Abstract
In general, no more than 1 g of metal sulfide can be completely digested in 20 mL of inverse aqua regia using the Carius tube technique. In this study, the sample weight increased after adding HClO4 to inverse aqua regia while the volume of acid concurrently decreased significantly. Three grams of metal sulfide were digested in 12 mL of acid (3 mL of HClO4 and 9 mL of inverse aqua regia) via the HClO4-inverse aqua regia method. The results using molybdenite reference materials JDC and HLP mixed with 3 g of pyrite were consistent with certified values. Compared to the traditional method, the HClO4-inverse aqua regia method could dissolve a larger sample mass (3 g) with a smaller volume of acids (12 mL). We simultaneously found that the oxidation of digestion acids greatly affected the Os signal but had no influence on the equilibrium of isotope exchange between 185Re and 190Os spikes in Re and Os samples. Remarkably, the heating temperature was the most significant factor influencing the equilibrium of isotope exchange, and the Os in a sample was not equilibrated with the spike until the heating temperature reached 190 °C.
1. Introduction
Re-Os isotopes are an important part of isotopic geochronology. Re and Os are highly siderophile elements (HSE) indicating a strong preference for a metal or sulfide phase over forming silicate minerals [1,2,3]. Therefore, the Re and Os could be enriched in metal sulfides such as molybdenite, pyrite, chalcopyrite, and arsenopyrite. Some minerals have economic value (e.g., molybdenite and chalcopyrite) while others coexist with minerals of economic value (e.g., pyrite and arsenopyrite). Therefore, the application of Re-Os isotope systems on a metal sulfide can be a powerful tool for directly dating the metallogenic epoch [4,5,6,7,8].
The most successful application of the Re-Os isotopic system for dating metal sulfides is molybdenite: the mineralization epoch of deposits is easily dated if molybdenite develops in a mining area [9,10,11]. However, besides molybdenite, other sulfide metals have lower probabilities of success on Re-Os dating because sulfide metals have low levels of Re and Os. For example, the content of Re and Os in pyrite is distributed between ppb to sub-ppb [12], and these minerals are denoted as low-content Re-Os metal sulfides including pyrite, chalcopyrite, and arsenopyrite. In the case of molybdenite, the application of the Re-Os isotopic system on low-content Re-Os metal sulfides can be used to date many more types of deposits [13,14,15,16,17,18]. Unfortunately, low-content Re-Os metal sulfides have a low probability of success when using Re-Os dating, and the inability to guarantee the stability of the measured signal is one of the reasons that leads to an inaccurate determination of the Re and Os content in metal sulfides.
Generally, 0.4 g of metal sulfide can be completely digested with 11 mL of inverse aqua regia [18,19]. In order to increase the Os signal, some researchers have dissolved larger samples (~1 g) in larger Carius tubes with nearly 20 mL of inverse aqua regia [20,21]. However, with low levels of Os in metal sulfides, this sample weight is not sufficient for Re-Os dating. For sample weights greater than 1 g, the dissolving capacity of acids might not be sufficiently strong to oxidize Os to OsO4. OsO4 plays an important role in Os separation techniques. For ICP-MS analysis, the sensitivity of Os (VIII) is much higher than other valence states of Os [22]. All Os separation techniques, including distillation, micro-distillation, and solvent extraction methods are based on the volatility and non-polarity of OsO4 [23,24]. Furthermore, Shirey and Walker [25] indicated that the oxidation of acids leads to the oxidation of Re and Os to their highest valence states and promotes the complete chemical equilibration of 185Re and 190Os in a sample. Qi et al. [26] improved the Carius method in that the samples were first dissolved with HNO3 in a 120 mL Carius tube to release gas with a small portion of volatile Os released at the same time (6% of total Os). They then collected Os using HCl and transferred these back to the Carius tube after the sulfides were reacted with HNO3. This method allowed the sample mass to be increased to 3 g using nearly 30 mL of acids, but the procedure appeared to be very complex.
Gao et al. [27] demonstrated that the HClO4-inverse aqua regia method could dissolve 0.8 g of sample with only 9 mL of acids (8 mL of inverse aqua regia and 1 mL of HClO4). In this paper, we focused on the effectiveness of the HClO4-inverse aqua regia method for sample weights greater than 1 g. The conditions were optimized to maximize the amount of sample tested. The influencing factors during the dissolution process for Re-Os dating are also discussed in this paper. This refined method was verified using several standard reference samples.
2. Material and Methods
2.1. Instrumentation
A Thermo Scientific Element I inductively coupled plasma-mass spectrometry (ICP-MS) instrument was used to analyze the sample. The Carius tubes were based on those described by Shirey and Walker [25], but the inner volume of the tube was approximately 100 mL.
2.2. Reagents and Solutions
Compared with other low-content Re-Os metal sulfides, pyrite is a low Re-Os sulfide commonly found in many types of sulfide deposits. Hence, it is always used as a preferred metal sulfide in applications of the Re-Os isotopic system. In our experiments, pyrite was used as a low-content Re-Os metal sulfide. HCl was prepared by sub-boiling distillation. HNO3 was further purified by boiling to four-fifths to remove volatile OsO4 after sub-boiling distillation. HClO4 was obtained from MERCK (Germany). The 185Re and 190Os spike samples were obtained from Oak Ridge National Laboratory.
2.3. Analytical Methods
The details of this procedure have been described by Shirey and Walker [25]. The process is summarized as follows: the spiked sample, HCl, HNO3, and HClO4 were added to the Carius tube through a thin-neck long funnel after which acids were frozen with ethanol-liquid nitrogen slush (−80 °C to −50 °C). The top was sealed using an oxygen-propane torch. The tube was then placed in a stainless steel jacket and heated to 210 °C for 36 h. Since large amounts of gases are produced in the tube during the 24 h heating period, the air pressure inside was much greater than the atmospheric pressure outside. Therefore, outgassing was necessary before the commencement of the Re and Os separation procedure. The Os separation procedure in this study was reported by Jin et al. [28] in which an economic electrothermal timing steamer for the common Carius tube in situ Os distillation was employed. In this technique, the Carius tube was sealed with a rubber head, and two thin Teflon tubes were inserted through two holes in the upper part of the rubber head serving as an inlet and an outlet for clean air and OsO4, respectively. The Os was separated from the solution in Carius tube by distillation in the steam bath and trapped in 3 mL of Milli-Q (MQ) water. Re was extracted from the aqueous residue via an anionic resin (AG1x-8, 200–400 mesh) as reported by Morgan et al. [29].
3. Results and Discussion
3.1. Influence of HClO4 Addition on Sample Digestion
According to previous studies, 1 g of pyrite is usually digested in 20 mL of acids via the inverse aqua regia method [18,19,20,21]. Therefore, a series of experiments were conducted on the basis of this condition. The Os signal was chosen as an indicator to reflect the strength of the dissolving capacity of acids. Considering that the spike is composed of 190Os and 185Re, whereas the 187Os signal is all from the sample, the 187Os signal was used to reflect the strength of the dissolving capacity of acids. The 187Os signal was 3.0 × 104 cps from 1 g of pyrite (Figure 1) when the volume of acid was 20 mL using the inverse aqua regia method. The 187Os signal was 3.5 × 104 cps as the sample weight increased to 2 g. The signal did not increase linearly with increasing sample weight. When 3 g of sample weight was used, the 187Os signal was only about 1.2 × 103 cps. These results suggest that it was not possible to dissolve more than 1 g of pyrite in 20 mL of acids with the inverse aqua regia method.
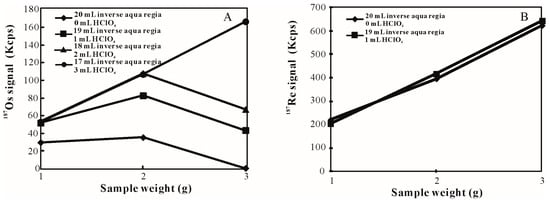
Figure 1.
Os (A) and Re (B) signal for different masses of pyrite obtained using the inverse aqua regia and HClO4-inverse aqua regia methods.
The 187Os signal increased significantly with the HClO4-inverse aqua regia method indicating that the dissolving capacity of acids was much stronger than that of the inverse aqua regia method. After 1 mL of HClO4 was added to 19 mL of inverse aqua regia, the 187Os signal reached 5.2 × 104 cps, which was 1.7 times higher than that of the inverse aqua regia method. The 187Os signal did not increase as the sample weight increased from 1 g to 3 g. The signal was about 75% of the intensity of the theoretical maximum, and no more than 1.0 × 104 cps of Os signal was obtained when the sample weight reached 3 g. When the volume of HClO4 was 2 mL, the 187Os signal from 2 g of pyrite was two-fold higher than that from 1 g of pyrite. However, the signal dropped obviously as the sample weight increased to 3 g. This result suggests that 2 g of pyrite could be completely digested via the HClO4-inverse aqua regia method, but the dissolving capacity of acids was too weak to dissolve 3 g of pyrite.
When the volume of HClO4 was 3 mL, we found a positive correlation between the sample weight and Os signal indicating that the dissolving capacity of acids was strong enough to dissolve 3 g of pyrite in total. When the sample weight was at/above 4 g or the volume of HClO4 was increased to 4 mL, the hazard of the Carius tube exploding obviously increased. Therefore, we recommend that the maximum desirable sample weight is 3 g for 20 mL of acids, and no more than 3 mL of HClO4 is added to inverse aqua regia.
In comparing the inverse aqua regia method with the HClO4-inverse aqua regia method, the addition of HClO4 could improve the dissolving capacity of acids significantly leading to an increased weight of the dissolved sample. However, gas would be formed because of the reaction between the pyrite and acids, and significantly more gas would be formed with an increased volume of acid and sample weight. Increased gas production could lead to higher pressures inside the tube and potential explosions [26]. To reduce this risk, it was necessary to find the minimal volume of digestion medium. When the maximum sample weight was fixed at 3 g and the addition of HClO4 was 3 mL, the 187Os signal was nearly unchanged when the volume of inverse aqua regia decreased from 17 mL to 9 mL (Figure 2). These data indicate that the dissolving capacity of acids was still strong enough to completely dissolve pyrite. The 187Os signal decreased to 1.1 × 105 cps when the volume of inverse aqua regia was 7 mL. Nearly 28% of the Os was not oxidized to OsO4, indicating that the dissolving capacity of acids was too weak to dissolve all of the sample. Thus, 3 mL of HClO4 and 9 mL of inverse aqua regia were chosen as the final oxidant composition.
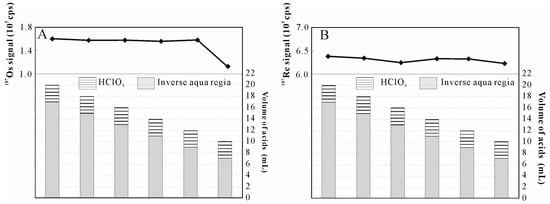
Figure 2.
Os (A) and Re (B) signal for 3 g of pyrite with different volumes of digestion medium obtained using the HClO4-inverse aqua regia method.
3.2. Digestion of Reference Materials and Procedural Blank
Because a natural reference material for low-content metal sulfides for Re-Os dating is unavailable, we used the molybdenite references GBW 04435 (JDC) and GBW 04436 (HLP). The references were mixed with 3 g of pyrite for monitoring the low-content metal sulfides of the reference (Table 1). The uncertainties of the ages for both reference material samples (Table 2) included the spike calibration uncertainties for both Re and Os [30], the weighing errors for spikes and samples, the ICP-MS measurement errors, the errors caused by mass bias, as well as the 1.02% uncertainty in the decay constant 1.666 × 10−10 a−1 for 187Re [2]. The total uncertainty at the 95% confidence level was calculated using the error propagation formula. The uncertainties for the weighted mean ages were calculated using ISOPLOT [31]. The results for JDC and HLP are in fairly good agreement with the certified values [32], which demonstrate that the proposed method is a reliable approach for determining Re and Os in metal sulfides. To keep a low procedural blank, all components in the setup of in situ distillation and Carius tube were only used once. This procedure was implemented to decrease the Os blank signal and avoid Os memory effect and cross contamination. The total procedural blanks are shown in Table 6. The blank values of Os were 1.0 pg for common Os and 0.1 pg for 187Os while the blank value of Re was 4.4 pg.

Table 1.
Analytical results for molybdenite RM, HLP, and JDC mixed with 3 g of pyrite.

Table 2.
Blank level.
3.3. Influence Factors during the Dissolution Process for Re-Os Dating
The Os signal was different when 1 g of pyrite was dissolved using two methods (Figure 1A). The intensity was 3.0 × 104 cps for the inverse aqua regia conditions, and the Os signal increased 1.7 times when 1 mL of HClO4 was added to the inverse aqua regia. The Os signal was unchanged as the volume of HClO4 increased. There are two possible explanations for this result: one is that the sample could not be totally dissolved with inverse aqua regia. The other is that the oxidizing power of inverse aqua regia was too weak to completely oxidize Os.
To further investigate this reaction, 1 g of pyrite mixed with 0.1 g of HLP was analyzed (Table 3). Since the concentrations of Re and Os in HLP were too high for the low level of Os in metal sulfides, an unknown sample (pyrite) was also analyzed (Table 4). Under inverse aqua regia conditions, the Re, 187Os, and common Os concentrations in pyrite were 26.60 ppb, 4.3 ppt, and 62.49 ppt, respectively. After the addition of 1 mL HClO4 to inverse aqua regia, the Re, 187Os, and common Os concentrations in pyrite were 26.67 ppb, 4.3 ppt, and 62.29 ppt, respectively. As the volume of HClO4 increased to 3 mL, the concentrations of Re and Os in pyrite were nearly unchanged. Simultaneously, the results for HLP were also in fairly good agreement with certified values.

Table 3.
Analytical results for 1 g of pyrite mixed with HLP in 20 mL of combined acids.

Table 4.
Analytical results for 1 g of unknown pyrite in 20 mL of combined acids.
The results from the HClO4-inverse aqua regia method were almost identical to the inverse aqua regia method, which suggest that the sample was totally dissolved and Re and Os in the sulfides were equilibrated with the spike by these two methods. We inferred that the oxidation of the acids may have been the reason for the large gap in the detected signal between these two methods. The oxidizing power of inverse aqua regia acids was not strong enough to completely oxidize Os to OsO4. Therefore, the concentration of OsO4 in the trapping solution was significantly lower than that obtained upon addition of HClO4. Thus, we suggest that the oxidizing power of acids did not influence the equilibrium of isotope exchange between 185Re and 190Os spikes within the Re and Os in the sample, but the Os signal was obviously affected.
During the digestion process, a yellow precipitate was observed when the sample weights were more than 2 g. A previous study suggested that the oxidation of the acids would dissipate as the precipitate appeared [12]. This effect was observed under inverse aqua regia conditions, and the 187Os signal decreased significantly as the sample weight increased beyond 2 g. However, the precipitate still appeared after the addition of 3 mL HClO4 but the 187Os signal increased with increasing sample weight. The yellow precipitate was formed as the volume of inverse aqua regia decreased from 17 mL to 9 mL; however, the 187Os signal remained unchanged (Figure 2). These results suggest that there was no obvious relationship between the yellow precipitate and the oxidation of the acids. The reason for the formation of the yellow precipitate remains unclear, but the precipitate did not influence the equilibrium of isotope exchange between 185Re and 190Os spikes within the Re and Os in the sample.
Shirey and Walker [33] indicated that the Os released from some matrices can be difficult to equilibrate with spikes at low temperatures (<200 °C); therefore, the tube is always heated to high temperatures (220 °C–240 °C). However, the risk of Carius tube explosion increases significantly as the heating temperature increases. For personal safety reasons, it is necessary to find the lowest temperature to ensure the equilibrium of isotope exchange between spikes and the sample. Before starting this experiment, the temperature of the oven was monitored to ensure that the temperature was stable during the heating period. The temperature of the oven was monitored every 2 h for 24 h, and the temperature was set at 200 °C, 190 °C, and 180 °C, respectively (Table 5). The results suggested that the actual temperature (AT) of the oven was consistent with set temperature (ST) during the heating period, which means that it can be used to research the relationship between the heating temperature and the equilibrium of isotope exchange. The JDC was dissolved using the HClO4-inverse aqua regia method at different temperatures (Table 6). As the heating temperature decreased from 210 °C to 190 °C, the results for JDC were in fairly good agreement with the certified value. These data indicate that the Re and Os in sulfides were equilibrated with the spike. However, there was a large degree of inconsistency with the results of JDC and the certified value when the heating temperature was 180 °C. The results of the Re concentration agreed well with the certified value while the Os values did not. This explains the large gap in the final results between the certified values. All of these data suggest that the Os released from the sample has difficulty equilibrating with spikes if the heating temperature is lower than 190 °C. We concurrently found that the Re in the sample more easily equilibrated with the spike than Os.

Table 5.
The stability of different temperature of the oven was monitored during 24 h.

Table 6.
Analytical results for molybdenite RM samples JDC at different temperatures.
4. Conclusions
In this study, the HClO4-inverse aqua regia method improved the digestion of larger masses of metal sulfide samples containing low concentrations of Os.
- Three grams of pyrite were completely dissolved with only 12 mL of mixed acids for Re-Os dating (3 mL of HClO4 and 9 mL of inverse aqua regia).
- The oxidation of acids may greatly affect the Os signal but has no influence on the equilibrium of isotope exchange between 185Re and 190Os spikes within Re and Os samples.
- The heating temperature has the greatest influence on the equilibrium of isotope exchange.
- The Os isotope system was not equilibrated between the spike and sample until the heating temperature reached 190 °C.
Author Contributions
All authors have read and agree to the published version of the manuscript. Conceptualization, B.G.; Methodology, B.G. and W.L.; writing—original draft preparation, B.G.; Writing—review and editing, W.L., L.Z., and J.G.
Funding
This study was financially funded by the National Natural Science General Project (Grant No. 41603016) and the National Key R&D Program of China (Grant No. 2016YFC0600106).
Acknowledgments
This study was financially supported by the National Natural Science General Project (Grant No. 41603016) and the National Key R&D Program of China (Grant No. 2016YFC0600106).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morgan, J.W.; Walker, R.J. Isotopic determinations of rhenium and osmium in meteorites by using fusion, distillation and ion-exchange separations. Anal. Chim. Acta 1989, 222, 291–300. [Google Scholar] [CrossRef]
- Smoliar, M.I.; Walker, R.J.; Morgan, J.W. Re-Os ages of group IIA, IIIA, IVA, and IVB iron meteorites. Science 1996, 271, 1099. [Google Scholar] [CrossRef]
- Luck, J.M.; Birck, J.L.; Allegre, C.J. 187 Re–187 Os systematics in meteorites: Early chronology of the Solar System and age of the Galaxy. Nature 1980, 283, 256–259. [Google Scholar] [CrossRef]
- Selby, D.; Creaser, R.A.; Stein, H.J.; Markey, R.J.; Hannah, J.L. Assessment of the 187Re decay constant by cross calibration of Re–Os molybdenite and U–Pb zircon chronometers in magmatic ore systems. Geochim. Cosmochim. Acta 2007, 71, 1999–2013. [Google Scholar] [CrossRef]
- Morgan, J.W. Rhenium-osmium dating method. In Geochemistry; Springer: Dordrecht, The Netherlands, 1998; pp. 547–550. [Google Scholar] [CrossRef]
- Gao, B.Y.; Zhang, L.C.; Jin, X.D.; Li, Z.Q.; Li, W.J. Rhenium-Osmium isotope systematics of an Early Mesoproterozoic SEDEX polymetallic pyrite deposit in the North China Craton: Implications for geological significance and the marine osmium isotopic record. Ore Geol. Rev. 2020, 117, 103331. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, L.; Jin, X.; Li, W.; Bai, Y.; Sakyi, P.A. Re-Os geochronology and trace element characteristics of the hydrothermally reworked pyrite of the Gaobanhe sediment-hosted polymetal pyrite deposit in Northern China and its geological significance. J. Geochem. Explor. 2020, 215, 106561. [Google Scholar] [CrossRef]
- Liu, Z.; Mao, X.; Ackerman, L.; Li, B.; Dick, J.M.; Yu, M.; Peng, J.; Shahzad, S.M. Two-stage gold mineralization of the Axi epithermal Au deposit, Western Tianshan, NW China: Evidence from Re–Os dating, S isotope, and trace elements of pyrite. Miner. Depos. 2019. [Google Scholar] [CrossRef]
- Lawley, C.; Selby, D.; Imber, J. Re-Os Molybdenite, Pyrite, and Chalcopyrite Geochronology, Lupa Goldfield, Southwestern Tanzania: Tracing Metallogenic Time Scales at Midcrustal Shear Zones Hosting Orogenic Au Deposits. Econ. Geol. 2013, 108, 1591–1613. [Google Scholar] [CrossRef]
- Barrapantoja, L.F. A Re-Os Study of Sulfides from the Bagdad Porphyry Cu-Mo Deposit, Northern Arizona, USA. Thermochim. Acta 2001, 371, 137–141. [Google Scholar]
- Barra, F.; Ruiz, J.; Mathur, R.; Titley, S. A Re–Os study of sulfide minerals from the Bagdad porphyry Cu–Mo deposit, northern Arizona, USA. Miner. Depos. 2003, 38, 585–596. [Google Scholar] [CrossRef]
- Qu, W.J.; Du, A.D.; Jing, R. Influence of H2O2 on the Signal Intensity of Rhenium, Osmium and Re-Os Age in the Process of Dissolution for Pyrite. Chin. J. Anal. Chem. 2008, 36, 223–226. [Google Scholar]
- Morelli, R.M. Re-Os Sulfide Geochronology of the Red Dog Sediment-Hosted Zn-Pb-Ag Deposit, Brooks Range, Alaska. Econ. Geol. 2004, 99, 1569–1576. [Google Scholar] [CrossRef]
- Stein, H.J.; Morgan, J.W.; Schersten, A. Re-Os Dating of Low-Level Highly Radiogenic (LLHR) Sulfides: The Harnas Gold Deposit, Southwest Sweden, Records Continental-Scale Tectonic Events. Econ. Geol. 2000, 95, 1657–1671. [Google Scholar] [CrossRef]
- Morgan, J.W.; Stein, H.J.; Hannah, J.L.; Markey, R.J.; Wiszniewska, J. Re-Os study of Fe-Ti-V oxide and Fe-Cu-Ni sulfide deposits, Suwałki Anorthosite Massif, northeast Poland. Miner. Depos. 2000, 35, 391–401. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, Y. Direct Re-Os dating of chalcopyrite from the Lala IOCG deposit in the Kangdian Copper Belt, China. Econ. Geol. 2013, 108, 871–882. [Google Scholar]
- Nozaki, T.; Suzuki, K.; Kato, Y. Re-Os geochronology of the Hitachi volcanogenic massive sulfide deposit: The oldest ore deposit in Japan. Econ. Geol. 2014, 109, 2023–2034. [Google Scholar] [CrossRef]
- Kelley, K.D.; Selby, D.; Falck, H.; Slack, J.F. Re-Os systematics and age of pyrite associated with stratiform Zn-Pb mineralization in the Howards Pass district, Yukon and Northwest Territories, Canada. Miner. Depos. 2017, 52, 1–19. [Google Scholar] [CrossRef]
- Kirk, J.D.; Ruiz, J.; Kesler, S.E.; Simon, A.; Muntean, J.L. Re-Os age of the pueblo Viejo epithermal deposit, Dominican Republic. Econ. Geol. 2013, 109, 503–512. [Google Scholar] [CrossRef]
- Zhu, M.T.; Zhang, L.C.; Wu, G.; Jin, X.D.; Xiang, P.; Li, W.J. Zircon U–Pb and pyrite Re–Os age constraints on pyrite mineralization in the Yinjiagou deposit, China. Int. Geol. Rev. 2013, 55, 1616–1625. [Google Scholar] [CrossRef]
- Gregory, M.J.; Schaefer, B.F.; Keays, R.R.; Wilde, A.R. Rhenium–osmium systematics of the Mount Isa copper orebody and the Eastern Creek Volcanics, Queensland, Australia: Implications for ore genesis. Miner. Depos. 2008, 43, 553–573. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, M.; Sun, M. Routine Os analysis by isotope dilution-inductively coupled plasma mass spectrometry: OsO4 in water solution gives high sensitivity. Amino Acids 2001, 16, 345–349. [Google Scholar] [CrossRef]
- Crocket, J.H.; Keays, R.R.; Hsieh, S.J. Determination of some precious metals by neutron activation analysis. Radioanal. Chem. 1968, 1, 487–507. [Google Scholar] [CrossRef]
- Cohen, A.S.; Waters, F.G. Separation of osmium from geological materials by solvent extraction for analysis by thermal ionisation mass spectrometry. Anal. Chim. Acta 1996, 332, 269–275. [Google Scholar] [CrossRef]
- Shirey, S.B.; Walker, R.J. Carius Tube Digestion for Low-Blank Rhenium-Osmium Analysis. Anal. Chem. 1995, 67, 2136–2141. [Google Scholar] [CrossRef]
- Qi, L.; Zhou, M.F.; Gao, J.F.; Zhao, Z.J. An improved Carius tube technique for determination of low concentrations of Re and Os in pyrites. Anal. At. Spectrom. 2010, 25, 585–589. [Google Scholar] [CrossRef]
- Gao, B.Y.; Li, W.J.; Jin, X.D.; Zhang, L.C. Application of addition HClO4 to improve the dissolution process and sensitivity of Os for low-concentration of Os in pyrite. Microchem. J. 2019, 150, 104165. [Google Scholar] [CrossRef]
- Jin, X.; Li, W.; Xiang, P.; Sakyi, P.A.; Zhu, M.; Zhang, L.J.; Dorrzapf, A. A contribution to common Carius tube distillation techniques. Anal. At. Spectrom. 2014, 396–404. [Google Scholar]
- Morgan, J.; Golightly, D. Methods for the separation of rhenium, osmium and molybdenum applicable to isotope geochemistry. Talanta 1991, 38, 259–265. [Google Scholar] [CrossRef]
- Markey, R.; Stein, H.; Morgan, J. Highly precise Re–Os dating for molybdenite using alkaline fusion and NTIMS. Talanta 1998, 45, 935–946. [Google Scholar] [CrossRef]
- Ludwig, K.R. User’s Manual for Isoplot 3.00: A Geochronological Toolkit for Microsoft Excel; Berkeley Geochronology Center Special Publication; Berkeley Geochronology Center: Berkeley, CA, USA, 2003. [Google Scholar]
- Du, A.; Wu, S.; Sun, D.; Wang, S.; Qu, W.; Markey, R.; Stain, H.; Morgan, J.; Malinovskiy, D. Preparation and Certification of Re-Os Dating Reference Materials: Molybdenites HLP and JDC. Geostand. Geoanal. Res. 2004, 28, 41–52. [Google Scholar] [CrossRef]
- Shirey, S.B. Walker, The Re-Os isotope system in cosmochemistry and high-temperature geochemistry. R.J. Annu. Rev. Earth Planet. Sci. 1998, 26, 423–500. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).