Evaluation of the Bond Strength and Cytotoxicity of Alkasite Restorative Material
Abstract
1. Introduction
2. Materials and Methods
2.1. μTBS
2.2. Cytotoxicity and Cell Morphology Analysis
2.3. Statistical Analysis
3. Results
3.1. μTBS
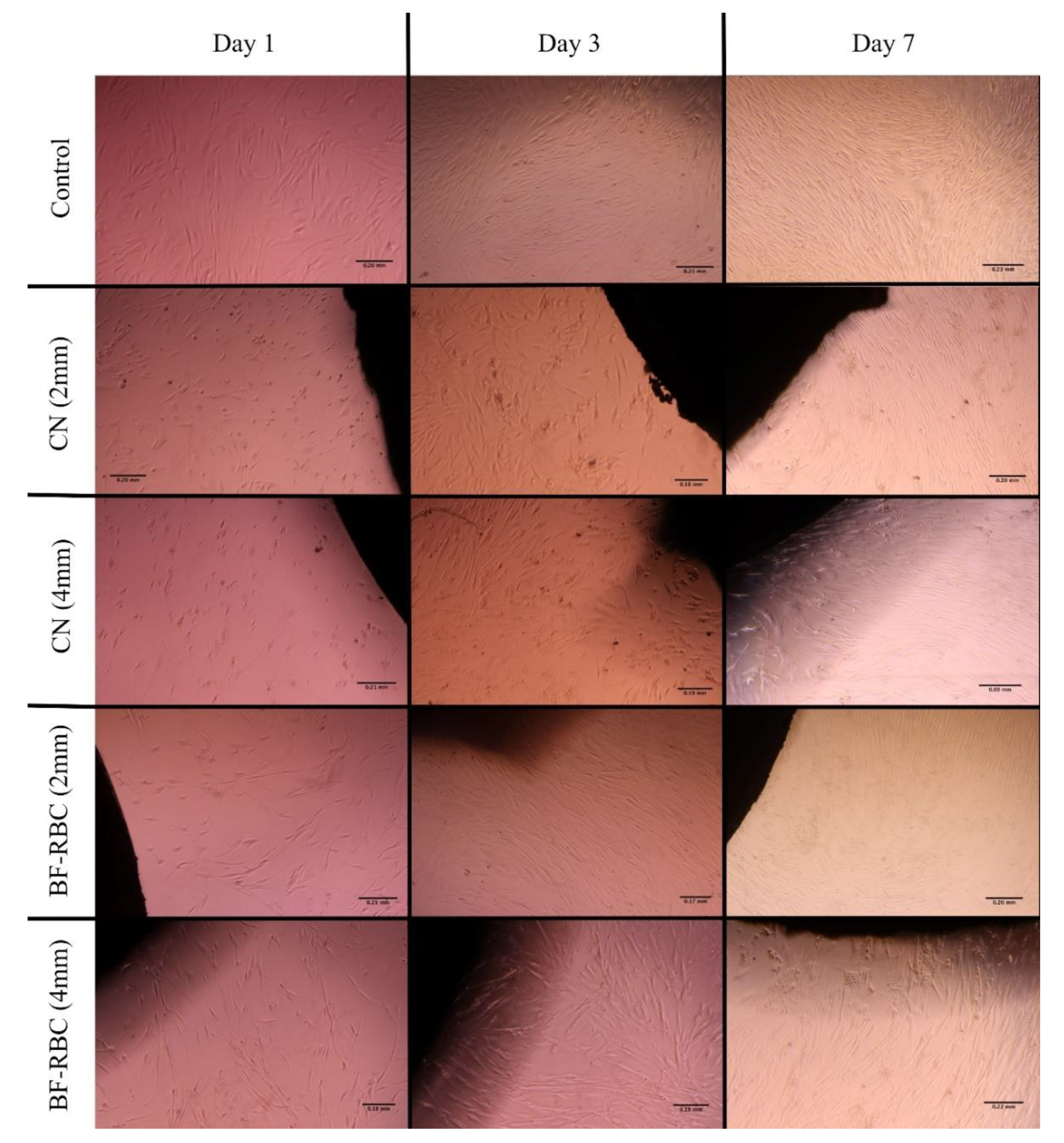
3.2. Cytotoxicity
3.3. Cell Morphology Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deliperi, S.; Bardwell, D.N. Multiple cuspal-coverage direct composite restorations: Functional and esthetic guidelines. J. Esthet. Restor. Dent. 2008, 20, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.D.; Opdam, N.J.; Hickel, R.; Brunton, P.A.; Gurgan, S.; Kakaboura, A.; Shearer, A.C.; Vanherle, G.; Wilson, N.H. Guidance on posterior resin composites: Academy of Operative Dentistry—European Section. J. Dent. 2014, 42, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Chesterman, J.; Jowett, A.; Gallacher, A.; Nixon, P. Bulk-fill resin-based composite restorative materials: A review. Br. Dent. J. 2017, 222, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.B.W.; Troconis, C.C.M.; Moreno, M.B.P.; Murillo-Gomez, F.; De Goes, M.F. Depth of cure of bulk fill resin composites: A systematic review. J. Esthet. Restor. Dent. 2018, 30, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Yap, A.U.; Lim, S.Y. In Vitro Biocompatibility of Contemporary Bulk-fill Composites. Oper. Dent. 2015, 40, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Gerula-Szymanska, A.; Kaczor, K.; Lewusz-Butkiewicz, K.; Nowicka, A. Marginal integrity of flowable and packable bulk fill materials used for class II restorations—A systematic review and meta-analysis of in vitro studies. Dent. Mater. J. 2020, 35, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Cidreira Boaro, L.C.; Pereira Lopes, D.; de Souza, A.S.C.; Lie Nakano, E.; Ayala Perez, M.D.; Pfeifer, C.S.; Goncalves, F. Clinical performance and chemical-physical properties of bulk fill composites resin—A systematic review and meta-analysis. Dent. Mater. 2019, 35, e249–e264. [Google Scholar] [CrossRef]
- Veloso, S.R.M.; Lemos, C.A.A.; de Moraes, S.L.D.; do Egito Vasconcelos, B.C.; Pellizzer, E.P.; de Melo Monteiro, G.Q. Clinical performance of bulk-fill and conventional resin composite restorations in posterior teeth: A systematic review and meta-analysis. Clin. Oral Investig. 2019, 23, 221–233. [Google Scholar] [CrossRef]
- Bellinaso, M.D.; Soares, F.Z.M.; Rocha, R.O. Do bulk-fill resins decrease the restorative time in posterior teeth? A systematic review and meta-analysis of in vitro studies. J. Investig. Clin. Dent. 2019, 10, e12463. [Google Scholar] [CrossRef]
- Reis, A.F.; Vestphal, M.; Amaral, R.C.D.; Rodrigues, J.A.; Roulet, J.F.; Roscoe, M.G. Efficiency of polymerization of bulk-fill composite resins: A systematic review. Braz. Oral. Res. 2017, 31, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N. Comparative Effect of Self- or Dual-Curing on Polymerization Kinetics and Mechanical Properties in a Novel, Dental-Resin-Based Composite with Alkaline Filler. Materials 2018, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Ak, A.T.; Alpoz, A.R.; Bayraktar, O.; Ertugrul, F. Monomer Release from Resin Based Dental Materials Cured with LED and Halogen Lights. Eur. J. Dent. 2010, 4, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Sigusch, B.W.; Pflaum, T.; Völpel, A.; Gretsch, K.; Hoy, S.; Watts, D.C.; Jandt, K.D. Resin-composite cytotoxicity varies with shade and irradiance. Dent. Mater. 2012, 28, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Gwinner, F.; Mitchell, J.C.; Pfeifer, C.; Ferracane, J.L. Cytotoxicity of resin composites containing bioactive glass fillers. Dent. Mater. 2015, 31, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi Chaharom, M.E.; Bahari, M.; Safyari, L.; Safarvand, H.; Shafaei, H.; Jafari Navimipour, E.; Alizadeh Oskoee, P.; Ajami, A.A.; Abed Kahnamouei, M. Effect of preheating on the cytotoxicity of bulk-fill composite resins. J. Dent. Res. Dent. Clin. Dent. Prospect. 2020, 14, 19–25. [Google Scholar] [CrossRef]
- Roulet, J.; Hussein, H.; Abdulhameed, N.; Shen, C. In vitro wear of two bioactive composites and a glass ionomer cement. Dtsch. Zahnärztl. Z. Int. 2019, 1, 24–30. [Google Scholar]
- Panpisut, P.; Toneluck, A. Monomer conversion, dimensional stability, biaxial flexural strength, and fluoride release of resin-based restorative material containing alkaline fillers. Dent. Mater. J. 2020, 39, 608–615. [Google Scholar] [CrossRef]
- Donly, K.J.; Liu, J.A. Dentin and enamel demineralization inhibition at restoration margins of Vitremer, Z 100 and Cention, N. Am. J. Dent. 2018, 31, 166–168. [Google Scholar]
- Roulet, J.F. Is in vitro research in restorative dentistry useless? J. Adhes. Dent. 2012, 14, 103–104. [Google Scholar]
- Wataha, J.C. Predicting clinical biological responses to dental materials. Dent. Mater. 2012, 28, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.; Geraldeli, S.; Maia, R.; Raposo, L.H.; Soares, C.J.; Yamagawa, J. Adhesion to tooth structure: A critical review of “micro” bond strength test methods. Dent. Mater. 2010, 26, e50–e62. [Google Scholar] [CrossRef] [PubMed]
- Naz, F.; Samad Khan, A.; Kader, M.A.; Al Gelban, L.O.S.; Mousa, N.M.A.; Asiri, R.S.H.; Hakeem, A.S. Comparative evaluation of mechanical and physical properties of a new bulk-fill alkasite with conventional restorative materials. Saudi Dent. J. 2020, in press. [Google Scholar]
- Yao, C.; Ahmed, M.H.; Zhang, F.; Mercelis, B.; Van Landuyt, K.L.; Huang, C.; Van Meerbeek, B. Structural/Chemical Characterization and Bond Strength of a New Self-Adhesive Bulk-fill Restorative. J. Adhes. Dent. 2020, 22, 85–97. [Google Scholar] [PubMed]
- Nakayama, G.; Caton, M.; Nova, M.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Method. 1997, 204, 205. [Google Scholar] [CrossRef]
- Eckert, G.J.; Platt, J.A. A statistical evaluation of microtensile bond strength methodology for dental adhesives. Dent. Mater. 2007, 23, 385–391. [Google Scholar] [CrossRef]
- Makhdoom, S.N.; Campbell, K.M.; Carvalho, R.M.; Manso, A.P. Effects of curing modes on depth of cure and microtensile bond strength of bulk fill composites to dentin. J. Appl. Oral. Sci. 2020, 28, e20190753. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, S.Y.; Kim, J.H.; Jun, S.K.; Kim, H.W.; Lee, J.H.; Lee, H.H. Depth-Dependent Cellular Response from Dental Bulk-Fill Resins in Human Dental Pulp Stem Cells. Stem. Cells Int. 2019, 2019, 1251536. [Google Scholar] [CrossRef]
- Pashley, D.H.; Carvalho, R.M.; Sano, H.; Nakajima, M.; Yoshiyama, M.; Shono, Y.; Fernandes, C.A.; Tay, F. The microtensile bond test: A review. J. Adhes. Dent. 1999, 1, 299–309. [Google Scholar]
- Sano, H.; Chowdhury, A.F.M.A.; Saikaew, P.; Matsumoto, M.; Hoshika, S.; Yamauti, M. The microtensile bond strength test: Its historical background and application to bond testing. Jpn. Dent. Sci. Rev. 2020, 56, 24–31. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Peumans, M.; Poitevin, A.; Mine, A.; Van Ende, A.; Neves, A.; De Munck, J. Relationship between bond-strength tests and clinical outcomes. Dent. Mater. 2010, 26, e100–e121. [Google Scholar] [CrossRef]
- Heintze, S.D.; Rousson, V.; Mahn, E. Bond strength tests of dental adhesive systems and their correlation with clinical results—A meta-analysis. Dent. Mater. 2015, 31, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Gale, M.S.; Darvell, B.W. Thermal cycling procedures for laboratory testing of dental restorations. J. Dent. 1999, 27, 89–99. [Google Scholar] [CrossRef]
- Sunada, N.; Ishii, R.; Shiratsuchi, K.; Shimizu, Y.; Tsubota, K.; Kurokawa, H.; Miyazaki, M. Ultrasonic measurement of the effects of adhesive application and power density on the polymerization behavior of core build-up resins. Acta Odontol. Scand. 2013, 71, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Sanares, A.M.E.; Itthagarun, A.; King, N.M.; Tay, F.R.; Pashley, D.H. Adverse surface interactions between one-bottle light-cured adhesives and chemical-cured composites. Dent. Mater. 2001, 17, 542–556. [Google Scholar] [CrossRef]
- Tay, F.R.; Suh, B.I.; Pashley, D.H.; Prati, C.; Chuang, S.F.; Li, F. Factors contributing to the incompatibility between simplified-step adhesives and self-cured or dual-cured composites. Part II. Single-bottle, total-etch adhesive. J. Adhes. Dent. 2003, 5, 91–105. [Google Scholar]
- Suh, B.I.; Feng, L.; Pashley, D.H.; Tay, F.R. Factors contributing to the incompatibility between simplified-step adhesives and chemically-cured or dual-cured composites. Part III. Effect of acidic resin monomers. J. Adhes. Dent. 2003, 5, 267–282. [Google Scholar]
- Endo, T.; Finger, W.J.; Hoffmann, M.; Kanehira, M.; Komatsu, M. The role of oxygen inhibition of a self-etch adhesive on self-cure resin composite bonding. Am. J. Dent. 2007, 20, 157–160. [Google Scholar]
- Gutierrez, M.F.; Sutil, E.; Malaquias, P.; de Paris Matos, T.; de Souza, L.M.; Reis, A.; Perdigao, J.; Loguercio, A.D. Effect of self-curing activators and curing protocols on adhesive properties of universal adhesives bonded to dual-cured composites. Dent. Mater. 2017, 33, 775–787. [Google Scholar] [CrossRef]
- Moszner, N.; Fischer, U.K.; Ganster, B.; Liska, R.; Rheinberger, V. Benzoyl germanium derivatives as novel visible light photoinitiators for dental materials. Dent. Mater. 2008, 24, 901–907. [Google Scholar] [CrossRef]
- Roulet, J.F.; Gummadi, S.; Hussein, H.S.; Abdulhameed, N.; Shen, C. In vitro wear of dual-cured bulkfill composites and flowable bulkfill composites. J. Esthet. Restor. Dent. 2020, 32, 512–520. [Google Scholar] [CrossRef]
- Ilie, N.; Hickel, R. Investigations on mechanical behaviour of dental composites. Clin. Oral. Investig. 2009, 13, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Soderholm, K.J.; Geraldeli, S.; Shen, C. What do microtensile bond strength values of adhesives mean? J. Adhes. Dent. 2012, 14, 307–314. [Google Scholar] [PubMed]
- Amaral, F.L.; Colucci, V.; Palma-Dibb, R.G.; Corona, S.A. Assessment of in vitro methods used to promote adhesive interface degradation: A critical review. J. Esthet. Restor. Dent. 2007, 19, 340–353. [Google Scholar] [CrossRef] [PubMed]
- De Munck, J.; Van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; Van Meerbeek, B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, S.T.; Dahl, J.E. Effect of thermal cycling on temperature changes and bond strength in different test specimens. Biomater. Investig. Dent. 2020, 7, 16–24. [Google Scholar] [CrossRef]
- Wiegand, C.; Hipler, U.C. Methods for the measurement of cell and tissue compatibility including tissue regeneration processes. GMS Krankenhhyg. Interdiszip. 2008, 3, 1–11. [Google Scholar]
- Schmalz, G.; Schuster, U.; Nuetzel, K.; Schweikl, H. An in vitro pulp chamber with three-dimensional cell cultures. J. Endod. 1999, 25, 24–29. [Google Scholar] [CrossRef]
- Durner, J.; Wellner, P.; Hickel, R.; Reichl, F.X. Synergistic interaction caused to human gingival fibroblasts from dental monomers. Dent. Mater. 2012, 28, 818–823. [Google Scholar] [CrossRef]
- Urcan, E.; Scherthan, H.; Styllou, M.; Haertel, U.; Hickel, R.; Reichl, F.X. Induction of DNA double-strand breaks in primary gingival fibroblasts by exposure to dental resin composites. Biomaterials 2010, 31, 2010–2014. [Google Scholar] [CrossRef]
- Mattioli-Belmonte, M.; Natali, D.; Tosi, G.; Torricelli, P.; Totaro, I.; Zizzi, A.; Fini, M.; Sabbatini, S.; Giavaresi, G.; Biagini, G. Resin-based dentin restorative materials under accelerated ageing: Bio-functional behavior. Int. J. Artif. Organ. 2006, 29, 1000–1011. [Google Scholar] [CrossRef]
- Kanjevac, T.; Milovanovic, M.; Volarevic, V.; Lukic, M.L.; Arsenijevic, N.; Markovic, D.; Zdravkovic, N.; Tesic, Z.; Lukic, A. Cytotoxic effects of glass ionomer cements on human dental pulp stem cells correlate with fluoride release. Med. Chem. 2012, 8, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Inkielewicz-Stepniak, I.; Santos-Martinez, M.J.; Medina, C.; Radomski, M.W. Pharmacological and toxicological effects of co-exposure of human gingival fibroblasts to silver nanoparticles and sodium fluoride. Int. J. Nanomed. 2014, 9, 1677–1687. [Google Scholar]
- Gonçalves, F.; Campos, L.M.P.; Rodrigues-Júnior, E.C.; Costa, F.V.; Marques, P.A.; Francci, C.E.; Braga, R.R.; Boaro, L.C.C. A comparative study of bulk-fill composites: Degree of conversion, post-gel shrinkage and cytotoxicity. Braz. Oral. Res. 2018, 32, e17. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.; Spinell, T.; Graf, A.; Wutzel, H.; Liska, R.; Watts, D.C.; Moritz, A.; Schedle, A. Cytotoxicity of post and core composites as a function of environmental conditions. Dent. Mater. 2014, 30, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.Y.; Bagheri, R.; Kim, Y.K.; Kim, K.H.; Burrow, M.F. Cure mechanisms in materials for use in esthetic dentistry. J. Investig. Clin. Dent. 2012, 3, 3–16. [Google Scholar] [CrossRef] [PubMed]




| Material and Manufacturer | Composition |
|---|---|
| Cention N, Ivoclar Vivadent, Schaan, Liechtenstein. | UDMA, tricyclodecan-dimethanol dimethacrtylate, polyethylene glycol dimethacrylate, inorganic fillers and customized fillers |
| Tetric N-Flow Bulk Fill, Ivoclar Vivadent, Schaan, Liechtenstein | Bis-GMA, UDMA, TEGDMA, Ivocerin, Barium glass, ytterbium trifluoride, mixed oxide, silicon dioxide |
| Tetric N-Bond Universal, Ivoclar Vivadent, Schaan, Liechtenstein | MDP, MCAP, HEMA, D3MA water, ethanol, silicon dioxide, initiators, and stabilizers |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, M.M.; Alshehri, T.; Alqarni, A.M.; Magdy, N.M.; Alhalabi, F.; Alotaibi, D.; Alrahlah, A. Evaluation of the Bond Strength and Cytotoxicity of Alkasite Restorative Material. Appl. Sci. 2020, 10, 6175. https://doi.org/10.3390/app10186175
Awad MM, Alshehri T, Alqarni AM, Magdy NM, Alhalabi F, Alotaibi D, Alrahlah A. Evaluation of the Bond Strength and Cytotoxicity of Alkasite Restorative Material. Applied Sciences. 2020; 10(18):6175. https://doi.org/10.3390/app10186175
Chicago/Turabian StyleAwad, Mohamed M., Turki Alshehri, Ahmed M. Alqarni, Nashaat M. Magdy, Feras Alhalabi, Dalal Alotaibi, and Ali Alrahlah. 2020. "Evaluation of the Bond Strength and Cytotoxicity of Alkasite Restorative Material" Applied Sciences 10, no. 18: 6175. https://doi.org/10.3390/app10186175
APA StyleAwad, M. M., Alshehri, T., Alqarni, A. M., Magdy, N. M., Alhalabi, F., Alotaibi, D., & Alrahlah, A. (2020). Evaluation of the Bond Strength and Cytotoxicity of Alkasite Restorative Material. Applied Sciences, 10(18), 6175. https://doi.org/10.3390/app10186175






