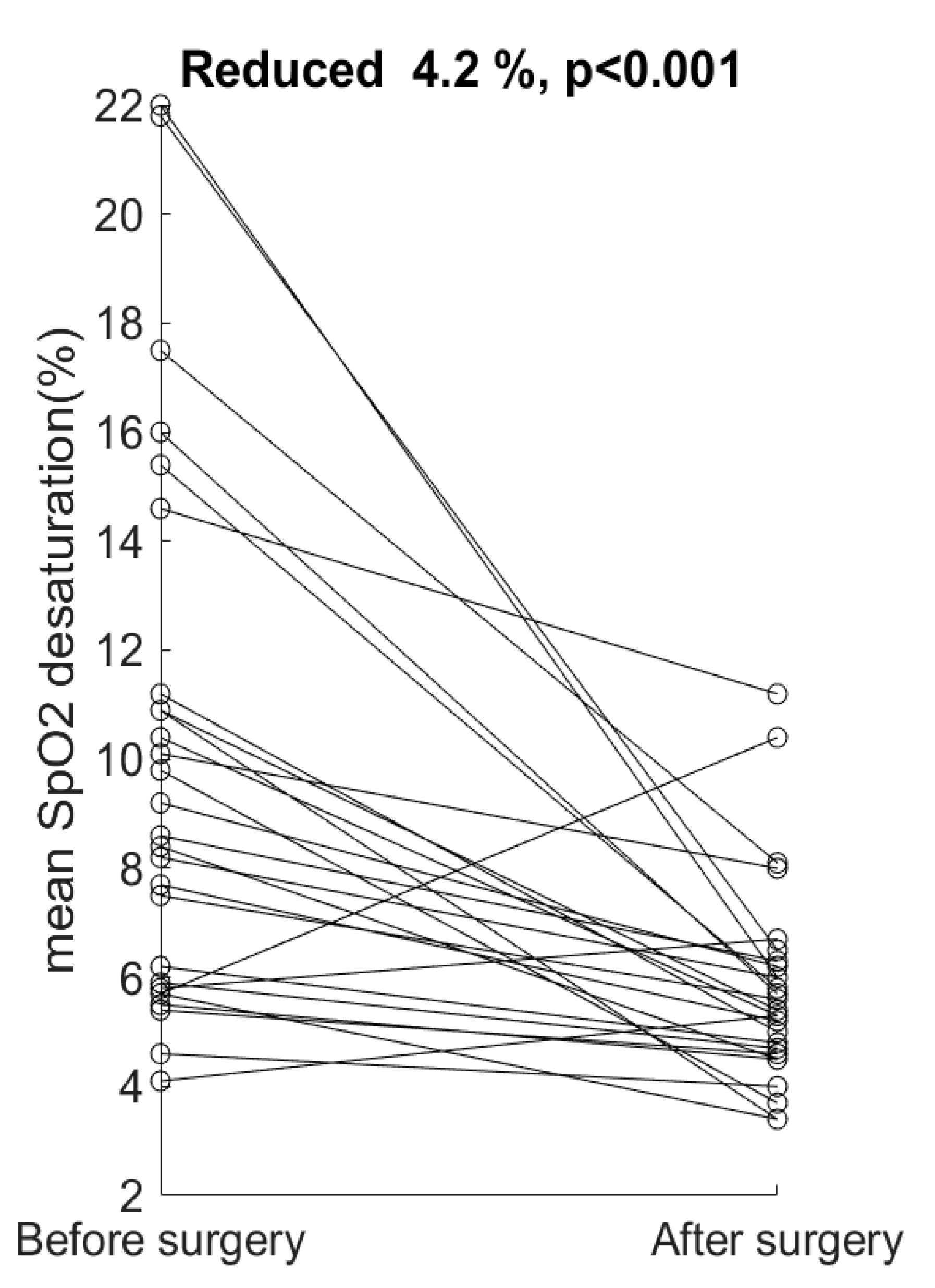
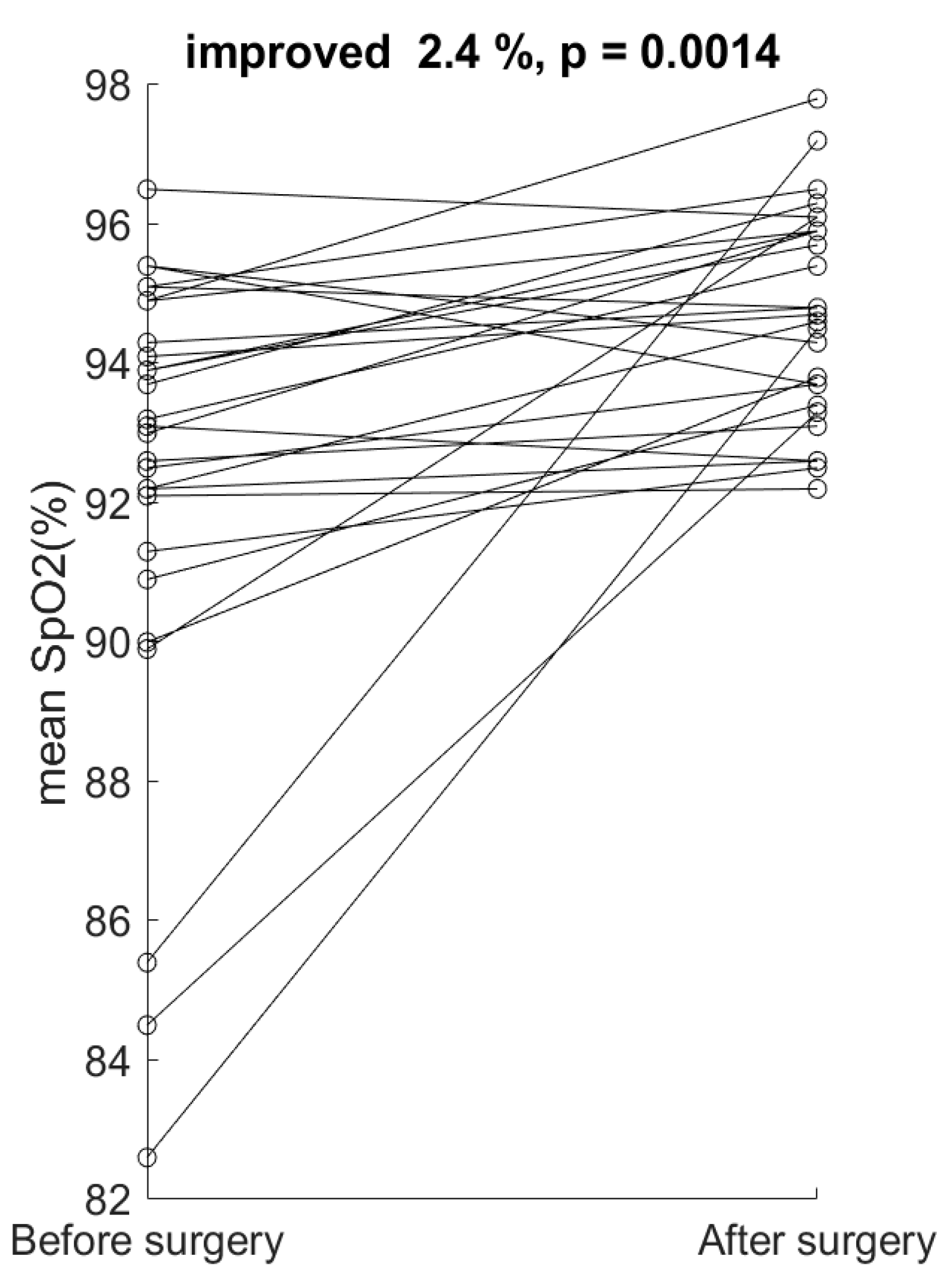
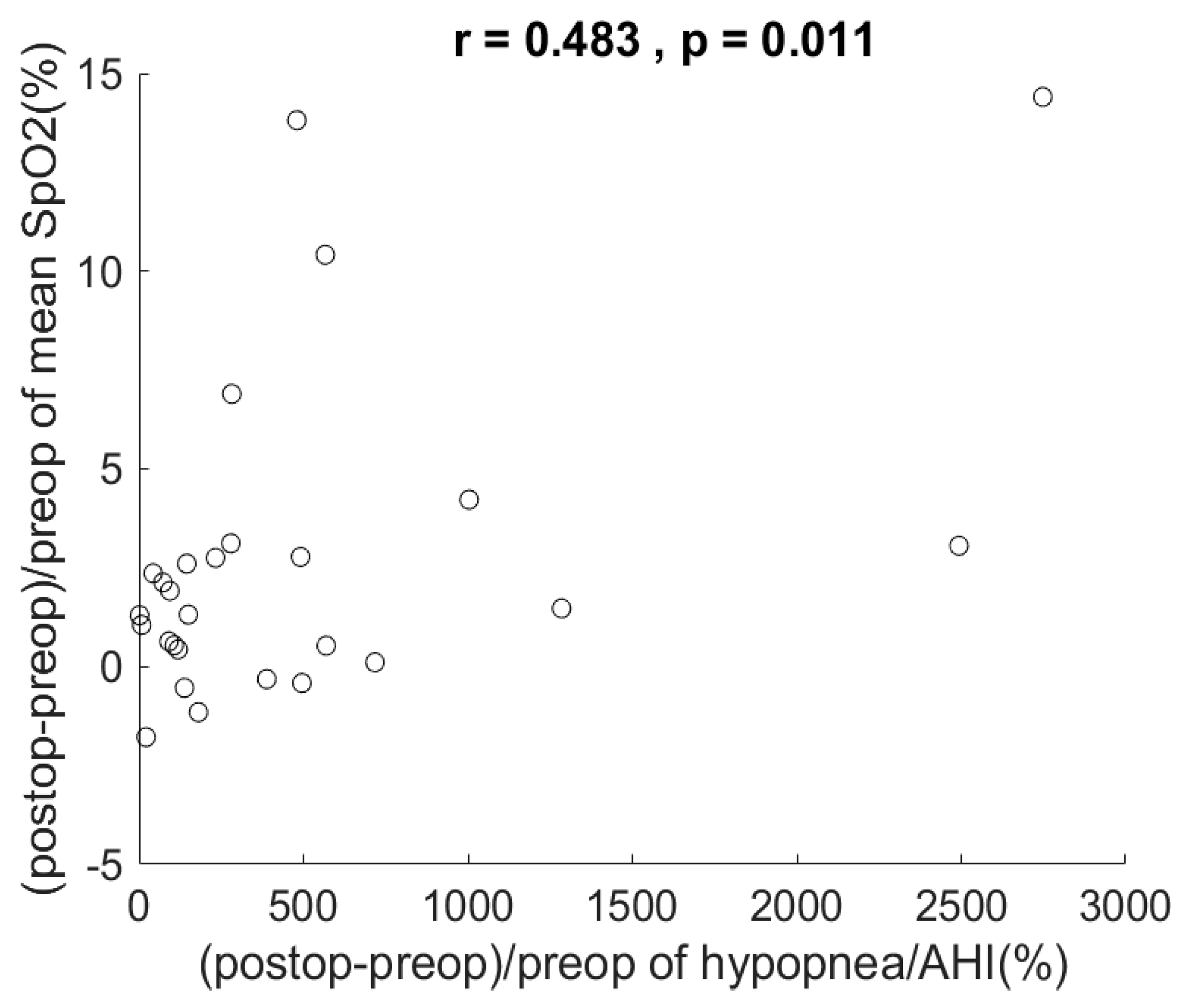
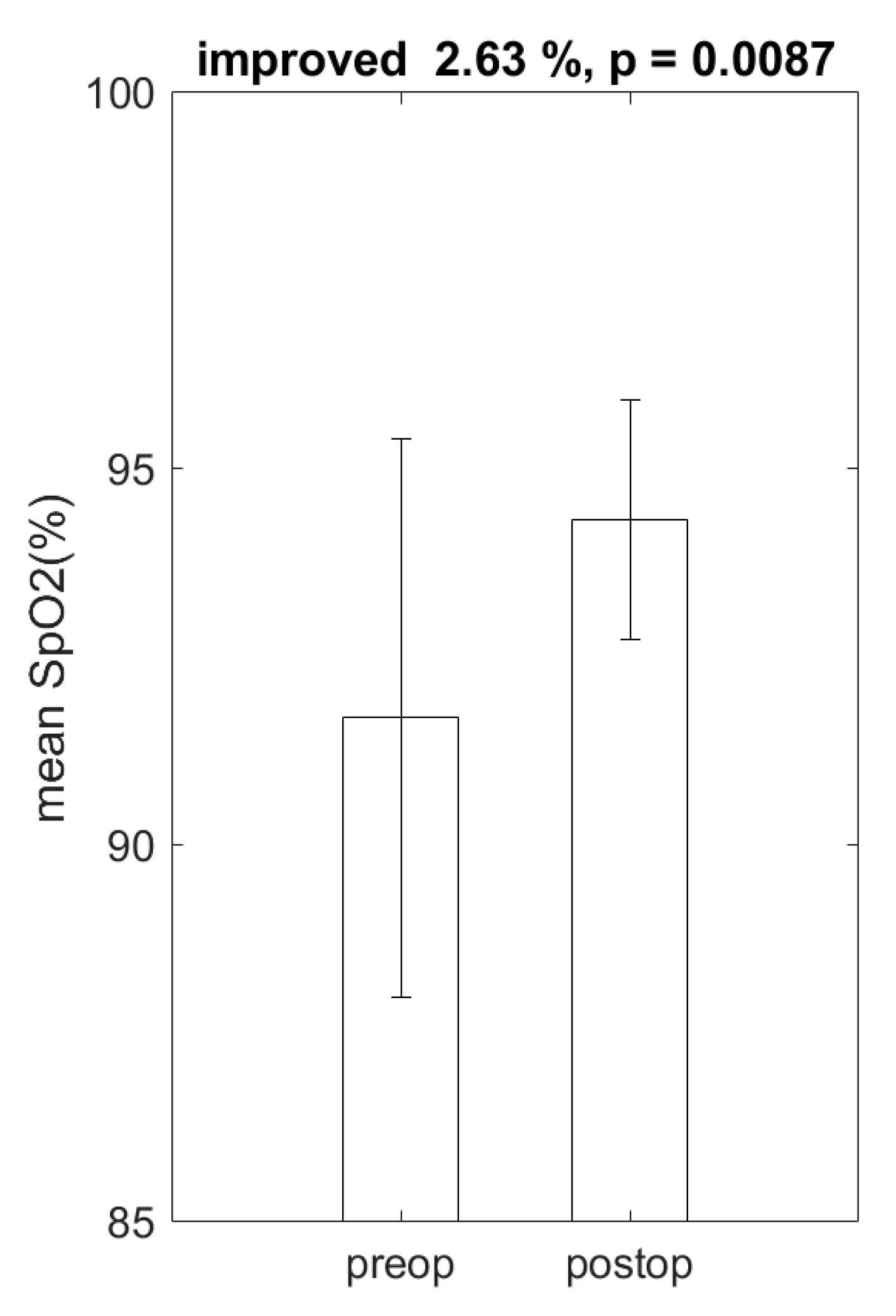
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Young, T.; Peppard, P.; Palta, M.; Hla, K.M.; Finn, L.; Morgan, B.; Skatrud, J. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch. Intern. Med. 1997, 157, 1746–1752. [Google Scholar] [CrossRef]
- Lavie, P.; Herer, P.; Hoffstein, V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: Population study. BMJ 2000, 320, 479–482. [Google Scholar] [CrossRef]
- Nieto, F.J.; Young, T.; Lind, B.K.; Shahar, E.; Samet, J.M.; Redline, S.; D’Agostino, R.B.; Newman, A.B.; Lebowitz, M.D.; Pickering, T.G.; et al. Association of Sleep-Disordered Breathing, Sleep Apnea, and Hypertension in a Large Community-Based Study. JAMA 2000, 283, 1829–1836. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective Study of the Association between Sleep-Disordered Breathing and Hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef]
- Cheshire, K.; Engleman, H.; Deary, I.; Shapiro, C.; Douglas, N.J. Factors Impairing Daytime Performance in Patients with Sleep Apnea/Hypopnea Syndrome. Arch. Intern. Med. 1992, 152, 538–541. [Google Scholar] [CrossRef]
- Kim, H.C.; Young, T.; Matthews, C.G.; Weber, S.M.; Woodard, A.R.; Palta, M. Sleep-disordered Breathing and Neuropsychological Deficits. Am. J. Respir. Crit. Care Med. 1997, 156, 1813–1819. [Google Scholar] [CrossRef]
- Perry, J.C.; Almeida, V.; Souza, F.G.; Schoorlemmer, G.H.; Colombari, E.; Tufik, S. Consequences of subchronic and chronic exposure to intermittent hypoxia and sleep deprivation on cardiovascular risk factors in rats. Respir. Physiol. Neurobiol. 2007, 156, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Bananian, S.; Lehrman, S.G.; Maguire, G.P. Cardiovascular Consequences of Sleep-related Breathing Disorders. Heart Dis. 2002, 4, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Shahar, E.; Whitney, C.W.; Redline, S.; Lee, E.T.; Newman, A.B.; Nieto, F.J.; O’Connor, G.T.; Boland, L.L.; Schwartz, J.E.; Samet, J.M. Sleep-disordered Breathing and Cardiovascular Disease. Am. J. Respir. Crit. Care Med. 2001, 163, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kulkas, A.; Tiihonen, P.; Julkunen, P.; Mervaala, E.; Töyräs, J. Novel parameters indicate significant differences in severity of obstructive sleep apnea with patients having similar apnea–hypopnea index. Med. Biol. Eng. 2013, 51, 697–708. [Google Scholar] [CrossRef]
- Kulkas, A.; Duce, B.; Hukins, C.; Töyräs, J.; Leppänen, T. Severity of desaturation events differs between hypopnea and obstructive apnea events and is modulated by their duration in obstructive sleep apnea. Sleep Breath. 2017, 21, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Bédard, M.-A.; Montplaisir, J.; Richer, F.; Malo, J. Nocturnal Hypoxemia as a Determinant of Vigilance Impairment in Sleep Apnea Syndrome. Chest 1991, 100, 367–370. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mediano, O.; Barcelò, A.; De La Peña, M.; Gozal, D.; Agusti, A.; Barbe, F. Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur. Respir. J. 2007, 30, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Wang, P.-C.; Lee, L.-A.; Chen, N.-H.; Fang, T.-J. Prediction of uvulopalatopharyngoplasty outcome: Anatomy-based staging system versus severity-based staging system. Sleep 2006, 29, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Campanini, A.; De Vito, A.; Frassineti, S.; Vicini, C. Role of skin-lined tracheotomy in obstructive sleep apnoea syndrome: Personal experience. Acta Otorhinolaryngol. Ital. 2004, 24, 68–74. [Google Scholar] [PubMed]
- Haviv, Y.; Bachar, G.; Aframian, D.; Almoznino, G.; Michaeli, E.; Benoliel, R. A 2-year mean follow-up of oral appliance therapy for severe obstructive sleep apnea: A cohort study. Oral Dis. 2014, 21, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Lavie, P.; Lavie, L.; Herer, P. All-cause mortality in males with sleep apnoea syndrome: Declining mortality rates with age. Eur. Respir. J. 2005, 25, 514–520. [Google Scholar] [CrossRef]
- Veasey, S.C.; Guilleminault, C.; Strohl, K.P.; Sanders, M.H.; Ballard, R.D.; Magalang, U.J. Medical therapy for obstructive sleep apnea: A review by the Medical Therapy for Obstructive Sleep Apnea Task Force of the Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep 2006, 29, 1036–1044. [Google Scholar] [CrossRef]
- Maimon, N.; Hanly, P.J. Does Snoring Intensity Correlate with the Severity of Obstructive Sleep Apnea? J. Clin. Sleep Med. 2010, 6, 475–478. [Google Scholar] [CrossRef]
- Brown, E.C.; Cheng, S.; McKenzie, D.K.; Butler, J.E.; Gandevia, S.C.; Bilston, L.E. Respiratory Movement of Upper Airway Tissue in Obstructive Sleep Apnea. Sleep 2013, 36, 1069–1076. [Google Scholar] [CrossRef]
- Olaithe, M.; Bucks, R. Executive Dysfunction in OSA Before and After Treatment: A Meta-Analysis. Sleep 2013, 36, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R. Obstructive sleep apnea. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1998, 85, 388–392. [Google Scholar] [CrossRef]
- Vilaseca, I.; Morelló, A.; Montserrat, J.M.; Santamaría, J.; Iranzo, A. Usefulness of uvulopalatopharyngoplasty with genioglossus and hyoid advancement in the treatment of obstructive sleep apnea. Arch. Otolaryngol.-Head Neck Surg. 2002, 128, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.-A.; Huang, C.-G.; Chen, N.-H.; Wang, C.-L.; Fang, T.-J.; Li, H.-Y. Severity of Obstructive Sleep Apnea Syndrome and High-Sensitivity C-Reactive Protein Reduced After Relocation Pharyngoplasty. Otolaryngol. Neck Surg. 2011, 144, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.-A.; Yu, J.-F.; Lo, Y.-L.; Chen, Y.-S.; Wang, D.-L.; Cho, C.-M.; Ni, Y.-L.; Chen, N.-H.; Fang, T.-J.; Huang, C.-G.; et al. Energy Types of Snoring Sounds in Patients with Obstructive Sleep Apnea Syndrome: A Preliminary Observation. PLoS ONE 2012, 7, e53481. [Google Scholar] [CrossRef]
- Shiba, T.; Takahashi, M.; Sato, Y.; Onoda, Y.; Hori, Y.; Sugiyama, T.; Bujo, H.; Maeno, T. Relationship between Severity of Obstructive Sleep Apnea Syndrome and Retinal Nerve Fiber Layer Thickness. Am. J. Ophthalmol. 2014, 157, 1202–1208. [Google Scholar] [CrossRef]
- Huynh, N.; Emami, E.; Helman, J.; Chervin, R. Interactions between sleep disorders and oral diseases. Oral Dis. 2013, 20, 236–245. [Google Scholar] [CrossRef]
- Gay, P.C.; Herold, D.L.; Olson, E.J. A randomized, double-blind clinical trial comparing continuous positive airway pressure with a novel bilevel pressure system for treatment of obstructive sleep apnea syndrome. Sleep 2003, 26, 864–869. [Google Scholar] [CrossRef][Green Version]
- Lacedonia, D.; Carpagnano, G.E.; Sabato, R.; Storto, M.M.L.; Palmiotti, G.A.; Capozzi, V.; Barbaro, M.F.; Gallo, C. Characterization of obstructive sleep apnea-hypopnea syndrome (OSA) population by means of cluster analysis. J. Sleep Res. 2016, 25, 724–730. [Google Scholar] [CrossRef]
- Anderson, F.E.; Kingshott, R.N.; Taylor, D.R.; Jones, D.R.; Kline, L.R.; Whyte, K.F. A randomized crossover efficacy trial of oral CPAP (Oracle) compared with nasal CPAP in the management of obstructive sleep apnea. Sleep 2003, 26, 721–726. [Google Scholar] [CrossRef]
- Levendowski, D.J.; Seagraves, S.; Popovic, D.; Westbrook, P.R. Assessment of a Neck-Based Treatment and Monitoring Device for Positional Obstructive Sleep Apnea. J. Clin. Sleep Med. 2014, 10, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Otake, K.; Sasanabe, R.; Hasegawa, R.; Banno, K.; Hori, R.; Okura, Y.; Yamanouchi, K.; Shiomi, T. Glucose Intolerance in Japanese Patients with Obstructive Sleep Apnea. Intern. Med. 2009, 48, 1863–1868. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Becker, H.F.; Koehler, U.; Stammnitz, A.; Peter, J.H. Heart block in patients with sleep apnoea. Thorax 1998, 53, S29–S32. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.B.; Frith, R.W.; Harding, D.A.; Cant, B.R. Uvulopalatopharyngoplasty in severe idiopathic obstructive sleep apnoea syndrome. Thorax 1989, 44, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Mickelson, S.A.; Rosenthal, L. Midline glossectomy and epiglottidectomy for obstructive sleep apnea syndrome. Laryngoscope 1997, 107, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.E.; Schechtman, K.B.; Piccirillo, J.F. The Efficacy of Surgical Modifications of the Upper Airway in Adults with Obstructive Sleep Apnea Syndrome. Sleep 1996, 19, 156–177. [Google Scholar] [CrossRef]
- Huang, E.I.; Kuo, C.-L.; Chou, Y.-T.; Lin, Y.-C.; Huang, S.-Y. Modified Z-palatoplasty with one-layer closure in one-stage multilevel surgery for severe obstructive sleep apnea. Auris Nasus Larynx 2018, 45, 791–795. [Google Scholar] [CrossRef]
- Caples, S.M.; Rowley, J.A.; Prinsell, J.R.; Pallanch, J.F.; Elamin, M.B.; Katz, S.G.; Harwick, J.D. Surgical Modifications of the Upper Airway for Obstructive Sleep Apnea in Adults: A Systematic Review and Meta-Analysis. Sleep 2010, 33, 1396–1407. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, E.I.; Huang, S.-Y.; Lin, Y.-C.; Lin, C.-M.; Lin, C.-K.; Huang, Y.-C.; Su, J.-A. Increasing Hypopnea in Sleep Breathing Disturbance Improves Postoperative Oxygen Saturation in Patients with Very Severe Obstructive Sleep Apnea. Appl. Sci. 2020, 10, 6539. https://doi.org/10.3390/app10186539
Huang EI, Huang S-Y, Lin Y-C, Lin C-M, Lin C-K, Huang Y-C, Su J-A. Increasing Hypopnea in Sleep Breathing Disturbance Improves Postoperative Oxygen Saturation in Patients with Very Severe Obstructive Sleep Apnea. Applied Sciences. 2020; 10(18):6539. https://doi.org/10.3390/app10186539
Chicago/Turabian StyleHuang, Ethan I., Shu-Yi Huang, Yu-Ching Lin, Chieh-Mo Lin, Chin-Kuo Lin, Ying-Chih Huang, and Jian-An Su. 2020. "Increasing Hypopnea in Sleep Breathing Disturbance Improves Postoperative Oxygen Saturation in Patients with Very Severe Obstructive Sleep Apnea" Applied Sciences 10, no. 18: 6539. https://doi.org/10.3390/app10186539
APA StyleHuang, E. I., Huang, S.-Y., Lin, Y.-C., Lin, C.-M., Lin, C.-K., Huang, Y.-C., & Su, J.-A. (2020). Increasing Hypopnea in Sleep Breathing Disturbance Improves Postoperative Oxygen Saturation in Patients with Very Severe Obstructive Sleep Apnea. Applied Sciences, 10(18), 6539. https://doi.org/10.3390/app10186539




