The Protective Effect of Static Magnetic Fields with Different Magnetic Inductions against Fluoride Toxicity Is Related to the NRF2 Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. RNA Extraction
2.3. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR) Assay
2.4. Apoptosis Assay
2.5. Statistical Analyses
3. Results
3.1. Transcriptional Activity of the Cellular Antioxidant System-Related Genes
3.2. Transcriptional Activity of the Apoptosis-Related Genes
3.3. Apoptosis Assay
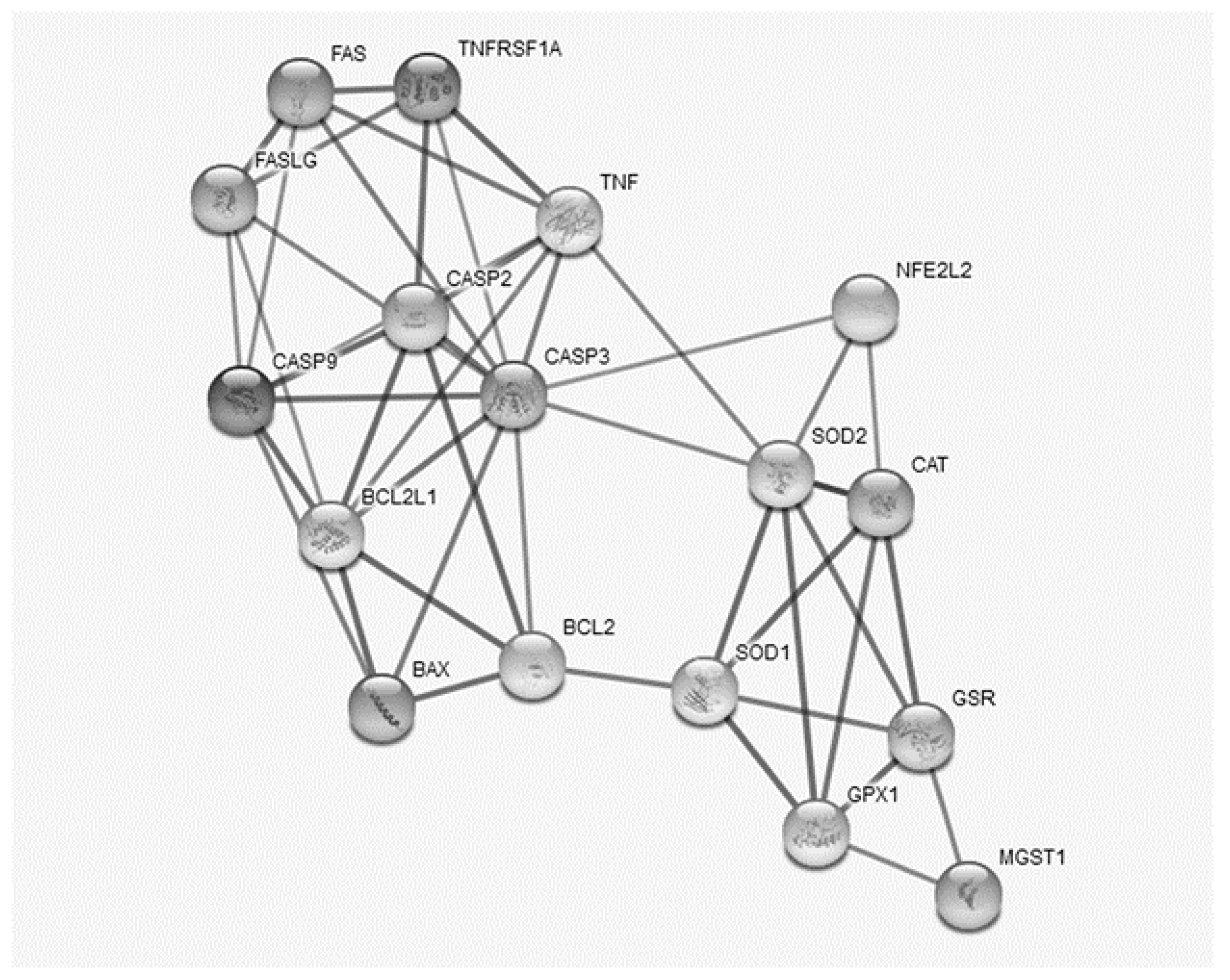
3.4. Relationships between Oxidative Stress and the Apoptosis Process
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Wu, J.; Cheng, M.; Liu, Q.; Yang, J.; Wu, S.; Lu, X.; Jin, C.; Ma, H.; Cai, Y. Protective Role of tert-Butylhydroquinone Against Sodium Fluoride-Induced Oxidative Stress and Apoptosis in PC12 Cells. Cell Mol. Neurobiol. 2015, 35, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, H.; Ba, Y.; Cheng, X.M.; Cui, L.X. Effect of oxidative stress on fluoride-induced apoptosis in primary cultured Sertoli cells of rats. Int. J. Environ. Health Res. 2015, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur Lekarski. 2020, 48, 124–127. [Google Scholar]
- Tan, S.N.; Sim, S.P.; Khoo, A.S. Potential role of oxidative stress-induced apoptosis in mediating chromosomal rearrangements in nasopharyngeal carcinoma. Cell Biosci. 2016, 6, 35. [Google Scholar] [CrossRef]
- Ozben, T. Oxidative stress and apoptosis: Impact on cancer therapy. J. Pharm. Sci. 2007, 96, 2181–2196. [Google Scholar] [CrossRef] [PubMed]
- Ghodbane, S.; Lahbib, A.; Sakly, M.; Abdelmelek, H. Bioeffects of static magnetic fields: Oxidative stress, genotoxic effects, and cancer studies. Biomed. Res. Int. 2013, 2013, 602987. [Google Scholar] [CrossRef]
- Kheifets, L.I.; Greenberg, R.S.; Neutra, R.R.; Hester, G.L.; Poole, C.L.; Rall, D.P.; Lundell, G. Electric and magnetic fields and cancer: Case study. Am. J. Epidemiol. 2001, 154, S50–S59. [Google Scholar] [CrossRef][Green Version]
- Albuquerque, W.W.; Costa, R.M.; de Fernandes, T.S.; Porto, A.L. Evidences of the static magnetic field influence on cellular systems. Prog. Biophys. Mol. Biol. 2016, 121, 16–28. [Google Scholar] [CrossRef]
- Coballase-Urrutia, E.; Navarro, L.; Ortiz, J.L.; Verdugo-Díaz, L.; Gallardo, J.M.; Hernández, M.E.; Estrada-Rojo, F. Static Magnetic Fields Modulate the Response of Different Oxidative Stress Markers in a Restraint Stress Model Animal. Biomed. Res. Int. 2018, 2018, 3960408. [Google Scholar] [CrossRef]
- Sirmatel, O.; Sert, C.; Sirmatel, F.; Selek, S.; Yokus, B. Total antioxidant capacity, total oxidant status and oxidative stress index in the men exposed to 1.5 T static magnetic field. Gen. Physiol. Biophys. 2007, 26, 86–90. [Google Scholar]
- Kimsa-Dudek, M.; Synowiec-Wojtarowicz, A.; Derewniuk, M.; Gawron, S.; Paul-Samojedny, M.; Kruszniewska-Rajs, C.; Pawłowska-Góral, K. Impact of fluoride and a static magnetic field on the gene expression that is associated with the antioxidant defense system of human fibroblasts. Chem. Biol. Interact. 2018, 287, 13–19. [Google Scholar] [CrossRef]
- Kimsa-Dudek, M.; Synowiec-Wojtarowicz, A.; Derewniuk, M.; Paul-Samojedny, M.; Pawłowska-Góral, K. The effect of simultaneous exposure of human fibroblasts to fluoride and moderate intensity static magnetic fields. Int. J. Radiat. Biol. 2019, 95, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Yadav, A.; Singh, S.V.; Mishra, P.; Rath, S.K. Isoniazid induces apoptosis: Role of oxidative stress and inhibition of nuclear translocation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Life Sci. 2018, 199, 23–33. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Xu, F.; Zhang, Y.; Li, Z.; Ju, X.; Wang, L. Insoluble-bound polyphenols of adlay seed ameliorate H2O2-induced oxidative stress in HepG2 cells via Nrf2 signalling. Food Chem. 2020, 325, 126865. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef]
- Sun, P.; Nie, X.; Chen, X.; Yin, L.; Luo, J.; Sun, L.; Wan, C.; Jiang, S. Nrf2 Signaling Elicits a Neuroprotective Role Against PFOS-mediated Oxidative Damage and Apoptosis. Neurochem. Res. 2018, 43, 2446–2459. [Google Scholar] [CrossRef]
- Zhang, W.; Li, M.; Li, N.; Liu, Z. Regulation of Keap-1/Nrf2 Signaling Pathway Is Activated by Oxidative Stress in Patients with Premature Rupture of Membranes. Med. Sci. Monit. 2020, 26, e921757. [Google Scholar] [CrossRef]
- Gawron, S.; Glinka, M.; Wolnik, T. Magnetyczna komora badawcza dedykowana do hodowli komórek. Zeszyty Problemowe-Maszyny Elektryczne 2012, 4, 11–16. [Google Scholar]
- Glinka, M.; Gawron, S.; Sieroń, A.; Pawłowska-Góral, K.; Cieślar, G.; Sieroń-Stołtny, K. Test chambers for cell culture in static magnetic field. J. Magn. Mater. 2013, 331, 208–215. [Google Scholar] [CrossRef]
- Dini, L.; Abbro, L. Bioeffects of Moderate-Intensity Static Magnetic Fields on Cell Cultures. Micron 2005, 36, 195–217. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska-Góral, K.; Kimsa-Dudek, M.; Synowiec-Wojtarowicz, A.; Orchel, J.; Glinka, M.; Gawron, S. Effect of static magnetic fields and phloretin on antioxidant defense system of human fibroblasts. Environ. Sci. Pollut. Res. Int. 2016, 23, 14989–14996. [Google Scholar] [CrossRef] [PubMed]
- Kimsa-Dudek, M.; Synowiec-Wojtarowicz, A.; Krawczyk, A.; Kruszniewska-Rajs, C.; Gawron, S.; Paul-Samojedny, M.; Gola, J. Anti-apoptotic effect of a static magnetic field in human cells that had been treated with sodium fluoride. J. Environ. Sci. Health A Tox. Hazard. Substain. Environ. Eng. 2020, 1–8. [Google Scholar] [CrossRef]
- Dhar, V.; Bhatnagar, M. Physiology and toxity of fluoride. Indian J. Dent. Res. 2009, 20, 350–355. [Google Scholar] [CrossRef]
- Whitford, G.M. Acute toxity of ingested fluride. Monogr. Oral Sci. 2011, 22, 66–80. [Google Scholar]
- Wei, W.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Li, Y.; Wamg, X.; Zhao, L. A mini review of fluoride-induced apoptotic pathways. Environ. Sci. Pollut. Res. Int. 2018, 25, 33926–33935. [Google Scholar] [CrossRef]
- Xu, X.; Lai, Y.; Hua, Z.C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef]
- Turkmen, K. Inflammation, oxidative stress, apoptosis, and autophagy in diabetes mellitus and diabetic kidney disease: The Four Horsemen of the Apocalypse. Int. Urol. Nephrol. 2017, 49, 837–844. [Google Scholar] [CrossRef]
- Méndez-Armenta, M.; Nava-Ruiz, C.; Juárez-Rebollar, D.; Rodriguez-Martinez, E.; Gómez, P.Y. Oxidative stress associated with neuronal apoptosis in experimental models of epilepsy. Oxid. Med. Cell. Longev. 2014, 2014, 293689. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Javad-Moosavi, S.A.; Reiter, R.J.; Yarahmadi, R.; Ghaznavi, H.; Mehrzadi, S. Oxidative/nitrosative stress, autophagy and apoptosis as therapeutic targets of melatonin in idiopathic pulmonary fibrosis. Expert Opin. Ther. Targets 2018, 22, 1049–1061. [Google Scholar] [CrossRef]
- Blomgren, K.; Hagberg, H. Free radicals, mitochondria, and hypoxia-ischemia in the developing brain. Free Radic. Biol. Med. 2006, 40, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Liu, S.; Wang, C.; Wang, F.; Song, Y.; Yan, N.; Xi, S.; Liu, Z.; Sun, G. JNK and NADPH oxidase involved in fluoride-induced oxidative stress in BV-2 microglia cells. Mediators Inflamm. 2013, 2013, 895975. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.P.; Tiwari, S.K.; Shaik, A.P.; Alsaeed, A.; Sultana, A.; Reddy, P.K. Effect of sodium fluoride on neuroimmunological parameters, oxidative stress and antioxidative defenses. Toxicol. Mech. Methods 2014, 24, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Luo, Q.; Cui, H.; Deng, H.; Kuang, P.; Liu, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; et al. Sodium fluoride causes oxidative stress and apoptosis in the mouse liver. Aging 2017, 9, 1623–1639. [Google Scholar] [CrossRef]
- Ameeramja, J.; Perumal, E. Possible Modulatory Effect of Tamarind Seed Coat Extract on Fluoride-Induced Pulmonary Inflammation and Fibrosis in Rats. Inflammation 2018, 41, 886–895. [Google Scholar] [CrossRef]
- Zuo, H.; Chen, L.; Kong, M.; Qiu, L.; Lü, P.; Wu, P.; Yang, Y.; Chen, K. Toxic effects of fluoride on organisms. Life Sci. 2018, 198, 18–24. [Google Scholar] [CrossRef]
- Grandjean, P. Developmental fluoride neurotoxicity: An updated review. Environ. Health 2019, 18, 110. [Google Scholar] [CrossRef]
- Khan, N.M.; Ahmad, I.; Haqqi, T.M. Nrf2/ARE pathway attenuates oxidative and apoptotic response in human osteoarthritis chondrocytes by activating ERK1/2/ELK1-P70S6K-P90RSK signaling axis. Free Radic. Biol. Med. 2018, 116, 159–171. [Google Scholar] [CrossRef]
- Pan, H.; Wang, H.; Zhu, L.; Wang, X.; Cong, Z.; Sun, K.; Fan, Y. The involvement of Nrf2-ARE pathway in regulation of apoptosis in human glioblastoma cell U251. Neurol. Res. 2013, 35, 71–78. [Google Scholar] [CrossRef]
- Bonay, M.; Deramaudt, T.B. Nrf2: New insight in cell apoptosis. Cell Death Dis. 2015, 6, e1897. [Google Scholar] [CrossRef]
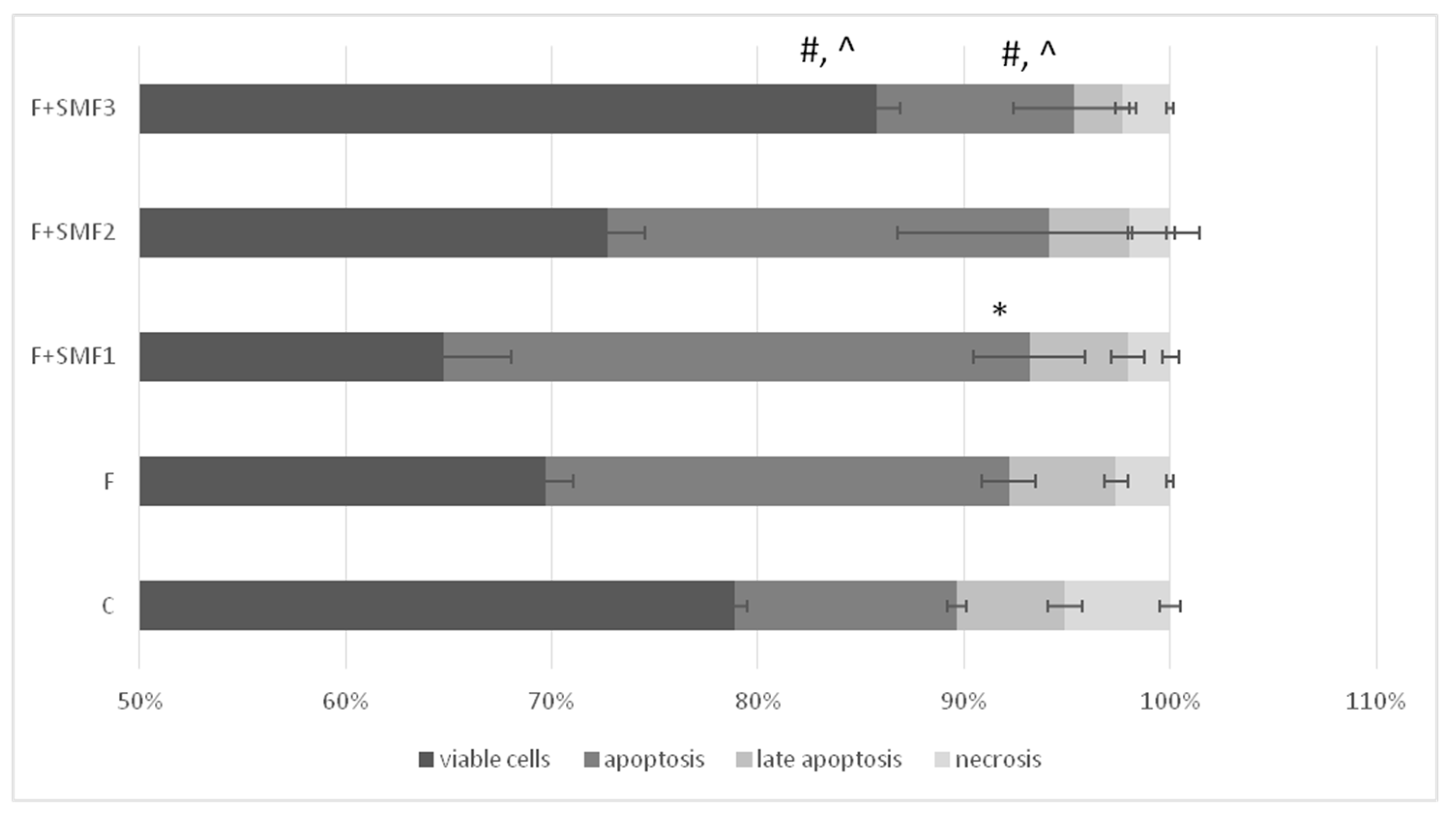
| C | F | F + SMF1 | F + SMF2 | F + SMF3 | * p | |
|---|---|---|---|---|---|---|
| mRNA Copy Numbers/µg RNA | ||||||
| SOD1 (superoxide dismutase 1) | 1,359,583 ± 114,105 | 1,438,688 ± 177,946 | 1,343,500 ± 19,0836 | 1,239,125 ± 163,142 | 1,764,375 ± 183,910 | 0.007 |
| SOD2 (superoxide dismutase 2) | 200,606 ± 21,341 | 206,831 ± 15,712 | 228,050 ± 13,736 | 216,919 ± 13,361 | 251,125 ± 22,574 | 0.009 |
| GSR (glutathione reductase) | 65,088 ± 4503 | 69,269 ± 11,353 | 63,300 ± 16,230 | 80,150 ± 4503 | 101,813 ± 11,462 | <0.001 |
| CAT (catalase) | 47,069 ± 15,632 | 51,825 ± 9008 | 58,769 ± 8490 | 61,044 ± 16,132 | 60,588 ± 10,691 | NS |
| GPx1 (glutathione peroxidase 1) | 544,500 ± 81,698 | 511,500 ± 48,140 | 522,750 ± 63,346 | 507,000 ± 30,043 | 473,500 ± 65,479 | NS |
| MGST1 (microsomal glutathione S-transferase 1) | 283,006 ± 56,731 | 339,250 ± 80,532 | 208,100 ± 117,469 | 420,725 ± 264,477 | 294,994 ± 229,390 | NS |
| NFE2L2 (nuclear factor erythroid 2-related factor 2) | 238,775 ± 89,756 | 28,271 ± 28,022 | 143,538 ± 78,523 | 129,013 ± 51,919 | 127,938 ± 25,088 | 0.006 |
| C | F | F + SMF1 | F + SMF2 | F + SMF3 | * p | |
|---|---|---|---|---|---|---|
| mRNA Copy Numbers/µg RNA | ||||||
| BAX (BCL2 associated X, apoptosis regulator) | 162,150 ± 56,390 | 1,057,813 ± 1,343,514 | 260,906 ± 90,299 | 165,288 ± 55,281 | 315,338 ± 278,153 | NS |
| BCL2 (B-cell lymphoma 2) | 75,243 ± 103,092 | 56,350 ± 9429 | 62,225 ± 34,664 | 23,101 ± 5323 | 15,527 ± 5319 | NS |
| BCLXL (B-cell lymphoma—extra large) | 197,181 ± 65,858 | 271,883 ± 47,827 | 257,775 ± 67,301 | 158,491 ± 42,901 | 112,387 ± 4472 | NS |
| FAS (Fas cell surface death receptor) | 334,825 ± 306,367 | 1,068,306 ± 1,029,560 | 1,087,450 ± 636,856 | 439,381 ± 298,303 | 227,213 ± 238,264 | NS |
| FASL (Fas ligand) | 356 ± 421 | 74 ± 89 | 149 ± 85 | 50 ± 50 | 51 ± 27 | NS |
| CASP9 (caspase 9) | 28,148 ± 13,279 | 37,381 ± 5907 | 31,013 ± 8629 | 14,529 ± 4178 | 16,955 ± 2902 | 0.004 |
| CASP2 (caspase 2) | 116,188 ± 30,287 | 186,388 ± 15,879 | 178,963 ± 14,011 | 140,131 ± 24,821 | 108,763 ± 21,211 | <0.001 |
| CASP3 (caspase 3) | 299,713 ± 137,632 | 480,000 ± 94,438 | 511,438 ± 167,428 | 283,938 ± 43,039 | 244,044 ± 168,991 | NS |
| TNF (tumor necrosis factor) | 17,930 ± 4651 | 37,638 ± 4096 | 40,500 ± 6992 | 14,412 ± 6424 | 23,328 ± 8232 | <0.001 |
| TNFR1 (tumor necrosis factor receptor 1) | 6,600,625 ± 4,298,333 | 8,998,125 ± 2,632,911 | 8,421,875 ± 1,566,133 | 6,648,750 ± 2,222,046 | 3,122,875 ± 1,240,915 | 0.049 |
| SOD1 | SOD2 | GSR | NFE2L2 | CASP2 | CASP9 | TNF | TNFR1 | |
|---|---|---|---|---|---|---|---|---|
| SOD1 | ||||||||
| SOD2 | 0.72 | |||||||
| GSR | 0.41 | 0.45 | ||||||
| NFE2L2 | 0.02 | −0.38 | −0.11 | |||||
| CASP2 | −0.53 | −0.36 | −0.71 | 0.29 | ||||
| CASP9 | −0.33 | −0.08 | −0.62 | −0.29 | 0.69 | |||
| TNF | 0.03 | −0.01 | −0.58 | −0.30 | 0.51 | 0.66 | ||
| TNFR1 | −0.54 | −0.45 | −0.84 | 0.22 | 0.89 | 0.61 | 0.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimsa-Dudek, M.; Krawczyk, A.; Synowiec-Wojtarowicz, A. The Protective Effect of Static Magnetic Fields with Different Magnetic Inductions against Fluoride Toxicity Is Related to the NRF2 Signaling Pathway. Appl. Sci. 2020, 10, 6509. https://doi.org/10.3390/app10186509
Kimsa-Dudek M, Krawczyk A, Synowiec-Wojtarowicz A. The Protective Effect of Static Magnetic Fields with Different Magnetic Inductions against Fluoride Toxicity Is Related to the NRF2 Signaling Pathway. Applied Sciences. 2020; 10(18):6509. https://doi.org/10.3390/app10186509
Chicago/Turabian StyleKimsa-Dudek, Magdalena, Agata Krawczyk, and Agnieszka Synowiec-Wojtarowicz. 2020. "The Protective Effect of Static Magnetic Fields with Different Magnetic Inductions against Fluoride Toxicity Is Related to the NRF2 Signaling Pathway" Applied Sciences 10, no. 18: 6509. https://doi.org/10.3390/app10186509
APA StyleKimsa-Dudek, M., Krawczyk, A., & Synowiec-Wojtarowicz, A. (2020). The Protective Effect of Static Magnetic Fields with Different Magnetic Inductions against Fluoride Toxicity Is Related to the NRF2 Signaling Pathway. Applied Sciences, 10(18), 6509. https://doi.org/10.3390/app10186509





