EVAR-eaSE: An Easy-to-Use Software for Planning Sac Embolization in EndoVascular Aneurysm Repair Procedure
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
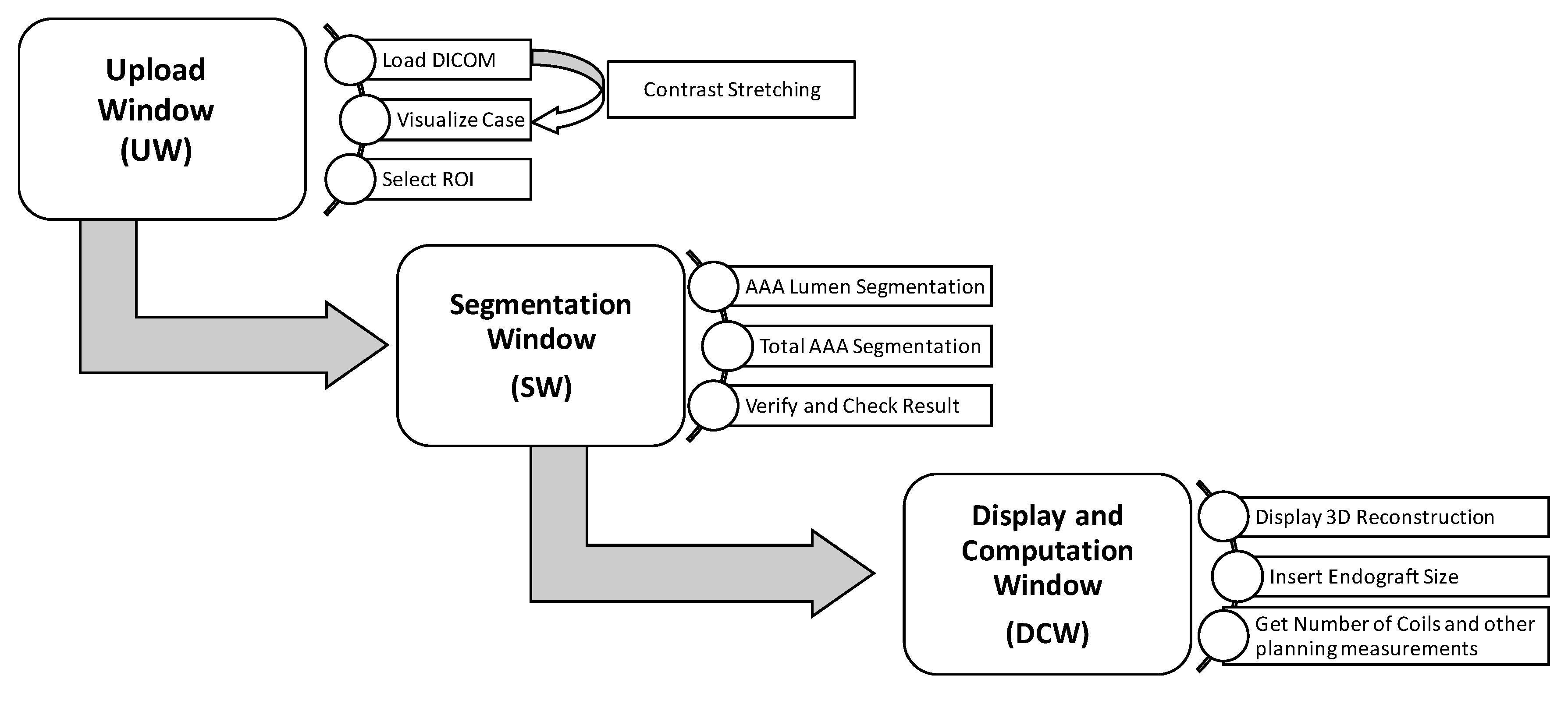
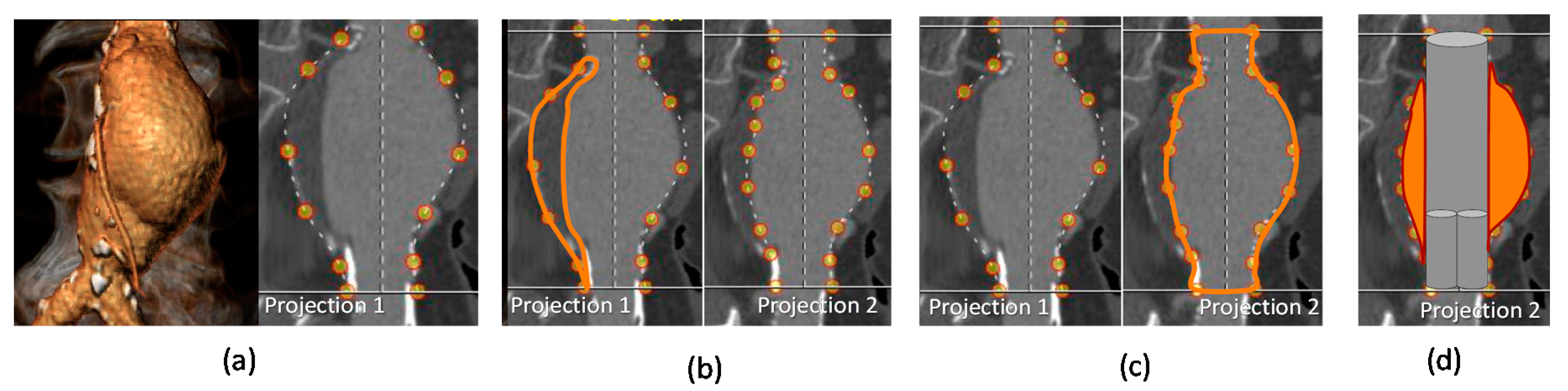
2.1. EVAR-eaSE Description
- 1.
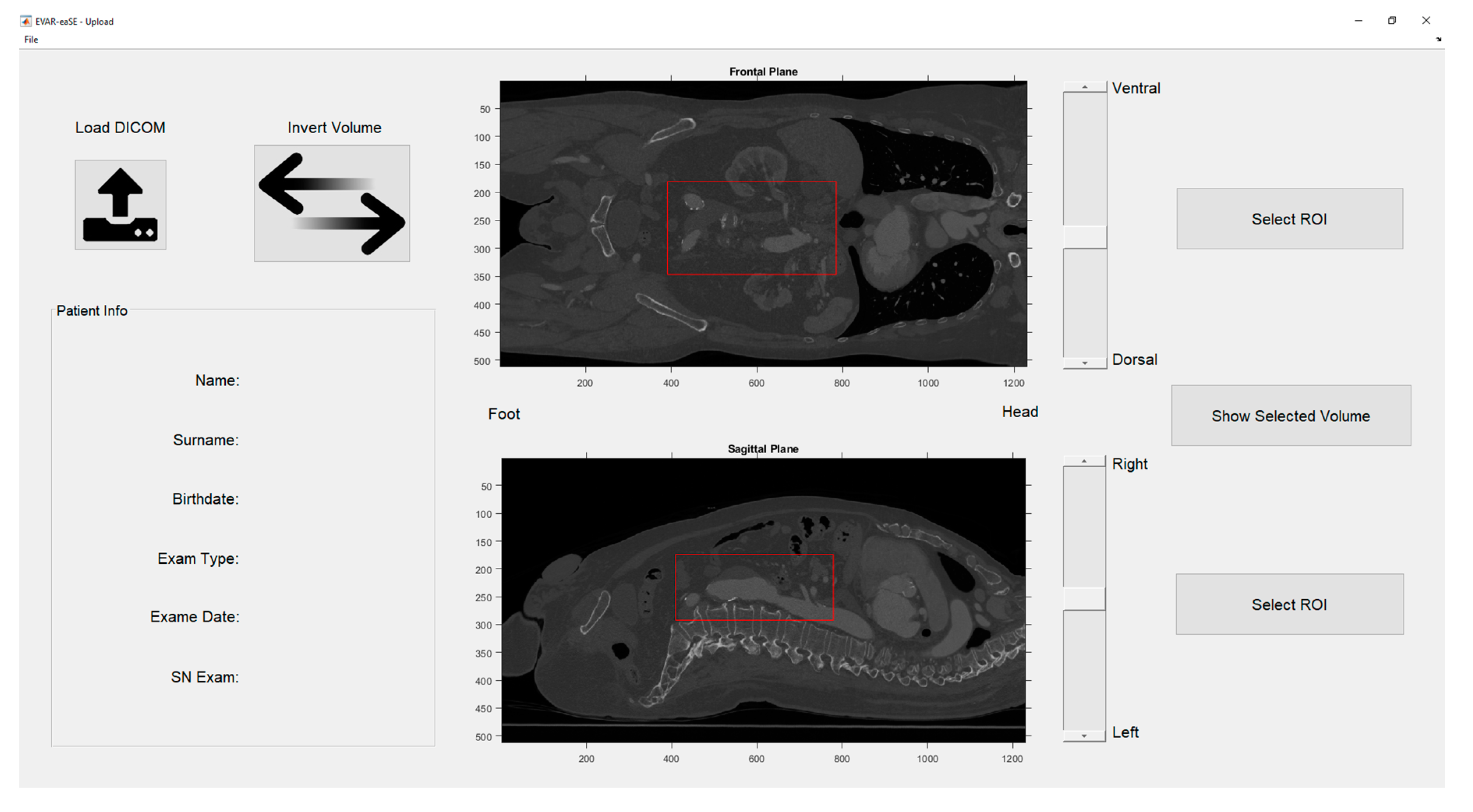
- Upload Window (UW): handles the uploading of DICOM (Digital Imaging and Communications in Medicine) files, e.g., computed tomography angiography (CTA) scans, the image pre-processing, the visualization of CTA volume rendering, and the selection of a region of interest (ROI).
- 2.
- Segmentation Window (SW): shows a contrast-enhanced version of the ROI selected in the previous window, and provides the ROI segmentation both for the pervious (lumen) and thrombotic part of the AAA; segmentation results are displayed in real time as contours on multi-planar volume reconstruction.
- 3.
- Display and Computation Window (DCW): displays the 3D models resulting from AAA segmentation and provides measurements useful for planning, including the optimal number of coils for SE.
2.1.1. Upload Window (UW)
2.1.2. Segmentation Window (SW)
2.1.3. Display and Computation Window (DCW)
2.2. EVAR-eaSE Evaluation
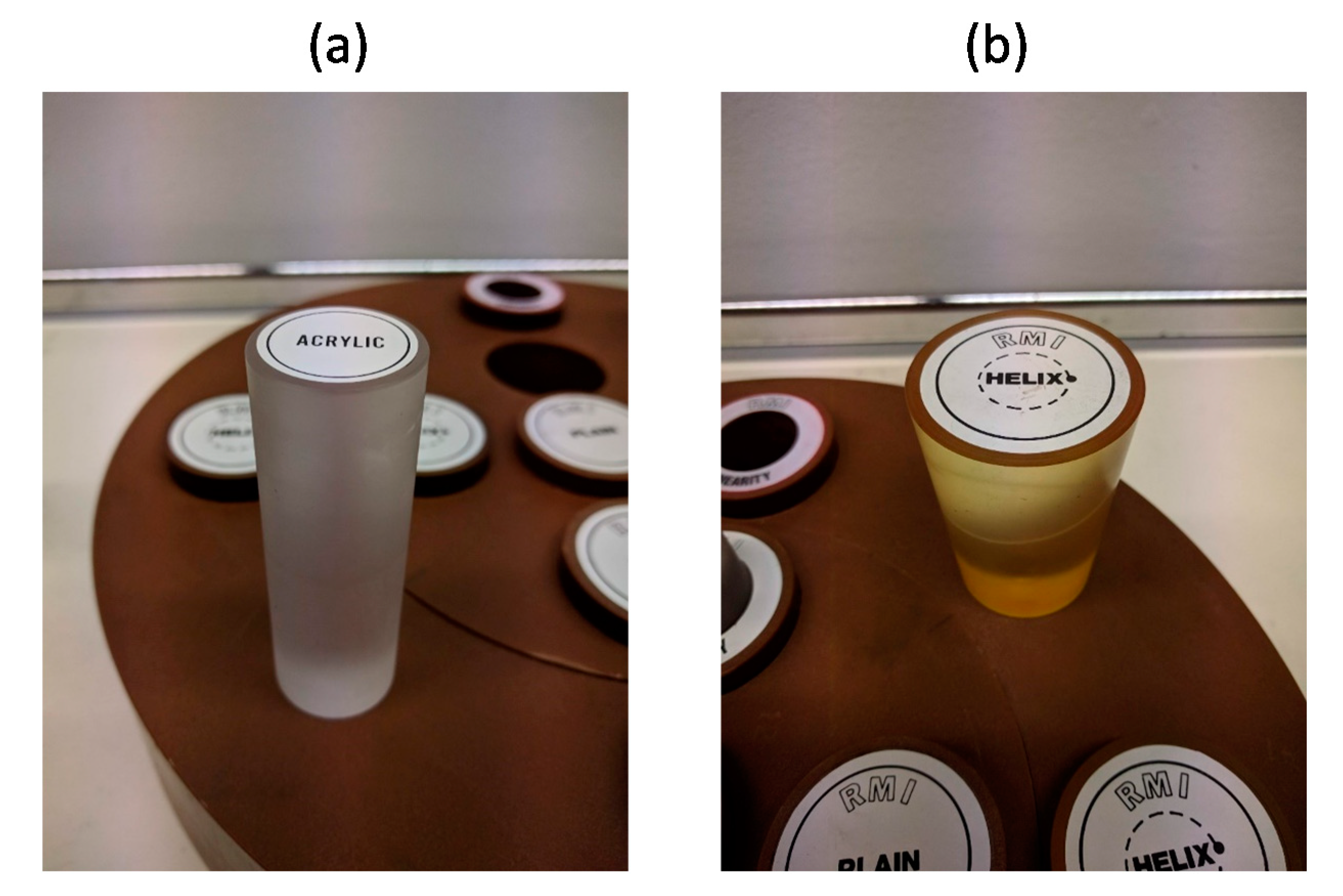
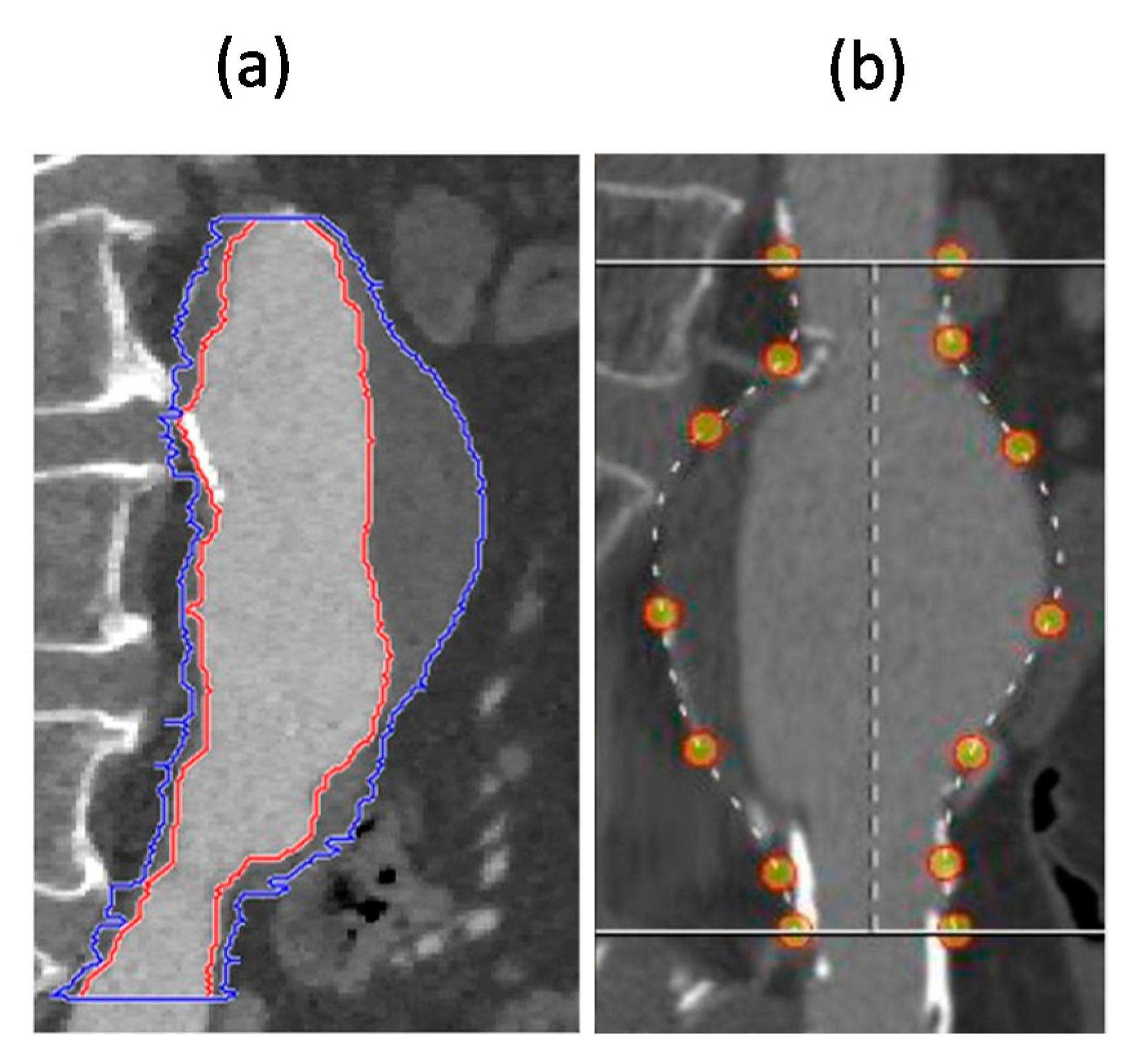
2.2.1. Phantom Tests
2.2.2. Comparative Analysis with 3Mensio
2.2.3. Data Analysis and Statistics
3. Results
3.1. Results of Phantom Tests
3.2. Results of Comparative Analysis
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Patel, R.; Sweeting, M.; Powell, J. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): A randomised controlled trail. J. Vasc. Surg. 2017, 65, 585. [Google Scholar] [CrossRef][Green Version]
- Pini, R.; Faggioli, G.; Longhi, M.; Mauro, R.; Freyrie, A.; Gargiulo, M.; Gallitto, E.; Mascoli, C.; Stella, A. The influence of study design on the evaluation of ruptured abdominal aortic aneurysm treatment. Ann. Vasc. Surg. 2014, 28, 1568–1580. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.E.; Atkins, M.D.; Brewster, D.C.; Chung, T.K.; Kwolek, C.J.; Lamuraglia, G.M.; Hodgman, T.M.; Cambria, R.P. Persistent type 2 endoleak after endovascular repair of abdominal aortic aneurysm is associated with adverse late outcomes. J. Vasc. Surg. 2007, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abularrage, C.J.; Crawford, R.S.; Conrad, M.F.; Lee, H.; Kwolek, C.J.; Brewster, D.C.; Cambria, R.P.; Lamuraglia, G.M. Preoperative variables predict persistent type 2 endoleak after endovascular aneurysm repair. J. Vasc. Surg. 2010, 52, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Lo, R.C.; Buck, D.B.; Herrmann, J.; Hamdan, A.D.; Wyers, M.; Patel, V.I.; Fillinger, M.; Schermerhorn, M.L. Vascular Study Group of New England Risk factors and consequences of persistent type II endoleaks. J. Vasc. Surg. 2016, 63, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Piazza, M.; Squizzato, F.; Zavatta, M.; Menegolo, M.; Ricotta, J.J.; Lepidi, S.; Grego, F.; Antonello, M. Outcomes of endovascular aneurysm repair with contemporary volume-dependent sac embolization in patients at risk for type II endoleak. J. Vasc. Surg. 2016, 63, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Fabre, D.; Fadel, E.; Brenot, P.; Hamdi, S.; Caro, A.G.; Mussot, S.; Becquemin, J.-P.; Angel, C. Type II endoleak prevention with coil embolization during endovascular aneurysm repair in high-risk patients. J. Vasc. Surg. 2015, 62, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dosluoglu, H.H.; Rivero, M.; Khan, S.Z.; Cherr, G.S.; Harris, L.M.; Dryjski, M.L. Pre-emptive nonselective perigraft aortic sac embolization with coils to prevent type II endoleak after endovascular aneurysm repair. J. Vasc. Surg. 2019, 69, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Mascoli, C.; Freyrie, A.; Gargiulo, M.; Gallitto, E.; Pini, R.; Faggioli, G.; Serra, C.; De Molo, C.; Stella, A. Selective Intra-procedural AAA sac Embolization During EVAR Reduces the Rate of Type II Endoleak. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 632–639. [Google Scholar] [CrossRef]
- Otsu, M.; Ishizaka, T.; Watanabe, M.; Hori, T.; Kohno, H.; Ishida, K.; Nakaya, M.; Matsumiya, G. Analysis of anatomical risk factors for persistent type II endoleaks following endovascular abdominal aortic aneurysm repair using CT angiography. Surg. Today 2015, 46, 48–55. [Google Scholar] [CrossRef]
- Gallitto, E.; Gargiulo, M.; Mascoli, C.; Freyrie, A.; De Matteis, M.; Serra, C.; Massoni, C.B.; Faggioli, G.; Stella, A. Persistent type II endoleak after EVAR: The predictive value of the AAA thrombus volume. J. Cardiovasc. Surg. 2015, 59, 79–86. [Google Scholar]
- Aoki, A.; Maruta, K.; Hosaka, N.; Omoto, T.; Masuda, T.; Gokan, T. Evaluation and coil embolization of the aortic side branches for prevention of type ii endoleak after endovascular repair of abdominal aortic aneurysm. Ann. Vasc. Dis. 2017, 10, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Mascoli, C.; Gargiulo, M.; Gallitto, E.; Longhi, M.; Pini, R.; Ancetti, S.; Faggioli, G.; Stella, A. IP071. Persistent type ii endoleak prevention by abdominal aortic aneurysm sac embolization during EVAR: An analysis of aneurysm volume and coils concentration. J. Vasc. Surg. 2017, 65, 75S–76S. [Google Scholar] [CrossRef]
- Reimerink, J.J.; Marquering, H.A.; Vahl, A.; Wisselink, W.; Schreve, M.A.; De Boo, D.W.; Reekers, J.A.; Legemate, D.A.; Balm, R. Semiautomatic sizing software in emergency endovascular aneurysm repair for ruptured abdominal aortic aneurysms. Cardiovasc. Interv. Radiol. 2013, 37, 623–630. [Google Scholar] [CrossRef]
- Van Keulen, J.W.; Moll, F.L.; Tolenaar, J.L.; Verhagen, H.J.M.; Van Herwaarden, J.A. Validation of a new standardized method to measure proximal aneurysm neck angulation. J. Vasc. Surg. 2010, 51, 821–828. [Google Scholar] [CrossRef]
- La Garma, V.H.E.-D.; Zenteno, M.; Padilla-Vázquez, F.; San-Juan, D.; Cerón-Morales, A. Comparative analysis of aneurysm volume by different methods based on angiography and computed tomography angiography. Neurosurg. Rev. 2018, 41, 1013–1019. [Google Scholar] [CrossRef]
- Woodward, K.; Forsberg, D.A. AngioSuite: An accurate method to calculate aneurysm volumes and packing densities. J. NeuroInterv. Surg. 2012, 5, 28–32. [Google Scholar] [CrossRef]
- Chan, T.F.; Vese, L. Active contours without edges. IEEE Trans. Image Process. 2001, 10, 266–277. [Google Scholar] [CrossRef]
- Oliveira, R.; Filho, M.E.; Ma, Z.; Papa, J.P.; Pereira, A.S.; Tavares, J.M.R. Computational methods for the image segmentation of pigmented skin lesions: A review. Comput. Methods Programs Biomed. 2016, 131, 127–141. [Google Scholar] [CrossRef]
- Bescós, J.O.; Slob, M.J.; Slump, C.H.; Sluzewski, M.; Van Rooij, W. Volume measurement of intracranial aneurysms from 3D rotational angiography: Improvement of accuracy by gradient edge detection. Am. J. Neuroradiol. 2005, 26, 2569–2572. [Google Scholar]
- Piotin, M.; Daghman, B.; Mounayer, C.; Spelle, L.; Moret, J. Ellipsoid approximation versus 3D rotational angiography in the volumetric assessment of intracranial aneurysms. Am. J. Neuroradiol. 2006, 27, 839–842. [Google Scholar] [PubMed]
- Sluzewski, M.; Van Rooij, W.J.; Slob, M.J.; Bescós, J.O.; Slump, C.H.; Wijnalda, D. Relation between Aneurysm Volume, Packing, and Compaction in 145 Cerebral Aneurysms Treated with Coils. Radiology 2004, 231, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Sadato, A.; Hayakawa, M.; Tanaka, T.; Hirose, Y. Comparison of cerebral aneurysm volumes as determined by digitally measured 3D rotational angiography and approximation from three diameters. Interv. Neuroradiol. 2011, 17, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, F.; Köse, C.; Sarı, A. An inverse approach for automatic segmentation of carotid and vertebral arteries in CTA. Expert Syst. Appl. 2018, 93, 358–375. [Google Scholar] [CrossRef]
- Moccia, S.; De Momi, E.; El Hadji, S.; Mattos, L.S. Blood vessel segmentation algorithms—Review of methods, datasets and evaluation metrics. Comput. Methods Progr. Biomed. 2018, 158, 71–91. [Google Scholar] [CrossRef]

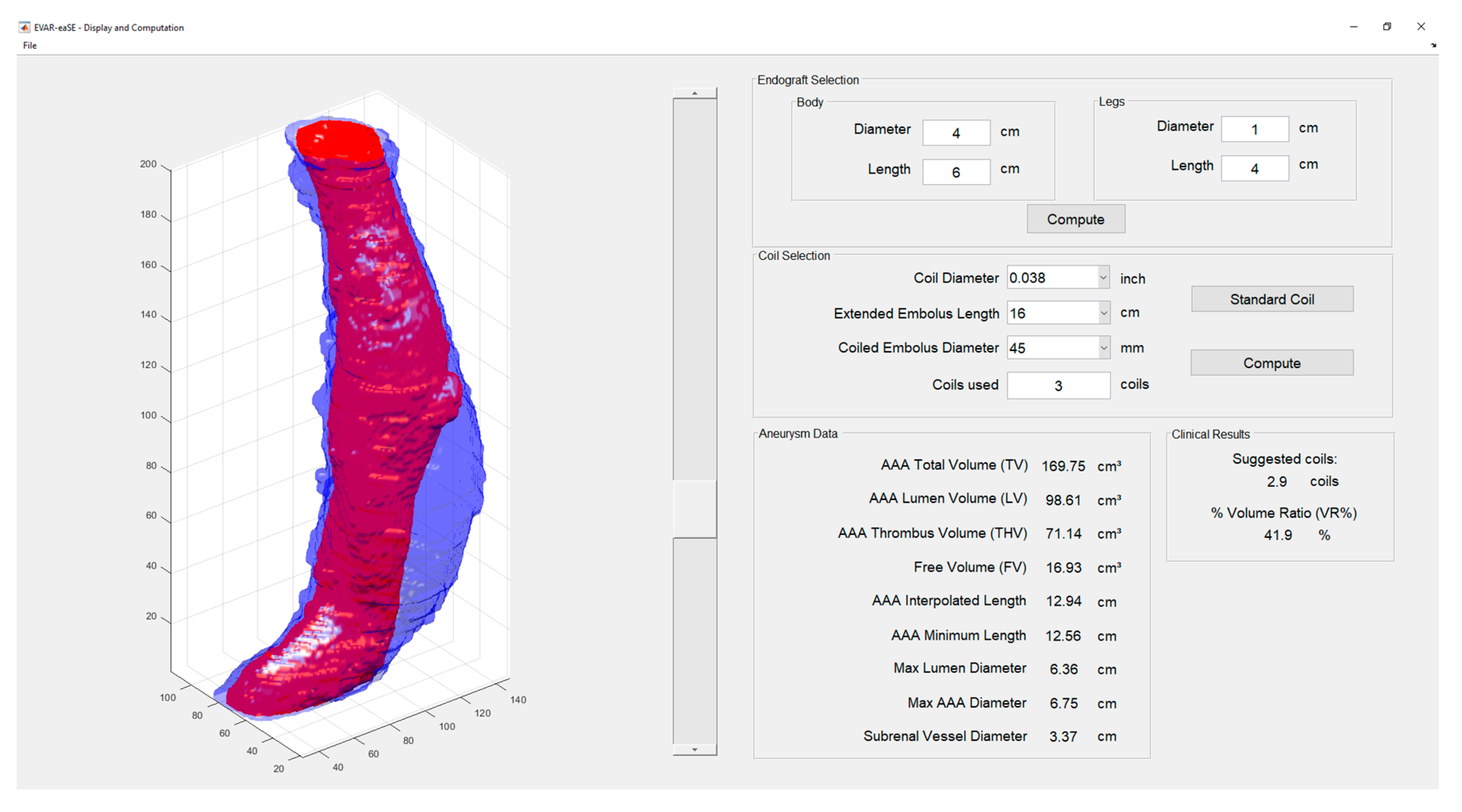

| Measurement | Description |
|---|---|
| AAA-Total Volume (TV) | Volume (in cm3) computed for the segmented total AAA, also including the thrombus. |
| AAA-Lumen Volume (LV) | Volume (in cm3) computed for the segmented AAA lumen. |
| AAA-Thrombus Volume (THV) | Volume (in cm3) computed for the segmented thrombus, calculated as difference between AAA-Total Volume (TV) and AAA-Lumen Volume (LV). |
| % Volume Ratio (%VR) | Percentual volumetric ratio between the thrombus volume (THV) and the total AAA volume, calculated as (THV/TV) *100. |
| Free Volume (FV) | Volume (in cm3) computed as the difference between the LV and the estimated endograft volume in the AAA |
| AAA Axis Length | Center line length, i.e., the line passing by the centroid of the AAA along each slice |
| Minimum AAA Length | Straight distance between the first and the last selected slices |
| Max Lumen Diameter | Maximum diameter for the segmented AAA lumen |
| Max AAA Diameter | Maximum diameter for the segmented entire AAA. |
| Subrenal Vessel Diameter | Diameter of the aorta just under the renal arteries, calculated as mean aorta diameter in the first five slices of the vessel, starting from the first selected slice. |
| Number of coils | The computed optimal number of coils, starting from the computed FV. |
| Phantom 1 | Slice Thickness 0.67 mm | Slice Thickness 2 mm |
|---|---|---|
| Mean Volume (cm3) | 50.03 | 50.09 |
| SD (cm3) | 2.05 | 2.33 |
| p-value | 0.950 | |
| PR = 0.5 overlap | PR = 1 No overlap | |
| Mean Volume (cm3) | 50.07 | 50.05 |
| SD (cm3) | 2.11 | 2.28 |
| p-value | 0.987 | |
| Phantom 2 | Slice Thickness 0.67 mm | Slice Thickness 2 mm |
|---|---|---|
| Mean Volume (cm3) | 107.18 | 107.94 |
| SD (cm3) | 5.18 | 5.05 |
| p-value | 0.813 | |
| PR = 0.5 overlap | PR = 1 No overlap | |
| Mean Volume (cm3) | 106.41 | 107.09 |
| SD (cm3) | 4.62 | 4.03 |
| p-value | 0.760 | |
| Computed Volume [cm3] (by EVAR-eaSE) | Real Volume [cm3] (by Gauge) | % Difference | |
|---|---|---|---|
| Phantom 1 | 50.06 | 54.24 | −7.7% |
| Phantom 2 | 107.53 | 110.33 | −2.5% |
| AAA-Lumen Volume (LV) | ||
|---|---|---|
| 3Mensio | EVAR-eaSE | |
| Mean (cm3) | 89.73 | 85.38 |
| SD (cm3) | 25.70 | 25.25 |
| Correlation (r) | 0.940 | |
| p-value | <0.001 | |
| AAA-Total Volume (TV) | ||
| 3Mensio | EVAR-eaSE | |
| Mean TV (cm3) | 142.19 | 127.96 |
| SD (cm3) | 44.88 | 40.65 |
| Correlation (r) | 0.954 | |
| p-value | <0.001 | |
| Software tools | |||||
|---|---|---|---|---|---|
| Feature | EVAR-eaSE | 3Mensio | AngioSuite | AngioCalc | Leonardo |
| Main field of application | Vascular | Vascular | Neurovascular | Neurovascular/Peripheral | Neurovascular |
| Imaging source | CTA | CTA | Biplane angiograms | Not applicable (use of ellipsoid and multi-lobular geometric models for the aneurysm) [16] | 3D rotational angiograms |
| Image segmentation | YES (semi-automatic segmentation of AAA lumen and AAA total volume) | YES (segmentation based on manual selection of fiduciary markers along the aneurysm shape) [14,15] | YES (segmentation based on a calibration performed from 2D aneurysm measurements, 3D measurements and fiduciary markers) [17] | Not applicable | YES (delineation of aneurysm contour in every slice in axial views) [16] |
| Computation of aneurysm volumes | YES | YES | YES | YES (shape model selection and input of 2D aneurysm measurements) | YES |
| Input of endograft and/or coils size | YES | NO | YES | YES | NO |
| Computation of planning parameters | YES (see Table 1) | YES [14,15] | YES (packing density) [17] | YES (packing density) [16,17] | NO |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cercenelli, L.; Lodi, S.; Bortolani, B.; Mascoli, C.; Gargiulo, M.; Marcelli, E. EVAR-eaSE: An Easy-to-Use Software for Planning Sac Embolization in EndoVascular Aneurysm Repair Procedure. Appl. Sci. 2020, 10, 6252. https://doi.org/10.3390/app10186252
Cercenelli L, Lodi S, Bortolani B, Mascoli C, Gargiulo M, Marcelli E. EVAR-eaSE: An Easy-to-Use Software for Planning Sac Embolization in EndoVascular Aneurysm Repair Procedure. Applied Sciences. 2020; 10(18):6252. https://doi.org/10.3390/app10186252
Chicago/Turabian StyleCercenelli, Laura, Simone Lodi, Barbara Bortolani, Chiara Mascoli, Mauro Gargiulo, and Emanuela Marcelli. 2020. "EVAR-eaSE: An Easy-to-Use Software for Planning Sac Embolization in EndoVascular Aneurysm Repair Procedure" Applied Sciences 10, no. 18: 6252. https://doi.org/10.3390/app10186252
APA StyleCercenelli, L., Lodi, S., Bortolani, B., Mascoli, C., Gargiulo, M., & Marcelli, E. (2020). EVAR-eaSE: An Easy-to-Use Software for Planning Sac Embolization in EndoVascular Aneurysm Repair Procedure. Applied Sciences, 10(18), 6252. https://doi.org/10.3390/app10186252






