The Implications of HClO4 for Dissolving Large Masses of Low Level Os in Metal Sulfides and Factors that Influence Re-Os Dating
Abstract
1. Introduction
2. Material and Methods
2.1. Instrumentation
2.2. Reagents and Solutions
2.3. Analytical Methods
3. Results and Discussion
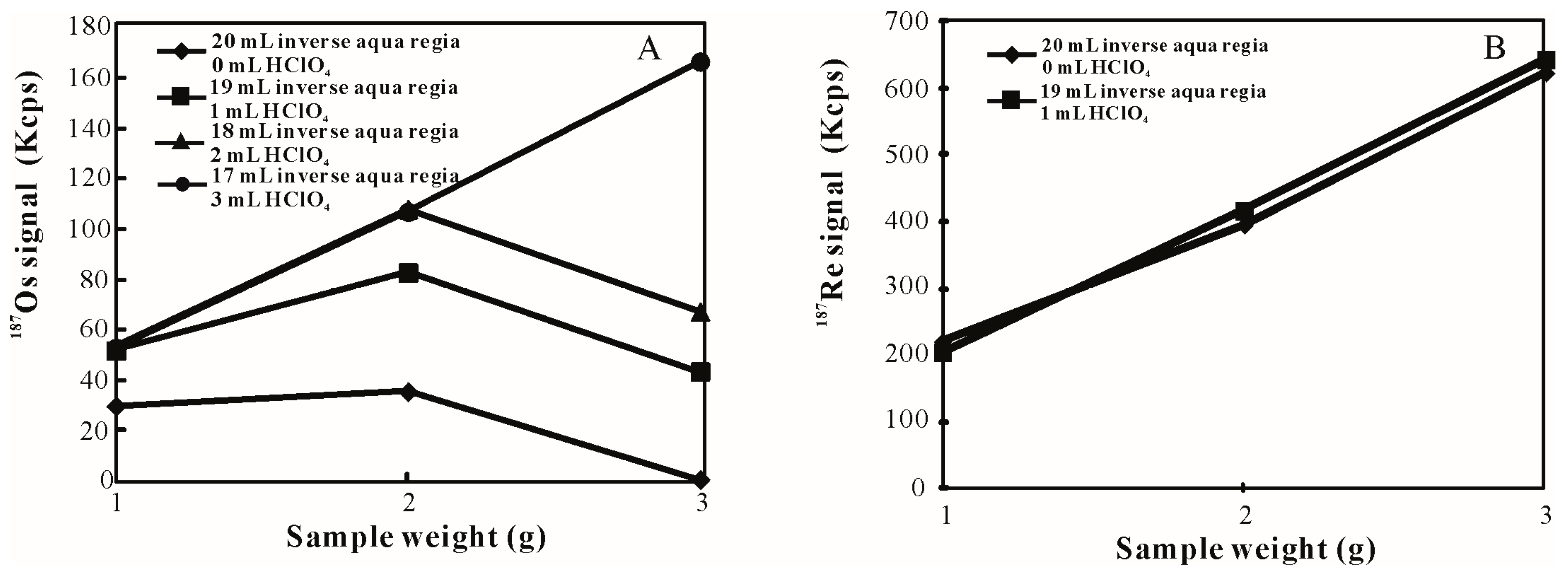
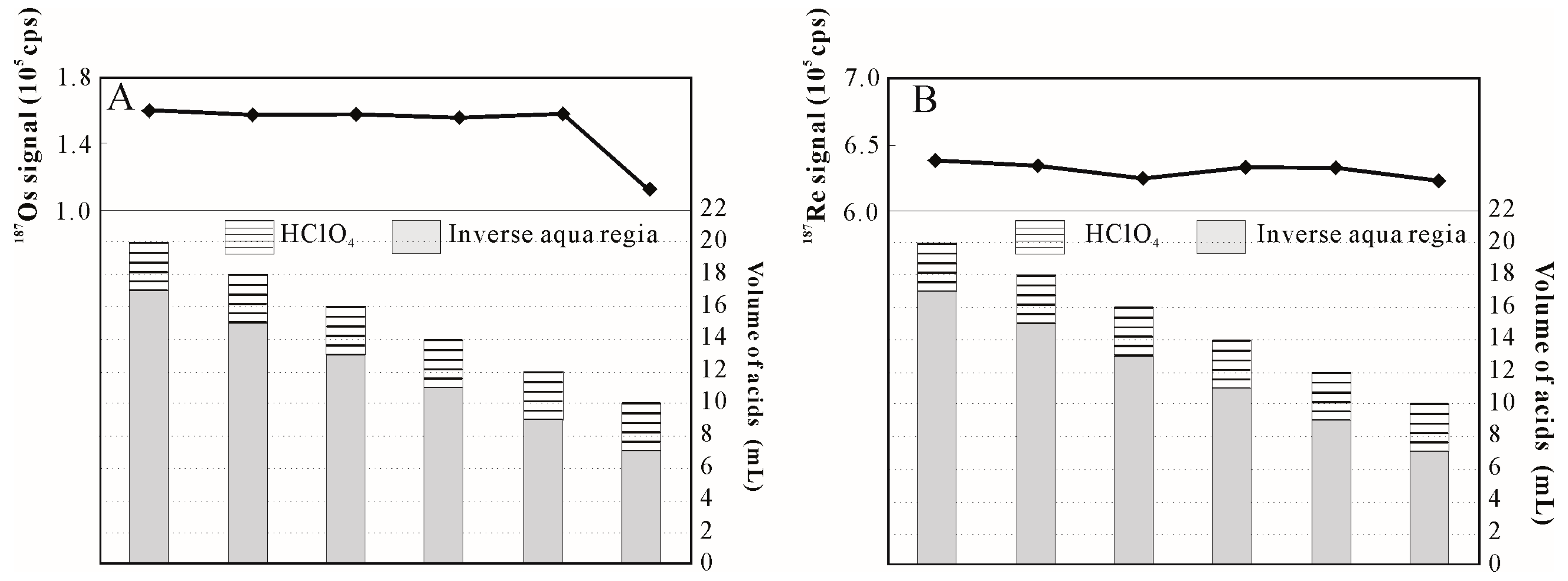
3.1. Influence of HClO4 Addition on Sample Digestion
3.2. Digestion of Reference Materials and Procedural Blank
3.3. Influence Factors during the Dissolution Process for Re-Os Dating
4. Conclusions
- Three grams of pyrite were completely dissolved with only 12 mL of mixed acids for Re-Os dating (3 mL of HClO4 and 9 mL of inverse aqua regia).
- The oxidation of acids may greatly affect the Os signal but has no influence on the equilibrium of isotope exchange between 185Re and 190Os spikes within Re and Os samples.
- The heating temperature has the greatest influence on the equilibrium of isotope exchange.
- The Os isotope system was not equilibrated between the spike and sample until the heating temperature reached 190 °C.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morgan, J.W.; Walker, R.J. Isotopic determinations of rhenium and osmium in meteorites by using fusion, distillation and ion-exchange separations. Anal. Chim. Acta 1989, 222, 291–300. [Google Scholar] [CrossRef]
- Smoliar, M.I.; Walker, R.J.; Morgan, J.W. Re-Os ages of group IIA, IIIA, IVA, and IVB iron meteorites. Science 1996, 271, 1099. [Google Scholar] [CrossRef]
- Luck, J.M.; Birck, J.L.; Allegre, C.J. 187 Re–187 Os systematics in meteorites: Early chronology of the Solar System and age of the Galaxy. Nature 1980, 283, 256–259. [Google Scholar] [CrossRef]
- Selby, D.; Creaser, R.A.; Stein, H.J.; Markey, R.J.; Hannah, J.L. Assessment of the 187Re decay constant by cross calibration of Re–Os molybdenite and U–Pb zircon chronometers in magmatic ore systems. Geochim. Cosmochim. Acta 2007, 71, 1999–2013. [Google Scholar] [CrossRef]
- Morgan, J.W. Rhenium-osmium dating method. In Geochemistry; Springer: Dordrecht, The Netherlands, 1998; pp. 547–550. [Google Scholar] [CrossRef]
- Gao, B.Y.; Zhang, L.C.; Jin, X.D.; Li, Z.Q.; Li, W.J. Rhenium-Osmium isotope systematics of an Early Mesoproterozoic SEDEX polymetallic pyrite deposit in the North China Craton: Implications for geological significance and the marine osmium isotopic record. Ore Geol. Rev. 2020, 117, 103331. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, L.; Jin, X.; Li, W.; Bai, Y.; Sakyi, P.A. Re-Os geochronology and trace element characteristics of the hydrothermally reworked pyrite of the Gaobanhe sediment-hosted polymetal pyrite deposit in Northern China and its geological significance. J. Geochem. Explor. 2020, 215, 106561. [Google Scholar] [CrossRef]
- Liu, Z.; Mao, X.; Ackerman, L.; Li, B.; Dick, J.M.; Yu, M.; Peng, J.; Shahzad, S.M. Two-stage gold mineralization of the Axi epithermal Au deposit, Western Tianshan, NW China: Evidence from Re–Os dating, S isotope, and trace elements of pyrite. Miner. Depos. 2019. [Google Scholar] [CrossRef]
- Lawley, C.; Selby, D.; Imber, J. Re-Os Molybdenite, Pyrite, and Chalcopyrite Geochronology, Lupa Goldfield, Southwestern Tanzania: Tracing Metallogenic Time Scales at Midcrustal Shear Zones Hosting Orogenic Au Deposits. Econ. Geol. 2013, 108, 1591–1613. [Google Scholar] [CrossRef]
- Barrapantoja, L.F. A Re-Os Study of Sulfides from the Bagdad Porphyry Cu-Mo Deposit, Northern Arizona, USA. Thermochim. Acta 2001, 371, 137–141. [Google Scholar]
- Barra, F.; Ruiz, J.; Mathur, R.; Titley, S. A Re–Os study of sulfide minerals from the Bagdad porphyry Cu–Mo deposit, northern Arizona, USA. Miner. Depos. 2003, 38, 585–596. [Google Scholar] [CrossRef]
- Qu, W.J.; Du, A.D.; Jing, R. Influence of H2O2 on the Signal Intensity of Rhenium, Osmium and Re-Os Age in the Process of Dissolution for Pyrite. Chin. J. Anal. Chem. 2008, 36, 223–226. [Google Scholar]
- Morelli, R.M. Re-Os Sulfide Geochronology of the Red Dog Sediment-Hosted Zn-Pb-Ag Deposit, Brooks Range, Alaska. Econ. Geol. 2004, 99, 1569–1576. [Google Scholar] [CrossRef]
- Stein, H.J.; Morgan, J.W.; Schersten, A. Re-Os Dating of Low-Level Highly Radiogenic (LLHR) Sulfides: The Harnas Gold Deposit, Southwest Sweden, Records Continental-Scale Tectonic Events. Econ. Geol. 2000, 95, 1657–1671. [Google Scholar] [CrossRef]
- Morgan, J.W.; Stein, H.J.; Hannah, J.L.; Markey, R.J.; Wiszniewska, J. Re-Os study of Fe-Ti-V oxide and Fe-Cu-Ni sulfide deposits, Suwałki Anorthosite Massif, northeast Poland. Miner. Depos. 2000, 35, 391–401. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, Y. Direct Re-Os dating of chalcopyrite from the Lala IOCG deposit in the Kangdian Copper Belt, China. Econ. Geol. 2013, 108, 871–882. [Google Scholar]
- Nozaki, T.; Suzuki, K.; Kato, Y. Re-Os geochronology of the Hitachi volcanogenic massive sulfide deposit: The oldest ore deposit in Japan. Econ. Geol. 2014, 109, 2023–2034. [Google Scholar] [CrossRef]
- Kelley, K.D.; Selby, D.; Falck, H.; Slack, J.F. Re-Os systematics and age of pyrite associated with stratiform Zn-Pb mineralization in the Howards Pass district, Yukon and Northwest Territories, Canada. Miner. Depos. 2017, 52, 1–19. [Google Scholar] [CrossRef]
- Kirk, J.D.; Ruiz, J.; Kesler, S.E.; Simon, A.; Muntean, J.L. Re-Os age of the pueblo Viejo epithermal deposit, Dominican Republic. Econ. Geol. 2013, 109, 503–512. [Google Scholar] [CrossRef]
- Zhu, M.T.; Zhang, L.C.; Wu, G.; Jin, X.D.; Xiang, P.; Li, W.J. Zircon U–Pb and pyrite Re–Os age constraints on pyrite mineralization in the Yinjiagou deposit, China. Int. Geol. Rev. 2013, 55, 1616–1625. [Google Scholar] [CrossRef]
- Gregory, M.J.; Schaefer, B.F.; Keays, R.R.; Wilde, A.R. Rhenium–osmium systematics of the Mount Isa copper orebody and the Eastern Creek Volcanics, Queensland, Australia: Implications for ore genesis. Miner. Depos. 2008, 43, 553–573. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, M.; Sun, M. Routine Os analysis by isotope dilution-inductively coupled plasma mass spectrometry: OsO4 in water solution gives high sensitivity. Amino Acids 2001, 16, 345–349. [Google Scholar] [CrossRef]
- Crocket, J.H.; Keays, R.R.; Hsieh, S.J. Determination of some precious metals by neutron activation analysis. Radioanal. Chem. 1968, 1, 487–507. [Google Scholar] [CrossRef]
- Cohen, A.S.; Waters, F.G. Separation of osmium from geological materials by solvent extraction for analysis by thermal ionisation mass spectrometry. Anal. Chim. Acta 1996, 332, 269–275. [Google Scholar] [CrossRef]
- Shirey, S.B.; Walker, R.J. Carius Tube Digestion for Low-Blank Rhenium-Osmium Analysis. Anal. Chem. 1995, 67, 2136–2141. [Google Scholar] [CrossRef]
- Qi, L.; Zhou, M.F.; Gao, J.F.; Zhao, Z.J. An improved Carius tube technique for determination of low concentrations of Re and Os in pyrites. Anal. At. Spectrom. 2010, 25, 585–589. [Google Scholar] [CrossRef]
- Gao, B.Y.; Li, W.J.; Jin, X.D.; Zhang, L.C. Application of addition HClO4 to improve the dissolution process and sensitivity of Os for low-concentration of Os in pyrite. Microchem. J. 2019, 150, 104165. [Google Scholar] [CrossRef]
- Jin, X.; Li, W.; Xiang, P.; Sakyi, P.A.; Zhu, M.; Zhang, L.J.; Dorrzapf, A. A contribution to common Carius tube distillation techniques. Anal. At. Spectrom. 2014, 396–404. [Google Scholar]
- Morgan, J.; Golightly, D. Methods for the separation of rhenium, osmium and molybdenum applicable to isotope geochemistry. Talanta 1991, 38, 259–265. [Google Scholar] [CrossRef]
- Markey, R.; Stein, H.; Morgan, J. Highly precise Re–Os dating for molybdenite using alkaline fusion and NTIMS. Talanta 1998, 45, 935–946. [Google Scholar] [CrossRef]
- Ludwig, K.R. User’s Manual for Isoplot 3.00: A Geochronological Toolkit for Microsoft Excel; Berkeley Geochronology Center Special Publication; Berkeley Geochronology Center: Berkeley, CA, USA, 2003. [Google Scholar]
- Du, A.; Wu, S.; Sun, D.; Wang, S.; Qu, W.; Markey, R.; Stain, H.; Morgan, J.; Malinovskiy, D. Preparation and Certification of Re-Os Dating Reference Materials: Molybdenites HLP and JDC. Geostand. Geoanal. Res. 2004, 28, 41–52. [Google Scholar] [CrossRef]
- Shirey, S.B. Walker, The Re-Os isotope system in cosmochemistry and high-temperature geochemistry. R.J. Annu. Rev. Earth Planet. Sci. 1998, 26, 423–500. [Google Scholar] [CrossRef]
| Sample Name | Re μg/g−1 | 187Os ng/g | Age (Ma) |
|---|---|---|---|
| HLP | 283.0 ± 3.0 | 658.5 ± 4.7 | 221.8 ± 3.6 |
| HLP | 288.3 ± 2.5 | 667.3 ± 6.7 | 220.7 ± 3.6 |
| HLP | 279.5 ± 3.3 | 641.5 ± 6.1 | 218.8 ± 4.0 |
| HLP | 289.0 ± 2.8 | 668.6 ± 4.8 | 220.5 ± 3.4 |
| AV | 284.9 ± 4.5 | 659.0 ± 12.5 | 220.5 ± 1.2 |
| CV | 283.8 ± 6.2 | 659.0 ± 14.0 | 221.4 ± 5.6 |
| JDC | 17.26 ± 0.22 | 25.21 ± 0.20 | 139.3 ± 2.5 |
| JDC | 17.54 ± 0.31 | 25.78 ± 0.27 | 140.2 ± 3.2 |
| JDC | 17.23 ± 0.20 | 24.93 ± 0.24 | 138.0 ± 2.5 |
| JDC | 17.92 ± 0.42 | 26.49 ± 0.27 | 141.0 ± 3.9 |
| AV | 17.49 ± 0.32 | 25.60 ± 0.69 | 139.6 ± 1.3 |
| CV | 17.39 ± 0.32 | 25.46 ± 0.60 | 139.6 ± 3.8 |
| Sample Name | Re (ng) | 187Os (ng) | Common Os (ng) | Total Os |
|---|---|---|---|---|
| Blank | 0.0067 | 0.0002 | 0.0008 | 0.0010 |
| Blank | 0.0032 | 0.0001 | 0.0008 | 0.0009 |
| Blank | 0.0033 | 0.0001 | 0.0010 | 0.0010 |
| Blank | 0.0043 | 0.0001 | 0.0013 | 0.0014 |
| AV(ng) | 0.0044 | 0.0001 | 0.0010 | 0.0011 |
| Sample Name | Re μg/g | 187Os ng/g | Age (Ma) | Dissolution Medium |
|---|---|---|---|---|
| HLP | 283.6 ± 2.9 | 653.7 ± 4.7 | 219.7 ± 3.5 | 20 mL of inverse aqua regia |
| HLP | 281.5 ± 3.6 | 647.6 ± 5.4 | 219.3 ± 4.0 | 1 mL of HClO4, 19 mL of inverse aqua regia |
| HLP | 285.8 ± 2.7 | 661.0 ± 4.0 | 220.4 ± 3.3 | 2 mL of HClO4, 18 mL of inverse aqua regia |
| HLP | 281.6 ± 2.2 | 649.9 ± 4.8 | 220.0 ± 3.2 | 3 mL of HClO4, 17 mL of inverse aqua regia |
| CV | 283.8 ± 6.2 | 659.0 ± 14.0 | 221.4 ± 5.6 |
| Re ng/g | Common Os pg/g | 187Os pg/g | Dissolution Medium |
|---|---|---|---|
| 26.20 ± 0.21 | 7.586 ± 0.144 | 63.37 ± 0.44 | 20 mL of inverse aqua regia |
| 26.56 ± 0.18 | 2.062 ± 0.089 | 63.88 ± 0.45 | 1 mL of HClO4, 19 mL of inverse aqua regia |
| 26.20 ± 0.23 | 2.582 ± 0.037 | 63.29 ± 0.39 | 2 mL of HClO4, 18 mL of inverse aqua regia |
| 26.49 ± 0.23 | 2.621 ± 0.142 | 63.70 ± 0.38 | 3 mL of HClO4, 17 mL of inverse aqua regia |
| ST | 200 °C | 190 °C | 180 °C | ||
|---|---|---|---|---|---|
| AT | |||||
| t | |||||
| 2 h | 201 °C | 192 °C | 181 °C | ||
| 4 h | 203 °C | 191 °C | 180 °C | ||
| 6 h | 202 °C | 190 °C | 181 °C | ||
| 8 h | 200 °C | 192 °C | 182 °C | ||
| 10 h | 202 °C | 191 °C | 180 °C | ||
| 12 h | 201 °C | 191 °C | 182 °C | ||
| 14 h | 201 °C | 190 °C | 183 °C | ||
| 16 h | 202 °C | 191 °C | 180 °C | ||
| 18 h | 200 °C | 193 °C | 182 °C | ||
| 20 h | 200 °C | 190 °C | 181 °C | ||
| 22 h | 202 °C | 192 °C | 183 °C | ||
| 24 h | 200 °C | 192 °C | 181 °C | ||
| Sample Name | Re μg/g | 187Os ng/g | Age (Ma) | Heating Temperature |
|---|---|---|---|---|
| JDC | 16.90 ± 0.21 | 24.78 ± 0.17 | 139.9 ± 2.4 | |
| JDC | 17.01 ± 0.13 | 24.71 ± 0.15 | 138.6 ± 1.9 | 200 °C |
| JDC | 17.09 ± 0.14 | 24.93 ± 0.17 | 139.2 ± 2.0 | |
| JDC | 16.88 ± 0.12 | 24.89 ± 0.21 | 140.7 ± 2.1 | |
| JDC | 16.94 ± 0.14 | 24.89 ± 0.20 | 140.2 ± 2.1 | 190 °C |
| JDC | 16.95 ± 0.14 | 24.62 ± 0.29 | 138.5 ± 2.4 | |
| JDC | 16.91 ± 0.20 | 19.89 ± 0.16 | 112.2 ± 1.9 | |
| JDC | 17.73 ± 0.21 | 20.44 ± 0.25 | 110.0 ± 2.2 | 180 °C |
| JDC | 17.64 ± 0.18 | 19.50 ± 0.15 | 105.5 ± 1.7 | |
| CV | 17.39 ± 0.32 | 25.46 ± 0.60 | 139.6 ± 3.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, B.; Li, W.; Zhang, L.; Gao, J. The Implications of HClO4 for Dissolving Large Masses of Low Level Os in Metal Sulfides and Factors that Influence Re-Os Dating. Appl. Sci. 2020, 10, 6218. https://doi.org/10.3390/app10186218
Gao B, Li W, Zhang L, Gao J. The Implications of HClO4 for Dissolving Large Masses of Low Level Os in Metal Sulfides and Factors that Influence Re-Os Dating. Applied Sciences. 2020; 10(18):6218. https://doi.org/10.3390/app10186218
Chicago/Turabian StyleGao, Bingyu, Wenjun Li, Lianchang Zhang, and Jun Gao. 2020. "The Implications of HClO4 for Dissolving Large Masses of Low Level Os in Metal Sulfides and Factors that Influence Re-Os Dating" Applied Sciences 10, no. 18: 6218. https://doi.org/10.3390/app10186218
APA StyleGao, B., Li, W., Zhang, L., & Gao, J. (2020). The Implications of HClO4 for Dissolving Large Masses of Low Level Os in Metal Sulfides and Factors that Influence Re-Os Dating. Applied Sciences, 10(18), 6218. https://doi.org/10.3390/app10186218




