A Detailed One-Dimensional Hydrodynamic and Kinetic Model for Sorption Enhanced Gasification
Abstract
1. Introduction
2. SEG Process Description and Model Development
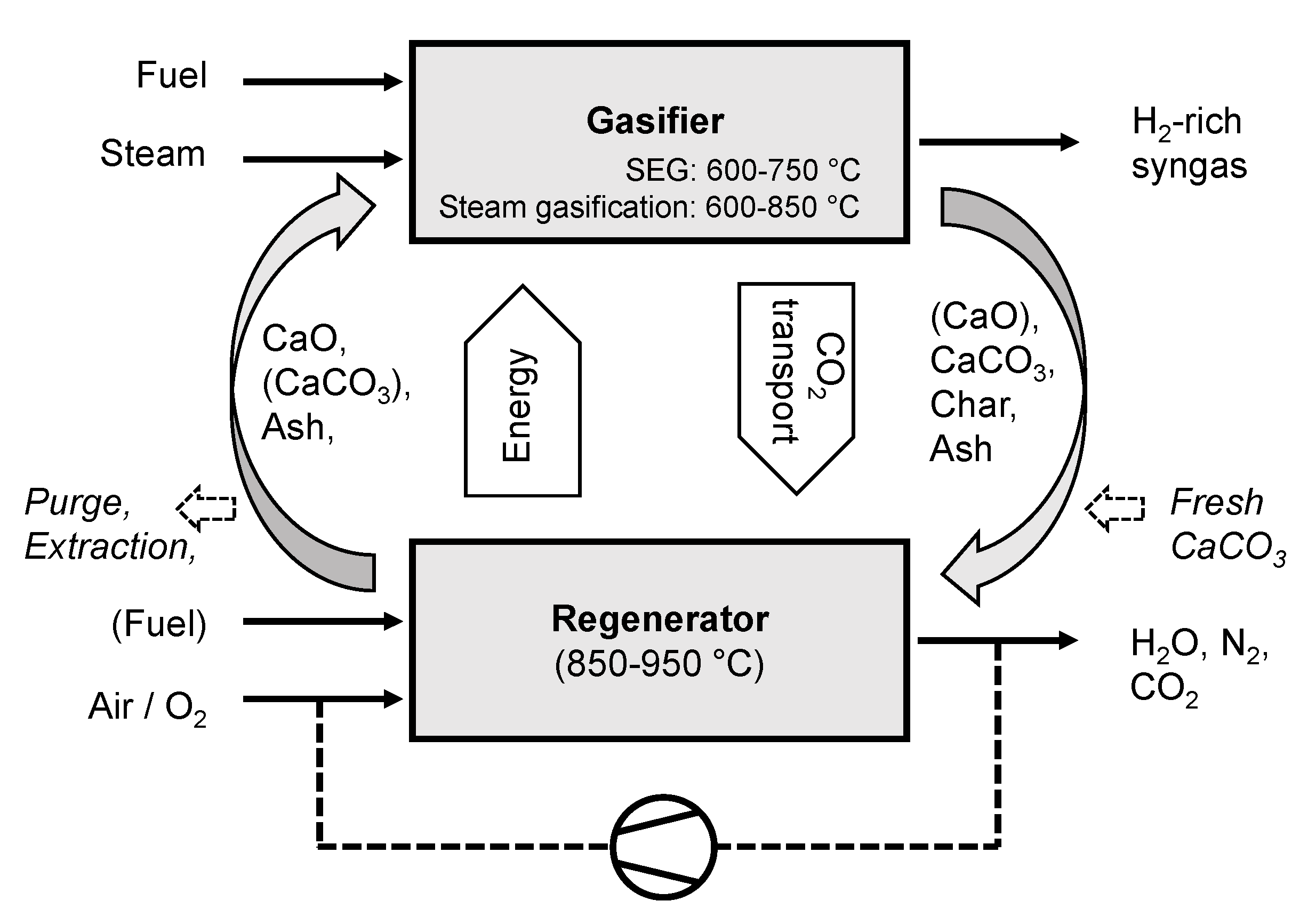
2.1. Description of the SEG Process
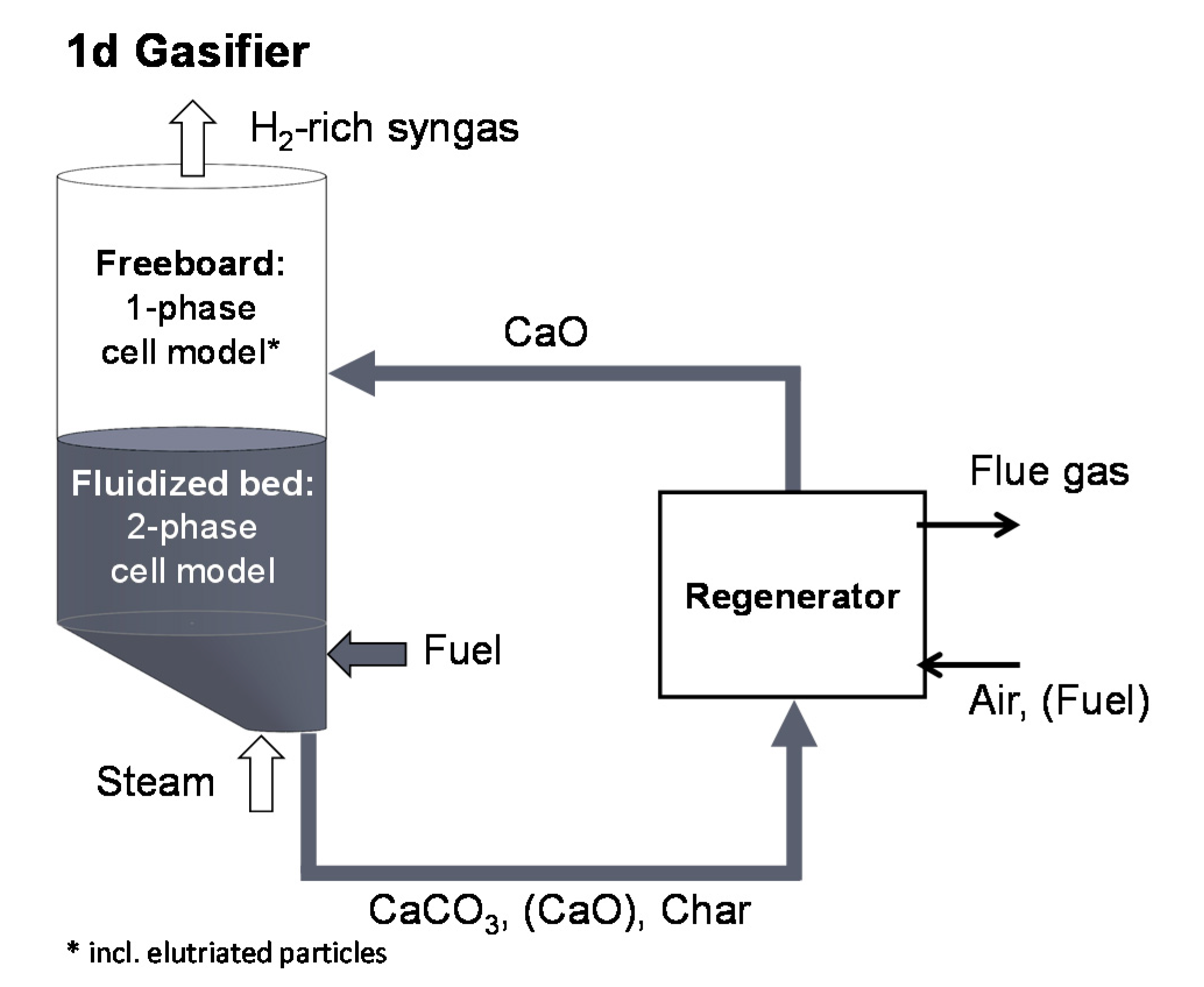
2.2. Development of a Dual Fluidized Bed Gasifier Model
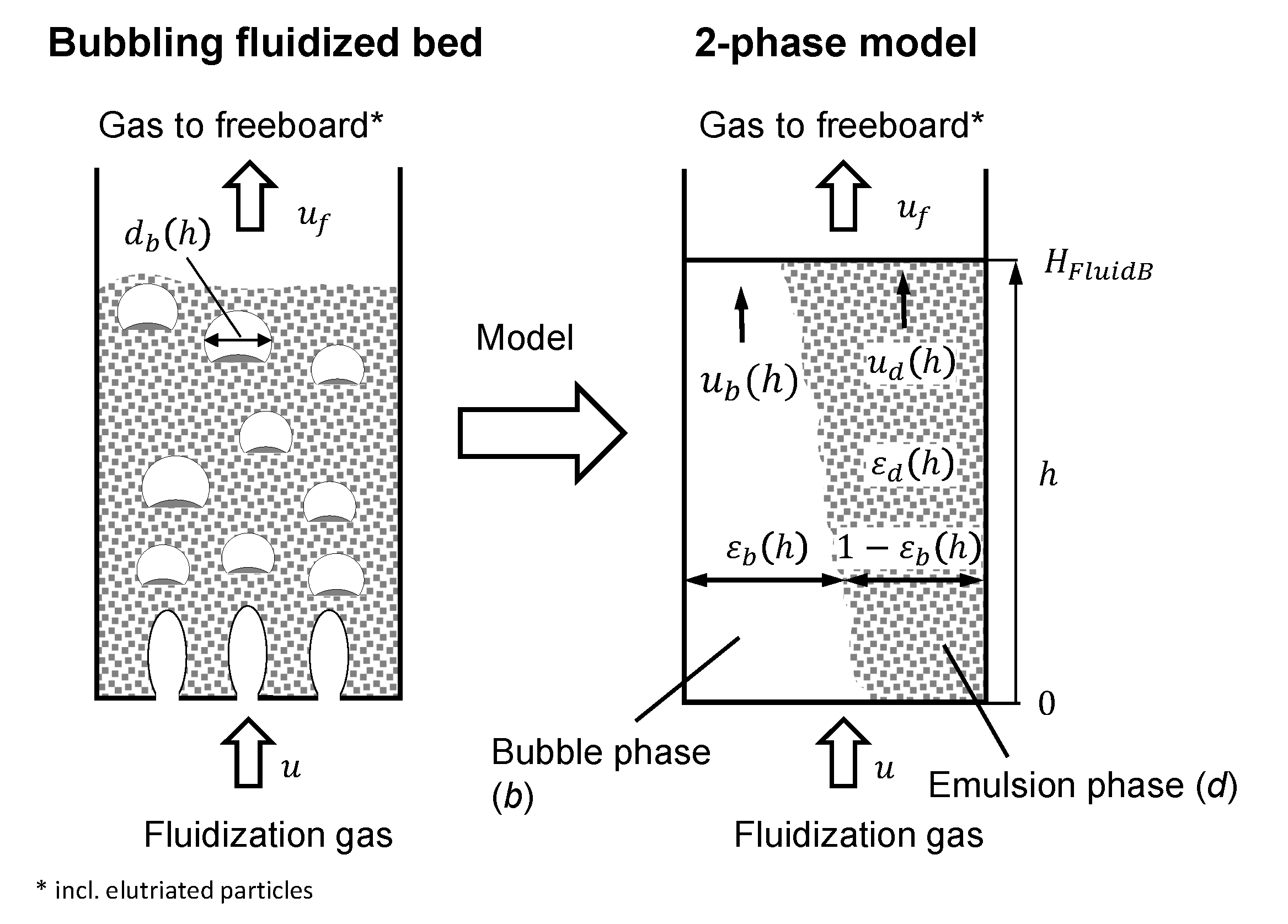
2.3. Hydrodynamics
2.3.1. Dense Phase
2.3.2. Bubble Phase
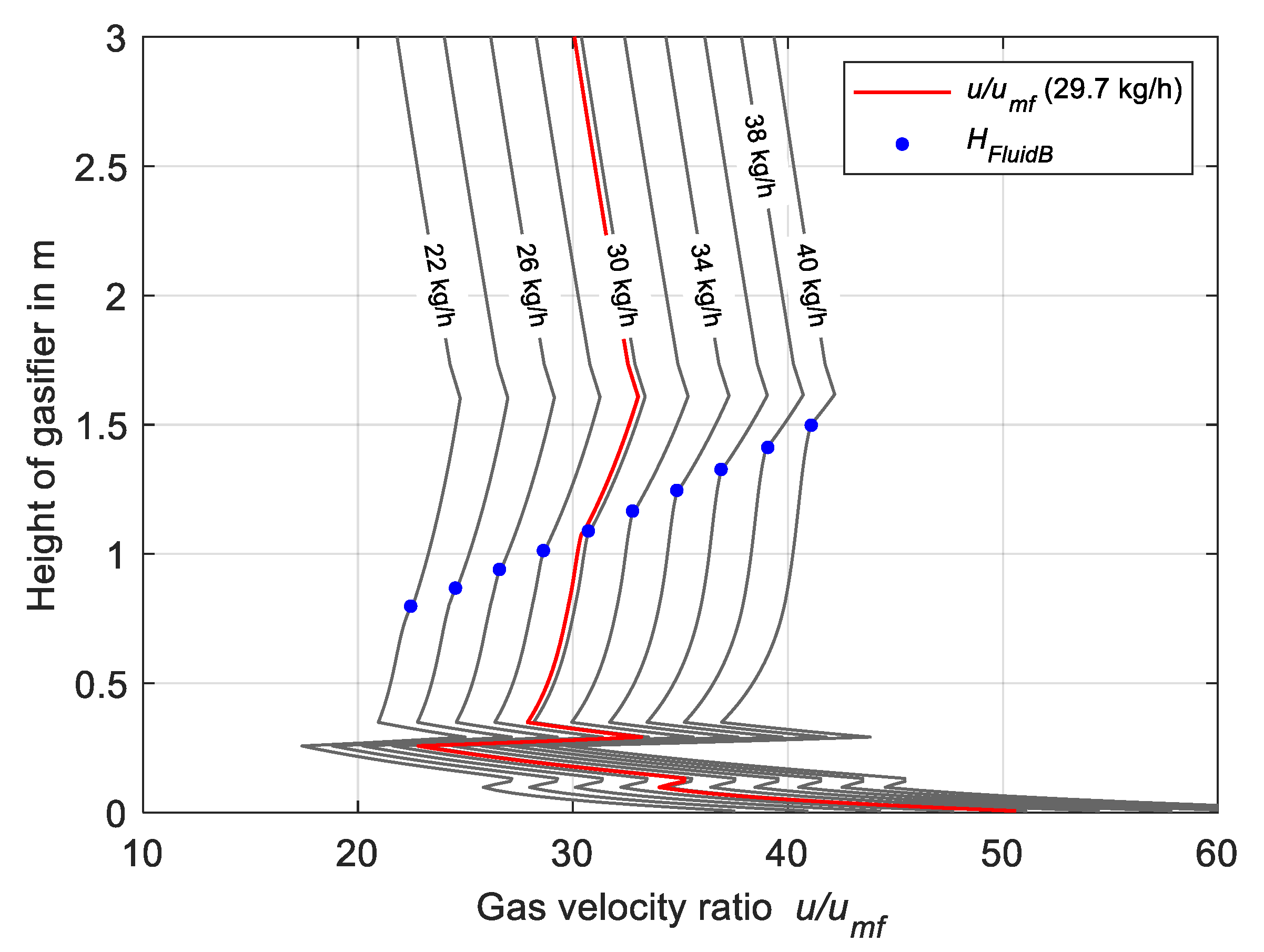
2.3.3. Fluidized Bed Height
2.3.4. Elutriation Rate
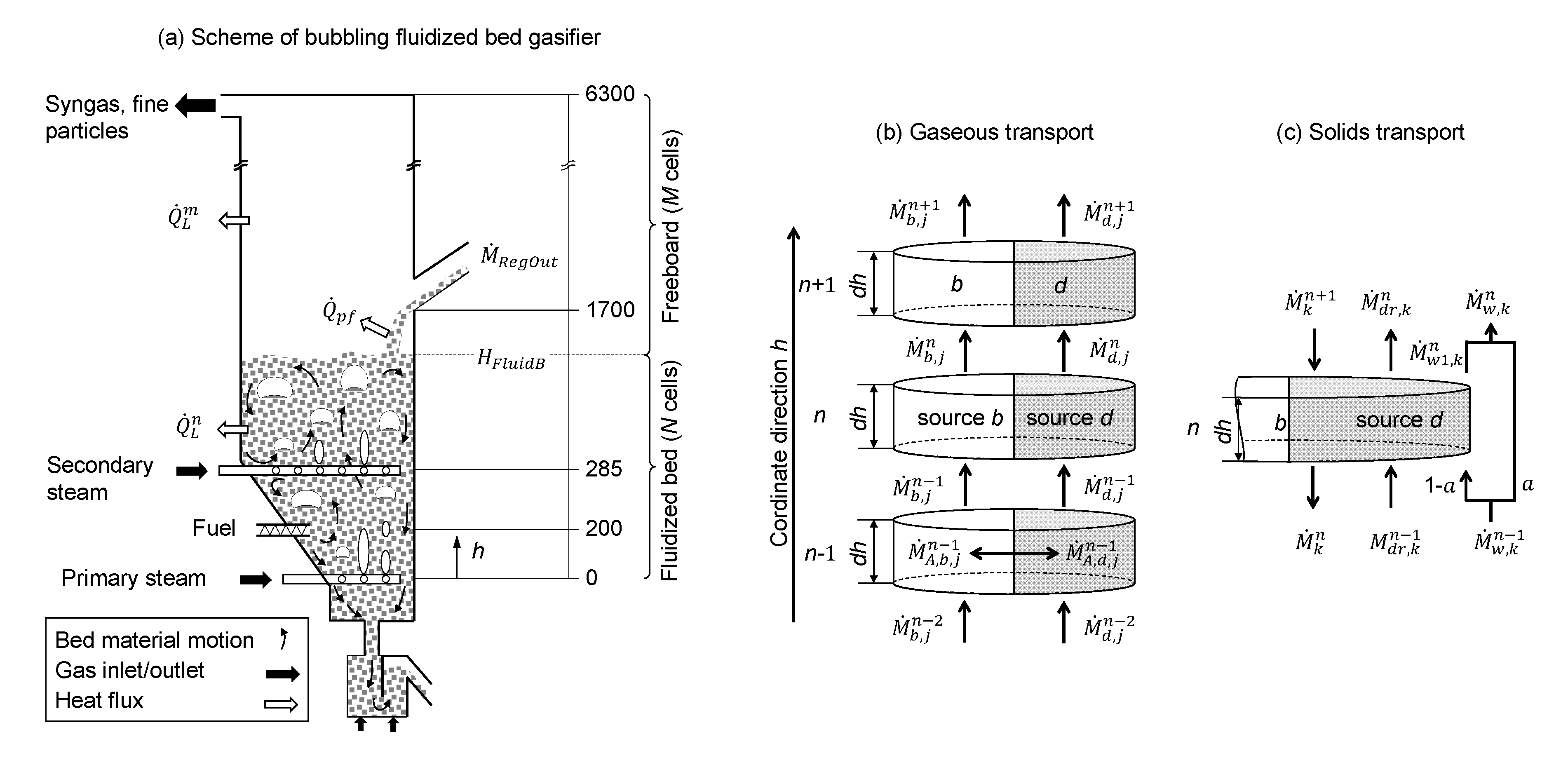
2.4. Gasifier Dimensions
2.5. Mass Balance
2.5.1. Fluidized Bed
2.5.2. Freeboard
2.6. Energy Balances
2.6.1. Fluidized Bed
2.6.2. Freeboard
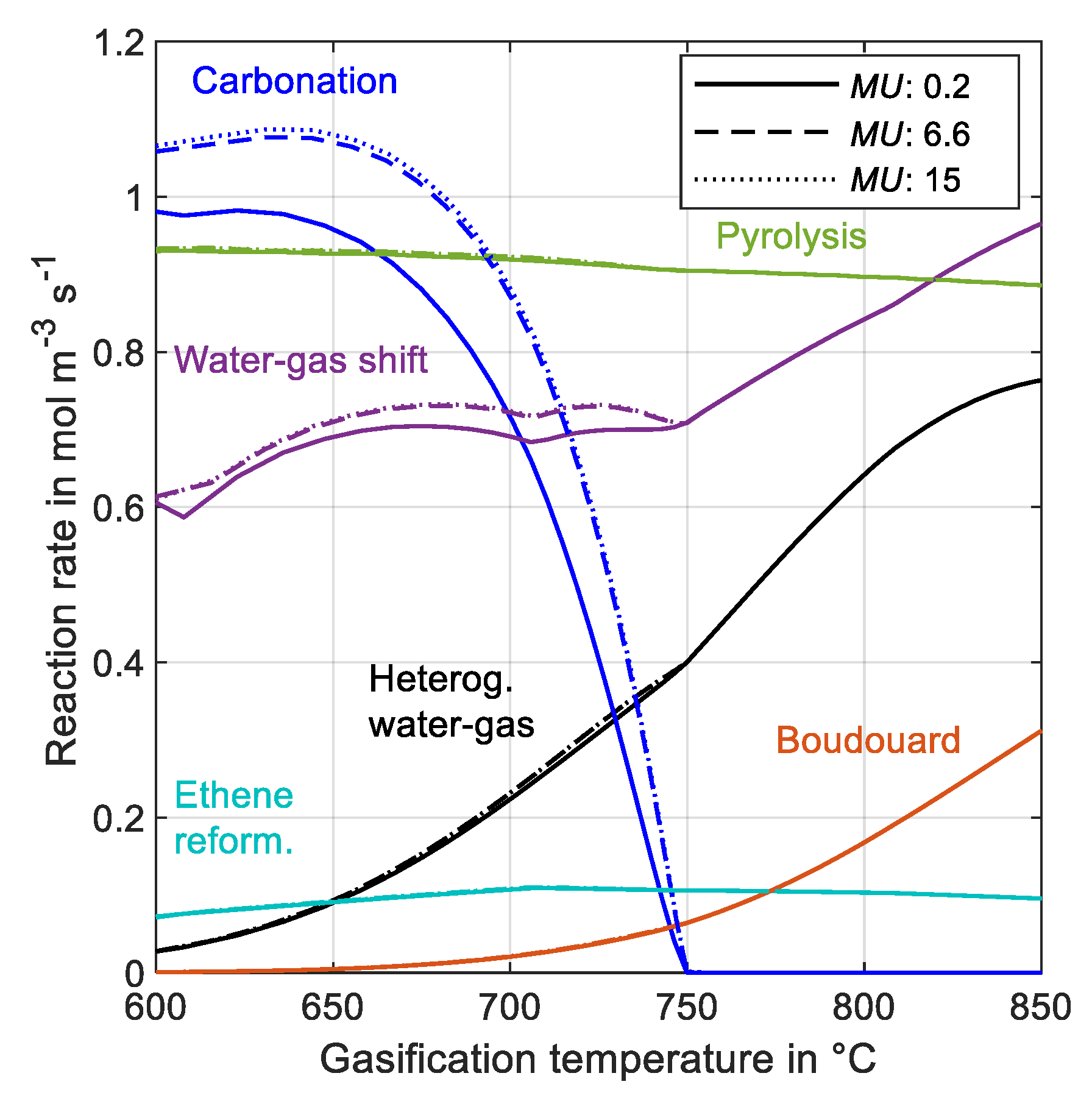
2.7. Chemical Reactions
2.8. Sorbent Deactivation
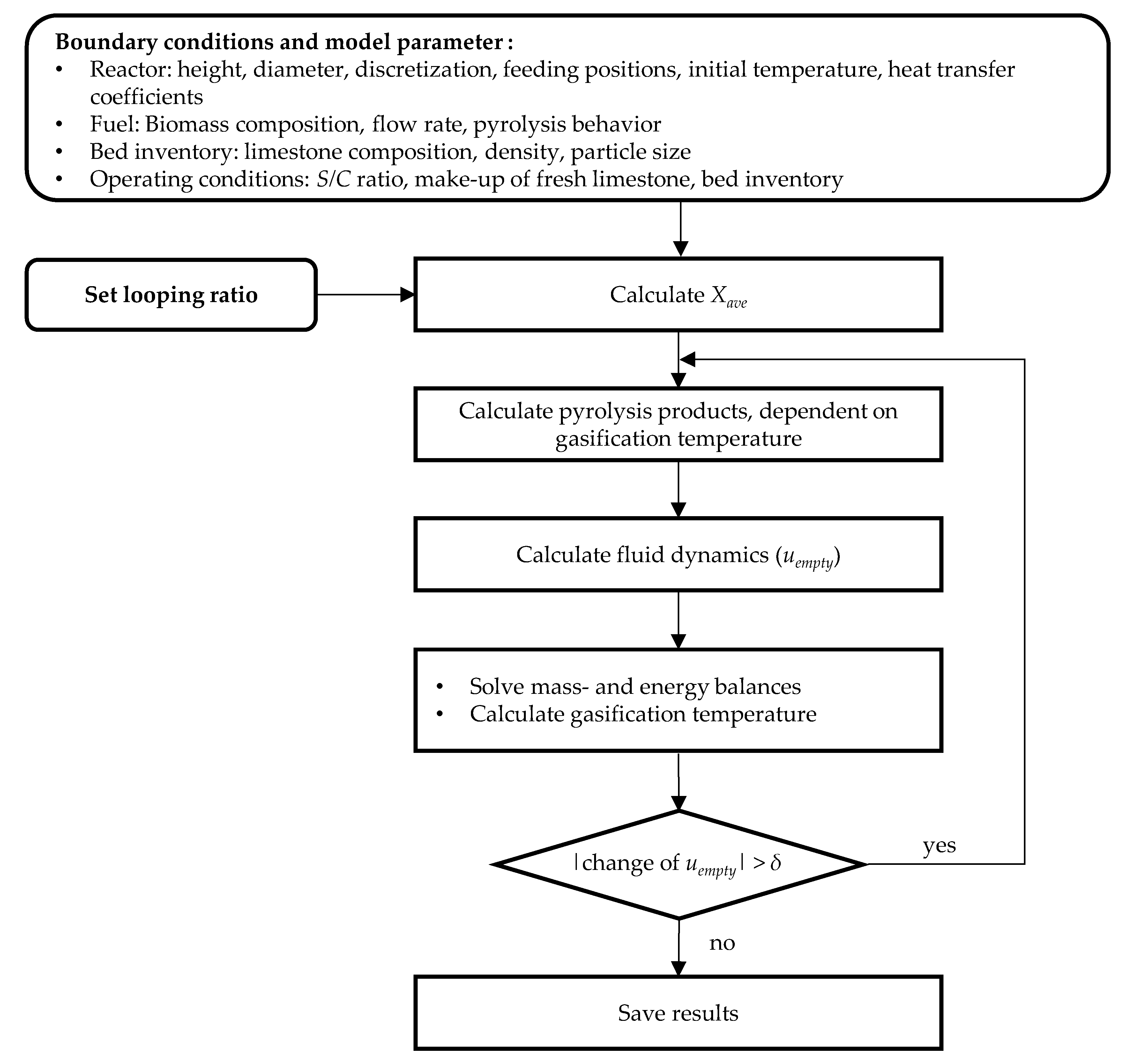
2.9. Simulation Algorithm
3. Results and Discussion
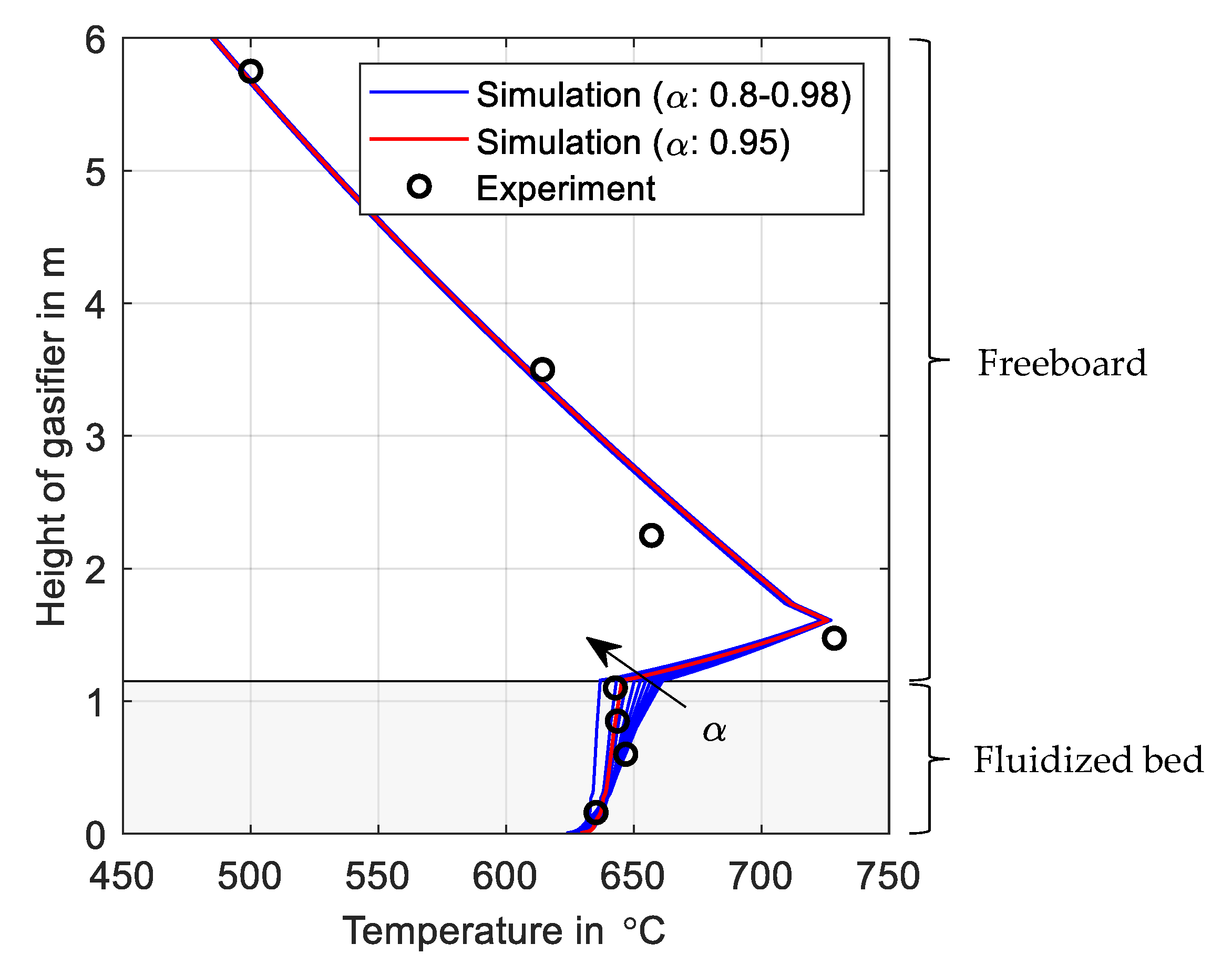
3.1. Verification of Model Parameter
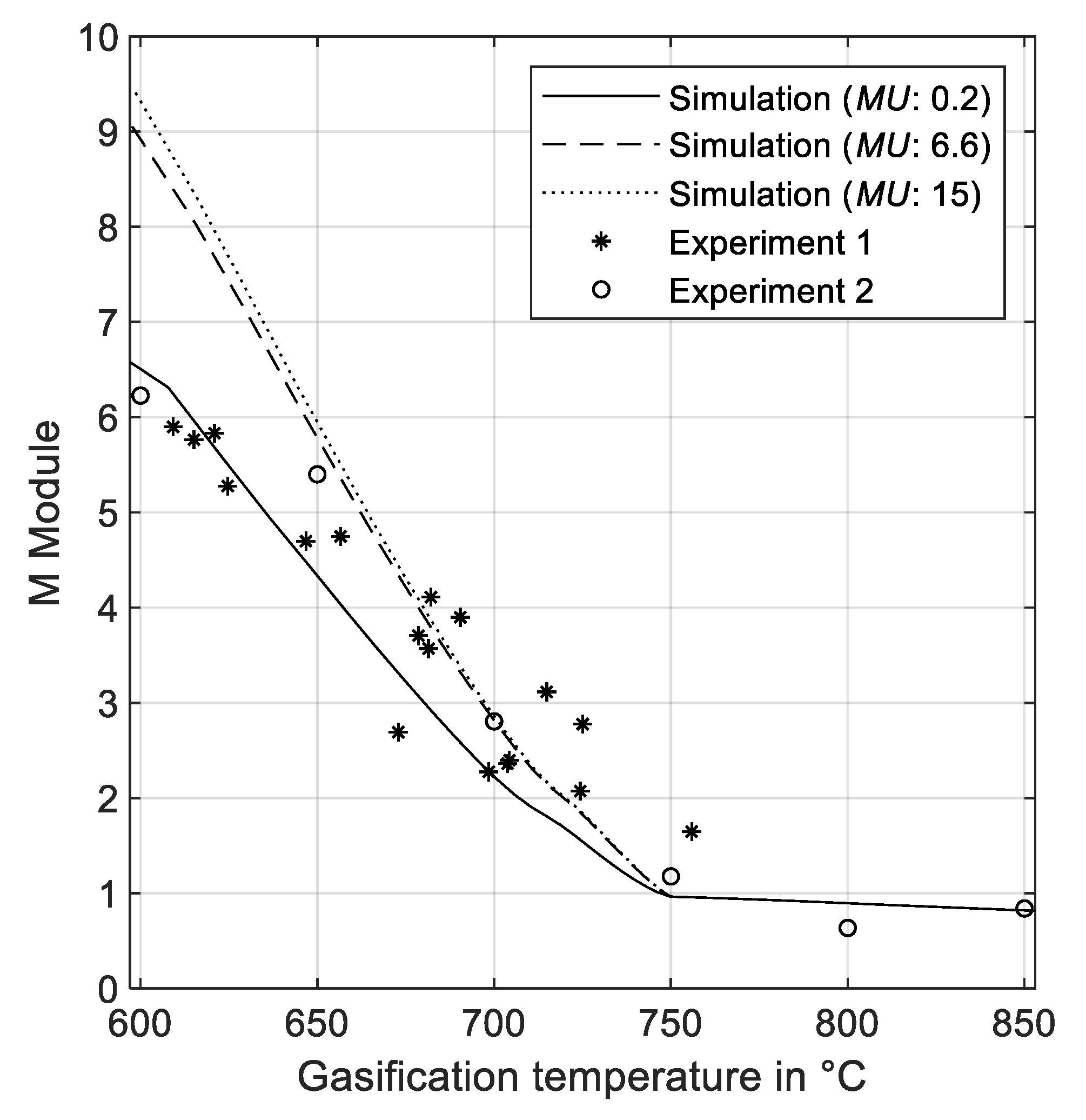
3.2. Validation with Experimental Data
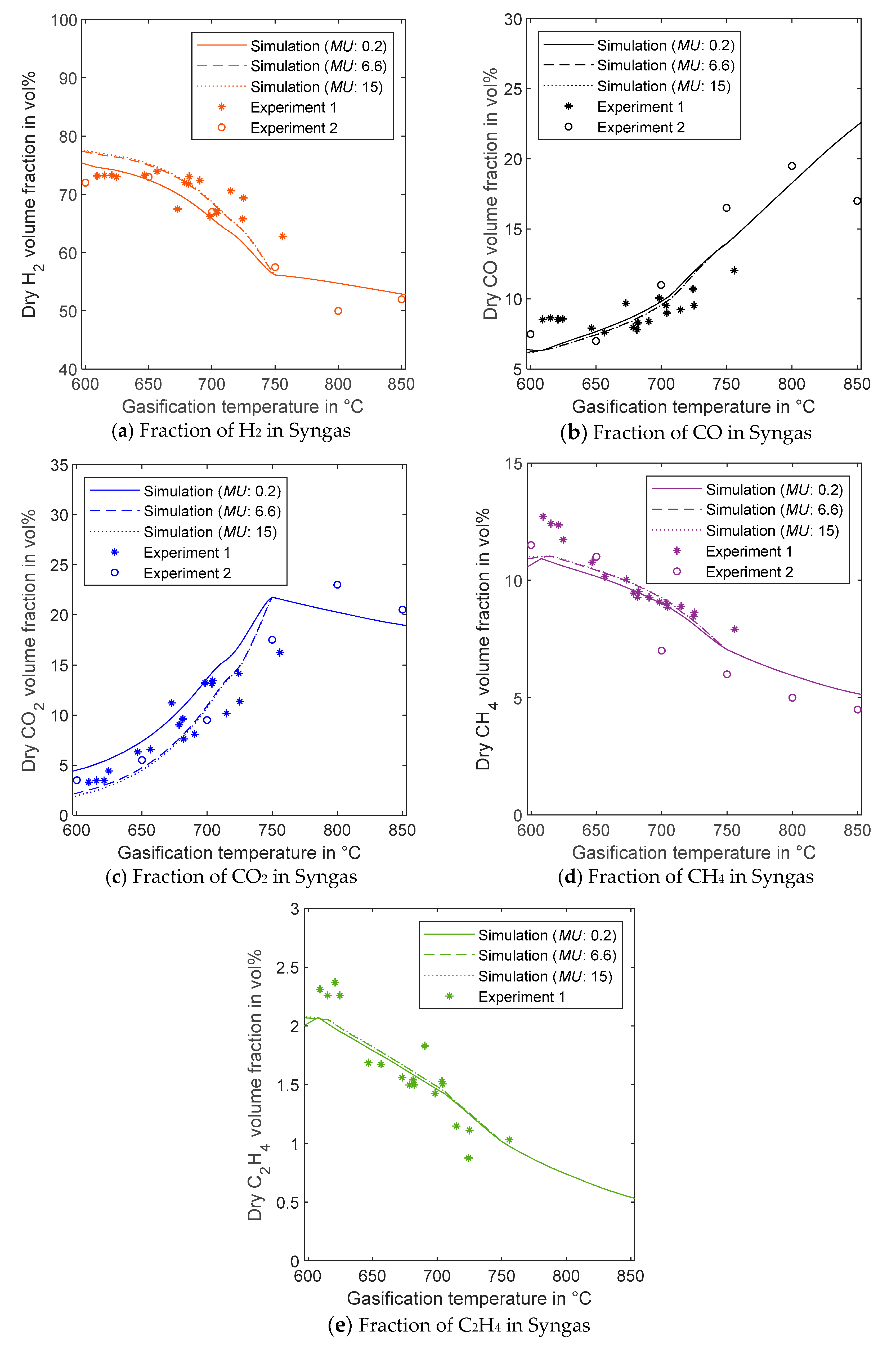
3.2.1. Effect of Gasification Temperature on Fractions of Syngas Components
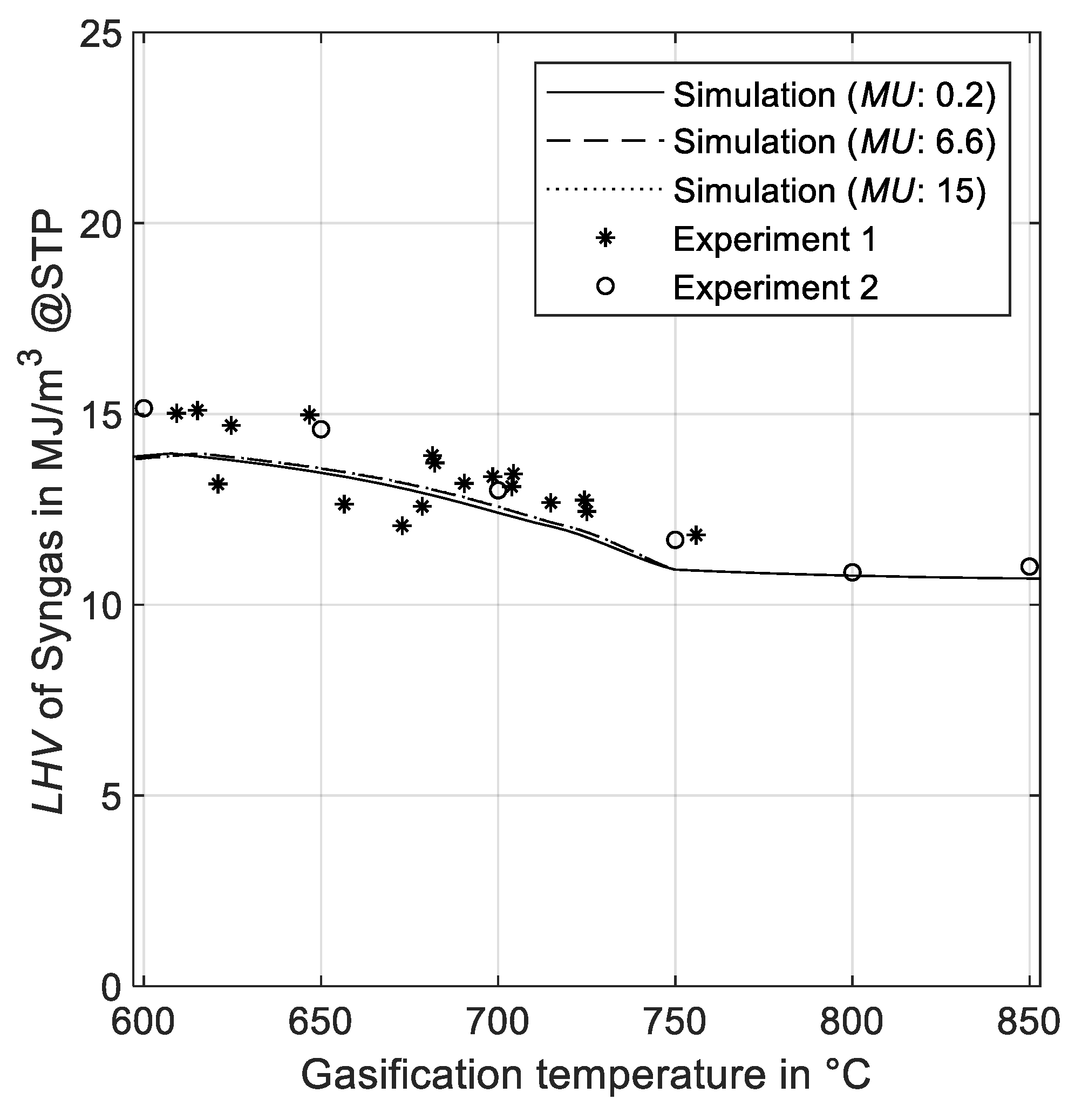
3.2.2. Effect of Gasification Temperature on LHV
3.2.3. Effect of Make-Up Flow on Reaction Rate
3.2.4. Effect of Gasification Temperature and Make-Up of Fresh Limestone on M Module
4. Sensitivity Analysis of Fuel Feeding Rate
4.1. Effect of Biomass Feeding Rate on Bed Height and Gas Velocity Ratio
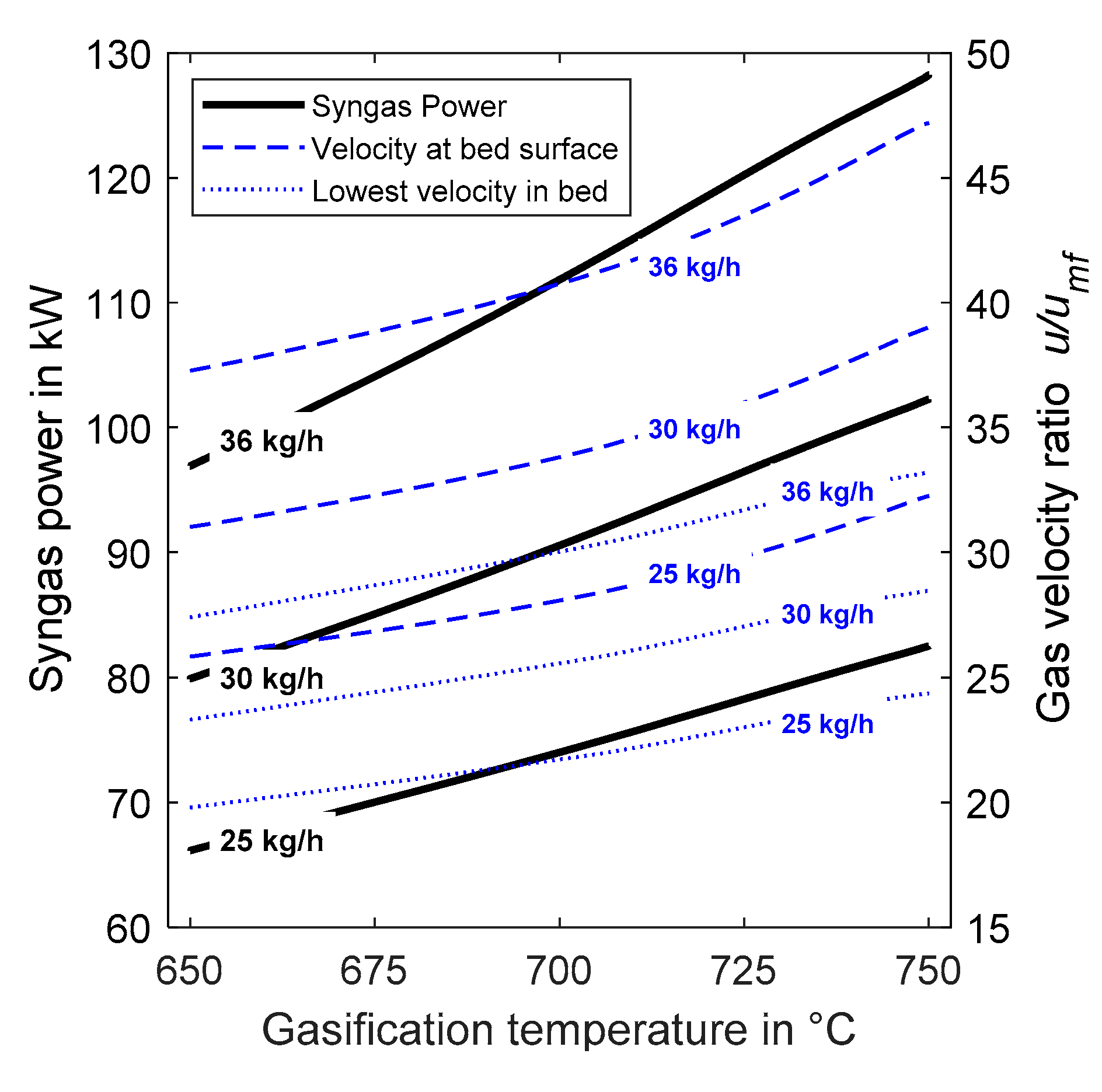
4.2. Performance Diagram for Gasifier Operation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | area (m2) |
| Ar | Archimedes number (-) |
| CD,b | drag coefficient of a bubble (-) |
| concentration of component j (kg/m3) | |
| concentration of component j (mol/m3) | |
| specific heat capacity (J kg−1K−1) | |
| Sauter diameter (m) | |
| diameter (m) | |
| mean Error (-) | |
| flow of fresh CaCO3 (mol/s) | |
| flow of circulating CaO (mol/s) | |
| flow of elemental carbon in fuel flow (mol/s) | |
| g | acceleration of gravity (m/s2) |
| H | height (m) |
| enthalpy flow (J/s) | |
| h | enthalpy (J/kg) |
| h | reactor co-ordinate (m) |
| I | maximum number of chemical reactions (-) |
| J | maximum number of components (-) |
| mass transfer coefficient (m/s) | |
| k | heat transfer coefficient (W m−2K−1) |
| k | empirical constant for sorbent deactivation (-) |
| kelut | elutriation rate constant (here in g/s) |
| LHV | lower heating value (MJ/m3) |
| M | mass (kg) |
| M | syngas module (mol/mol) |
| M | maximum number of cells in freeboard (-) |
| mass flow (kg/h, g/s) | |
| MU | make-up flow of fresh limestone (kg/h) |
| MW | molar weight (kg/kmol) |
| m | discretization cell in freeboard (-) |
| N | number of calcination-carbonation cycles (-) |
| N | maximum number of cells in fluidized bed (-) |
| n | discretization cell in fluidized bed (-) |
| parameter for descent rate of single particle in suspension phase | |
| partial pressure of component j (-) | |
| heat flux (J/s) | |
| Re | Reynolds number (-) |
| R | reaction rate (mol/s, kg/s) |
| r | reaction rate (mol s−1m−3, kg s−1m−3) |
| S/C | steam-to-carbon ratio (mol H2O/mol carbon) |
| T | Temperature (K) |
| t | time (s) |
| u | superficial gas velocity (m/s) |
| minimum fluidization gas velocity (m/s) | |
| ascent velocity of bubble (m/s) | |
| volume flow (m3/h) | |
| particle falling velocity (m/s) | |
| WHSV | weight hourly space velocity (1/h) |
| average CO2 carrying capacity (mol CaCO3/mol Ca) | |
| CO2 carrying capacity after N cycles (mol CaCO3/mol Ca) | |
| Xr | empirical constant for sorbent deactivation (-) |
| x | mass fraction (-) |
| y | volume fraction (-) |
| α | fraction of mass exchange in calculation cell (-) |
| δ | precision of numerical calculation |
| ε | porosity (-) |
| ν | kinematic viscosity (m2/s) |
| ν | stoichiometric coefficient (-) |
| ρ | density (kg/m3) |
| ψ | sphericity of particles (-) |
| ωj | mass fraction of pyrolysis product j (-) |
| BM | biomass |
| b | bubble phase |
| c | cross section in reactor |
| d | emulsion phase |
| dr | drift |
| eq | equilibrium |
| elut | elutriation |
| empty | condition in empty reactor tube |
| f | freeboard |
| g | gas |
| i | index of chemical reaction |
| in | inlet flow via boundary condition |
| j | index of gas component |
| k | index of solid component |
| mf | condition at minimum fluidization |
| p | particle |
| r | reactor |
| w | wake |
| wf | water-free |
Appendix A
| Component | 600 °C | 650 °C | 700 °C | 750 °C | 800 °C | 850 °C |
|---|---|---|---|---|---|---|
| Ash | 0.0032 | 0.0032 | 0.0032 | 0.0032 | 0.0032 | 0.0032 |
| Char | 0.2473 | 0.2483 | 0.2451 | 0.2435 | 0.2412 | 0.2324 |
| H2O | 0.1654 | 0.1508 | 0.1330 | 0.1312 | 0.1291 | 0.1243 |
| CO2 | 0.2475 | 0.2433 | 0.2807 | 0.2514 | 0.2293 | 0.1890 |
| CO | 0.1706 | 0.2017 | 0.1851 | 0.2246 | 0.2556 | 0.3145 |
| CH4 | 0.0523 | 0.0581 | 0.0632 | 0.0631 | 0.0629 | 0.0619 |
| H2 | 0.0251 | 0.0269 | 0.0284 | 0.0296 | 0.0308 | 0.0326 |
| C2H4 | 0.0399 | 0.0457 | 0.0509 | 0.0482 | 0.0453 | 0.0407 |
| C10H8 | 0.0488 | 0.0221 | 0.0104 | 0.0052 | 0.0027 | 0.0014 |
| Simulated Values | Mean Error in %, MU: 0.2 kg/h | Mean Error in %, MU: 6.6 kg/h | Mean Error in %, MU: 15 kg/h |
|---|---|---|---|
| H2—Composition vs. Temperature (°C) | 5.5 | 4.9 | 5.0 |
| CO—Composition vs. Temperature (°C) | 15.7 | 15.3 | 15.3 |
| CO2—Composition vs. Temperature (°C) | 33.9 | 22.8 | 25.1 |
| CH4—Composition vs. Temperature (°C) | 7.2 | 6.6 | 6.5 |
| C2H4—Composition vs. Temperature (°C) | 14.1 | 14.3 | 14.2 |
| LHV vs. Temperature (°C) | 6.7 | 5.9 | 5.9 |
| M module vs. Temperature (°C) | 23.4 | 29.9 | 32.2 |
References
- Statistics, I.E.A. CO2 Emissions from Fuel Combustion: Highlights; International Energy Agency: Paris, France, 2017. [Google Scholar]
- Olivier, J.G.J.; Schure, K.M.; Peters, J.A.H.W. Trends in Global CO2 and Total Greenhouse Gas Emissions; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2017. [Google Scholar]
- Göransson, K.; Söderlind, U.; He, J.; Zhang, W. Review of syngas production via biomass DFBGs. Renew. Sustain. Energy Rev. 2011, 15, 482–492. [Google Scholar] [CrossRef]
- Rauch, R.; Hofbauer, H. Wirbelschicht-Wasserdampf-Vergasung in der Anlage Güssing (A); Betriebserfahrungen aus zwei Jahren Demonstrationsbetrieb. In Proceedings of the 9th International Symposium “Energetische Nutzung nachwachsender Rohstoffe”, Freiberg, Germany, 4–5 September 2003; FAU: Nuremberg, Germany, 2003. [Google Scholar]
- Thunman, H.; Larsson, A.; Hedenskog, M. Commissioning of the GoBiGas 20 MW Biomethane Plant. Proceedings of Tcbiomass 2015, Chicago, IL, USA, 2–5 November 2015; GTI: Des Plaines, IL, USA, 2015. [Google Scholar]
- Fiaschi, D.; Michelini, M. A two-phase one-dimensional biomass gasification kinetics model. Biomass Bioenergy 2001, 21, 121–132. [Google Scholar] [CrossRef]
- Lü, P.; Kong, X.; Wu, C.; Yuan, Z.; Ma, L.; Chang, J. Modeling and simulation of biomass air-steam gasification in a fluidized bed. Front. Chem. Eng. China 2008, 2, 209–213. [Google Scholar] [CrossRef]
- Puig-Arnavat, M.; Bruno, J.C.; Coronas, A. Review and analysis of biomass gasification models. Renew. Sustain. Energy Rev. 2010, 14, 2841–2851. [Google Scholar] [CrossRef]
- Gordillo, E.D.; Belghit, A. A two phase model of high temperature steam-only gasification of biomass char in bubbling fluidized bed reactors using nuclear heat. Int. J. Hydrog. Energy 2011, 36, 374–381. [Google Scholar] [CrossRef]
- Agu, C.E.; Pfeifer, C.; Eikeland, M.; Tokheim, L.-A.; Moldestad, B.M.E. Detailed one-dimensional model for steam-biomass gasification in a bubbling fluidized bed. Energy Fuels 2019, 33, 7385–7397. [Google Scholar] [CrossRef]
- Hawthorne, C.; Poboss, N.; Dieter, H.; Gredinger, A.; Zieba, M.; Scheffknecht, G. Operation and results of a 200-kWth dual fluidized bed pilot plant gasifier with adsorption-enhanced reforming. Biomass Convers. Biorefinery 2012, 2, 217–227. [Google Scholar] [CrossRef]
- Schweitzer, D.; Albrecht, F.G.; Schmid, M.; Beirow, M.; Spörl, R.; Dietrich, R.-U.; Seitz, A. Process simulation and techno-economic assessment of SER steam gasification for hydrogen production. Int. J. Hydrog. Energy 2018, 43, 569–579. [Google Scholar] [CrossRef]
- Rauch, R.; Hrbek, J.; Hofbauer, H. Biomass gasification for synthesis gas production and applications of the syngas. WIREs Energy Environ. 2014, 3, 343–362. [Google Scholar] [CrossRef]
- Inayat, A.; Ahmad, M.M.; Yusup, S.; Mutalib, M.I.A. Biomass Steam Gasification with In-Situ CO2 Capture for Enriched Hydrogen Gas. Production: A Reaction Kinetics Modelling Approach. Energies 2010, 3, 1472–1484. [Google Scholar] [CrossRef]
- Sreejith, C.C.; Muraleedharan, C.; Arun, P. Air–steam gasification of biomass in fluidized bed with CO2 absorption: A kinetic model for performance prediction. Fuel Process. Technol. 2015, 130, 197–207. [Google Scholar] [CrossRef]
- Hejazi, B.; Grace, J.R.; Bi, X.; Mahecha-Botero, A. Kinetic model of steam gasification of biomass in a bubbling fluidized bed reactor. Energy Fuels 2017, 31, 1702–1711. [Google Scholar] [CrossRef]
- Hejazi, B.; Grace, J.R.; Mahecha-Botero, A. Kinetic modeling of lime-enhanced biomass steam gasification in a dual fluidized bed reactor. Ind. Eng. Chem. Res. 2019, 58, 12953–12963. [Google Scholar] [CrossRef]
- Pitkäoja, A.; Ritvanen, J.; Hafner, S.; Hyppänen, T.; Scheffknecht, G. Simulation of a sorbent enhanced gasification pilot reactor and validation of reactor model. Energy Convers. Manag. 2020, 204, 112318. [Google Scholar] [CrossRef]
- Poboß, N. Experimentelle Untersuchung der Sorptionsunterstützten Reformierung. Ph.D. Thesis, Universität Stuttgart, Stuttgart, Germany, 18 May 2016. [Google Scholar]
- Wang, C.; Zhou, X.; Jia, L.; Tan, Y. Sintering of limestone in calcination/carbonation cycles. Ind. Eng. Chem. Res. 2014, 53, 16235–16244. [Google Scholar] [CrossRef]
- Pfeifer, C.; Puchner, B.; Hofbauer, H. Comparison of dual fluidized bed steam gasification of biomass with and without selective transport of CO2. Chem. Eng. Sci. 2009, 64, 5073–5083. [Google Scholar] [CrossRef]
- Koppatz, S.; Pfeifer, C.; Rauch, R.; Hofbauer, H.; Marquard-Moellenstedt, T.; Specht, M. H2 rich product gas by steam gasification of biomass with in situ CO2 absorption in a dual fluidized bed system of 8 MW fuel input. Fuel Process. Technol. 2009, 90, 914–921. [Google Scholar] [CrossRef]
- Schmid, M.; Beirow, M.; Schweitzer, D.; Waizmann, G.; Spörl, R.; Scheffknecht, G. Product gas composition for steam-oxygen fluidized bed gasification of dried sewage sludge, straw pellets and wood pellets and the influence of limestone as bed material. Biomass Bioenergy 2018, 117, 71–77. [Google Scholar] [CrossRef]
- Soukup, G.; Pfeifer, C.; Kreuzeder, A.; Hofbauer, H. In situ CO2 capture in a dual fluidized bed biomass steam gasifier—Bed material and fuel variation. Chem. Eng. Technol. 2009, 32, 348–354. [Google Scholar] [CrossRef]
- Schweitzer, D.; Beirow, M.; Gredinger, A.; Armbrust, N.; Waizmann, G.; Dieter, H.; Scheffknecht, G. Pilot-scale demonstration of Oxy-SER steam gasification: Production of syngas with pre-combustion CO2 capture. Energy Procedia 2016, 86, 56–68. [Google Scholar] [CrossRef]
- Hilligardt, K. Zur Strömungsmechanik von Grobkornwirbelschichten. Ph.D. Thesis, Technische Universität Hamburg, Hamburg, Germany, 1986. [Google Scholar]
- Tepper, H. Zur Vergasung von Rest-und Abfallholz in Wirbelschichtreaktoren für Dezentrale Energieversorgungsanlagen. Ph.D. Thesis, Universität Magdeburg, Magdeburg, Germany, 2005. [Google Scholar]
- Richardson, J.F.; Zaki, W.N. Sedimentation and fluidisation: Part I. Chem. Eng. Res. Des. 1997, 75, S82–S100. [Google Scholar] [CrossRef]
- Werther, J. Experimentelle Untersuchungen zur Hydrodynamik von Gas./Festoff-Wirbelschichten. Ph.D. Thesis, Technische Fakultät der Universität Erlangen-Nürnberg, Erlangen, Germany, 1972. [Google Scholar]
- Davidson, J.F. Bubble formation at an orifice in an inviscid liquid. Trans. Inst. Chem. Eng. 1960, 38, 335–342. [Google Scholar]
- Wen, C.Y.; Chen, L.H. Fluidized bed freeboard phenomena: Entrainment and elutriation. AIChE J. 1982, 28, 117–128. [Google Scholar] [CrossRef]
- Dietz, S. Wärmeübergang in Blasenbildenden Wirbelschichten. Ph.D. Thesis, Technische Fakultät der Universität Erlangen-Nürnberg, Erlangen, Germany, 1994. [Google Scholar]
- Zhang, D.; Koksal, M. Heat transfer in a pulsed bubbling fluidized bed. Powder Technol. 2006, 168, 21–31. [Google Scholar] [CrossRef]
- Fagbemi, L.; Khezami, L.; Capart, R. Pyrolysis products from different biomasses: Application to the thermal cracking of tar. Appl. Energy 2001, 69, 293–306. [Google Scholar] [CrossRef]
- Roberts, A.F.; Clough, G. Thermal decomposition of wood in an inert atmosphere. In Proceedings of the Ninth Symposium (International) on Combustion, New York, NY, USA, 27 August–1 September 1963. [Google Scholar]
- Hemati, M.; Laguerie, C. Determination of the kinetics of the wood sawdust steam gasification of charcoal in a thermobalance. Entropie 1988, 142, 29–40. [Google Scholar]
- Kramb, J.; Konttinen, J.; Gómez-Barea, A.; Moilanen, A.; Umeki, K. Modeling biomass char gasification kinetics for improving prediction of carbon conversion in a fluidized bed gasifier. Fuel 2014, 132, 107–115. [Google Scholar] [CrossRef]
- Charitos, A.; Rodríguez, N.; Hawthorne, C.; Alonso, M.; Zieba, M.; Arias, B.; Kopanakis, G.; Scheffknecht, G.; Abanades, J.C. Experimental Validation of the calcium looping CO2 capture process with two circulating fluidized bed carbonator reactors. Ind. Eng. Chem. Res. 2011, 50, 9685–9695. [Google Scholar] [CrossRef]
- Stanmore, B.R.; Gilot, P. Review—calcination and carbonation of limestone during thermal cycling for CO2 sequestration. Fuel Process. Technol. 2005, 86, 1707–1743. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling wood gasification in a countercurrent fixed-bed reactor. AIChE J. 2004, 50, 2306–2319. [Google Scholar] [CrossRef]
- Mostafavi, E.; Pauls, J.H.; Lim, C.J.; Mahinpey, N. Simulation of high-temperature steam-only gasification of woody biomass with dry-sorption CO2 capture. Can. J. Chem. Eng. 2016, 94, 1648–1656. [Google Scholar] [CrossRef]
- Dong, J.; Nzihou, A.; Chi, Y.; Weiss-Hortala, E.; Ni, M.; Lyczko, N.; Tang, Y.; Ducousso, M. Hydrogen-rich gas production from steam gasification of bio-char in the presence of CaO. Waste Biomass Valorization 2017, 8, 2735–2746. [Google Scholar] [CrossRef]
- Alvarez, D.; Abanades, J.C. Pore-size and shape effects on the recarbonation performance of calcium oxide submitted to repeated calcination/recarbonation cycles. Energy Fuels 2005, 19, 270–278. [Google Scholar] [CrossRef]
- Grasa, G.S.; Abanades, J.C. CO2 capture capacity of CaO in long series of carbonation/calcination cycles. Ind. Eng. Chem. Res. 2006, 45, 8846–8851. [Google Scholar] [CrossRef]
- Hawthorne, C.; Charitos, A.; Perez-Pulido, C.A.; Bing, Z.; Scheffknecht, G. Design of a dual fluidised bed system for the post-combustion removal of CO2 using CaO. Part. I: CFB carbonator reactor model. In Proceedings of the 9th International Conference on Circulating Fluidized Beds, Hamburg, Germany, 13–16 May 2008; pp. 759–764. [Google Scholar]
- Zhen-shan, L.; Ning-sheng, C.; Croiset, E. Process analysis of CO2 capture from flue gas using carbonation/calcination cycles. AIChE J. 2008, 54, 1912–1925. [Google Scholar] [CrossRef]
- Abanades, J.C. The maximum capture efficiency of CO2 using a carbonation/calcination cycle of CaO/CaCO3. Chem. Eng. J. 2002, 90, 303–306. [Google Scholar] [CrossRef]
- Pauls, J.H.; Mahinpey, N.; Mostafavi, E. Simulation of air-steam gasification of woody biomass in a bubbling fluidized bed using Aspen Plus: A comprehensive model including pyrolysis, hydrodynamics and tar production. Biomass Bioenergy 2016, 95, 157–166. [Google Scholar] [CrossRef]
- Nikoo, M.B.; Mahinpey, N. Simulation of biomass gasification in fluidized bed reactor using ASPEN PLUS. Biomass Bioenergy 2008, 32, 1245–1254. [Google Scholar] [CrossRef]
- Dybkjær, I.; Aasberg-Petersen, K. Synthesis gas technology large-scale applications. Can. J. Chem. Eng. 2016, 94, 607–612. [Google Scholar] [CrossRef]
- Poboss, N.; Zieba, M.; Scheffknecht, G. Experimental investigation of affecting parameters on the gasification of biomass fuels in a 20 kWth dual fluidized bed. In Proceedings of the International Conference on Polygeneration Strategies (ICPS10), Leipzig, Germany, 7–9 September 2010. [Google Scholar]
| Reaction | Chemical Reaction and Kinetics | Reference |
|---|---|---|
| 1. Pyrolysis | BMwf → ωAsh Ash + ωChar Char + ωH2O H2O + ωCO2 CO2 + ωCO CO + ωCH4 CH4 + ωH2 H2 + ωC2H4 C2H4 + ωC10H8 C10H8 in kgs−1m−3 | [35] |
| 2. Heterogeneous water–gas | C + H2O → CO + H2 in mol s−1 m−3 | [36] |
| 3. Boudouard | C + CO2 → 2CO in mol s−1m−3 | [37] |
| 4. Carbonation | CO2 + CaO → CaCO3 in mol s−1m−3 | [38] [39] |
| 5. Water–gas shift | CO + H2O ⟷ CO2 + H2 in mol s−1m−3 | [40] |
| 6. Ethene reformation | C2H4 + 2H2O → 2CO + 4H2 in mol s−1m−3 | [41] 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beirow, M.; Parvez, A.M.; Schmid, M.; Scheffknecht, G. A Detailed One-Dimensional Hydrodynamic and Kinetic Model for Sorption Enhanced Gasification. Appl. Sci. 2020, 10, 6136. https://doi.org/10.3390/app10176136
Beirow M, Parvez AM, Schmid M, Scheffknecht G. A Detailed One-Dimensional Hydrodynamic and Kinetic Model for Sorption Enhanced Gasification. Applied Sciences. 2020; 10(17):6136. https://doi.org/10.3390/app10176136
Chicago/Turabian StyleBeirow, Marcel, Ashak Mahmud Parvez, Max Schmid, and Günter Scheffknecht. 2020. "A Detailed One-Dimensional Hydrodynamic and Kinetic Model for Sorption Enhanced Gasification" Applied Sciences 10, no. 17: 6136. https://doi.org/10.3390/app10176136
APA StyleBeirow, M., Parvez, A. M., Schmid, M., & Scheffknecht, G. (2020). A Detailed One-Dimensional Hydrodynamic and Kinetic Model for Sorption Enhanced Gasification. Applied Sciences, 10(17), 6136. https://doi.org/10.3390/app10176136





