Study of the Healing Properties of Natural Sources of Georgia and Modeling of Their Purification Processes
Abstract
:1. Introduction
- ➢
- Borjomi—Medicinal-table hydrocarbonate sodium mineral water of medium mineralization. The springs are in the Borjomi Gorge, in eastern Georgia. Unlike many other similar mineral waters, Borjomi does not have time to cool underground and comes to the surface warm (38–41 °C), the water “undergoes” natural filtration and is enriched with 60 different minerals, which make its composition unique. The mineral water treats the digestive system, diseases of the gastrointestinal tract, biliary ducts, kidneys and much more [32,33,34,35,36].
- ➢
- Likani—A unique natural medicinal-table water, saturated with minerals, contains a mineral-ion complex—a combination of magnesium and calcium hydrocarbonates. Likani is richly saturated with natural gas, therefore it perfectly quenches thirst. Carbon dioxide helps lift mineral water from a depth of 1500 m and gives it a light taste. The water springs are located in the Borjomi Valley at an altitude of 810 m above sea level.
- ➢
- Nabeglavi—Healing-table, carbonate–hydrocarbonate, sodium–calcium sparkling water. The spring is located at an altitude of 475 m above sea level. By its healing properties it is not inferior to the famous Borjomi mineral water—it is recommended both for balneology and drinking, regular use as table water helps to cleanse the body of harmful substances and enrich it with essential minerals. The increased content of magnesium ion has a beneficial effect on the cardiovascular and nervous systems. A small chlorine content significantly increases the therapeutic effect of water, and an increased number of sulfates is important for the treatment of diseases of the gastrointestinal tract. Due to the unique complex of minerals that make up Nabeglavi, the mineral water acts as an “inside shower” and perfectly cleanses the body. In addition, it enhances the immune system and is effective in the prevention and treatment of diseases of the digestive system and metabolism, also contributing to the removal of waste [37].
- ➢
- Sairme—Natural table, healing-table and healing mineral waters which are formed in the deep zones of the earth’s crust, where they are saturated with natural carbon dioxide and come to the surface in the form of natural ascending (nongravity) spring. They are located in the west of Georgia, at an altitude of 950 m above sea level. The springs come from several sources and differ from each other in chemical composition and healing properties.
- ➢
- Bakuriani—Natural very low mineral content water, saturated with mineral complexes, regulates the body’s water balance. Frequent use of this water activates the physiological state of a person. The springs are in the east of Georgia. This water is characterized by a minimal concentration of calcium, potassium, sodium and magnesium, and contains a small amount of fluorine and iodine ions. It is recommended for use in baby food, and, with its unique composition, it is the best for people leading an active lifestyle [38].
- ➢
- Healing water Lugela is unique not only in Georgia, but throughout the world. This is calcium chloride 9% mineral water and is transparent and odorless with a slightly bitter taste, which does not freeze even at the lowest possible temperature. The springs of the Lugela mineral waters are located in Western Georgia. It is a healing calcium chloride water that treats diseases of the skeletal system and joints, allergies, nephritis, stomatitis and bleeding [39,40,41].
- ➢
- Mineral water Zvare is rich in iodine. It is used to treat the digestive system, chronic gastritis, pancreatitis, liver and biliary tract diseases, metabolic disorders and to balance acidity. The springs are located in Imereti (Western Georgia), at an altitude of 800 m above sea level.
- ➢
- Living water—This is the name of the thermal radon mineral waters of Tskhaltubo located at an altitude of 100 m above sea level. Biologically active trace elements were found in the mineral springs—iodine, bromine, manganese, lithium, boron, zinc, strontium, copper—which play an important role in the life of the body. The physical and chemical properties of water are unique: it is warm (35 °C), light, clean and odorless. The mineral springs were mainly used for bathing and inhalation, but in recent years natural radon waters have been increasingly used for drinking treatment. Radon therapy is used for diseases of the cardiovascular system, musculoskeletal system, digestive organs, central and peripheral nervous systems, skin diseases, metabolic disorders [42].
- ➢
- ➢
- Bahmaro is fresh water with a very low salinity, the chemical composition of which is dominated by magnesium and calcium hydrocarbonates, it is saturated with oxygen and has an unusually mild taste. The water is approved and recommended for use in baby food [45].
- ➢
2. Materials and Methods
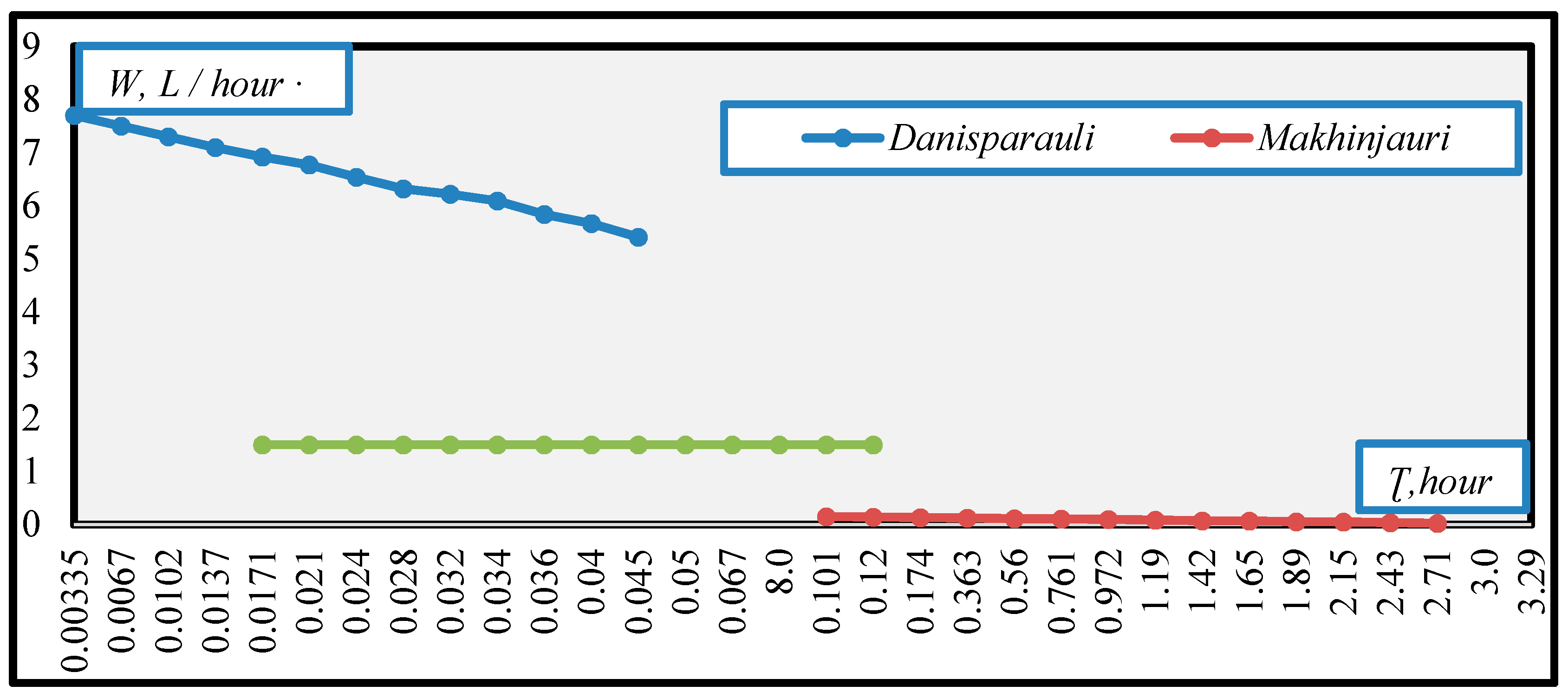
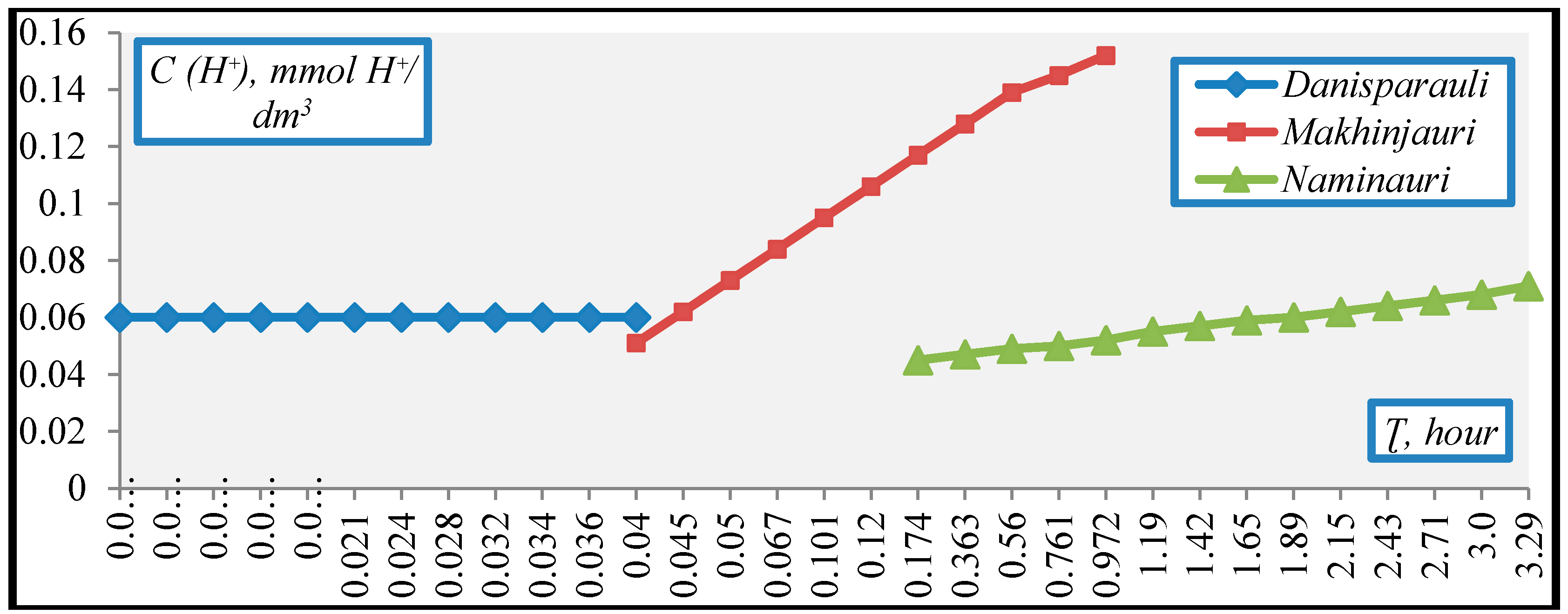
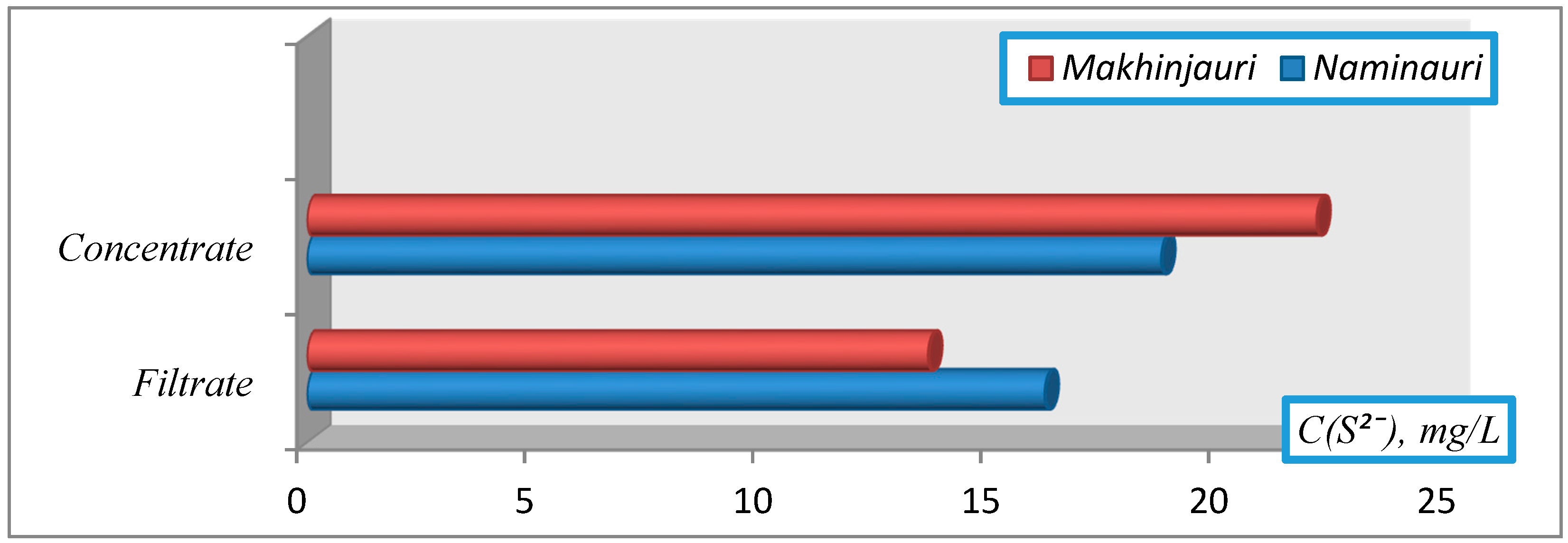
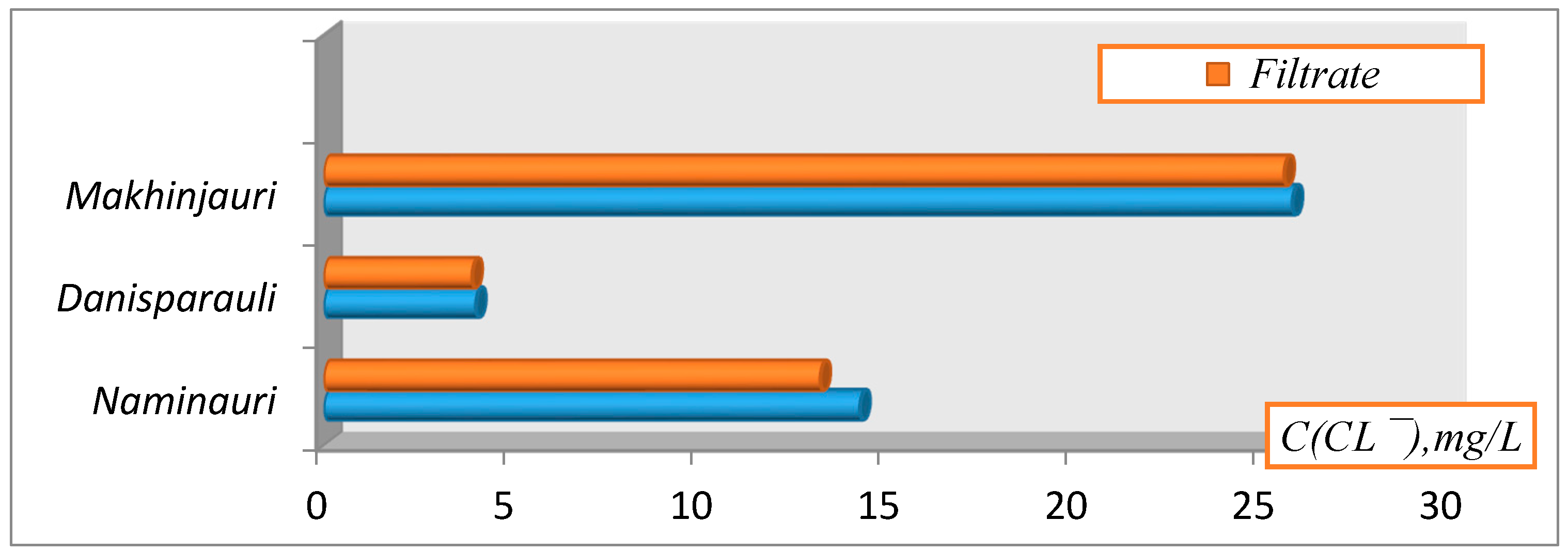
3. Results and Discussion
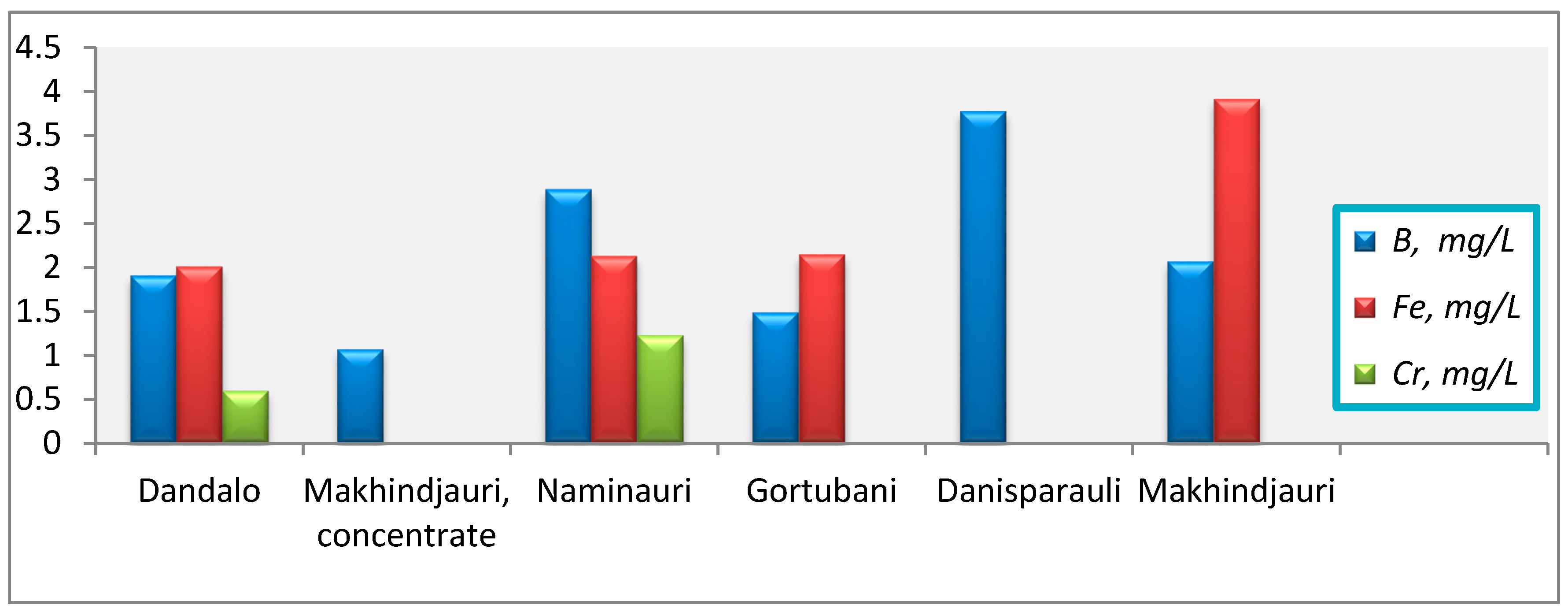
- B (mg/L): Makhinjauri concentrate (1.07)→Gortubani (1.49)→Dandalo (1.91)→Makhinjauri (2.07)→Naminauri (2.89)→Danisparauli (3.78);
- Fe (mg/L): Dandalo (2.01)→Naminauri (2.13)→Gortubani (2.15)→Makhinjauri (3.92);
- Cr (mg/L): Dandalo (0.598)→Naminauri (1.23).
- K(mg/L): Makhinjauri (44.5)→Danisparauli (47.1)→Makhinjauri concentrate (56.6)→Gortubani (59.0)→Naminauri (59)→Dandalo (71.4);
- Ca(mg/L): Naminauri (25.5)→Makhinjauri concentrate (54.5)→Dandalo (10.7)→Danisparauli(12.1);
- Mg(mg/L): Makhinjauri (42.0)→Naminauri (52.1)→Gortubani (57.1)→Danisparauli (72.7)→Makhinjauri concentrate (77.8).
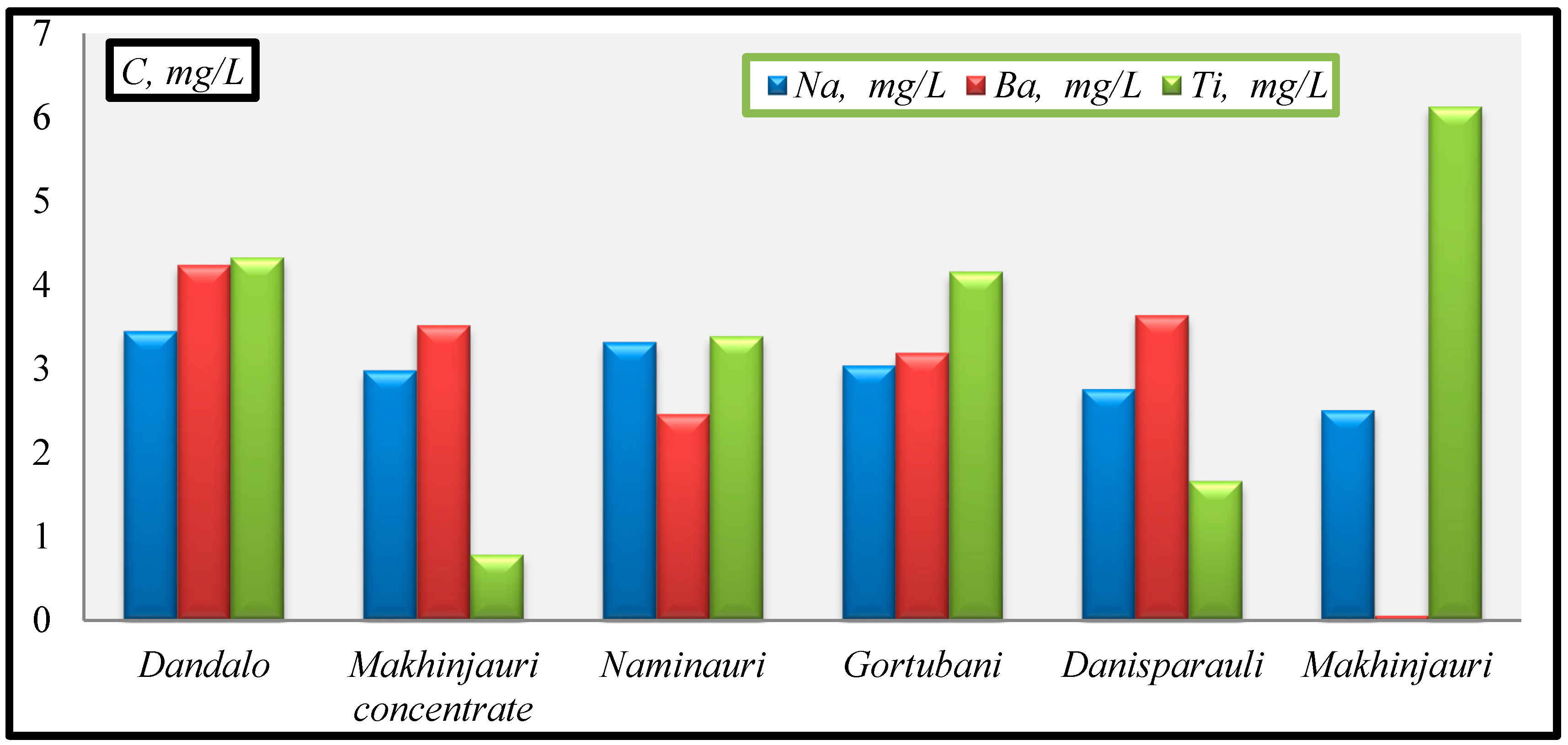
- Na(mg/L): Makhinjauri (2.51)→Danisparauli (2.76)→Makhinjauri concentrate (2.98)→Gortubani (3.04)→Naminauri (3.32)→Dandalo(3.45);
- Ba(mgL): Makhinjauri (0.0567)→Naminauri (2.46)→Gortubani (3.19)→Makhinjauri concentrate (3.52)→Danisparauli (3.64)→Dandalo.(4.24);
- Ti(mg/L): Makhinjauri concentrate (0.786)→Danisparauli (1.66)→Naminauri (3.39)→Gortubani (4.16)→Dandalo (4.33)→Makhinjauri (6.13).
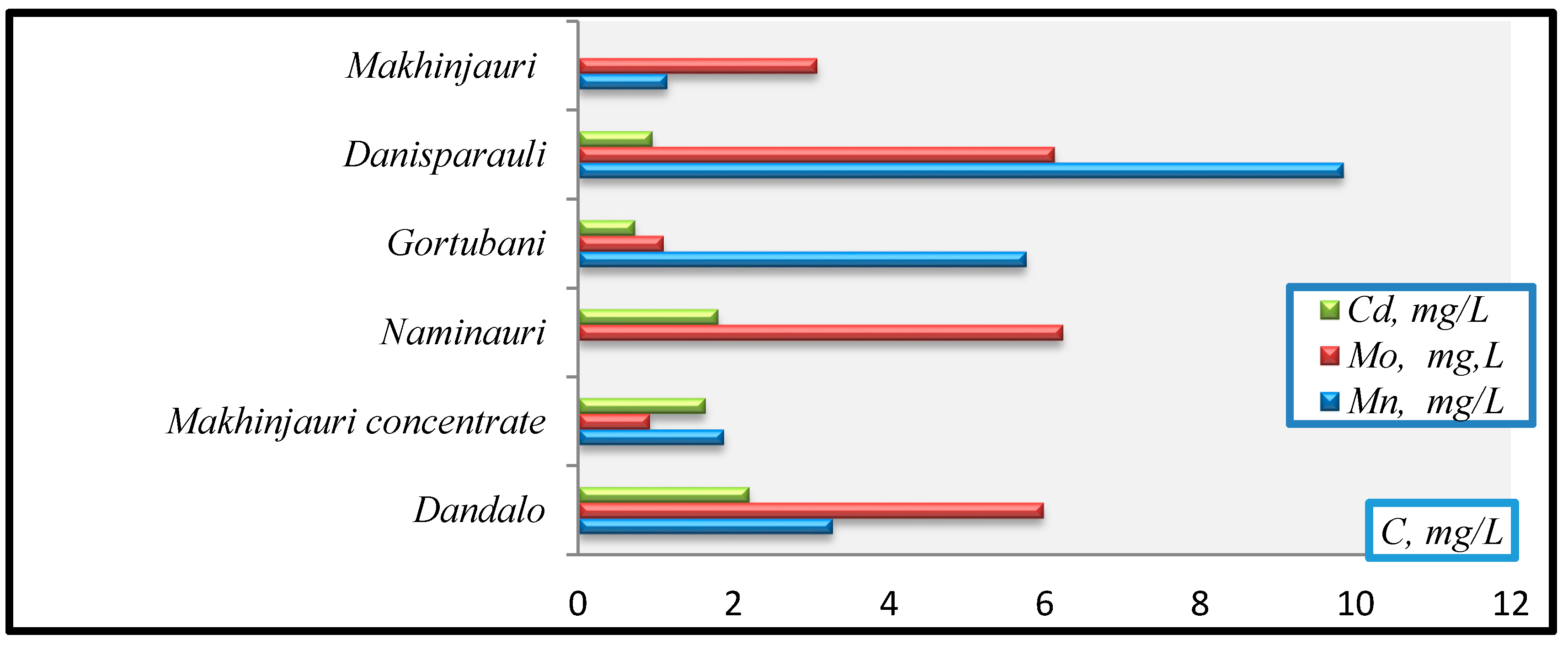
- Cd(mg/L): Gortubani (0.72)→Danisparauli (0.944)→Makhinjauri concentrate (1.63→Naminauri (1.79)→Dandalo (2.19).
- Mo (mg/L): Makhinjauri concentrate (0.918)→Gortubani (1.09)→Makhinjauri (3.07)→Dandalo (5.98)→Danisparauli (6.12)→Naminauri (6.23).
- Mn (mg/L): Makhinjauri (1.14)→Makhinjauri concentrate (1.87)→Dandalo (3.27)→Gortubani (5.76)→Danisparauli (9.84).
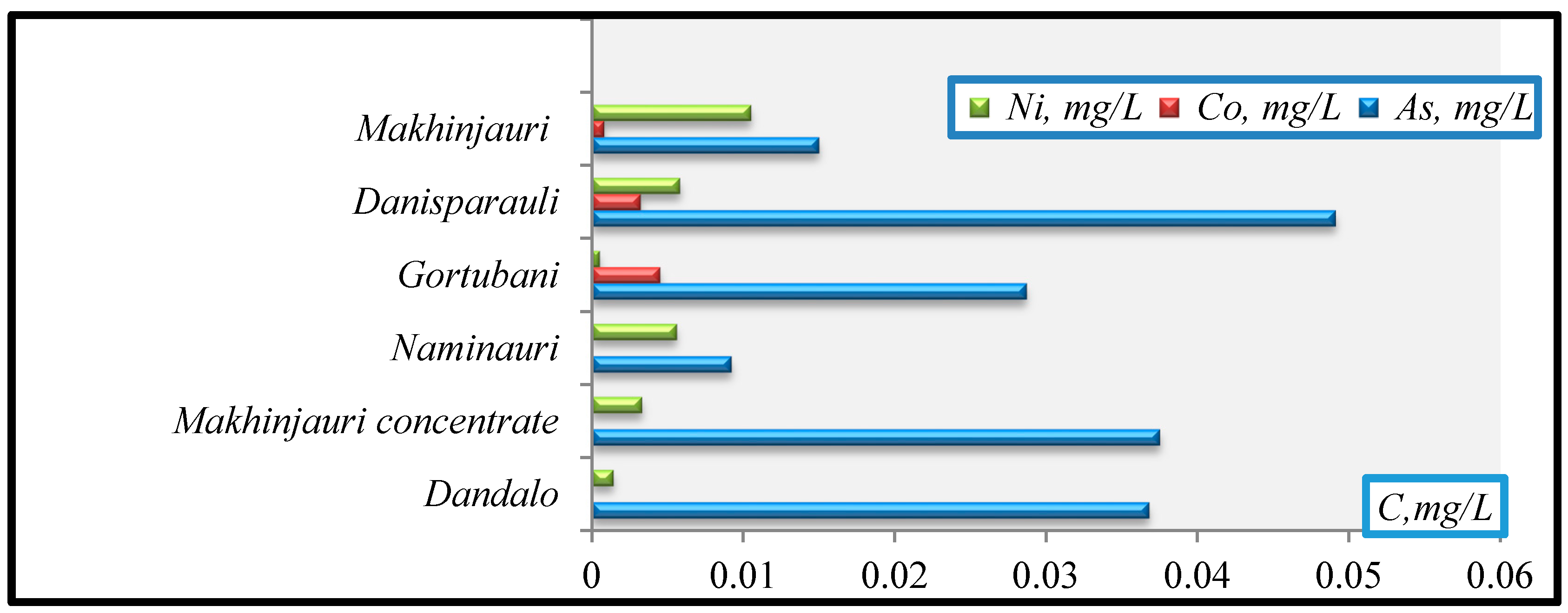
- Ni(mg/L): Gortubani (0.0005)→Dandalo (0.0014)→Makhinjauri concentrate(0.0033)→Naminauri (0.0056)→Danisparauli (0.0058)→Makhinjauri (0.0105);
- Co (mg/L): Makhinjauri (0.0008)→Danisparauli (0.0032)→Gortubani (0.0045), Dandalo.
- As(mg/L): Naminauri (0.0092)→Makhinjauri (0.015)→Gortubani (0.0287)→Dandalo (0.036)→Makhinjauri concentrate (0.0375)→Danisparauli (0.0491).
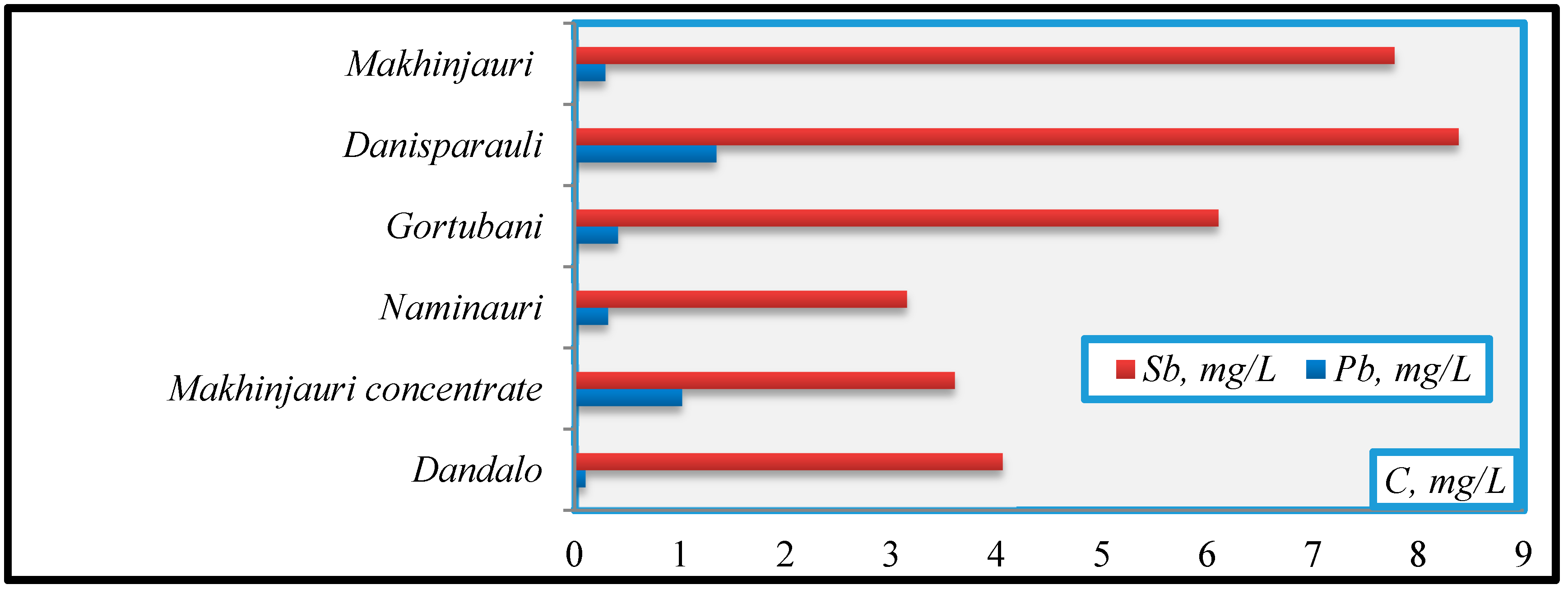
- Sb (mg/L): Naminauri (3.15)→Makhinjauri concentrate (3.6)→Dandalo (4.06)→Gortubani (6.1)→Makhinjauri (7.77)→Danisparauli (8.38);
- Pb(mg/L): Dandalo(0.096)→Naminauri(0.317)→Gortubani(0.412)→Makhinjauri(0.298)→Makhinjauri concentrate (1.02)→Danisparauli (1.34).
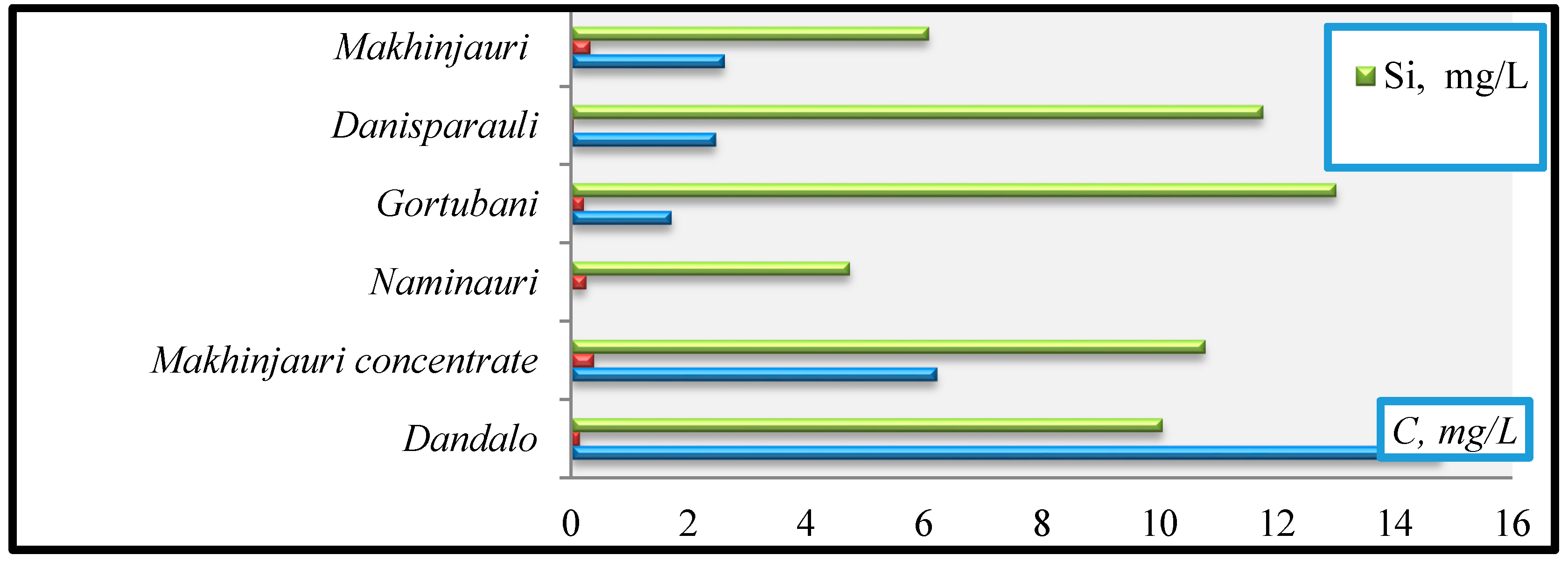
- Si (mg/L): Naminauri (4.73)→Makhinjauri (6.07)→Dandalo (10.05)→Makhinjauri concentrate (10.77)→Danisparauli (11.75)→Gortubani (13).
- Hg (mg/L): Danisparauli (0.0267)→Dandalo (0.132)→Gortubani (0.208)→Naminauri (0.243)→Makhinjauri (0.315)→Makhinjauri concentrate (0.376).
- V(mg/L): Gortubani (1.7)→Danisparauli (2.45)→Makhinjaur (2.6)→Makhinjauri concentrate (6.21)→Dandalo (14.8). In the water of Naminauri were not found.
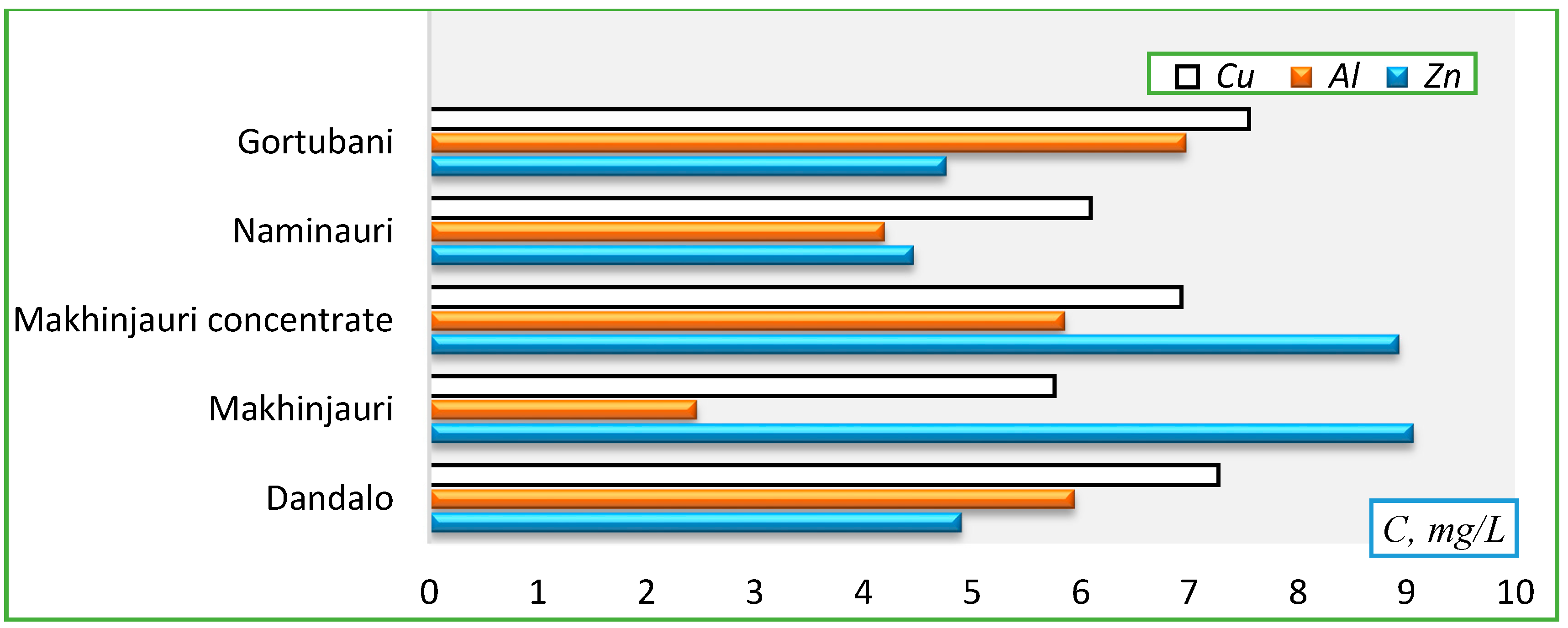
- Zn (mg/L): Naminauri (4.46)→Gortubani (4.76)→Dandalo (4.9)→Danisparauli (8.65)→Makhinjauri concentrate (8.93)→Makhinjauri (9.06).
- Al (mg/L): Makhinjauri (2.46)→Naminauri (4.19)→Dandalo (5.65)→Makhinjauri concentrate (5.85)→Gortubani (6.97)→Danisparauli (8.16).
- Cu(mg/L): Makhinjauri (5.76)→Naminauri (6.09)→Makhinjauri concentrate (9.93)→Danisparauli (7.02)→Dandalo (7.27)→Gortubani (7.55).
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prokopenko, O.; Eremenko, Y.; Omelyanenko, V. Role of international factor in innovation ecosystem formation. Econ. Ann. XXI 2014, 3–4, 4–7. [Google Scholar]
- Prokopenko, O.; Kysly, V.; Shevchenko, H. Peculiarities of the natural resources economic estimation under the transformational conditions. Econ. Ann. XXI 2014, 7–8, 40–43. [Google Scholar]
- Ishchenko, V.; Pohrebennyk, V.; Borowik, B.; Falat, P.; Shaikhanova, A. Toxic substances in hazardous household waste. In Proceedings of the International Multidisciplinary Scientific GeoConference SGEM, Albena, Bulgaria, 2–8 July 2018; Volume 18, pp. 223–230. [Google Scholar]
- Mitryasova, O.; Pohrebennyk, V. Hydrochemical indicators of water system analysis as factors of the environmental quality state. In Sustainable Production: Novel Trends in Energy, Environment and Material Systems. Studies in Systems, Decision and Control; Królczyk, G., Wzorek, M., Król, A., Kochan, O., Su, J., Kacprzyk, J., Eds.; Springer: Cham, Switzerland, 2020; Volume 198, pp. 91–104. [Google Scholar]
- Mitryasova, O.; Pohrebennyk, V. Integrated environmental assessment of the surface waters pollution: Regional aspect. In Proceedings of the International Multidisciplinary Scientific GeoConference SGEM, Vienna, Austria, 27–29 November 2017; Volume 33, pp. 235–242. [Google Scholar]
- Pohrebennyk, V.; Petryk, A. The degree of pollution with heavy metals of fallow soils in rural administrative units of Psary and Płoki in Poland. In Proceedings of the 17th International Multidisciplinary Scientific GeoConference, SGEM, Albena, Bulgaria, 29 June–5 July 2017; Volume 17, pp. 967–974. [Google Scholar]
- Mitryasova, O.; Pohrebennyk, V.; Cygnar, M.; Sopilnyak, I. Environmental natural water quality assessment by method of correlation analysis. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, Albena, Bulgaria, 30 June–6 July 2016; Volume 2, pp. 317–324. [Google Scholar]
- Cherniuk, V.V.; Ivaniv, V.V.; Bihun, I.V.; Wojtowicz, J.M. Coeffificient of flow rate of inlet cylindrical nozzles with lateral orthogonal Inflflow. In Proceedings of the CEE International Conference Current Issues of Civil and Environmental Engineering, Lviv–Košice–Rzeszów, Lviv, Ukraine, 11–13 September 2019; pp. 50–57. [Google Scholar] [CrossRef]
- Bosak, N.; Cherniuk, V.; Matlai, I.; Bihun, I. Studying the mutual interaction of hydraulic characteristics of water distributing pipelines and their spraying devices in the coolers at energy units. East. Eur. J. Enterp. Technol. 2019, 3, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Mitryasova, O.; Pohrebennyk, V.; Kardasz, P.; Pohrebennyk, V.; Kardasz, P. Hydrochemical aspects of surface water quality assessment. In Proceedings of the 18th International Multidisciplinary Scientific Geoconference, SGEM, Albena, Bulgaria, 2–8 July 2018; Volume 18, pp. 513–520. [Google Scholar]
- Bejanidze, I.; Pohrebennyk, V.; Kharebava, T.; Koncelidze, Z.; Jun, S. Development of waste-free, eco-pure combined technology for fruit processing. In Proceedings of the International Multidisciplinary Scientific GeoConference SGEM, Albena, Bulgaria, 30 June–6 July 2019; Volume 19, pp. 173–180. [Google Scholar]
- Bejanidze, I.; Pohrebennyk, V.; Kharebava, T.; Koncelidze, L.; Sun, J. Correction of the chemical composition of the washing waters received as a result of h cation exchange of ionex change resin. In Proceedings of the International International Multidisciplinary Scientific GeoConference SGEM, Albena, Bulgaria, 30 June–6 July 2019; Volume 19, pp. 133–140.
- Bejanidze, I.; Kardash, P.; Pohrebennyk, V.; Kharebava, T.; Didmanidze, N. Influence of technological parameters of ultrafiltration process of vegetable juices on their quality. In Proceedings of the International Multidisciplinary Scientific GeoConference SGEM, Albena, Bulgaria, 30 June–6 July 2019; Volume 19, pp. 321–328. [Google Scholar]
- Bejanidze, I.; Tavdgiridze, G.; Kharebava, T. Microbiological estimation of electrodialistic process of obtain potable water. Eur. Sci. Rev. 2017, 7–8, 137–139. [Google Scholar]
- Crompton, T.R. Determination of Toxic Organic Chemicals in Natural Waters, Sediments and Soils. Determination and Analysis; Academic Press: Cambridge, MA, USA, 2019; p. 422. ISBN 9780128165300. [Google Scholar]
- Raimer, A.A. Characteristic of the composition of mineral water. New Sci. Exp. Tradit. Innov. 2016, 591-2, 23–25. (In Russia) [Google Scholar]
- Charis, M.; Galanakis, E.A. Sustainable Water and Wastewater Processing; Elsevier: Amsterdam, The Netherlands, 2010; p. 393. ISBN 978012816170. [Google Scholar]
- Prasad, M.N.V. Disinfection By-products in Drinking Water. Detection and Treatment; Butterworth-Heinemann: Oxford, UK, 2020; p. 490. ISBN 9780081029770. [Google Scholar]
- Crompton, T.R. Determination of Metals in Natural Waters, Sediments, and Soils; Elsevier Science: Amsterdam, The Netherlands, 2015; p. 318. ISBN 9780128026540. [Google Scholar]
- Pavlova, A.; Krylova, O.; Vasnetsova, O. Mineral waters as a pharmaceutical product. Russia. Pharmacy 2018, 4, 7–12. [Google Scholar]
- Romanova, O. Treatment Wthin Live and Dead Water; Minsk: Vector, Belarus, 2007. [Google Scholar]
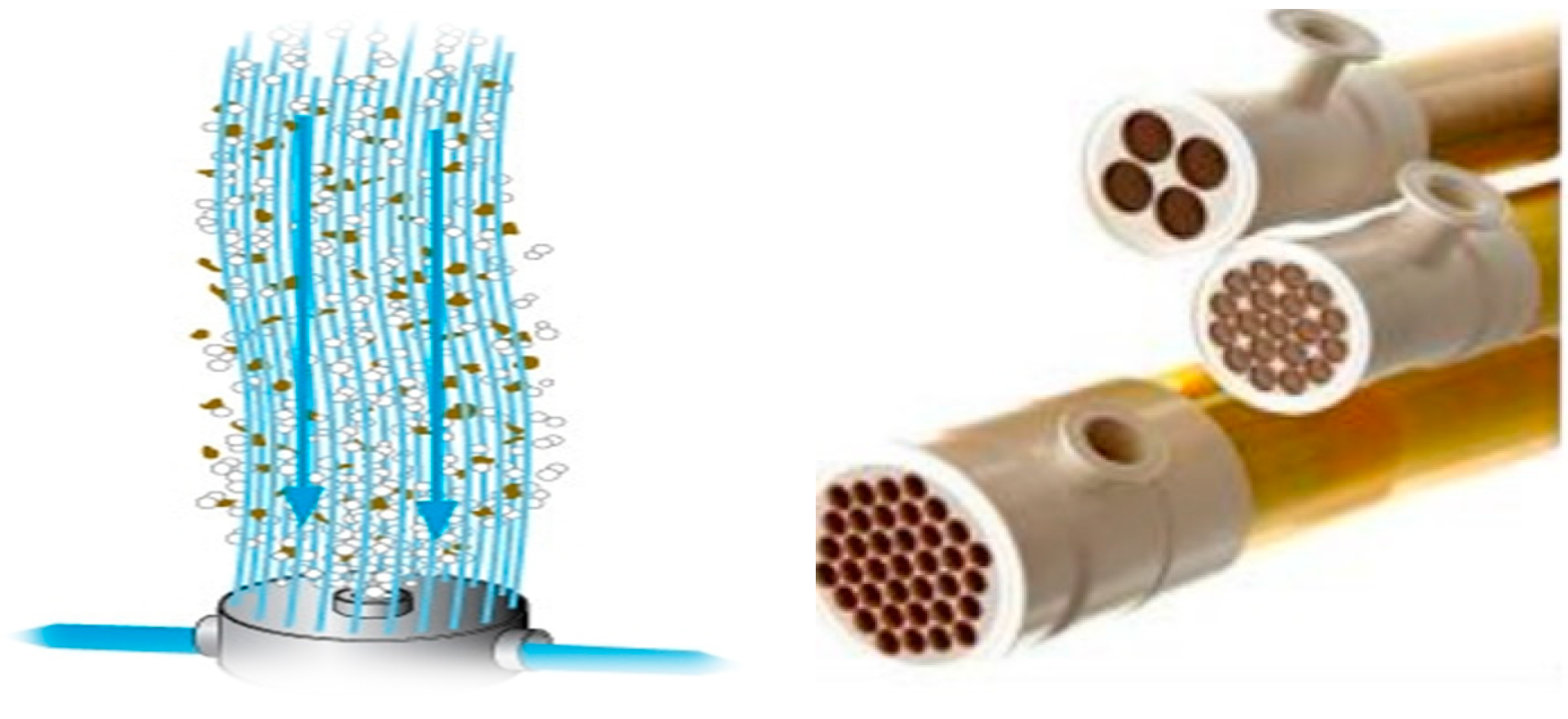
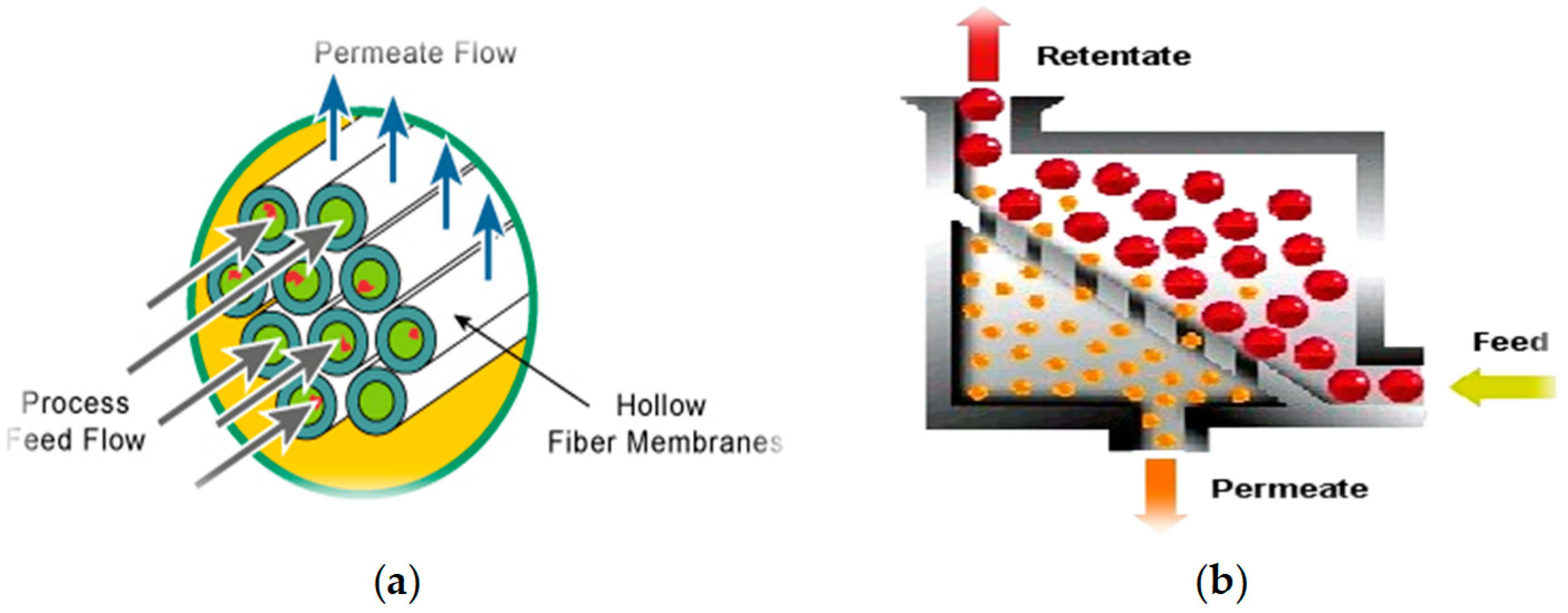
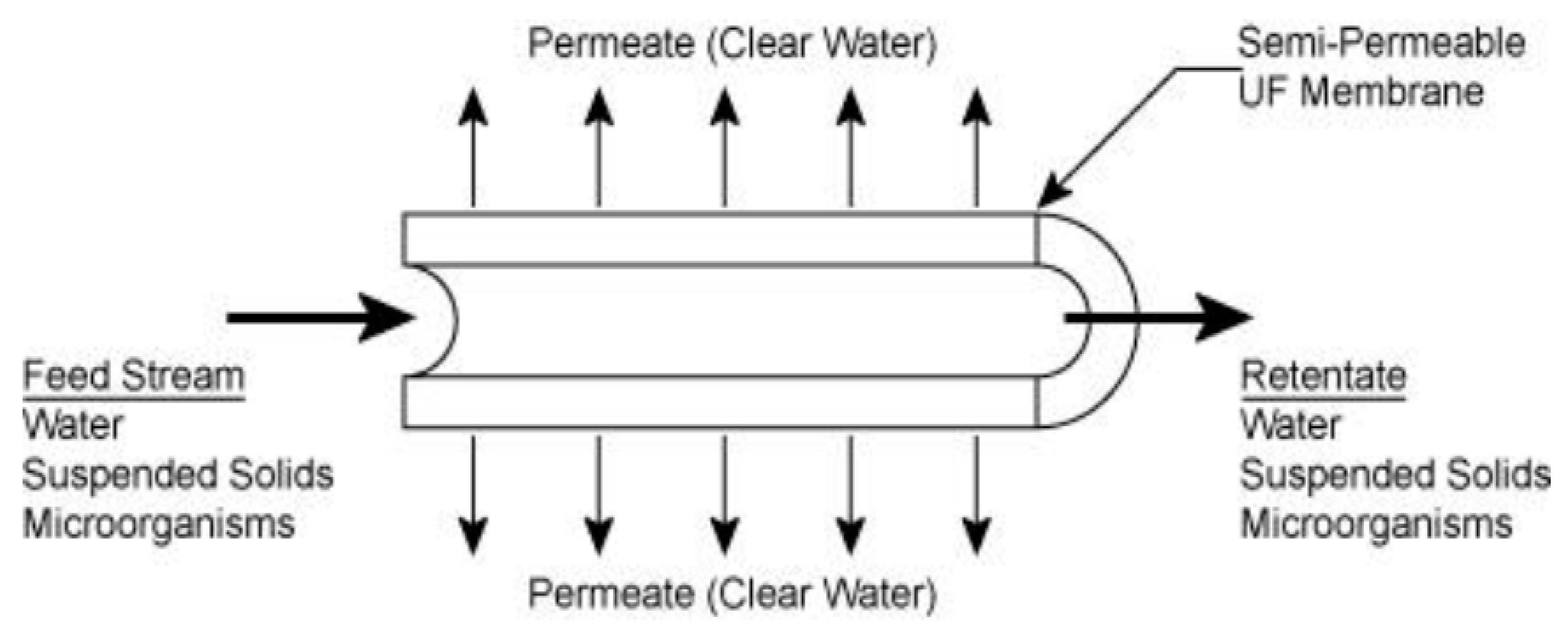
- Feng, Y.; Chung, T.-S. Hollow Fiber Membranes. Fabrication and Applications; Elsevier: Amsterdam, The Netherlands, 2020; p. 412. ISBN 9780128218761. [Google Scholar]
- Lidin, A. Mineral Waters; Publishing House: Quezon, Philippines, 2009. [Google Scholar]
- Bejanidze, I.; Kharebava, T.; Davitadze, N.; Koncelidze, Z.; Koncelidze, L. Monograph: Purification of natural and wastewater by electromembrane methods; BSU: Bulacan, Philippines, 2019; p. 178. [Google Scholar]
- Mitache, M.M.; Neagu, L.C.; Carmen, M. Microbiological and chemical characterization of bottled water. Sci. Beverages 2019, 4, 209–226. [Google Scholar]
- Huertos, M.L. Ecology and Management of Inland Waters. A Californian Perspective with Global Applications; Elsevier: Amsterdam, The Netherlands, 2020; p. 554. ISBN 9780128142660. [Google Scholar]
- Bejanidze, I.; Kharebava, T.; Kekutia, L.; Davitadze, N. The reagent-free, eco-pure technology for purification of natural waters from heavy metals. In Proceedings of the Third International Conference of European Academy of Science, Bonn, Germany, 20–30 December 2018; pp. 79–81. [Google Scholar]
- Zainchkovskiy, A.; Kushnirenko, A. Market of mineral water in ukraine: problems of quality and safety. Scientific Works of NUFT 2018, 24, 120–129. [Google Scholar] [CrossRef]
- Quattrini, S.; Pampaloni, B.; Brandi, M.L. Natural mineral waters: Chemical characteristics and health effects. Clin. Cases Miner Bone Metab. 2016, 13, 173–180. [Google Scholar] [CrossRef]
- Ahmad, A.; Azam, T. Water purification technologies bottled and packaged water. Sci. Beverages 2019, 4, 83–120. [Google Scholar]
- Bacciottini, L.; Tanini, A.; Falchetti, A.; Masi, L.; Franceschelli, F.; Pampaloni, B.; Giorgi, G.; Brandi, M.L. Calcium bioavailability from a calcium-rich mineral water, with some observations on method. J. Clin. Gastroenterol. 2004, 38, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Chau, N.D.; Tomaszewska, B. Bottled and packaged water. Sci. Beverages 2019, 4, 1–38. [Google Scholar]
- Bertoni, M.; Olivieri, F.; Manghetti, M.; Boccolini, E.; Bellomini, M.G.; Blandizzi, C.; Bonino, F.; del Tacca, M. Effects of a bicarbonate-alkaline mineral water on gastric functions and functional dyspepsia: A preclinical and clinical study. Pharmacol. Res. 2002, 46, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A. Water Purification; Academic Press: Cambridge, MA, USA, 2017; p. 772. ISBN 9780128043004. [Google Scholar]
- Schnug, E.; Hassoun, R.; Haneklaus, S. Contribution of mineral and tap water to the dietary intake of as, B, Ca, Ce, Cu, F, La, Li, Mo, Ni, P, Pb, Sr, U, and Zn by humans water purification technologies bottled and packaged water. Sci. Beverages 2019, 4, 241–276. [Google Scholar]
- Singh, R.; Hankins, N. Emerging Membrane Technology for Sustainable Water Treatment; Elsevier: Amsterdam, The Netherlands, 2016; p. 480. ISBN 9780444633125. [Google Scholar]
- Sparling, D. Wildlife, Fisheries, Forests and Parks. Natural Resource. Administration; Academic Press: Cambridge, MA, USA, 2014; p. 374. ISBN 9780124046474. [Google Scholar]
- Gupta, V.K.; Imran, A. Environmental Water: Advances in Treatment, Remediation and Recycling; Elsevier Science: Amsterdam, The Netherlands, 2012; p. 426. ISBN 9780444594037. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 1997; pp. 5–7. [Google Scholar]
- Pavlova, A.; Krylova, O.; Vasnetsova, O. Classification of mineral waters. Russia. Pharmacy 2018, 1, 8–13. [Google Scholar]
- Bothe, G.; Coh, A.; Auinger, A. Efficacy and safety of a natural mineral water rich in magnesium and sulphate for bowel function: A double-blind, randomized, placebo-controlled study. Eur. J. Nutr. Springer Nat. 2015, 56, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Karagülle, O.; Kleczka, T.; Vidal, C.; Candir, F.; Gundermann, G.; Külpmann, W.; Gehrke, A.; Gutenbrunner, C. Magnesium Absorption from Mineral Waters of Different Magnesium Content in Healthy Subjects; Complementary Medicine Research Karger AG: Basel, Switzerland, 2006; p. 13. ISSN 2504–2106. [Google Scholar]
- Rylander, R.; Maurice, J. Mineral water intake reduces blood pressure among subjects with low urinary magnesium and calcium levels. BMC Public Health Springer Nat. 2004, 56, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Greupner, T.; Schneider, I.; Hahn, A. Calcium bioavailability from mineral waters with different mineralization in comparison to milk and a supplement. J. Am. Coll. Nutr. 2017, 36, 386–390. [Google Scholar] [CrossRef]
- Meunier, P.; Jenvrin, C.; Munoz, M.; Gueronnière, V.; Garnero, P.; Menz, M. Consumption of a high calcium mineral water lowers biochemical indices of bone remodeling in postmenopausal women with low calcium intake. Osteoporos. Int. 2005, 16, 1203–1209. [Google Scholar] [CrossRef]
- GOST R 54316-2011. National standard of the Russian Federation. Natural Mineral Drinking Waters; General technical conditions; National Standard of the Russian Federation: Moskva, Russia, 2011. [Google Scholar]
- Tvalchrelidze, A.; Silagadze, A. Freshwater mineral water of Georgia for Europe. Cauc. Glob. 2011, 5, 53–64. [Google Scholar]
- EU’s Drinking Water Standards. Lenntech: Water Treatment Solutions. Available online: http://www.lenntech.com/ applications/drinking/standards/eu-s-drinking-water-standards.htm (accessed on 17 September 2020).
- Tarkhan-Mouravi, I. Resorts of Georgia; Balneological Resort Publishing House: Tbilisi, Georgia, 2009; p. 83. [Google Scholar]
- Janelidze, A. Treatment by Sairme; Springs: Tbilisi, Georgia, 2006; p. 46. [Google Scholar]
- Petrova, N.G.; Alibaev, S.G.; Shupineva, A.V. The therapeutic properties of water. Beer Drink. 2013, 6, 60–61. [Google Scholar]
- Sekerina, I.N. Monitoring of borjomi deposit of carbon dioxide mineral water as the informational basis for the study and revaluation of reserves. Geoecol. Eng. Geol. Hydrogeol. Geocryol. 2019, 3, 83–89. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bejanidze, I.; Petrov, O.; Kharebava, T.; Pohrebennyk, V.; Davitadze, N.; Didmanidze, N. Study of the Healing Properties of Natural Sources of Georgia and Modeling of Their Purification Processes. Appl. Sci. 2020, 10, 6529. https://doi.org/10.3390/app10186529
Bejanidze I, Petrov O, Kharebava T, Pohrebennyk V, Davitadze N, Didmanidze N. Study of the Healing Properties of Natural Sources of Georgia and Modeling of Their Purification Processes. Applied Sciences. 2020; 10(18):6529. https://doi.org/10.3390/app10186529
Chicago/Turabian StyleBejanidze, Irina, Oleksandr Petrov, Tina Kharebava, Volodymyr Pohrebennyk, Nazi Davitadze, and Nato Didmanidze. 2020. "Study of the Healing Properties of Natural Sources of Georgia and Modeling of Their Purification Processes" Applied Sciences 10, no. 18: 6529. https://doi.org/10.3390/app10186529
APA StyleBejanidze, I., Petrov, O., Kharebava, T., Pohrebennyk, V., Davitadze, N., & Didmanidze, N. (2020). Study of the Healing Properties of Natural Sources of Georgia and Modeling of Their Purification Processes. Applied Sciences, 10(18), 6529. https://doi.org/10.3390/app10186529



