Marine Microalgae for Potential Lutein Production
Abstract
1. Introduction
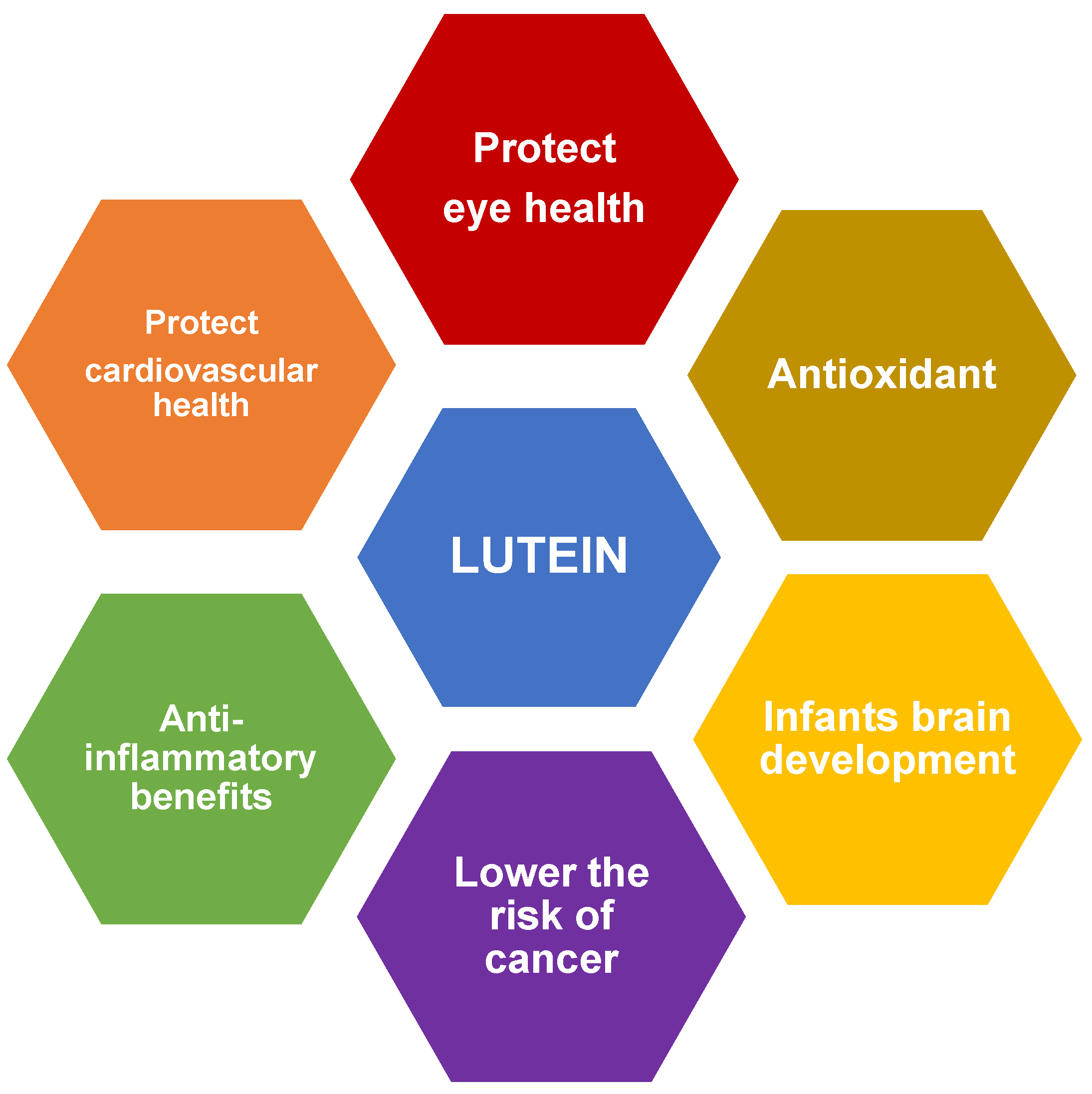
2. Health Benefits of Lutein
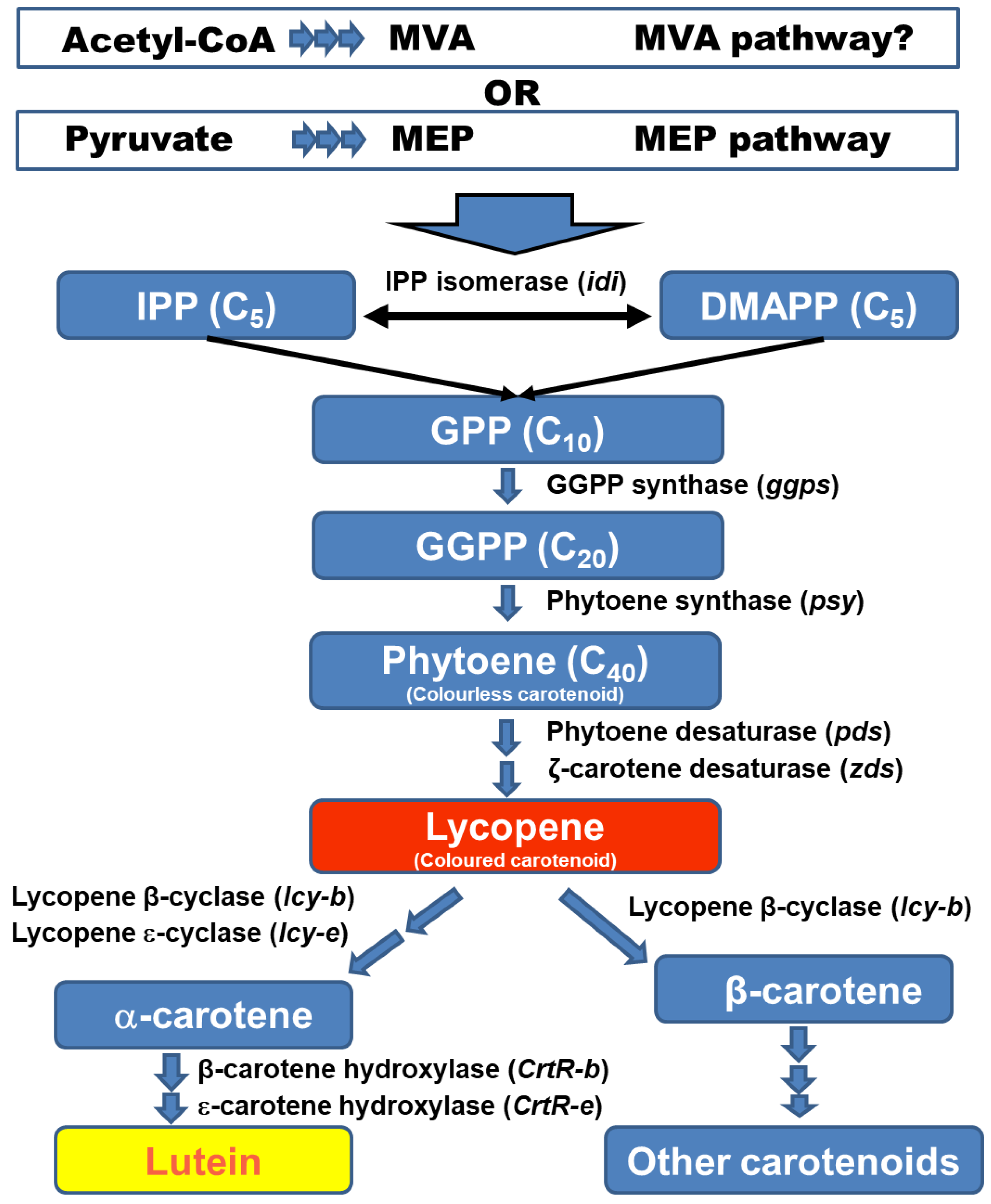
3. Putative Biosynthetic Pathway of Lutein in Microalgae
4. Engineering of Biosynthetic Pathways in Microalgae for Lutein Production
5. Synthetic Production of Lutein
6. Microalgae Cultivation for Commercial Lutein Production and Challenges
7. Extraction of Lutein from Microalgae and Challenges
8. Current Market Demand, Value and Sources
9. Overall Discussion and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernandes, A.S.; do Nascimento, T.C.; Jacob-Lopes, E.; De Rosso, V.V.; Zepka, L.Q. Introductory Chapter: Carotenoids—A Brief Overview on Its Structure, Biosynthesis, Synthesis, and Applications. Prog. Carotenoid Res. 2018, 1–16. [Google Scholar] [CrossRef][Green Version]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef]
- Roberts, J.E.; Dennison, J. The Photobiology of Lutein and Zeaxanthin in the Eye. J. Ophthalmol. 2015, 2015, 687173. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.; Casella, P.; Sangiorgio, P.; Verardi, A.; Ferraro, A.; Hristoforou, E.; Molino, A.; Musmarra, D. Natural beta-carotene: A microalgae derivate for nutraceutical applications. Chem. Eng. Trans. 2020, 79, 103–108. [Google Scholar] [CrossRef]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 1–21. [Google Scholar] [CrossRef]
- Saha, S.K.; Murray, P. Exploitation of microalgae species for nutraceutical purposes: Cultivation aspects. Fermentation 2018, 4, 46. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Ahmed, I.; Iqbal, H.M.N. High-value compounds from microalgae with industrial exploitability—A review. Front. Biosci. Sch. 2017, 9, 319–342. [Google Scholar] [CrossRef]
- Saha, S.K.; Mchugh, E.; Murray, P.; Walsh, D.J. Microalgae as a source of nutraceuticals. In Phycotoxins: Chemistry and Biochemistry, 2nd ed.; Botana, L.M., Alfonso, A., Eds.; JohnWiley & Sons Ltd.: Chichester, UK, 2015; pp. 255–292. [Google Scholar]
- Roberts, R.L.; Green, J.; Lewis, B. Lutein and zeaxanthin in eye and skin health. Clin. Dermatol. 2009, 27, 195–201. [Google Scholar] [CrossRef]
- Miller, D.L.; Papayannopoulos, I.A.; Styles, J.; Bobin, S.A.; Lin, Y.Y.; Biemann, K.; Iqbal, K. Peptide Compositions of the Cerebrovascular and Senile Plaque Core Amyloid Deposits of Alzheimer′s Disease. Arch. Biochem. Biophys. 1993, 301, 41–52. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. Letter to the editor: Current results on the potential health benefits of lutein. EXCLI J. 2016, 15, 308–314. [Google Scholar] [PubMed]
- Giordano, E.; Quadro, L. Lutein, zeaxanthin and mammalian development: Metabolism, functions and implications for health. Arch. Biochem. Biophys. 2018, 647, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, E.; Dhawan, A. Scanning the scars: The utility of transient elastography in young children. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 551. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Ochoa Becerra, M.; Mojica Contreras, L.; Hsieh Lo, M.; Mateos Díaz, J.; Castillo Herrera, G. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Barredo, J.-L. Microbial Carotenoids from Bacteria and Microalgae. Methods and Protocols. Methods Mol. Biol. 2012, 892, 133–141. [Google Scholar] [CrossRef]
- Gwak, Y.; Hwang, Y.S.; Wang, B.; Kim, M.; Jeong, J.; Lee, C.G.; Hu, Q.; Han, D.; Jin, E. Comparative analyses of lipidomes and transcriptomes reveal a concerted action of multiple defensive systems against photooxidative stress in Haematococcus pluvialis. J. Exp. Bot. 2014, 65, 4317–4334. [Google Scholar] [CrossRef]
- Hunter, W.N. The non-mevalonate pathway of isoprenoid precursor biosynthesis. J. Biol. Chem. 2007, 282, 21573–21577. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Lee, S.; Jin, E.S. Development of a Chlorella vulgaris mutant by chemical mutagenesis as a producer for natural violaxanthin. Algal Res. 2020, 46, 101790. [Google Scholar] [CrossRef]
- Lee, P.C.; Schmidt-Dannert, C. Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl. Microbiol. Biotechnol. 2002, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.D.; Hou, Y.; Huang, X.X.; Qiu, T.Q.; Jiang, J.G. Ultrasound-enhanced subcritical CO2 extraction of lutein from Chlorella pyrenoidosa. J. Agric. Food Chem. 2015, 63, 4597–4605. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Akgün, İ.H.; Conk Dalay, M. Converted carotenoid production in Dunaliella salina by using cyclization inhibitors 2-methylimidazole and 3-amino-1,2,4-triazole. Turkish J. Biol. 2017, 41, 213–219. [Google Scholar] [CrossRef]
- Liang, M.H.; Hao, Y.F.; Li, Y.M.; Liang, Y.J.; Jiang, J.G. Inhibiting Lycopene Cyclases to Accumulate Lycopene in High β-Carotene-Accumulating Dunaliella bardawil. Food Bioprocess Technol. 2016, 9, 1002–1009. [Google Scholar] [CrossRef]
- Ishikawa, E.; Abe, H. Lycopene accumulation and cyclic carotenoid deficiency in heterotrophic Chlorella treated with nicotine. J. Ind. Microbiol. Biotechnol. 2004, 31, 585–589. [Google Scholar] [CrossRef]
- Fazeli, M.R.; Tofighi, H.; Madadkar-Sobhani, A.; Shahverdi, A.R.; Nejad-Sattari, T.; Sako, M.; Jamalifar, H. Nicotine inhibition of lycopene cyclase enhances accumulation of carotenoid intermediates by Dunaliella salina CCAP 19/18. Eur. J. Phycol. 2009, 44, 215–220. [Google Scholar] [CrossRef]
- Cordero, B.F.; Obraztsova, I.; Couso, I.; Leon, R.; Vargas, M.A.; Rodriguez, H. Enhancement of lutein production in Chlorella sorokiniana (chorophyta) by improvement of culture conditions and random mutagenesis. Mar. Drugs 2011, 9, 1607–1624. [Google Scholar] [CrossRef]
- Kamath, B.S.; Vidhyavathi, R.; Sarada, R.; Ravishankar, G.A. Enhancement of carotenoids by mutation and stress induced carotenogenic genes in Haematococcus pluvialis mutants. Bioresour. Technol. 2008, 99, 8667–8673. [Google Scholar] [CrossRef]
- Huang, W.; Lin, Y.; He, M.; Gong, Y.; Huang, J. Induced high-yield production of zeaxanthin, lutein, and β-carotene by a mutant of Chlorella zofingiensis. J. Agric. Food Chem. 2018, 66, 891–897. [Google Scholar] [CrossRef]
- Gimpel, J.A.; Henríquez, V.; Mayfield, S.P. In metabolic engineering of eukaryotic microalgae: Potential and challenges come with great diversity. Front. Microbiol. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Coll, J.M. Methodologies for transferring DNA into eukaryotic microalgae. Span. J. Agric. Res. 2006, 4, 316–330. [Google Scholar] [CrossRef]
- Terashima, M.; Specht, M.; Hippler, M. The chloroplast proteome: A survey from the Chlamydomonas reinhardtii perspective with a focus on distinctive features. Curr. Genet. 2011, 57, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Neu, T.R.; Lawrence, J.R. Investigation of Microbial Biofilm Structure by Laser Scanning Microscopy. Adv. Biochem. Eng. Biotechnol. 2014, 123, 127–141. [Google Scholar] [CrossRef]
- Lizzul, A.M.; Lekuona-Amundarain, A.; Purton, S.; Campos, L.C. Characterization of Chlorella sorokiniana, UTEX 1230. Biology 2018, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Cordero, B.F.; Couso, I.; León, R.; Rodríguez, H.; Vargas, M.Á. Enhancement of carotenoids biosynthesis in Chlamydomonas reinhardtii by nuclear transformation using a phytoene synthase gene isolated from Chlorella zofingiensis. Appl. Microbiol. Biotechnol. 2011, 91, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gerken, H.; Huang, J.; Chen, F. Engineering of an endogenous phytoene desaturase gene as a dominant selectable marker for Chlamydomonas reinhardtii transformation and enhanced biosynthesis of carotenoids. Process Biochem. 2013, 48, 788–795. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Gerken, H.; Huang, J.; Jiang, Y.; Chen, F. Genetic engineering of the green alga Chlorella zofingiensis: A modified norflurazon-resistant phytoene desaturase gene as a dominant selectable marker. Appl. Microbiol. Biotechnol. 2014, 98, 5069–5079. [Google Scholar] [CrossRef]
- Rathod, J.P.; Vira, C.; Lali, A.M.; Prakash, G. Metabolic Engineering of Chlamydomonas reinhardtii for Enhanced β-Carotene and Lutein Production. Appl. Biochem. Biotechnol. 2020, 190, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Majer, E.; Llorente, B.; Rodríguez-Concepción, M.; Daròs, J.A. Rewiring carotenoid biosynthesis in plants using a viral vector. Sci. Rep. 2017, 7, 41645. [Google Scholar] [CrossRef] [PubMed]
- Bogacz-Radomska, L.; Harasym, J. β-Carotene-properties and production methods. Food Qual. Saf. 2018, 2, 69–74. [Google Scholar] [CrossRef]
- El-Gawad, E.A.A.; Wang, H.P.; Yao, H. Diet Supplemented With Synthetic Carotenoids: Effects on Growth Performance and Biochemical and Immunological Parameters of Yellow Perch (Perca flavescens). Front. Physiol. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F.; Chang, A.N. Total synthesis of (3R,3′R,6′R)-lutein and its stereoisomers. J. Org. Chem. 2009, 74, 3875–3885. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Wang, R.; Wu, X.; Zhang, Y.; Ge, J.; Liu, Z. Synthesis of lutein esters by using a reusable lipase-Pluronic conjugate as the catalyst. Catal. Lett. 2015, 145, 1825–1829. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Chan, M.C.; Liu, C.C.; Chen, C.Y.; Lee, W.L.; Lee, D.J.; Chang, J.S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef]
- Ho, S.H.; Xie, Y.; Chan, M.C.; Liu, C.C.; Chen, C.Y.; Lee, D.J.; Huang, C.C.; Chang, J.S. Effects of nitrogen source availability and bioreactor operating strategies on lutein production with Scenedesmus obliquus FSP-3. Bioresour. Technol. 2015, 184, 131–138. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, R.; Liu, X.; Ho, S.H.; Xie, Y.; Chen, J. Strategies related to light quality and temperature to improve lutein production of marine microalga Chlamydomonas sp. Bioprocess Biosyst. Eng. 2019, 42, 435–443. [Google Scholar] [CrossRef]
- Schüler, L.M.; Santos, T.; Pereira, H.; Duarte, P.; Katkam, N.G.; Florindo, C.; Schulze, P.S.C.; Barreira, L.; Varela, J.C.S. Improved production of lutein and β-carotene by thermal and light intensity upshifts in the marine microalga Tetraselmis sp. CTP4. Algal Res. 2020, 45, 101732. [Google Scholar] [CrossRef]
- Ma, R.; Zhao, X.; Xie, Y.; Ho, S.H.; Chen, J. Enhancing lutein productivity of Chlamydomonas sp. via high-intensity light exposure with corresponding carotenogenic genes expression profiles. Bioresour. Technol. 2019, 275, 416–420. [Google Scholar] [CrossRef]
- Chen, W.C.; Hsu, Y.C.; Chang, J.S.; Ho, S.H.; Wang, L.F.; Wei, Y.H. Enhancing production of lutein by a mixotrophic cultivation system using microalga Scenedesmus obliquus CWL-1. Bioresour. Technol. 2019, 291, 121891. [Google Scholar] [CrossRef]
- Flórez-Miranda, L.; Cañizares-Villanueva, R.O.; Melchy-Antonio, O.; Martínez-Jerónimo, F.; Flores-Ortíz, C.M. Two stage heterotrophy/photoinduction culture of Scenedesmus incrassatulus: Potential for lutein production. J. Biotechnol. 2017, 262, 67–74. [Google Scholar] [CrossRef]
- Xie, Y.; Li, J.; Ma, R.; Ho, S.H.; Shi, X.; Liu, L.; Chen, J. Bioprocess operation strategies with mixotrophy/photoinduction to enhance lutein production of microalga Chlorella sorokiniana FZU60. Bioresour. Technol. 2019, 290, 121798. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.M.; Jiang, Y.; Chen, F. High-yield production of lutein by the green microalga Chlorella protothecoides in heterotrophic fed-batch culture. Biotechnol. Prog. 2002, 18, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; Lele, S. Statistical media optimization for lutein production from microalgae Auxenochlorella protothecoides SAG 211-7A. Int. J. Adv. Biotechnol. Res. 2010, 1, 104–114. [Google Scholar]
- Borowitzka, M.A.; Borowitzka, L.J.; Kessly, D. Effects of salinity increase on carotenoid accumulation in the green alga Dunaliella salina. J. Appl. Phycol. 1990, 2, 111–119. [Google Scholar] [CrossRef]
- Blanco, A.M.; Moreno, J.; Del Campo, J.A.; Rivas, J.; Guerrero, M.G. Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl. Microbiol. Biotechnol. 2007, 73, 1259–1266. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Kumar, S.P.J.; Kumar, G.V.; Dash, A.; Scholz, P.; Banerjee, R. Sustainable green solvents and techniques for lipid extraction from microalgae: A review. Algal Res. 2017, 21, 138–147. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Fernandez-Sevilla, J.M.; Fernández, F.G.A.; García, M.C.C.; Grima, E.M. Supercritical fluid extraction of carotenoids from Scenedesmus almeriensis. Food Chem. 2010, 123, 928–935. [Google Scholar] [CrossRef]
- Araya, B.; Gouveia, L.; Nobre, B.; Reis, A.; Chamy, R.; Poirrier, P. Evaluation of the simultaneous production of lutein and lipids using a vertical alveolar panel bioreactor for three Chlorella species. Algal Res. 2014, 6, 218–222. [Google Scholar] [CrossRef]
- Chen, C.Y.; Jesisca; Hsieh, C.; Lee, D.J.; Chang, C.H.; Chang, J.S. Production, extraction and stabilization of lutein from microalga Chlorella sorokiniana MB-1. Bioresour. Technol. 2016, 200, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Nonomura, A.M. Process for Producing a Naturally-Derived Carotene/Oil Composition by Direct Extraction from Algae. U.S. Patent 4,680,314A, 14 July 1987. [Google Scholar]
- Low, K.L.; Idris, A.; Mohd Yusof, N. Novel protocol optimized for microalgae lutein used as food additives. Food Chem. 2020, 307, 125631. [Google Scholar] [CrossRef]
- Cerón, M.C.; Campos, I.; Sánchez, J.F.; Acién, F.G.; Molina, E.; Fernández-Sevilla, J.M. Recovery of lutein from microalgae biomass: Development of a process for Scenedesmus almeriensis biomass. J. Agric. Food Chem. 2008, 56, 11761–11766. [Google Scholar] [CrossRef]
- Gong, M.; Li, X.; Bassi, A. Investigation of simultaneous lutein and lipid extraction from wet microalgae using Nile Red as solvatochromic shift probe. J. Appl. Phycol. 2018, 30, 1617–1627. [Google Scholar] [CrossRef]
- Li, H.B.; Chen, F. Preparative isolation and purification of astaxanthin from the microalga Chlorococcum sp. by high-speed counter-current chromatography. J. Chromatogr. A 2001, 925, 133–137. [Google Scholar] [CrossRef]
- Yen, H.W.; Chiang, W.C.; Sun, C.H. Supercritical fluid extraction of lutein from Scenedesmus cultured in an autotrophical photobioreactor. J. Taiwan Inst. Chem. Eng. 2012, 43, 53–57. [Google Scholar] [CrossRef]
- Miguel, F.; Martín, A.; Mattea, F.; Cocero, M.J. Precipitation of lutein and co-precipitation of lutein and poly-lactic acid with the supercritical anti-solvent process. Chem. Eng. Process. Process Intensif. 2008, 47, 1594–1602. [Google Scholar] [CrossRef]
- Di Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from Haematococcus pluvialis microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef]
- Mehariya, S.; Iovine, A.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Casella, P.; Karatza, D.; Marino, T.; Musmarra, D.; et al. Supercritical fluid extraction of lutein from Scenedesmus almeriensis. Molecules 2019, 24, 1324. [Google Scholar] [CrossRef]
- Ruen-Ngam, D.; Shotipruk, A.; Pavasant, P.; Machmudah, S.; Goto, M. Selective extraction of lutein from alcohol treated Chlorella vulgaris by supercritical CO2. Chem. Eng. Technol. 2012, 35, 255–260. [Google Scholar] [CrossRef]
- Zhengyun, W.; Wu, S.; Xianming, S. Supercritical fluid extraction and determination of lutein in heterotrophically cultivated Chlorella pyrenoidosa. J. Food Process Eng. 2007, 30, 174–185. [Google Scholar] [CrossRef]
- Ishida, B.K.; Chapman, M.H. Carotenoid Extraction from Plants Using a Novel, Environmentally Friendly Solvent. J. Agric. Food Chem. 2009, 57, 1051–1059. [Google Scholar] [CrossRef]
- Ma, R.; Zhao, X.; Ho, S.H.; Shi, X.; Liu, L.; Xie, Y.; Chen, J.; Lu, Y. Co-production of lutein and fatty acid in microalga Chlamydomonas sp. JSC4 in response to different temperatures with gene expression profiles. Algal Res. 2020, 47, 101821. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, K.; Zhao, X.; Ma, R.; Chen, J.; Ho, S.H. Manipulating Nutritional Conditions and Salinity-Gradient Stress for Enhanced Lutein Production in Marine Microalga Chlamydomonas sp. Biotechnol. J. 2019, 14, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Garbayo, I.; Cuaresma, M.; Vílchez, C.; Vega, J.M. Effect of abiotic stress on the production of lutein and β-carotene by Chlamydomonas acidophila. Process Biochem. 2008, 43, 1158–1161. [Google Scholar] [CrossRef]
- Gayathri, S.; Radhika, S.R.R.; Suman, T.Y.; Aranganathan, L. Ultrasound-assisted microextraction of β, ε-carotene-3, 3′-diol (lutein) from marine microalgae Chlorella salina: Effect of different extraction parameters. Biomass Convers. Biorefinery 2018, 8, 791–797. [Google Scholar] [CrossRef]
- Serejo, M.L.; Posadas, E.; Boncz, M.A.; Blanco, S.; García-Encina, P.; Muñoz, R. Influence of biogas flow rate on biomass composition during the optimization of biogas upgrading in microalgal-bacterial processes. Environ. Sci. Technol. 2015, 49, 3228–3236. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.Á.; Rivas, J.; Guerrero, M.G. Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J. Biotechnol. 2001, 85, 289–295. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Dhanarajan, G.; Dash, S.K.; Sen, R. An advanced hybrid medium optimization strategy for the enhanced productivity of lutein in Chlorella minutissima. Algal Res. 2015, 7, 24–32. [Google Scholar] [CrossRef]
- Shi, X.M.; Liu, H.J.; Zhang, X.W.; Chen, F. Production of biomass and lutein by Chlorella protothecoides at various glucose concentrations in heterotrophic cultures. Process Biochem. 1999, 34, 341–347. [Google Scholar] [CrossRef]
- Chen, C.Y.; Liu, C.C. Optimization of lutein production with a two-stage mixotrophic cultivation system with Chlorella sorokiniana MB-1. Bioresour. Technol. 2018, 262, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Deenu, A.; Naruenartwongsakul, S.; Kim, S.M. Optimization and economic evaluation of ultrasound extraction of lutein from Chlorella vulgaris. Biotechnol. Bioprocess Eng. 2013, 18, 1151–1162. [Google Scholar] [CrossRef]
- Xin, C.; Addy, M.M.; Zhao, J.; Cheng, Y.; Cheng, S.; Mu, D.; Liu, Y.; Ding, R.; Chen, P.; Ruan, R. Comprehensive techno-economic analysis of wastewater-based algal biofuel production: A case study. Bioresour. Technol. 2016, 211, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.Á.; Rivas, J.; Guerrero, M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, X.; Chen, J.; Yang, X.; Ho, S.H.; Wang, B.; Chang, J.S.; Shen, Y. Enhancing cell growth and lutein productivity of Desmodesmus sp. F51 by optimal utilization of inorganic carbon sources and ammonium salt. Bioresour. Technol. 2017, 244, 664–671. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Moreno, J.; Rodríguez, H.; Angeles Vargas, M.; Rivas, J.; Guerrero, M.G. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Ram, S.; Paliwal, C.; Mishra, S. Growth medium and nitrogen stress sparked biochemical and carotenogenic alterations in Scenedesmus sp. CCNM 1028. Bioresour. Technol. Rep. 2019, 7, 100194. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Karatza, D.; Chianese, S.; Iovine, A.; Casella, P.; Marino, T.; Musmarra, D. Bench-scale cultivation of microalgae Scenedesmus almeriensis for CO2 capture and lutein production. Energies 2019, 12, 2806. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández, J.M.; Acién, F.G.; Rueda, A.; Pérez-Parra, J.; Molina, E. Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem. 2008, 43, 398–405. [Google Scholar] [CrossRef]
- Lutein Market to Reach USD 454.8 Million by 2026|Reports And Data. Available online: https://www.prnewswire.com/news-releases/lutein-market-to-reach-usd-454-8-million-by-2026--reports-and-data-300941985.html (accessed on 4 September 2020).
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
| Microalgae | Biomass | Cultivation Conditions | Lutein Yield | Stress Conditions | Extraction Methodologies | References |
|---|---|---|---|---|---|---|
| Marine cultures | ||||||
| Chlamydomonas sp. JSC4 | 1271 mg L−1 d−1 | 1-L glass photobioreactor | 3.27 mg L−1 d−1 | Temperature (35 °C) | Solvent extraction | [75] |
| Chlamydomonas sp. | 1500 mg L−1 d−1 | 1-L glass photobioreactor | 5.08 mg L−1 d−1 | Light intensity (625 μmol photons m−2 s−1) | Solvent extraction | [50] |
| Chlamydomonas sp. JSC4 | 560 mg L−1 d−1 | 1-L glass photobioreactor | 3.42 mg g−1 | Salinity gradient | Solvent extraction | [76] |
| Chlamydomonas sp. JSC4 | 490 mg L−1 d−1 | 1-L glass photobioreactor | 2.95 mg g−1 | Light wavelengths (blue light) | Solvent extraction | [50] |
| Chlamydomonas acidophila | - | Batch growth | 20 mg L−1 | UV-A radiation (10 μmol photons m−2 s−1), or heated at 40 °C. | Solvent extraction | [77] |
| Chlorella salina | - | 3-L glass flask | 2.92 mg g−1 | - | Microextraction coupled with ultrasonication | [78] |
| Dunaliella salina | 2.2 g m−2 d−1 | Tubular photobioreactor | 15.4 mg m−2 d−1 | None | Solvent extraction | [79] |
| Muriellopsis sp. | 40 g m−2 d−1 | Outdoor tubular photobioreactor | 6 mg g−1 | None | Solvent extraction | [80] |
| Muriellopsis sp. | 12.9 g m−2 d−1 | Open ponds | 100 mg m−2 d−1 | None | Solvent extraction | [57] |
| Tetraselmis sp. CTP4 | - | 5-L reactors | 3.17 mg g−1 | Light intensity (170 and 280 μmol photons m−2 s−1) and temperature (35 °C) | Solvent extraction | [49] |
| Freshwater cultures | ||||||
| Chlorella minutissima | 0.117 g L−1 d−1 | 2-L airlift photobioreactor | 5.58 mg g−1 | None | Solvent extraction | [81] |
| C. vulgaris, C. zofingiensis and C. protothecoides | 0.131, 0.122, 0.103 g L−1 d−1 (respectively) | In indoor vertical alveolar panel photobioreactor | 3.86, 4.38 and 3.59 mg g−1 (respectively) | None | Glass bead vortexing and ball mill grinding | [61] |
| Chlorella protothecoides | 31.2 g L−1 | Heterotrophic growth in a 3.7-L fermenter | 1.90 mg g−1 | 80 g L−1 glucose addition | Solvent extraction | [82] |
| Chlorella pyrenoidosa | - | - | 1.24 mg g−1 | None | Ultrasound-enhanced subcritical CO2 extraction | [23] |
| Chlorella sorokiniana | 1.98 g L−1 d−1 | Two-stage mixotrophic cultivation | 7.62 mg L−1 d−1 | None | Solvent extraction | [83] |
| Chlorella vulgaris | - | Batch | 3.36 mg g−1 | None | Ultrasound extraction with enzymatic pretreatment | [84] |
| Chlorella sorokiniana | 2.4 g L−1 | Semi-batch mixotrophic cultivation. | 5.21 mg g−1 | None | Reduced pressure extraction method. | [85] |
| Chlorella protothecoides | 28.4 g L−1 | Heterotrophic batch growth in a 3.7-L fermenter | 0.27 mg g−1 | Nitrogen limitation and high temperature | Mechanical method | [54] |
| Chlorella zofingiensis | 7 g L−1 | Batch growth | 4 mg g−1 | None | Solvent extraction | [86] |
| Desmodesmus sp. | 939 mg L−1 d−1 | 1-L glass vessel | 5.22 mg L−1 d−1 | Different C/N ratios (1:1 and 150 mg L−1) | Solvent extraction | [87] |
| Muriellopsis sp. | 5.37 g L−1 | Batch growth | 29.8 mg L−1 | None | Solvent extraction | [88] |
| Scenedesmus incrassatulus | 17.98 g L−1 | Two-stage heterotrophy photoinduction culture | 1.49 mg g−1 | Glucose concentration increase (30.3 g L−1) | Solvent extraction | [52] |
| Scenedesmus sp. CCNM 1028 | 0.47 g L−1 | Batch growth (1L) | 2.12 mg g−1 | Two-stage nitrogen starvation | Solvent extraction | [89] |
| Scenedesmus obliquus CWL-1 | 9.88 g L−1 | Mixotrophic cultivation | 1.78 mg g−1 | Light-related strategies (12/12 L/D, blue to red light) | Solvent extraction | [51] |
| Scenedesmus almeriensis | 0.95 g L−1 | Vertical bubble column photo-bioreactor | 8.54 mg g−1 | Different CO2 Content (3.0% v/v) | Accelerated solvent extraction | [90] |
| Scenedesmus sp. | 1.1 g L−1 | 20 L photobioreactor | 1.794 mg g−1 | Different pressure and temperature in the SFE operation (400 bar, 70 °C and ethanol as the co-solvent) | Supercritical CO2 extraction | [68] |
| Scenedesmus almeriensis | 0.63 g L−1 | Bubble column photobioreactors (2.0 L) | 3.6 mg L−1 | Salinity (5 g L−1) | Solvent extraction | [91] |
| Scenedesmus obliquus | 2.44 g L−1 | 1-L glass vessel | 3.63 mg g−1 | Light-related strategies | Solvent extraction | [46] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, S.K.; Ermis, H.; Murray, P. Marine Microalgae for Potential Lutein Production. Appl. Sci. 2020, 10, 6457. https://doi.org/10.3390/app10186457
Saha SK, Ermis H, Murray P. Marine Microalgae for Potential Lutein Production. Applied Sciences. 2020; 10(18):6457. https://doi.org/10.3390/app10186457
Chicago/Turabian StyleSaha, Sushanta Kumar, Hande Ermis, and Patrick Murray. 2020. "Marine Microalgae for Potential Lutein Production" Applied Sciences 10, no. 18: 6457. https://doi.org/10.3390/app10186457
APA StyleSaha, S. K., Ermis, H., & Murray, P. (2020). Marine Microalgae for Potential Lutein Production. Applied Sciences, 10(18), 6457. https://doi.org/10.3390/app10186457







