Structure of Bacterial Communities in Phosphorus-Enriched Rhizosphere Soils
Abstract
1. Introduction
2. Materials and Methods
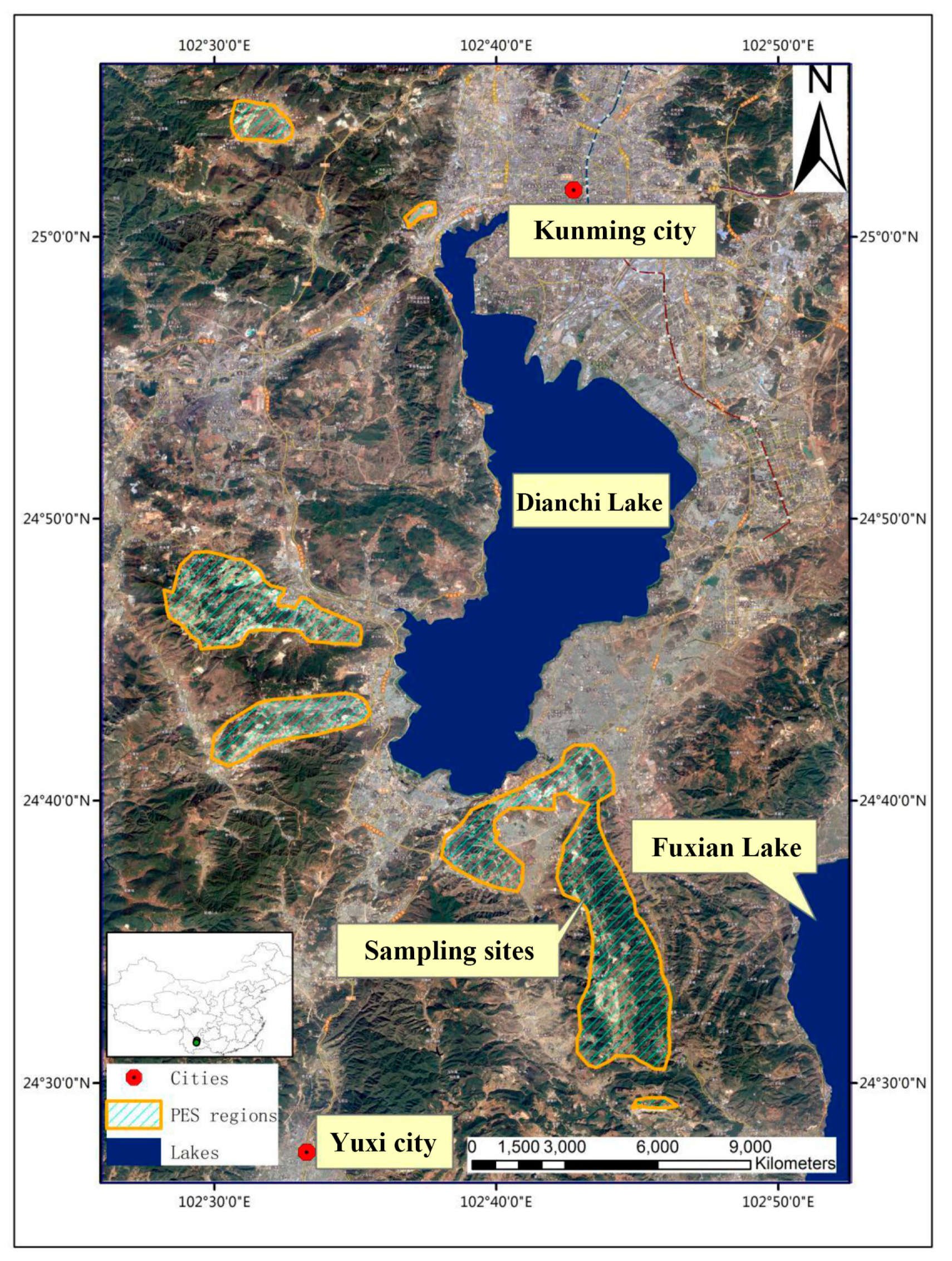
2.1. Study Area
2.2. Soil Sampling
2.3. Soil Physicochemical Analysis
2.4. Illumina MiSeq for High-Throughput Sequencing of 16S rRNA
2.5. Data Analysis
3. Results
3.1. Physicochemical Properties
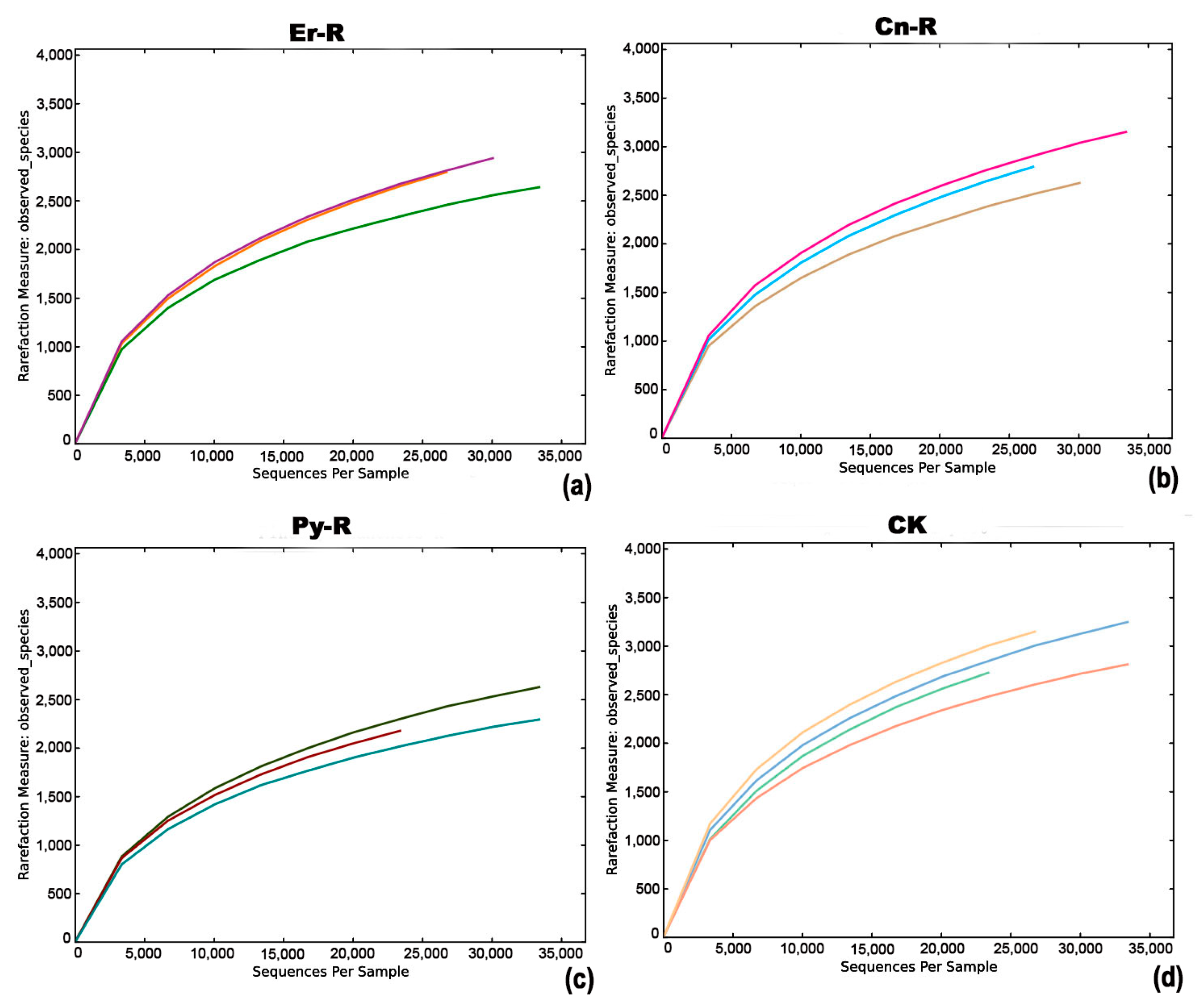
3.2. Sequencing Results
3.3. Differences in Bacterial Diversity
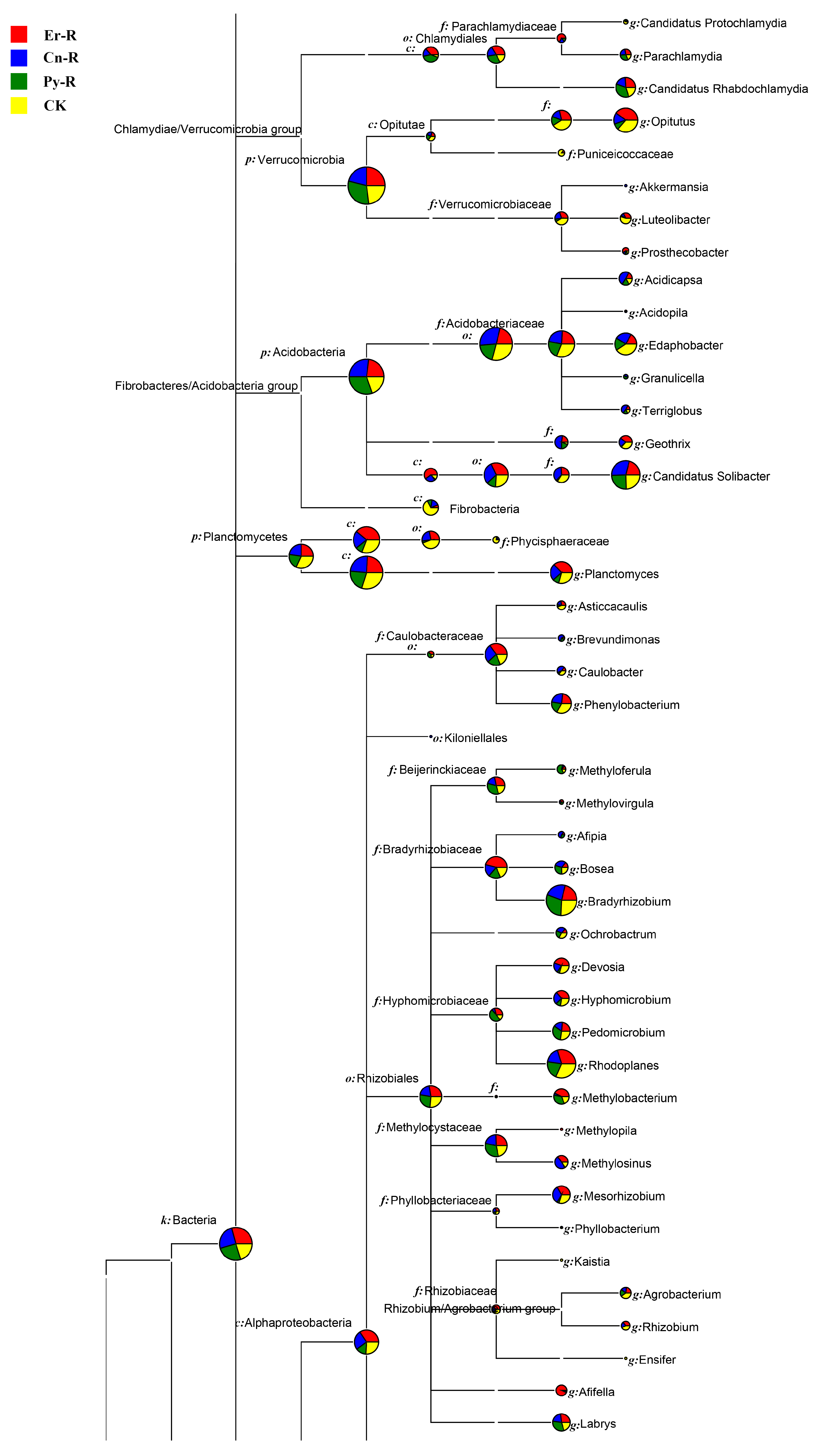
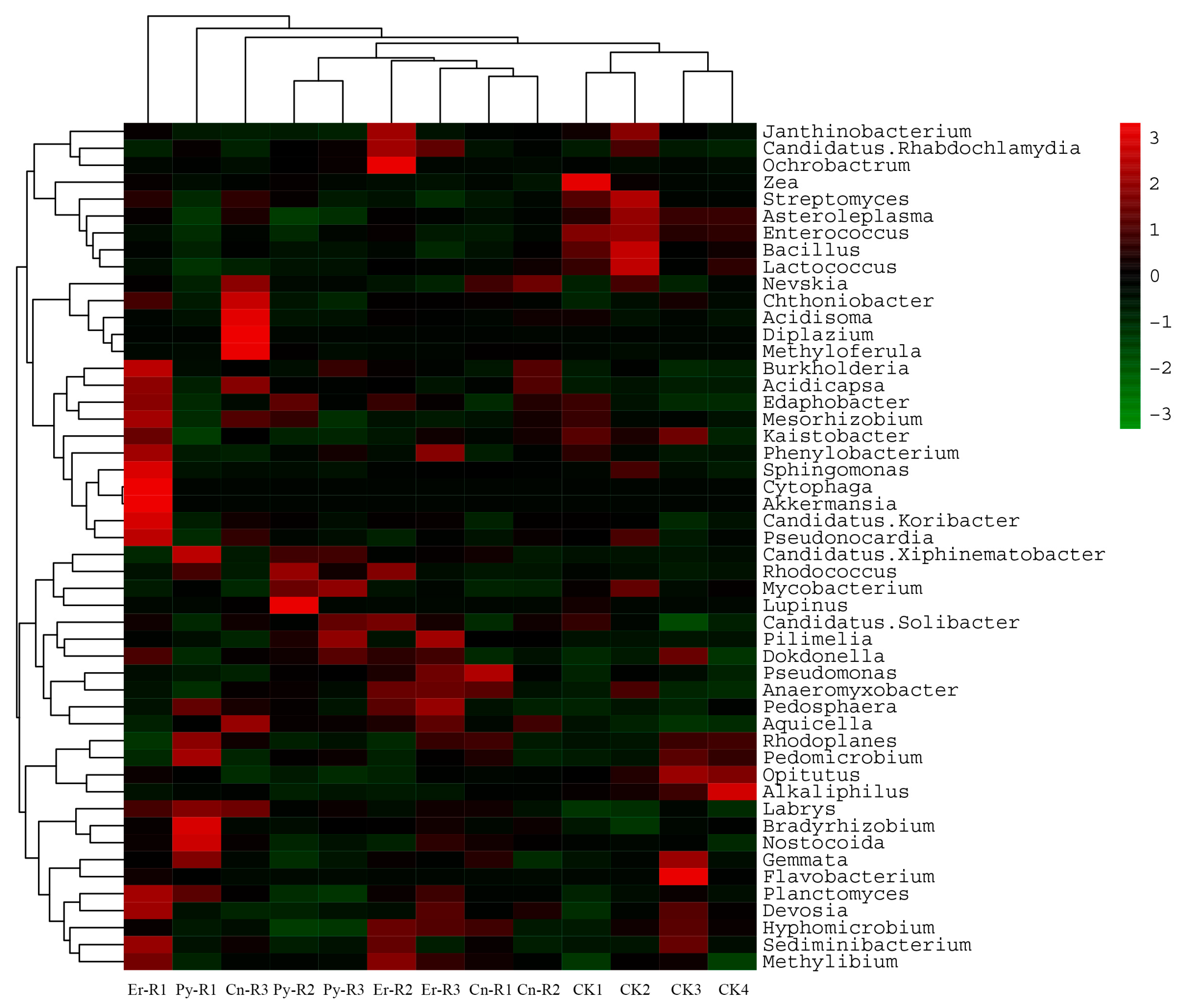
3.4. Bacterial Communities and Diversity
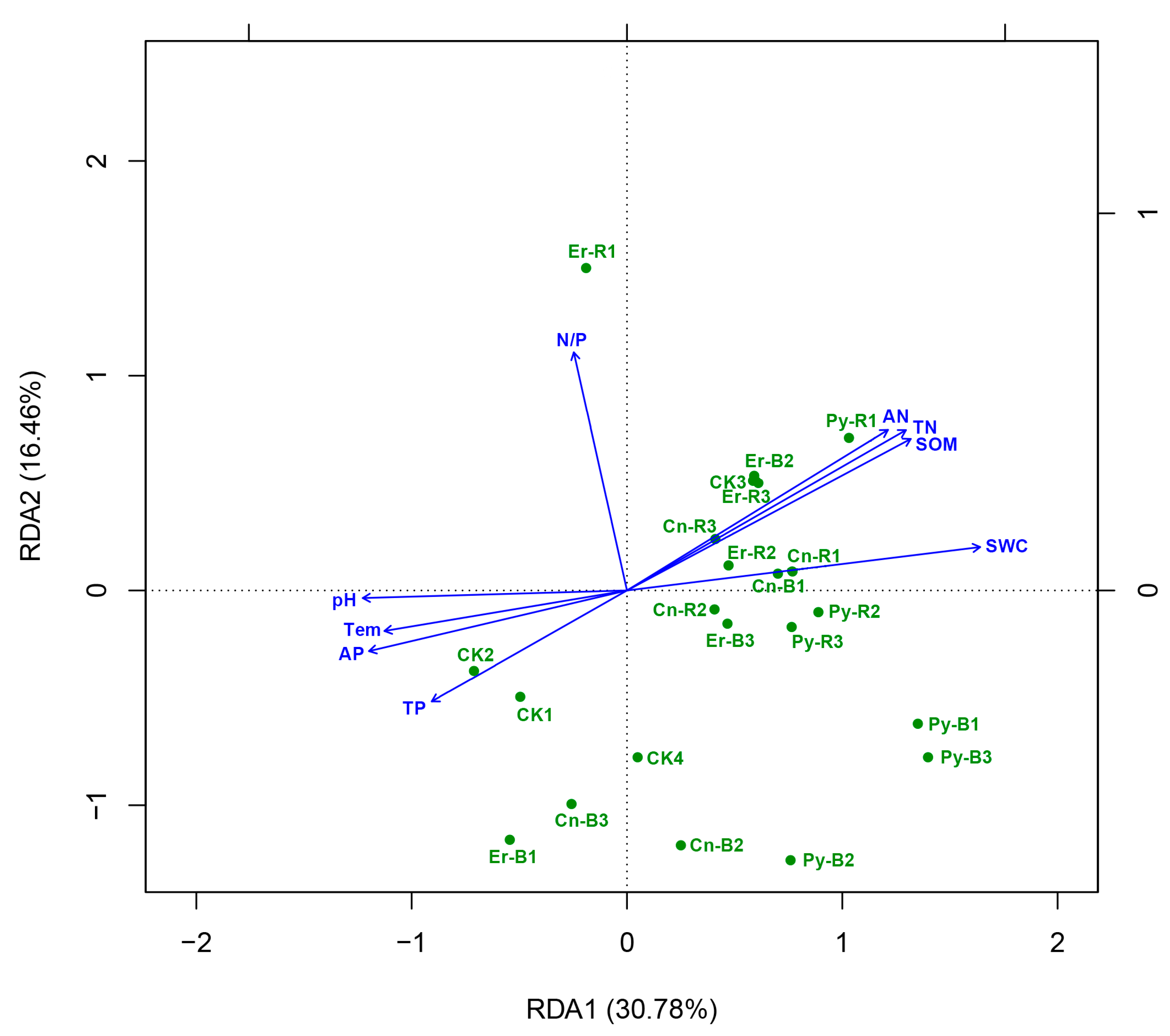
3.5. Impact of Soil Properties on the Relative Abundances of Bacterial Taxa
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| No | Samples | DNA Concentration (ng/μL) | 260/280 Value | 260/230 Value | Volume (μL) | Total Amount (μg) |
|---|---|---|---|---|---|---|
| 1 | Er-R1 | 13.84 | 1.80 | 0.56 | 50.00 | 0.69 |
| 2 | Er-R2 | 23.54 | 1.65 | 0.62 | 50.00 | 1.18 |
| 3 | Er-R3 | 22.43 | 1.79 | 0.65 | 50.00 | 1.12 |
| 4 | Er-B1 | 12.87 | 1.92 | 0.53 | 50.00 | 0.64 |
| 5 | Er-B2 | 26.76 | 1.76 | 0.74 | 50.00 | 1.34 |
| 6 | Er-B3 | 11.39 | 1.64 | 0.45 | 50.00 | 0.57 |
| 7 | Cn-R1 | 12.26 | 1.72 | 0.43 | 50.00 | 0.61 |
| 8 | Cn-R2 | 14.58 | 1.69 | 0.58 | 50.00 | 0.73 |
| 9 | Cn-R3 | 24.89 | 1.82 | 0.73 | 50.00 | 1.24 |
| 10 | Cn-B1 | 11.73 | 1.69 | 0.46 | 50.00 | 0.59 |
| 11 | Cn-B2 | 5.57 | 1.71 | 0.24 | 50.00 | 0.28 |
| 12 | Cn-B3 | 5.75 | 1.53 | 0.25 | 50.00 | 0.29 |
| 13 | Py-R1 | 15.96 | 1.52 | 0.51 | 50.00 | 0.80 |
| 14 | Py-R2 | 16.00 | 1.55 | 0.49 | 50.00 | 0.80 |
| 15 | Py-R3 | 17.73 | 1.64 | 0.55 | 50.00 | 0.89 |
| 16 | Py-B1 | 10.41 | 1.51 | 0.40 | 50.00 | 0.52 |
| 17 | Py-B2 | 9.87 | 1.45 | 0.35 | 50.00 | 0.49 |
| 18 | Py-B3 | 10.92 | 1.54 | 0.40 | 50.00 | 0.55 |
| 19 | CK1 | 5.44 | 1.39 | 0.29 | 50.00 | 0.27 |
| 20 | CK2 | 10.35 | 1.79 | 0.48 | 50.00 | 0.52 |
| 21 | CK3 | 9.49 | 1.72 | 0.40 | 50.00 | 0.47 |
| 22 | CK4 | 11.41 | 1.71 | 0.38 | 50.00 | 0.57 |
References
- McGuire, K.L.; Treseder, K.K. Microbial communities and their relevance for ecosystem models: Decomposition as a case study. Soil Biol. Biochem. 2010, 42, 529–535. [Google Scholar] [CrossRef]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Ravi, R.K.; Anusuya, S.; Balachandar, M.; Muthukumar, T. Microbial Interactions in Soil Formation and Nutrient Cycling. In Mycorrhizosphere and Pedogenesis; Varma, A., Choudhary, D.K., Eds.; Springer: Singapore, 2019; pp. 368–382. [Google Scholar] [CrossRef]
- Berg, G.; Grube, M.; Schloter, M.; Smalla, K. Unraveling the plant microbiome: Looking back and future perspectives. Front. Microbiol. 2014, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Dessaux, Y.; Hinsinger, P.; Lemanceau, P. Rhizosphere: So many achievements and even more challenges. Plant Soil 2009, 321, 1–3. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Kopriva, S. Metabolic niches in the rhizosphere microbiome: New tools and approaches to analyse metabolic mechanisms of plant–microbe nutrient exchange. J. Exp. Bot. 2019, 70, 1087–1094. [Google Scholar] [CrossRef]
- Pii, Y.; Penn, A.; Terzano, R.; Crecchio, C.; Mimmo, T.; Cesco, S. Plant-microorganism-soil interactions influence the Fe availability in the rhizosphere of cucumber plants. Plant Physiol. Biochem. 2015, 87, 45–52. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579. [Google Scholar] [CrossRef]
- O’Brien, S.L.; Gibbons, S.M.; Owens, S.M.; Hampton–Marcell, J.; Johnston, E.R.; Jastrow, J.D.; Gilbert, J.A.; Meyer, F.; Antonopoulos, D.A. Spatial scale drives patterns in soil bacterial diversity. Environ. Microbiol. 2016, 18, 2039–2051. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, S.; Gao, Q.; Liu, S.; Ganjurjav, H.; Wang, X.; Su, X.; Wu, X. Soil bacterial and fungal diversity differently correlated with soil biochemistry in alpine grassland ecosystems in response to environmental changes. Sci. Rep. 2017, 7, 43077. [Google Scholar] [CrossRef]
- Shen, P.; Murphy, D.V.; George, S.J.; Lapis-Gaza, H.; Xu, M.; Gleeson, D.B. Increasing the size of the microbial biomass altered bacterial community structure which enhances plant phosphorus uptake. PLoS ONE 2016, 11, e0166062. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Yu, W.; Turak, A.; Chen, D.; Huang, Y.; Ao, J.; Jiang, Y.; Huang, Z. Effects of nitrogen and phosphorus inputs on soil bacterial abundance, diversity, and community composition in Chinese fir plantations. Front. Microbiol. 2018, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xiang, W.; Ouyang, S.; Forrester, D.I.; Zhou, B.; Chen, L.; Ge, T.; Lei, P.; Chen, L.; Zeng, Y.; et al. Linkage between tree species richness and soil microbial diversity improves phosphorus bioavailability. Funct. Ecol. 2019, 33, 1549–1560. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Samaddar, S.; Chatterjee, P.; Truu, J.; Anandham, R.; Kim, S.; Sa, T. Long-term phosphorus limitation changes the bacterial community structure and functioning in paddy soils. Appl. Soil Ecol. 2019, 134, 111–115. [Google Scholar] [CrossRef]
- Xie, J.; Xue, W.; Li, C.; Yan, Z.; Li, D.; Li, G.; Chen, X.; Chen, D. Water-soluble phosphorus contributes significantly to shaping the community structure of rhizospheric bacteria in rocky desertification areas. Sci. Rep. 2019, 9, 18408. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Ranjitkar, S.; Zhai, D.; Li, Y.; Xu, J.; Li, B.; Lu, Y. Current re-vegetation patterns and restoration issues in degraded geological phosphorus-rich mountain areas: A synthetic analysis of Central Yunnan, SW China. Plant Divers. 2017, 39, 140–148. [Google Scholar] [CrossRef]
- Sharpley, A.N.; McDowell, R.W.; Kleinman, P.J. Phosphorus loss from land to water: Integrating agricultural and environmental management. Plant Soil 2001, 237, 287–307. [Google Scholar] [CrossRef]
- Yan, K.; Duan, C.; Fu, D.; Li, J.; Wong, M.H.G.; Qian, L.; Tian, Y. Leaf nitrogen and phosphorus stoichiometry of plant communities in geochemically phosphorus-enriched soils in a subtropical mountainous region, SW China. Environ. Earth Sci. 2015, 74, 3867–3876. [Google Scholar] [CrossRef]
- Yan, K.; Xu, J.-C.; Gao, W.; Li, M.-J.; Li, Y.-J.; Zhao, Z.-X.; Yuan, Z.-W.; Zhang, F.-S.; Elser, J. Human Perturbation on Phosphorus Cycles of Lake Dianchi in Southwest China, Yunnan Agricultural University: Kunming, China, 2020; Unpublished work.
- Yan, K.; Yuan, Z.; Goldberg, S.; Gao, W.; Ostermann, A.; Xu, J.; Zhang, F.; Elser, J. Phosphorus mitigation remains critical in water protection: A review and meta-analysis from one of China’s most eutrophicated lakes. Sci. Total Environ. 2019, 689, 1336–1347. [Google Scholar] [CrossRef]
- Yan, K.; Wong, M.H.G.; Zhang, L.; Xiong, H.; Fu, D.; Duan, C.; Guo, Z.; Wang, C. Is Phytoextraction Efficient for Remediating Phosphorus-Enriched Soils in Mountainous Region? A Case Study of Lake Dianchi Watershed of Southwestern China. Appl. Mech. Mater. 2014, 448–453, 488–493. [Google Scholar] [CrossRef]
- Ahkami, A.H.; White III, R.A.; Handakumbura, P.P.; Jansson, C. Rhizosphere engineering: Enhancing sustainable plant ecosystem productivity. Rhizosphere 2017, 3, 233–243. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O. Plant health: Feedback effect of root exudates–rhizobiome interactions. Appl. Microbiol. 2019, 103, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Gu, X.; Zhu, T.; Pan, X.; Zhu, Y.; Long, X.; Shao, H.; Liu, M.; Rengel, Z. Industrial crop Jerusalem artichoke restored coastal saline soil quality by reducing salt and increasing diversity of bacterial community. Appl. Soil Ecol. 2019, 138, 195–206. [Google Scholar] [CrossRef]
- Galaviz, C.; Lopez, B.R.; de–Bashan, L.E.; Hirsch, A.M.; Maymon, M.; Bashan, Y. Root growth improvement of mesquite seedlings and bacterial rhizosphere and soil community changes are induced by inoculation with plant growth–promoting bacteria and promote restoration of eroded desert soil. Land Degrad. Dev. 2018, 29, 1453–1466. [Google Scholar] [CrossRef]
- Mesa, V.; Navazas, A.; González–Gil, R.; González, A.; Weyens, N.; Lauga, B.; Gallego, J.L.R.; Sánchez, J.; Peláez, A.I. Use of endophytic and rhizosphere bacteria to improve phytoremediation of arsenic–contaminated industrial soils by autochthonous Betula celtiberica. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef]
- Yang, P.-X.; Ma, L.; Chen, M.-H.; Xi, J.-Q.; He, F.; Duan, C.-Q.; Mo, M.-H.; Fang, D.-H.; Duan, Y.-Q.; Yang, F.-X. Phosphate solubilizing ability and phylogenetic diversity of bacteria from P-rich soils around Dianchi Lake drainage area of China. Pedosphere 2012, 22, 707–716. [Google Scholar] [CrossRef]
- Tang, C.Q.; Zhao, M.-H.; Li, X.-S.; Ohsawa, M.; Ou, X.-K. Secondary succession of plant communities in a subtropical mountainous region of SW China. Ecol. Res. 2010, 25, 149–161. [Google Scholar] [CrossRef]
- Tang, C.Q.; Hou, X.; Gao, K.; Xia, T.; Duan, C.; Fu, D. Man-made versus natural forests in mid-Yunnan, southwestern China. Mt. Res. Dev. 2007, 27, 242–249. [Google Scholar] [CrossRef]
- Zhu, A.X.; Yang, L.; Li, B.; Qin, C.; Pei, T.; Liu, B. Construction of membership functions for predictive soil mapping under fuzzy logic. Geoderma 2010, 155, 164–174. [Google Scholar] [CrossRef]
- Jarrell, W.M.; Armstrong, D.E.; Grigal, D.F.; Kelly, E.F.; Monger, H.C.; Wedin, D.A. Soil water and temperature status. In Standard Soil Methods for Long-Term Ecological Research; Robertson, G.P., Coleman, D.C., Bledsoe, C.S., Sollins, P., Eds.; Oxford University Press: New York, NY, USA, 1999; Volume 2, pp. 55–73. [Google Scholar]
- Robertson, G.P.; Sollins, P.; Ellis, B.G.; Lajtha, K. Exchangeable ions, pH, and cation exchange capacity. In Standard Soil Methods for Long-Term Ecological Research; Robertson, G.P., Coleman, D.C., Bledsoe, C.S., Sollins, P., Eds.; Oxford University Press: New York, NY, USA, 1999; Volume 2, pp. 106–114. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, Part 3: Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1996; Volume 14, pp. 961–1010. [Google Scholar]
- Bremner, J.M. Nitrogen—Total. In Methods of Soil Analysis, Part 3: Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1996; Volume 14, pp. 1085–1121. [Google Scholar]
- Mulvaney, R.L.; Khan, S.A.; Stevens, W.B.; Mulvaney, C.S. Improved diffusion methods for determination of inorganic nitrogen in soil extracts and water. Biol. Fertil. Soils 1997, 24, 413–420. [Google Scholar] [CrossRef]
- Fu, D.; Wu, X.; Duan, C.; Zhao, L.; Li, B. Different life-form plants exert different rhizosphere effects on phosphorus biogeochemistry in subtropical mountainous soils with low and high phosphorus content. Soil Tillage Res. 2020, 199, 104516. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Gotelli, N.J.; Colwell, R.K. Estimating species richness. In Biological Diversity: Frontiers in Measurement and Assessment; Magurran, A.E., McGill, B.J., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 39–54. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Huson, D.H.; Mitra, S.; Ruscheweyh, H.J.; Weber, N.; Schuster, S.C. Integrative analysis of environmental sequences using MEGAN4. Genome Res. 2011, 21, 1552–1560. [Google Scholar] [CrossRef]
- Ihaka, R.; Gentleman, R. R: A language for data analysis and graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar]
- Asnicar, F.; Weingart, G.; Tickle, T.L.; Huttenhower, C.; Segata, N. Compact graphical representation of phylogenetic data and metadata with GraPhlAn. PeerJ 2015, 3, e1029. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Meena, V.D. Rhizosphere effect on nutrient availability in soil and its uptake by plants: A review. Proc. Natl. Acad. Sci. India Sect B Biol. Sci. 2015, 85, 1–12. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, Y.; Tan, Y.; Wu, Z.; Lu, P.; Zhang, G.; Yu, F. Restoration with pioneer plants changes soil properties and remodels the diversity and structure of bacterial communities in rhizosphere and bulk soil of copper mine tailings in Jiangxi Province, China. Environ. Sci. Pollut. Res. 2018, 25, 22106–22119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B.; Ma, M.; Chen, S.; Li, L.; Cao, F.; Li, J. Long-term fertilization changes bacterial diversity and bacterial communities in the maize rhizosphere of Chinese Mollisols. Appl. Soil Ecol. 2017, 125, 88–96. [Google Scholar] [CrossRef]
- Cheng, J.; Lee, X.; Tang, Y.; Zhang, Q. Long-term effects of biochar amendment on rhizosphere and bulk soil microbial communities in a karst region, southwest China. Appl. Soil Ecol. 2019, 140, 126–134. [Google Scholar] [CrossRef]
- Haichar, F.E.Z.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Lanoue, A.; Strecker, T.; Scheu, S.; Steinauer, K.; Thakur, M.P.; Mommer, L. Root biomass and exudates link plant diversity with soil bacterial and fungal biomass. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Wang, Z.; Li, T.; Wen, X.; Liu, Y.; Han, J.; Liao, Y.; DeBruyn, J.M. Fungal communities in rhizosphere soil under conservation tillage shift in response to plant growth. Front. Microbiol 2017, 8, 1301. [Google Scholar] [CrossRef]
- Liu, J.; Ha, V.N.; Shen, Z.; Zhu, H.; Zhao, F.; Zhao, Z. Characteristics of bulk and rhizosphere soil microbial community in an ancient Platycladus orientalis forest. Appl. Soil Ecol. 2018, 132, 91–98. [Google Scholar] [CrossRef]
- Li, M.; Hu, Z.; Zhu, X.; Zhou, G. Risk of phosphorus leaching from phosphorus-enriched soils in the Dianchi catchment, Southwestern China. Environ. Sci. Pollut. Res. 2015, 22, 8460–8470. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.W.; Kuramae, E.E.; Navarrete, A.A.; Van Veen, J.A.; Tsai, S.M. Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J. 2014, 8, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, L.; Wen, H. Changes in the composition and diversity of bacterial communities 13 years after soil reclamation of abandoned mine land in eastern China. Ecol. Res. 2015, 30, 357–366. [Google Scholar] [CrossRef]
- Wei, Z.; Hao, Z.; Li, X.; Guan, Z.; Cai, Y.; Liao, X. The effects of phytoremediation on soil bacterial communities in an abandoned mine site of rare earth elements. Sci. Total Environ. 2019, 670, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Bargali, K.; Tewari, A. Growth and water relation parameters in drought-stressed Coriaria nepalensis seedlings. J. Arid Environ. 2004, 58, 505–512. [Google Scholar] [CrossRef]
- Fu, D.; He, F.; Guo, Z.; Yan, K.; Wu, X.; Duan, C. Assessment of ecological restoration function of the Coriaria nepalensis-Erianthus rufipilus community in the phosphorus-enriched degraded mountain area in the Lake Dianchi Watershed, Southwestern China. Chin. J. Plant Ecol. 2013, 37, 326–334. (In Chinese) [Google Scholar] [CrossRef]
- Tarlera, S.; Jangid, K.; Ivester, A.H.; Whitman, W.B.; Williams, M.A. Microbial community succession and bacterial diversity in soils during 77 000 years of ecosystem development. FEMS Microbiol. Ecol. 2008, 64, 129–140. [Google Scholar] [CrossRef]
- Wang, X.-H.; Yang, Q.-H.; Li, F.-S.; He, L.-L.; He, S.-C. Characterization of the chromosomal transmission of intergeneric hybrids of Saccharum spp. and Erianthus fulvus by genomic in situ hybridization. Crop Sci. 2010, 50, 1642–1648. [Google Scholar] [CrossRef]
- Evans, D.L.; Joshi, S.V.; Wang, J. Whole chloroplast genome and gene locus phylogenies reveal the taxonomic placement and relationship of Tripidium (Panicoideae: Andropogoneae) to sugarcane. BMC Evol. Biol. 2019, 19, 33. [Google Scholar] [CrossRef]
- Kohn, T.; Heuer, A.; Jogler, M.; Vollmers, J.; Boedeker, C.; Bunk, B.; Rast, P.; Borchert, D.; Glöckner, I.; Freese, H.M.; et al. Fuerstia marisgermanicae gen. nov., sp. nov., an unusual member of the phylum Planctomycetes from the German Wadden Sea. Front. Microbiol. 2016, 7, 2079. [Google Scholar] [CrossRef]
- Andrei, A.Ş.; Salcher, M.M.; Mehrshad, M.; Rychtecký, P.; Znachor, P.; Ghai, R. Niche-directed evolution modulates genome architecture in freshwater Planctomycetes. ISME J. 2019, 13, 1056–1071. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, K.; Yu, Y.; Hu, T.; Wei, J. Impact of climate change and drought regime on water footprint of crop production: The case of Lake Dianchi Basin, China. Nat. Hazards 2015, 79, 549–566. [Google Scholar] [CrossRef]
- Ma, F.; Zhao, C.; Milne, R.; Ji, M.; Chen, L.; Liu, J. Enhanced drought-tolerance in the homoploid hybrid species Pinus densata: Implication for its habitat divergence from two progenitors. New Phytol. 2010, 185, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Lu, S.; Liu, S.; Guo, H.; Wang, T.; Zhou, J.; Peng, X. Changes in Plant Rhizosphere Microbial Communities under Different Vegetation Restoration Patterns in Karst and Non–karst Ecosystems. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Tian, J.; Lu, X.; Chen, Q.; Kuang, X.; Liang, C.; Deng, L.; Lin, D.; Cai, K.; Tian, J. Phosphorus fertilization affects soybean rhizosphere phosphorus dynamics and the bacterial community in karst soils. Plant Soil 2020, 1–16. [Google Scholar] [CrossRef]
- Cortois, R.; De Deyn, G.B. The curse of the black box. Plant Soil 2012, 350, 27–33. [Google Scholar] [CrossRef]
- Weidner, S.; Koller, R.; Latz, E.; Kowalchuk, G.; Bonkowski, M.; Scheu, S.; Jousset, A. Bacterial diversity amplifies nutrient–based plant–soil feedbacks. Funct. Ecol. 2015, 29, 1341–1349. [Google Scholar] [CrossRef]
- Emmett, B.D.; Buckley, D.H.; Drinkwater, L.E. Plant growth rate and nitrogen uptake shape rhizosphere bacterial community composition and activity in an agricultural field. New Phytol. 2020, 225, 960–973. [Google Scholar] [CrossRef]
- Xiao, G.; Li, T.; Zhang, X.; Yu, H.; Huang, H.; Gupta, D.K. Uptake and accumulation of phosphorus by dominant plant species growing in a phosphorus mining area. J. Hazard. Mater. 2009, 171, 542–550. [Google Scholar] [CrossRef]
- Fu, D.; Wu, X.; Huang, N.; Duan, C. Effects of the invasive herb Ageratina adenophora on understory plant communities and tree seedling growth in Pinus yunnanensis forests in Yunnan, China. J. For. Res. 2018, 23, 112–119. [Google Scholar] [CrossRef]
- De Marco, A.; Esposito, F.; Berg, B.; Zarrelli, A.; Virzo De Santo, A. Litter Inhibitory Effects on Soil Microbial Biomass, Activity, and Catabolic Diversity in Two Paired Stands of Robinia pseudoacacia L. and Pinus nigra Arn. Forests 2018, 9, 766. [Google Scholar] [CrossRef]

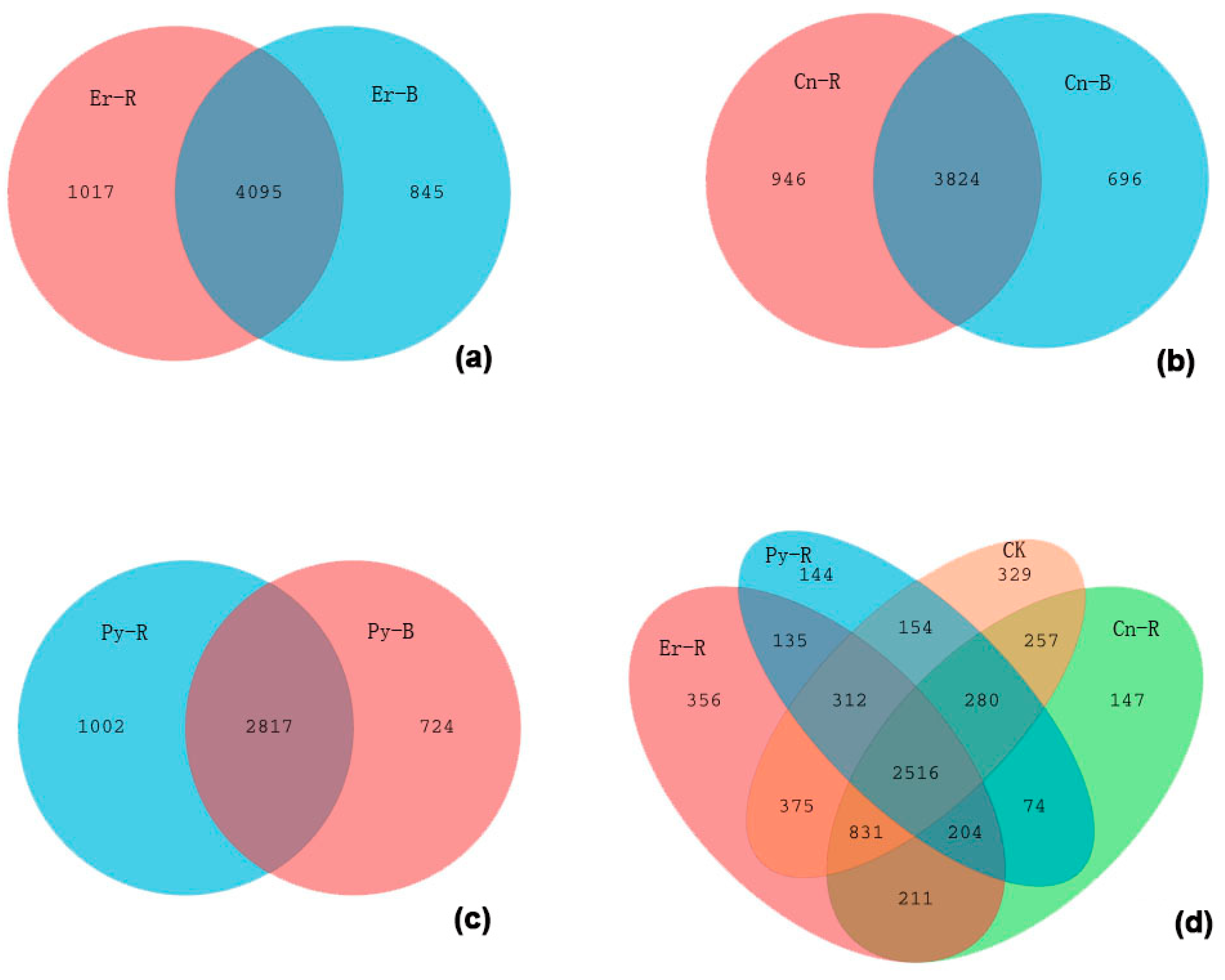
| Abbreviation | Vegetation | Soil Location | Number of Samples |
|---|---|---|---|
| Er-R | Erianthus rufipilus | Rhizosphere | 3 |
| Er-B | Bulk | 3 | |
| Cn-R | Coriaria nepalensis | Rhizosphere | 3 |
| Cn-B | Bulk | 3 | |
| Py-R | Pinus yunnanensis | Rhizosphere | 3 |
| Py-B | Bulk | 3 | |
| CK | Grassland plots | Bulk | 4 |
| Soil Samples | Tem (℃) | SWC (%) | pH | SOM (g/kg) | TN (g/kg) | AN (mg/kg) | TP (g/kg) | AP (mg/kg) | N/P |
|---|---|---|---|---|---|---|---|---|---|
| Er-R | 15.45 ± 0.87 a | 30.70 ± 5.16 a | 4.48 ± 0.28 a | 52.45 ± 15.68 b | 2.79 ± 0.58 ab | 94.06 ± 14.62 bc | 5.13 ± 0.79 b | 117.34 ± 44.06 b | 0.55 ± 0.14 a |
| Er-B | 15.61 ± 1.49 a | 29.27 ± 2.34 a | 4.34 ± 0.02 a | 36.83 ± 13.75 bc | 2.22 ± 0.41 ab | 65.55 ± 19.15 c | 4.57 ± 0.65 b | 72.96 ± 30.43 b | 0.49 ± 0.10 a |
| Cn-R | 14.61 ± 1.16 a | 28.35 ± 2.98 ab | 4.34 ± 0.04 a | 34.21 ± 5.72 bc | 2.24 ± 0.11 ab | 72.58 ± 14.81 c | 3.93 ± 0.22 b | 64.20 ± 24.64 b | 0.57 ± 0.06 a |
| Cn-B | 15.22 ± 1.48 a | 26.95 ± 3.33 ab | 4.32 ± 0.09 a | 27.82 ± 8.39 c | 1.93 ± 0.40 b | 58.43 ± 18.33 c | 4.20 ± 0.22 b | 59.07 ± 20.74 b | 0.46 ± 0.11 a |
| Py-R | 11.09 ± 0.22 b | 33.47 ± 1.03 a | 4.36 ± 0.12 a | 74.66 ± 10.94 a | 2.99 ± 0.43 a | 166.30 ± 10.24 a | 11.36 ± 1.01 a | 253.10 ± 30.77 a | 0.27 ± 0.05 b |
| Py-B | 10.88 ± 0.50 b | 31.38 ± 0.95 a | 4.35 ± 0.05 a | 39.22 ± 1.57 bc | 1.83 ± 0.41 b | 115.51 ± 19.16 b | 12.09 ± 1.09 a | 287.92 ± 56.28 a | 0.15 ± 0.04 b |
| CK | 11.09 ± 0.35 b | 22.88 ± 1.15 b | 4.39 ± 0.19 a | 22.26 ± 2.24 c | 1.92 ± 0.21 b | 67.83 ± 2.06 c | 3.71 ± 0.43 b | 59.22 ± 11.56 b | 0.52 ± 0.06 a |
| Soil Sample | Chao1 | ACE | Simpson | Shannon |
|---|---|---|---|---|
| Er-R | 1868(1748,1953) | 2302(2135,2478) | 0.99151443 | 8.93(8.85,9.02) |
| Cn-R | 1817(1668,2068) | 2209(2010,2503) | 0.989189011 | 8.78(8.72,8.82) |
| Py-R | 1595(1428,1745) | 2036(1736,2193) | 0.981008466 | 8.11(7.72,8.36) |
| CK | 2012(1682,2333) | 2511(1682,3530) | 0.990400468 | 8.91(8.42,9.24) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Duan, C.; Fu, D.; Wu, X.; Yan, K.; Fernando, E.; Karunarathna, S.C.; Promputtha, I.; Mortimer, P.E.; Xu, J. Structure of Bacterial Communities in Phosphorus-Enriched Rhizosphere Soils. Appl. Sci. 2020, 10, 6387. https://doi.org/10.3390/app10186387
Hu Y, Duan C, Fu D, Wu X, Yan K, Fernando E, Karunarathna SC, Promputtha I, Mortimer PE, Xu J. Structure of Bacterial Communities in Phosphorus-Enriched Rhizosphere Soils. Applied Sciences. 2020; 10(18):6387. https://doi.org/10.3390/app10186387
Chicago/Turabian StyleHu, Yuwei, Changqun Duan, Denggao Fu, Xiaoni Wu, Kai Yan, Eustace Fernando, Samantha C. Karunarathna, Itthayakorn Promputtha, Peter E. Mortimer, and Jianchu Xu. 2020. "Structure of Bacterial Communities in Phosphorus-Enriched Rhizosphere Soils" Applied Sciences 10, no. 18: 6387. https://doi.org/10.3390/app10186387
APA StyleHu, Y., Duan, C., Fu, D., Wu, X., Yan, K., Fernando, E., Karunarathna, S. C., Promputtha, I., Mortimer, P. E., & Xu, J. (2020). Structure of Bacterial Communities in Phosphorus-Enriched Rhizosphere Soils. Applied Sciences, 10(18), 6387. https://doi.org/10.3390/app10186387








