Abstract
YCo5 permanent magnet exhibits high uniaxial magnetocrystalline anisotropy energy and has a high Curie temperature. These are good properties for a permanent magnet, but YCo5 has a low energy product, which is notably insufficient for a permanent magnet. In order to improve the energy product in YCo5, we suggest replacing cobalt with iron, which has a much bigger magnetic moment. With a combination of density-functional-theory calculations and thermodynamic CALculation of PHAse Diagrams (CALPHAD) modeling, we show that a new magnet, YFe3(Ni1-xCox)2, is thermodynamically stable and exhibits an improved energy product without significant detrimental effects on the magnetocrystalline anisotropy energy or the Curie temperature.
1. Introduction
Three principal material parameters define the intrinsic properties of hard magnetic materials: (i) spontaneous (saturation) magnetization, Ms, (ii) Curie temperature, Tc, and (iii) magnetocrystalline anisotropy energy (MAE), characterized by its first-order anisotropy coefficient, K1 [1]. These three parameters all need to be large for an economically effective permanent magnet, i.e., Tc ~≥ 550 K, Ms ~≥ 1 MA/m, K1 ~≥ 4 MJ/m3. By incorporating transition-metal (TM) with rare-earth-metal (RE) atoms in numerous intermetallic compounds [1,2], one produces a material with these fascinating magnetic properties.
In our previous papers [3,4], we suggested a new economically effective permanent magnet, SmFe3CoNi, that was developed from the well-known SmCo5 blueprint. More contemporary neodymium magnets of the Nd-Fe-B type are superior to the SmCo5 magnet because of their greater maximum energy product emerging from their iron-rich stoichiometry. Our proposed SmFe3CoNi magnet, however, eliminated most of the detriment of the SmCo5 magnet while maintaining its superior high-temperature effectiveness over neodymium magnets.
According to ref. [5], the price of Sm and Co is moderately high, which makes reducing the price of the SmCo5 magnet a dominant objective. The authors [5] claim that the rare-earth (RE) Y (225 RMB/kg) is cheaper than Sm (450 RMB/kg), and by limited substitution of Sm with Y atoms, a high performance (Sm1-xYx) Co5 magnet can be prepared. The YCo5 compound (the CaCu5-type structure) is isostructural with SmCo5. The YCo5 magnet exhibits significant MAE of K1 ~6.5 MJ/m3 (3.41 meV/f.u.) [6], which is excessive compared to that of the Nd2Fe14B (Neomax) magnets (K1 ~4.9 MJ/m3, [7]). It furthermore has a Curie temperature (Tc ~987 K, [6]) almost twice that of the Neomax (Tc ~588 K, [7]). However, Nd2Fe14B presently prevails the worlds wholesale for permanent magnets (~62%) [8,9] because it has the biggest energy production sustained by its record energy product (BH)max ~512 kJ/m3 [7], more than twice that of the YCo5 magnets, (BH)max = 224 kJ/m3 [10,11].
The YCo5 magnet is probably the most studied magnet with the CaCu5-type structure. It has been explored both experimentally and theoretically in the past due to its unique qualification as a “gap” permanent magnetic material [5,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84]. Actually, almost all experimental papers on the YCo5 magnet have been dedicated to studying MAE of the undoped magnet as well as doped with Sm, Nd, Ge, Fe, Cu, and Ni [5,10,11,12,13,14,15,16,17,18,19,20,22,23,24,25,26,27,28,29,31,32,33,34,35,41,42,43,52,53,54,62,66,70,72,73,74,77,80]. MAE, the Curie temperature, and the energy product, which are 5.7 MJ/m3, 921 K, and 224 kJ/m3, correspondingly, were first reported in refs. [10,11,12] Slightly higher values, K1 ~6.4 MJ/m3 and Tc ~987 K, were reported in ref. [15]. According to Schweitzer and Tasset [28], the Curie temperature is Tc ~920 K. Ermolenko [17] studied the temperature dependence of MAE of the YCo5 magnet. Later, similar measurements were performed by Al-Omari et al. [54]. Refs. [20,22] represent the first review papers dedicated to the experimental study of the YCo5 magnet, although Buschow [20] claimed that the total magnetic moment of YCo5 is equal to m(tot) = 7.52 μB/f.u. Higher values of the total magnetic moment for YCo5, m(tot) = 8.33 μB/f.u., and MAE, K1 ~7.38 MJ/m3, were also reported [29].
In regard to the SmCo5 magnet, the MAE is dominated by the 3D electron contribution from Co, although the localized Sm 4f electrons are believed to influence MAE somewhat [3]. For YCo5, on the other hand, magnetic anisotropy is entirely due to the Co 3d electrons because Y has no electrons occupying 4f states [16,18,19,23,24,25,28]. According to Refs. [16,18,23,25,28,70], the spin-polarized neutron scattering, inelastic spin flip-neuron scattering, nuclear magnetic resonance (NMR), neutron diffraction (ND), and x-ray magnetic circular dichroism (XMCD) measurements of YCo5 revealed a very large orbital contribution to the total magnetic moment from Co1(2c) sites, indicating that the large axial MAE of the YCo5 magnet arises from the Co1(2c) sites. The orbital moment on the Co2(3g) sites is smaller than the orbital moment on the Co1(2c) sites and contributes to the planar MAE of the YCo5 magnet [23,25].
Several experimental studies of doping the RECo5 compound with Fe or Ni [14,31] suggest that iron atoms prefer to occupy 3g sites because they are slightly larger than cobalt atoms and are more easily accommodated in these positions. On the other hand, there are vigorous manifestations that nickel atoms conversely occupy 2c sites. According to ref. [31], the compositional dependency of the lattice constant and the interatomic distances can be satisfactory justified by nickel atoms occupying 2c rather than 3g sites in the RECo5 compound. Copper atoms choose to occupy 3g sites because the atomic volume of copper is bigger than that of cobalt atoms and even bigger than that of iron atoms [31].
From a price standpoint, it is profitable to replace Co atoms with Fe because of the abundance of Fe in the Earth’s crust compared to Co (by a factor of ~2000) making a Fe-containing magnet economically more attractive. Furthermore, Fe is a ferromagnetic metal with a very substantial magnetization at room temperature (1.76 MA/m [7]). The YCo5 magnet doped with iron has been the subject of numerous experimental papers [13,18,20,34,35,41,42,52,72,73,80]. Replacing all cobalt with iron to enhance magnetization in the YFe5 compound produces a thermodynamically unstable phase, which is not experimentally observed within the equilibrium Y-Fe phase diagram. Contrarily, the Y(Co1–xFex)5 compounds (CaCu5-type) have been observed, with x ranging from 0.2 to 0.4 [42,52]. Moreover, the Curie temperature of the Y(Co1-xFex)5 alloys has been found to increase from ~930 K to ~1020 K when varying across increasing x from 0.0 to 0.2 [42], contrary to the Y2(Co1–xFex)17 alloys that display a monotonic reduction in the Curie temperature with rising Fe amount. The orbital moment is also bigger in cobalt compared to iron, and a decrease of the MAE is thus expected for x > 0. The lattice constant and magnetization also enhance with x from 0 to 0.4 [52] in the Y(Co1-xFex)5 alloys.
In addition to iron and nickel, copper has also been used to dope the YCo5 magnet [20,28,31,33,50,52,53]. In contrast to the Y(Co1–xFex)5 system, Y(Co1–xNix)5 and Y(Co1–xCux)5 compounds are stable across the entire composition domain (i.e., x = 0–1). Substituting cobalt atoms with nickel or copper atoms gradually decreases magnetization and magnetic anisotropy. By connecting diffraction techniques and electron microscopy, Colin et al. [66] studied the solubility restriction of Ge in the Y(Co1–xGex)5 alloys. A preferential substitution of Ge atoms that were larger than Co atoms on the 3g positions was observed.
Numerous theoretical approaches have been used to study the YCo5 magnets. These include augmented-plane-waves (APW), point charge, linearized muffin-tin orbitals within atomic sphere approximation (LMTO-ASA), Haydok recursion (HR), full-potential linearized augmented-plane-waves (FLAPW), linear combination of atomic orbitals (LCAO), pseudopotential projected augmented waves (VASP-PAW), augmented spherical waves (ASW-ASA), full-potential linear muffin-tin orbitals (FPLMTO), dynamical mean field theory in conjunction with density functional theory (LDA-DMFT), and Korringa–Kohn–Rostoker multiple-scattering formulation in conjunction with the coherent potential approximation and disordered local moment approximation (KKRASA-CPA-DLM) [21,26,27,30,37,38,39,40,44,45,46,47,48,49,50,51,55,56,57,58,59,60,61,62,63,64,65,67,68,70,71,74,75,76,77,78,79,81,82,83,84]. According to the self-consistent APW calculations [21], the total moment of the YCo5 magnet is equal to m(tot) = 7.31 μB/f.u., which is in accordance with the initially reported experimental data, m(tot) = 7.52 μB/f.u. [20]. Using the point charge model, Inomata [30] confirmed the conclusion presented in the experimental papers [8,16,19,23,25,28], namely, the orbital moments of Co atoms located on the 2c sites contribute to the axial (positive) MAE of YCo5, and the orbital moments of Co atoms located on the 3g sites contribute to its planar (negative) contribution. The importance of the orbital polarization (OP) has been emphasized in LMTO-ASA papers [37,38,39,40,45,46,47]. The magnetization of the YCo5 compound doped by Ni, Y(Co5-xNix) has been studied within the Haydok recursion method [44]. According to these calculations, when the amount of Ni dissolved in the YCo5 magnet reaches 60%, the YCo2Ni3 compound loses its magnetization. The LMTO-ASA formalism has also been applied to study the Y(Co1-xNix)5 alloys [48,51]. These calculations confirm that the nickel atoms choose to occupy 2c sites. LCAO calculations by Zhang et al. [49] reveal the total magnetic moment of the YCo5 magnet is equal to m(tot) = 8.70 μB/f.u. Steinbeck et al. [55,56] performed fully relativistic LCAO calculations of MAE of YCo5 and Y(Co1-xFex)5 compounds. Their calculated MAE of the YCo5 magnet is K1 = 1.11 MJ/m3 and K1 = 8.40 MJ/m3 for SOC and SOC+OP theory (where SOC means spin-orbit coupling), correspondingly. They furthermore obtained within the SOC+OP scheme the orbital moments that are 0.33 μB and 0.26 μB for 2c and 3g sites, correspondingly. These moments are in accordance with experimental measurements of 0.26 μB (2c) and 0.24 μB (3g) [28]. Calculated MAE for the Y(Co1-xFex)5 compounds show qualitative agreement with the experimental data [34]. The calculated Curie temperature of the YCo5 magnet Tc = 998 K [57] is in good accord with results of the previous calculations by Wohlfarth [26,27] and the experimental data, Tc ~987 K [6].
Using FLAPW (without OP), Larson et al. [58,59,60,61] calculated MAE of the YCo5 magnet (K1 = 2.83 MJ/m3) as well as Y(Co1-xFex)5 and Y(Co1-xCux)5 alloys. Calculated MAE of the Y(Co1-xFex)5 compounds is in qualitative accord with the results of the previously mentioned LCAO calculations [56]. MAE of the Y(Co1-xFex)5 magnets with 6–7 % doping increases by about 1.9 MJ/m3 before falling rapidly with a larger Fe doping. The total magnetic moment gradually increases from m(tot) = 7.2 μB/f.u. (YCo5) to ~10 μB/f.u. (Y(Co1Fe4)) then falls to 9 μB/f.u. for YFe5.
When doping the YCo5 magnet with copper, the total moment gradually decreases with increasing copper concentration, and finally the moment disappears for the Y(Co1Cu4) compound. The calculations performed by Larson and Mazin [61] show that, for the Y(Co1-xCux)5 alloys, the total magnetic moment decreases linearly with Cu doping, identical to the results of Ni doping of the Y(Co1-xNix)5 alloys [51]. However, in contrast to the Y(Co1-xNix)5 compounds, the magnetic moments of the cobalt atoms are reasonably local and barely impacted by Cu doping of the Y(Co1-xCux)5 alloys until x ~0.5, where the moment promptly falls to a small value before steadily decreasing [61]. The authors [61] concluded that cobalt in the doped YCo5 compounds can occur either in a high- or a low-spin state, depending on the local surroundings. A similar conclusion (the high spin to the low spin transition) is presented in the paper of Wu et al. [63], where VASP-PAW formalism is applied to study the Y(Co1-xAgx)5 alloys.
The ASW-ASA formalism has been applied for study magnetic and electronic properties of the Y(Co1-xFex)5 compounds: YCo5, Y(Fe2Co3), Y(Fe3Co2), and YFe5 [65]. FPLMTO calculations for the Y(Co1-xFex)5 compounds with x = 0, 0,1, 0.2, and 0.3 [67] confirm that iron atoms choose to occupy 3g sites, and total magnetization increases from 7.50 μB/f.u. to 8.97 μB/f.u. with increasing x from 0 to 0.3 because iron atoms have a much bigger magnetic moment (~2.4 μB) than the magnetic moment of cobalt atoms (~1.5 μB).
A real-space pseudopotential formalism with SOC incorporated by norm-conserving pseudopotentials has been used to compute MAE of the YCo5 magnet in different structures [78]. It appears that the authentic CaCu5-type structure (Space Group P6/mmm) possesses the largest total magnetic moment m(tot) = 7.13 μB/f.u. and MAE K1 = 2.68 MJ/m3. The latter is, however, significantly smaller than the experimental value of K1 = 6.5 MJ/m3 [6].
Nguyen et al. [79] studied the dependence of MAE of the YCo5 magnet with intra-atomic Coulomb interaction (the Hubbard U parameter, DFT+U scheme) and strength of SOC within the PAW-VASP method. These calculations showed that, for GGA+U calculations, with 1.4 eV ≤ U ≤ 1.47 eV and J = 0.8 eV, MAE of the YCo5 compound changes within the experimentally observed range. Similar results are presented in the work of Zhu et al. [70], where LDA+DMFT computational scheme is used to replace the OP correction [37]. The LDA+DMFT calculations suggest that electron correlation effects play an essential role in the formation and the enhancement of orbital moments and magnetic anisotropy in the YCo5 magnet. ND, XRD measurements, and FPLMTO-DMFT technique have been used [77] to describe an isomorphic (without change of the symmetry) lattice collapse and the electronically topological transition (ETT), which is the first order Lifshitz phase transition in the YCo5 magnet at pressure about 18 ± 2 GPa first reported in refs. [62,63] based on XRD measurements and FPLO calculations [66]. According to these calculations, the Curie temperature of the YCo5 magnet, Tc ≈ 987 K, is in the range of experimental measurements. These calculations reveal that, at ambient pressure, the orbital moments are 0.22 μB and 0.18 μB for 2c and 3g, correspondingly, and at pressure of 7.2 GPa, the orbital moments are 0.18 μB and 0.15 μB for 2c and 3g, correspondingly, which is in reasonable agreement with the experimental observation [28] (at ambient pressure) and also in agreement with results of LDA-DMFT calculations [70]. The atom-resolved Co1(2c) and Co2(3g) orbital moments reach the maximum values for the Coulomb parameter U ~2 eV. These results for the orbital moments are consistent with previous XMCD measurements [71], inelastic spin flip-neuron scattering experiments [16], and OP calculations [37,55,56].
The temperature dependence of MAE and the spontaneous magnetization of the YCo5 magnet has been calculated by utilizing the relativistic KKRASA-CPA-DLM approach [71,74,75,76,81,82]. The calculated total magnetic moment, m(tot), is 8.50 μB/f.u. 8.03 μB/f.u. at 0 K and around T = 100 K, correspondingly, [71], which is in an fair accord with the experimental data, 8.33 μB/f.u., at liquid-helium temperature [29], and 7.99 μB/f.u. at room temperature [28]. Calculated MAE is equal to K1 ~4.63 MJ/m3 at T ≈ 50 K [71], which is larger than reported in the previous calculations, K1 ~2.83 MJ/m3 at T = 0 K [58,59,60,61] (see Figure 1, [71]), but smaller than the experimental value of K1 ~7.38 MJ/m3 at T = 4.2 K [24], K1 ~6.40 MJ/m3 at T = 77 K [15] as well as K1 ~6.03 MJ/m3 [15], K1 ~5.50 MJ/m3 [69], and K1 ~5.00 MJ/m3 [17] at room temperature, T = 293 K. The calculated MAE decreases as temperature rises, for example, K1 ~1.1 MJ/m3 at 600 K [71]. Calculated Curie temperatures Tc ~965 K [71] and Tc ~885 K 74] compare well with experimental measurements Tc ~920 K [28] and Tc ~987 K [15]. KKRASA-CPA-DLM formalism has been applied to study temperature dependence of magnetization and the Curie temperature of the YCo5 magnet doped by Ni and Fe [74]. Both experiments and calculations show an increase or a decrease in magnetization with Fe or Ni replacement, correspondingly. The same KKRASA-CPA-DLM formalism has been applied to study the temperature dependence of magnetization and MAE for the Y(Co1-x-yFexCuy)5 magnets [81]. These calculations show that MAE of the YCo5 magnet could be increased by adding relatively minor amounts of Fe and/or Cu, with the larger MAE field detected for the composition Y(Co0.82Fe0.09Cu0.09)5 with the condition of a preferred replacement of Fe and Cu at 3g and 2c, correspondingly. The recent improvement of KKRASA-CPA-DLM methodology allowed Patrick and Staunton [82] to reproduce the experimental MAE of the YCo5 magnet, K1 ~5.0 MJ/m3 [17], at room temperature. Recent overviews of the first-principles studies of the doped YCo5 magnets are presented in refs. [83,84].
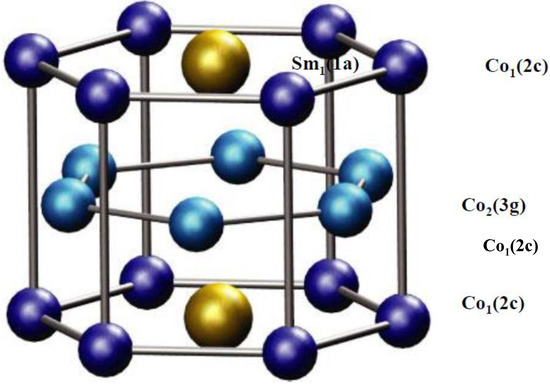
Figure 1.
Crystal structure of SmCo5. The schematic is taken from ref. [62].
In addition to the numerous first-principle studies of the YCo5 magnet, the phase stability and the thermodynamics of the Y-Co system have been assessed experimentally [85,86,87] and computationally, using the semi-empirical CALPHAD (CALculation of PHAse Diagrams) method [88,89,90]. Experimentally, YCo5 was reported to melt congruently at Tm ~1623 K, possess a small homogeneity range at high temperature, and decompose below T ~998 K [86]. In addition, the Y-Co-Ni ternary system evaluation, reported by Gupta [91], revealed a complete solubility from YCo5 to YNi5 at 1073 K and 1273 K. This ternary system was assessed using the CALPHAD method [92,93], and the solubility of the Y(Co,Ni)5 phase has been reproduced [92]. Experimental characterization of the Y-Co-Fe system demonstrated maximum solubility of Fe in Y(Co,Fe)5 of ~20 at.% [94] and ~5 at.% [95] at 1323 K and 1073 K, correspondingly. There are no reported CALPHAD assessments (parameters) for Y-Co-Fe and Y-Fe-Ni ternary systems (a maximum solubility of ~30 at.% Fe in Y(Fe,Ni)5 was reported by [95] at 873 K), and no available information exists regarding thermodynamic properties or phase stability of the Y(Co,Fe,Ni)5 compound.
Therefore, the principal goal of the present research is to examine the stabilizing effects of adding nickel to the Y(Co1–xFex)5 alloys. We employ ab initio calculations using two complementary techniques: (i) the fully relativistic exact muffin-tin orbital method (FREMTO) and (ii) the full-potential linear muffin-tin orbital method (FPLMTO). All methods account for all relativistic effects, the so-called spin-orbit coupling (SOC). The implementation of these particular methods ensures that our results are rigorous and independent of technical implementations while employing the most advantageous attributes and durability strengths of each method. Related details of the ab initio [96,97] and the CALPHAD computational methods are outlined in Section 2. Results of the density-functional-theory (DFT) calculations of the ground state properties of the Y(Co-Fe-Ni)5 alloys connected with CALPHAD calculations are outlined in Section 3. Next, we demonstrate results of the DFT calculations of the magnetic properties of the Y(Co-Fe-Ni)5 alloys in Section 4. Lastly, discussion and concluding remarks are outlined in Section 5.
2. Computational Methods
The calculations we denote as EMTO are performed while using the Green’s function formalism based on the improved screened Korringa–Kohn–Rostoker technique, where the one-electron potential is defined by the optimized overlapping muffin-tin (OOMT) potential spheres [98,99]. Inside the potential spheres, the potential is spherically symmetric, while it is permanent between the spheres. The radius of the potential spheres, the spherical potential inside these spheres, and the permanent value in the interstitial region are resolved by minimizing (i) the deviation between the exact and overlapping potentials and (ii) the errors that are caused by the overlap between the spheres. Within the EMTO formalism, the one-electron states are calculated exactly for the OOMT potentials. As a product of the EMTO calculation, one can determine the self-consistent Green’s function of the system and the complete, non-spherically symmetric charge density. Lastly, the total energy is calculated while using the full charge-density technique [100]. We consider, as the valence states, 6s, 5p, 5d, and 4f states for Sm and 4s and 3d states for Co and Fe. The corresponding Kohn–Sham orbitals are expanded in terms of spdf exact muffin-tin orbitals, i.e., we select an orbital momentum cutoff lmax = 3. The EMTO orbitals, consequently, consist of the spdf partial waves (solutions of the radial Schrödinger equation for the spherical OOMT potential wells) and the spdf screened spherical waves (solutions of the Helmholtz equation for the OOMT muffin-tin zero potential). The completeness of the muffin-tin basis was discussed in detail in ref. [99]. The generalized gradient approximation (GGA-PBE) [101] is selected for the electron exchange and the correlation energy functional. Integration over the Brillouin zone is performed using the special k-point technique [102] with 784 k-points in the irreducible wedge of the zone (IBZ). The moments of the density of states, needed for the kinetic energy and valence charge density, are computed by integrating the Green’s function over a complex energy contour with 2.2 Ry diameter while using a Gaussian integration technique with 30 points on a semi-circle enclosing the occupied states. In the case of the implementation of the FR-EMTO formalism, SOC is included through the four-component Dirac equation [103].
In order to treat compositional disorder, the EMTO method is coupled with the coherent potential approximation (CPA) [104,105]. The ground-state properties of the Y(Co1–x–yFexNiy)5 alloys are obtained from EMTO-CPA calculations that include the Coulomb screening potential and energy [106,107,108]. The paramagnetic state of the Y(Co1–x–yFexNiy)5 is modeled within the disordered local moment (DLM) approximation [109,110]. The equilibrium atomic density of the alloy is obtained from a Murnaghan fit to the total energy versus atomic volume curve [111].
The highest level of theory (least approximations) is implemented in a full-potential scheme, i.e., no structural approximations, which includes spin-orbit interaction as explained [112] for early and late lanthanides [96,113]. The full-potential linear muffin-tin orbital (FPLMTO) accomplishes this in ways that are detailed [114]. For Y, we use two energy parameters coupled with each basis function, and these parameters have different values for pseudocore states (4s and 4p) and valence states (5s, 5p, 4d, and 4f). Test calculations including the 3d states as semi-core states for Y do not significantly change any result. For the TM atom, we use a similar set-up. The spin–orbit interaction and the orbital polarization (OP) operate on the d and f orbital. OP is introduced in FPLMTO in a self-consistent fashion so there are no fixed parameters. Because of the way it is constructed, the orbital polarization often enhances spin–orbit coupling, leading to better orbital moments [114]. Both the generalized gradient and the local-density approximations (GGA-PBE and LDA) [101,115] are applied.
For the computation of the magnetic anisotropy energy, i.e., the difference in total energy between spins pointing in plane (100) or perpendicular to the plane along the z axis (001), one finds small differences. To resolve these small numbers, one has to be very careful calculating the total energy for the two cases as consistent as possible. Here, we decide to remove all crystal symmetry, thus, from a symmetry set-up standpoint, the calculations are identical. This approach is less efficient but in practice more accurate because errors associated with different k points, Brillouin zones, and other numerical approximations are minimized. Brillouin zone integration over 10,000 k points is carried out using Fermi-Dirac broadening corresponding to room temperature.
The CALPHAD method permits the prediction of phase stability and thermodynamics of multi-component systems based on the extrapolation of the phase diagram data and the thermodynamic properties of its constitutive binary and ternary systems [116,117,118]. The CALPHAD formalism consists of modeling Gibbs energy functions for each phase of the system (potentially including all elements) to reproduce the available thermodynamic (e.g., heat of formation) and phase diagram (e.g., melting point) data. Once the parameters of these functions are assessed for the constitutive systems, extrapolation (or prediction) can be made for multi-component systems. Here, we focus on heat of formation (thermodynamic property), phase diagram data (e.g., melting point, solubility limits), and CALPHAD assessment of the YTM5 compound with TM = Co, Fe, and Ni as a first step toward the establishment of a complete Y-Co-Fe-Ni database. Model parameters optimization and CALPHAD calculations are performed using the Thermo-Calc software package (2019b version) in combination with its PARROT module [119,120].
3. Thermodynamics Properties of the Y(Co-Fe-Ni)5 Alloys
The YCo5 compound crystallizes in the hexagonal CaCu5-type structure with three non-equivalent atomic sites: Y1-(1a), Co1-(2c), and Co2-(3g) (see Figure 1) with six atoms per formula unit and per computational cell.
Table 1 shows the equilibrium formula unit (f.u.) volume, bulk modulus, and the pressure derivative of the bulk modulus for the YCo5 compound calculated using each of the ab initio techniques. The results of the EMTO calculations are presented both without (“SREMTO”, “SR” means scalar-relativistic) and with (“FREMTO”) inclusion of SOC. The experimental value of the equilibrium f.u. volume is taken from ref. [29,55]—see further discussion below. Notice that the equilibrium volume, calculated by FPLMTO method, agrees very well with the experimental data and that incorporating the relativistic SOC in the calculation has only a minor effect.

Table 1.
Equilibrium formula unit volume, bulk modulus, and its pressure derivative of the YCo5 compound as functions of the ab initio technique. The unit cell volume is measured at T = 4.1 K.
Earlier neutron-diffraction studies of the Th(Co1–xFex)5 alloys (also based on the CaCu5-type structure) [14] show that the larger Fe atoms preoccupies the 3g-type sites, whereas the smaller Co atoms choose to occupy the 2c-type sites. This occupational inclination has been affirmed by DFT calculations for YCo5 [67] and SmCo5 [68] compounds. In line with these calculations, the total energy for Fe at the 3g site (E3g) is lower that than for Fe at the 2c site (E2c) by 0.21 eV/f.u. and 0.10 eV/f.u. for YCo5 and SmCo5 magnets, correspondingly. As was mentioned in refs. [19,28], if the YCo5 magnet is doped with Fe and Ni, Fe atoms occupy preferentially 3g sites, while Ni atoms favor 2c sites.
Figure 2a shows the heat of formation calculated within the EMTO-CPA technique of the pseudo-binary YFe3(Ni1–xCox)2 alloys where Fe atoms occupy all 3g-type sites and the occupation of the 2c-type sites continuously changes from pure Ni (the YFe3Ni2 compound) to pure Co (the YFe3Co2 compound). The current calculations show that the YFe3(Ni1–xCox)2 alloys could remain stable until almost all Ni atoms are replaced by Co atoms.
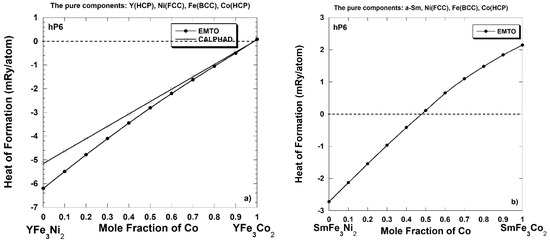
Figure 2.
(a) The heat of formation of the pseudo-binary YFe3(Ni1-xCox)2 alloys predicted via ab initio (0 K) and CALculation of PHAse Diagrams (CALPHAD) (298 K) calculations. (b) The heat of formation of the pseudo-binary SmFe3(Ni1-xCox)2 alloys predicted via ab initio calculations [4].
For comparison, we also show (Figure 2b) the heat of formation calculated within the EMTO-CPA formalism [3,4] of the pseudo-binary SmFe3(Ni1–xCox)2 alloys where Fe atoms occupy all 3g-type sites and the occupation of the 2c-type sites continuously changes from pure Ni (the SmFe3Ni2 compound) to pure Co (the SmFe3Co2 compound). These calculations show that the pseudo-binary SmFe3(Ni1–xCox)2 alloys could remain stable until almost half of Ni atoms are replaced by Co atoms.
In order to understand the difference between Figure 2a,b, one needs to take into consideration several of the following arguments. Nickel metal forms the stable CaCu5-type compounds with both yttrium and samarium metals. Calculated within EMTO formalism, the heat of formation of SmNi5 and YNi5 compounds (in the CaCu5-type structure) is −18.95 mRy/atom and −22.91 mRy/atom, correspondingly, which is in accord with the experimental measurements of −23.08 mRy/atom (−30.3 kJ/mole, SmNi5, [32]) and −25.98 mRy/atom (−34.1 kJ/mole, YNi5, [32]). As we mentioned in the Introduction, the YFe5 compound as well as the SmFe5 compound (see refs. [3,4]) do not exist in the equilibrium Y-Fe and Sm-Fe phase diagrams, correspondingly, thus no experimental information about the heat of formation of these hypothetical compounds is available. However, the EMTO calculations show that that the heat of formation of the YFe5 compound is positive, +6.46 mRy/atom, and is half of the calculated heat of formation of the SmFe5 compound, +12.68 mRy/atom. As a result, the calculated heat of formation of the YFe3Co2 compound, +0.09 mRy/atom, appears to be smaller than the calculated heat of formation of the SmFe3Co2 compound, +2.15 mRy/atom [4]. The computed heats of formation of the YFe3Ni2 and the SmFe3Ni2 compounds are both negative (stable compounds), however, the absolute value of the calculated heat of formation of the YFe3Ni2 compound, |−6.20| mRy/atom, is more than twice as high as the absolute value of the heat of formation of the SmFe3Ni2 compound, |−2.72| mRy/atom [4]. As a result, the region of stability of the pseudo-binary YFe3(Ni1–xCox)2 alloys appears to be almost twice as wide as the region of stability of the pseudo-binary SmFe3(Ni1–xCox)2 alloys.
The promising phase stability of the YFe3CoNi compound highlighted by the EMTO-predicted heat of formation of the pseudo-binary YFe3(Ni1-xCox)2 alloys in Figure 2a sparks interest in studying the thermodynamics and the stability of the Y(Co,Fe,Ni)5 compounds versus temperature in the broader Y-Co-Fe-Ni composition space. As mentioned in Section 2, a full CALPHAD assessment of the Co-Fe-Ni-Y database requires evaluation of the constitutive binary (i.e., Co-Fe, Co-Ni, Co-Y, Fe-Ni, Fe-Y, Ni-Y) and ternary (i.e., Co-Fe-Ni, Y-Co-Fe, Y-Co-Ni, Y-Fe-Ni) systems. This study is focused on the Y-containing binary systems to leverage and confirm the ab initio findings and serve as foundation for an upcoming CALPHAD-centric study.
The first Y-Co CALPHAD assessment, in which all intermetallic compounds were evaluated as stoichiometric compounds, was reported by Du et al. [88]. This assessment was revisited in [89] to consider solubility range in YCo5 and Y2Co17. As both structures of Y2Co17 (Th2Ni17-type structure at high temperature and Th2Zn17-type structure at low temperature) are similar to the hexagonal CaCu5-type structure (YCo5), Du and Lü [89] treated both Y2Co17 and YCo5 compounds as one phase with the formula (Y,Co2)1(Y,Co2)2Co15. The reasoning behind the use of a one-phase three-sublattice model is that homogeneity regions of Y2Co17 and YCo5 could be described by a CaCu5-type lattice in which part of the Ca sites is occupied by pairs of Co atoms [89]. However, as mentioned by Golumbfskie and Liu [90], modeling the YCo5 and the Y2Co17 compounds separately allows for the extension of this binary system to be more readily implemented into a multicomponent databases. Thus, YCo5 and Y2Co17 were modeled in [90] using (Y,Co2)1(Co)4(Co,Va) and (Y,Co2)1(Y,Co2)2(Co)15 formulas, correspondingly. Note that vacancies (Va) were introduced, and first-principles calculations for the end members of the YCo5 compound were used to parameterize the model. Finally, Golumbfskie and Liu [90] underlined that the Y2Co17 phase should be produced via a congruent melting reaction (as experimentally accepted) rather than the result of the peritectic reaction (as produced by the assessment of [89]). The most recent assessment [90] was used as a starting point to re-assess the Y-Co phase diagram using simpler (Y)2(Co)17 and (Y)1(Co)5 two-sublattice models for Y2Co17 and YCo5 compounds, correspondingly, to ease the extension of the database to multicomponent systems while conserving the main features of the system.
The re-assessed Y-Co phase diagram is presented in Figure 3 (with model parameters reported in Table 2) and exhibits good agreement with experiments: (i) congruent melting of Y2Co17 and YCo5 at 1630 K (exp.: 1630 K [86]) and 1615 K (exp.: 1623 K [86]), correspondingly; (ii) decomposition temperature of YCo5 at 914 K (exp.: ~998 K [86]); (iii) heat of formation of YCo5 equal to ‘−12.46 kJ/mole and ‘−13.61 kJ/mole at 298 and 1000 K, correspondingly, compared with ‘−12.20 ± 0.87 kJ/mole measured between 850 and 1200 K in [85]; (iv) heat of formation of Y2Co17 equal to ‘−8.74 kJ/mole and ‘−9.47 kJ/mole at 298 and 1000 K, correspondingly, compared with ‘−7.6 ± 0.80 kJ/mole measured between 850 and 1200 K in [8].
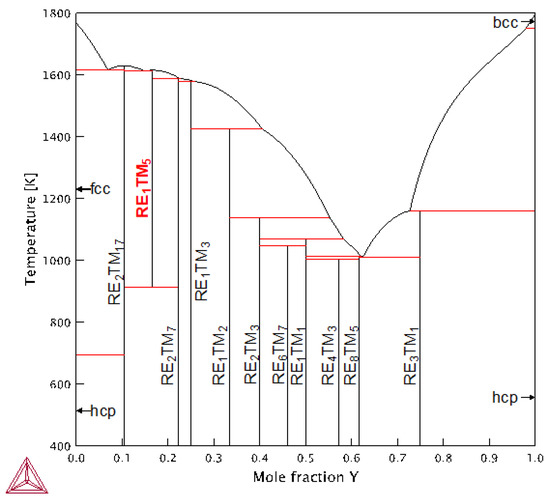
Figure 3.
The Y-Co phase diagram calculated using the CALPHAD assessment presented in this study. Here, RE = Y and TM = Co.

Table 2.
Optimized thermodynamic parameters of Y2TM17 and YTM5 compounds with TM = Co, Fe, Ni (J/mole).
The first CALPHAD assessment of the Y-Fe system was reported by Du et al. [121] and later refined by Lü et al. [122] to better replicate the ternary phase equilibria in the Y-Fe-Ni system. However, as mentioned by Konar et al. [123], the Gibbs energy of compounds reported in [121] was distinct from the experimental data, and neither the thermodynamic parameters for liquid or solid phases nor the invariant reactions were given in the updated assessment [122], making it impossible to evaluate. The Y-Fe system was re-assessed by Kardellass et al. [124,125] considering homogeneity range in the Y6Fe23 and the YFe2 phases compared to the stoichiometric compounds previously considered. Then, Konar et al. [123] published a new thermodynamic assessment of the Y-Fe system. However, the use of the modified quasichemical model for the liquid phase renders this work incompatible with the substitutional model used in other assessments. Thus, the latest assessment by Saenko et al. [126], which is the most comprehensive and compatible one, is used in the present study. In addition to the four reported compounds (i.e., Y2Fe17, Y6Fe23, YFe3, YFe2), we introduce the metastable YFe5 compound based on the ab initio heat of formation (+6.46 mRy/atom = +8.48 kJ/mole). The introduction of this end-member in the (Y)1(Co,Fe,Ni)5 model (Table 2) does not change the Y-Fe phase diagram but permits us to calculate the heat of formation of YFe3(Ni1-xCox)2 alloys when combined with the Y-Ni system.
The Y-Ni system was assessed by Du and Zhang [127] considering all intermetallics as line compounds. This work was slightly modified by Mattern et al. [128] to reproduce Y-Nb-Ni data. Later on, Du and Lü [94] re-assessed the Y-Ni system to make it compatible with the three sublattice model used for the Y-Co assessment. This last work was also modified by Huang et al. [129] to fit the Y-Al-Ni system. Finally, Mezbahul-Islam and Medraj [130] assessed the Y-Ni system using the modified quasichemical model to consider the prevalence of short-range ordering in the liquid phase. In the present study, the initial assessment of Du and Zhang [127] is considered as it agrees with most experimental data [32,131,132]. In particular, the heat of formation of YNi5 (−29.58 kJ/mole and −31.05 kJ/mole at 298 and 1000 K, correspondingly) [127] is in accord with the experimental data of −34.1 kJ/mole (298 K) [32] and −21.28 ± 0.42 kJ/mole (average between 887 and 1224 K) [131] and the calculated value of −30.09 kJ/mole (= −22.91 mRy/atom, present study).
The resulting CALPHAD model presented in Table 2 for the YTM5 phase can now be used to calculate the heat of formation of the pseudo-binary YFe3(Ni1-xCox)2 alloys with reference to hcp-Y, hcp-Co, bcc-Fe, and fcc-Ni at 298 K. As observed in Figure 2a, the CALPHAD results at 298 K are in excellent agreement with the ab initio predicted values at 0 K. Considering that the CALPHAD database is a combination from various sources (i.e., Y-Co re-assessed from [92], Y-Fe from [126] with new ab inito input, and Y-Ni from [127]), this agreement highlights both the predictive capability of ab initio methods and the flexibility and reliability of the CALPHAD method. The CALPHAD work performed in the present study on the Y-containing binary systems reveals a negative heat of formation across almost the entire YFe3(Ni1-xCox)2 pseudo-binary, confirming the great potential of stabilizing a YFe3(Ni1-xCox)2 magnet. However, additional assessments are required to include the experimental data gathered on the ternary Y-Co-Ni [91,95], Y-Fe-Ni [95], and Y-Co-Fe [94,95] systems to consistently represent the Gibbs energy landscape of the entire Y-Co-Fe-Ni composition space and propose alloy compositions and thermal treatments to stabilize the Fe-rich Y(Co,Ni,Fe)5 magnet. The goal of the current paper is to examine the stabilizing outcomes (enthalpy) of adding nickel to the Y(Co1–xFex)5 alloys and its impact on magnetic properties (Section 4); this additional CALPHAD work will be presented in a follow up paper.
4. Magnetic Properties of the Y(Co-Fe-Ni)5 Alloys
Table 3 presents the results of the FREMTO calculations of the site-projected spin, m(s), and orbital, m(o), moments of the YCo5 compound. According to [37], Y and Co spins should align in an antiparallel fashion (AF) that is predicted in the present self-consistent calculations. The calculated total moment, m(tot) = 7.82 μB/f.u., is slightly smaller than the experimentally reported value of 8.30 μB/f.u. [29]. The calculated spin moments are 1.55 μB and 1.47 μB for 2c and 3g sites, correspondingly, which are larger than the recorded experimental values of 1.44 μB and 1.31 μB [16,28]. The calculated orbital moments are 0.14 μB and 0.11 μB for 2c and 3g sites, correspondingly, which are smaller than recorded experimental data of 0.26 μB and 0.24 μB [16]. It is important to mention that the present FREMTO calculations reflect the experimental (spin flip-neuron scattering) observation; for the YCo5 compound, the orbital moment of Co1(2c) atoms is bigger than the orbital moment of Co2(3g) atoms [16,19,23,25], which was confirmed by the previous calculations [37,56,71]. The large MAE of the YCo5 compound comes from a big orbital allowance from Co1(2c) sites, which are located in the same plane as Y1(1a) sites [19,23,25]. Appropriately, Co1(2c) atoms have a big positive MAE allowance, while Co2(3g) atoms have a small negative MAE allowance. In line with ref. [71], the axial (positive) MAE of the YCo5 magnet can be achieved only if orbital moments on the Co1(2c) atoms are bigger than the orbital moments of the Co2(3g) atoms. The same outcome was reached in a past study [3,4]; in the SmCo5 magnet, the orbital moment on Co1(2c) atom is bigger than on Co2(3g) atom. Calculations [3] anticipated MAE of the SmCo5 magnet would be K1 = 19.63 MJ/m3, which is actually close to the experimental data K1 = 17.2 MJ/m3 [3].

Table 3.
Site-projected spin, m(s), and orbital, m(o), magnetic moments for the YCo5 compound: FREMTO calculations. m(tot) = 7.82 μB/f.u.
A mean-field treatment for the Curie temperature, Tc, can be formulated as [133,134]:
where is the difference among the ground state total energies of the DLM and the AF state, and kB is the Boltzmann constant. Principally, an assessment of the Curie temperature can be achieved from the total energy difference between the ferromagnetic (or antiferromagnetic) and the paramagnetic states. However, in line with {133], the difference between the total energies can be substituted by the difference between the effective single-particle (one atomic specie) energies, which are directly associated with AF and DLM states (the so-called mean-field treatment). In the present work, are calculated at the equilibrium volumes for DLM and AF states, correspondingly. According to the present EMTO-DLM and EMTO-AF calculations, Tc = 891.8 K for the YCo5 magnet, which is in good accord with the experimental data Tc = 920 K [28], which is essentially bigger than that of the commonly used Nd2Fe14B magnet (Tc = 588 K [7]). The similar EMTO calculations reveal Tc = 1149.3 K for the YFe5 compound, although, as was mentioned in the Introduction, this compound does not exist in the Y-Fe phase diagram. However, as was also mentioned in the Introduction, there is an experimentally observed [42] tendency of the Curie temperature to increase with Fe doping of the YCo5 magnet, i.e., from Tc = 930 K (the YCo5 compound) to Tc = 1020 K (the Y(Co0.8Fe0.2)5 compound). Our calculations qualitatively reflect this tendency.
Figure 4 shows the Curie temperature calculated within the EMTO-CPA technique of the pseudo-binary YFe3(Ni1–xCox)2 alloys where Fe atoms occupy all 3g-type sites and the occupation of the 2c-type sites continuously changes from pure Ni (the YFe3Ni2 compound) to pure Co (the YFe3Co2 compound). The dotted line corresponds to the calculated Curie temperature of the YCo5 magnet, Tc = 891.8 K. The calculated Curie temperature is equal to 572.6 K and 1097.7 K for the YFe3Ni2 and YFe3Co2 magnets, correspondingly. Although the calculated Curie temperature of the YFe3Ni2 compound lies about 320 K below of the Curie temperature of the YCo5 magnet, this deficiency can be removed by substituting 70 at.% of Ni by Co. The Curie temperature of the YFe3(Ni0.3Co0.7)2 magnet is equal to 899.9 K.
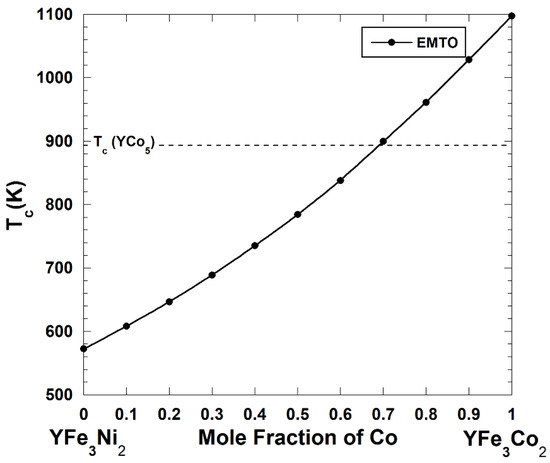
Figure 4.
The Curie temperature of the pseudo-binary YFe3(Ni1-xCox)2 alloys. The dotted line corresponds to the calculated Curie temperature of the YCo5 magnet.
According to the present calculations, the YFe3(Ni0.3Co0.7)2 magnet shows an enormous total moment of m(tot) ~9.79 μB, essentially due to the iron atoms that each contribute with 2.45 μB. The total moment of the YFe3(Ni0.3Co0.7)2 magnet is thus essentially bigger than that of the traditional YCo5 magnet that has a calculated total moment of m(tot) ~7.82 μB. The experimental values of saturation magnetization (Ms) and the maximum energy product ((BH)max) for the YCo5 magnet are 0.85 MA/m and 224 kJ/m3, correspondingly [6,10,11]. Because saturation magnetization and magnetic moment are approximately proportional, Ms ~m(tot), and the maximum energy product is approximately proportional to the square of the saturation magnetization, (BH)max ~(Ms)2 [135], one can evaluate that saturation magnetization for the YFe3(Ni0.3Co0.7)2 magnet is proportional to:
and the maximum energy product for the YFe3(Ni0.3Co0.7)2 magnet should be approximately:
which is ~69% of the record maximum energy product of the Nd2Fe14B magnet, (BH)max = 512 kJ/m3 [7]. Particularly, the YFe3(Ni0.3Co0.7)2 magnet, which has a Curie temperature similar to the YCo5 magnet, is a substantially steadier magnet than the YCo5 magnet (its maximum energy product should be ~57% larger).
As has been mentioned in the Introduction, the magnetic anisotropy energy (MAE) is one of the more important properties of an efficient magnet. In the quest to increase the saturation magnetic moment or energy product, by substituting cobalt for iron, one has to review the impact of the doping on the MAE as well.
In Table 4 we collect calculated MAEs for the YCo5-type magnets alloyed with iron, nickel, or both. Here, for the YFe3Co2 and the YFe3CoNi magnets, we keep the iron atoms on the energetically favorably 3g sites as discussed above. In the case of YFe3CoNi, we model Co and Ni on the 2c sites as two average atoms consistent with our modeling of SmFe3CoNi [3]. In all calculations, we relax the atomic volume and the c/a axial ratio of the hexagonal phase. We find some sensitivity (not shown) of the MAE to the axial ratios, suggesting that the structural relaxation is important.

Table 4.
FPLMTO results. GGA and LDA refer to generalized gradient approximation and local density approximation, correspondingly. K1 reflects the magnetic anisotropy energy.
According to the values calculated in Table 4, we realize that both YFe5 and YNi5 have relatively small magnetic anisotropy and, for that reason alone, they are not particularly good magnets. YFe5 is really only included in the table to provide context to the other magnets, as it does not exist in the hexagonal phase. YCo5 contrarily exists and is predicted to have significant magnetic anisotropy. We primarily rely on DFT-GGA calculations for these magnetic compounds because GGA performs better for the magnetic 3d transition metals relative to the LDA or even more modern approximations [136]. It is also a well-known fact that GGA reproduces the proper magnetic ground state of iron, as opposed to the LDA. Our GGA calculations reproduce the experimental atomic volume very well but overestimate the MAE for YCo5 relative to experimental data. DFT-GGA (T = 0 K) gives the unit cell volume Vcell = 82.65 Å3 and anisotropy K1 = 9.89 meV/cell (19.2 MJ/m3). These numbers shall be compared to experimental data at Т = 4.1 K, Vcell = 82.50 Å3 [29,55], K1 = 3.80 meV/cell (7.38 MJ/m3) [29,56] and at T = 293 K, Vcell = 83.99 Å3 [33], K1 = 3.04 meV/cell (5.80 MJ/m3) [29,56]. Here, the unit cell volume at T = 4.1 K, Vcell = 82.50 Å3 is identified using the experimental value of the MAE coefficient, K1, presented in the units of (MJ/m3), [29,56], and (meV/cell) [55]. Steinbeck et al. [56], on the other hand, calculated K1 = 4.4 meV/cell using LDA and orbital polarization in better agreement with experimental data. However, their calculation was performed at the experimental cell volume and not the LDA volume, which is drastically smaller. Their agreement with experiment is thus somewhat coincidental. We believe the difference in the calculated K1 numbers is mostly due to electron exchange and correlation approximation. In Table 4, we also show our LDA results, and for YCo5 (6.44 meV/cell), it is closer to experimental data (3.8 meV/cell) [29,55] than our GGA result.
Unfortunately, we realize that the MAE for these magnetic systems can be very sensitive to the particular DFT approximation (GGA or LDA) that is applied. Furthermore, there does not appear to be a simple systematic explanation for the difference in the MAE when comparing GGA and LDA methods. The results are very consistent for YFe5, YNi5 (small MAE), and YFe3CoNi (intermediate MAE ~4.5–5 meV/cell), while for YCo5, the GGA produces a value significantly greater than the LDA (9.89 and 6.44 meV/cell, correspondingly). For YFe3Co2 and YFe3Ni2, the LDA values are about three and two times larger than the GGA number, correspondingly. Apparently, the particular electron exchange and the correlation approximation are important for the anisotropy, and to explore this further, we applied several other popular formulations for YFe3Co2; the resulting MAE varied very sensitively. This remarkable sensitivity has not been recognized before for these types of magnets and certainly warrants further studies.
5. Discussion and Conclusions
According to our past calculations [3,4], replacing most of Co with Fe in the SmCo5 magnet and using Ni as a thermodynamic mediator results in an SmFe3CoNi magnet that has extraordinary magnetic properties, such as a very high Curie temperature, robust magnetic anisotropy about twice that of the Nd2Fe14B magnet, and a big maximum energy product (an estimated 70.5% of the record maximum energy product of the Nd2Fe14B magnet), which should be about 56% larger than that of the SmCo5 precursor. However, even if we substitute a relatively expensive metal Co (283 RMB/kg) by cheaper Ni (99 RMB/kg) and Fe (3 RMB/kg), the cost of the suggested SmFe3CoNi magnet is still mostly defined by the relatively expensive RE samarium metal (450 RMB/kg). Thus, in this paper, we expand on the fundamental idea of refs. [3,4] by using nickel metal as the stabilizing material in the YCo5 magnet (Y is almost half the price of Sm [5]) in order to accommodate the maximum amount of iron metal to favor a very high magnetization.
We find a significant difference between the regions of stability of the YFe3(Ni1-xCox)2 and SmFe3(Ni1-xCox)2 alloys. In order to stabilize the SmFe3(Ni1-xCox)2 alloys, one should have at least half of the 2c-type sites to be occupied by Ni atoms. The extreme case is the SmFe3CoNi magnet, where Fe atoms occupy all 3g-type sites, and Ni and Co atoms occupy the 2c-type sites with equal probability, which has been suggested in refs [3,4]. For the YFe3(Ni1-xCox)2 alloys, it is possible to have the stable solutions until approximately all Ni atoms are substituted by Co atoms. Our ab initio heat of formation predictions are confirmed by CALPHAD modeling at 298 K (Figure 2a). The combination of negative heat of formation and extended solubility limits experimentally observed in the YTM5 (TM = Co, Fe, Ni) magnets (i.e., complete solubility from YCo5 to YNi5 at 1073 K and 1273 K; solubility of ~20 at.% Fe in Y(Co,Fe)5 at 1323 K [94]; solubility of ~30 at.% Fe in Y(Fe,Ni)5 at 873 K [95]) is promising for experimentalists who would like to synthesize these new magnets. Based on a thorough literature review, theoretical calculations, and preliminary CALPHAD works presented in this study, an in-depth CALPHAD assessment of the Y-Co-Fe-Ni system has been initiated and will provide guidance on how to make these new magnets. Meanwhile, a specific example from this work is the YFe3Co2 magnet for which we calculated the Curie temperature, Tc, which is equal to 1097.7 K and the maximum energy product, (BH)max(YFe3Co2), as ~365 kJ/m3, which is ~71% of the record maximum energy product of the Nd2Fe14B magnet, (BH)max = 512 kJ/m3. Here, the maximum energy products of YFe3Co2 and YFe3CoNi magnets are estimated using the calculated total magnetic moments of YFe3Co2, YFe3CoNi, and YCo5 magnets as well as the experimental values of the saturated magnetization and the maximum energy product of the YCo5 magnet. Calculations are performed in the same fashion as for the YFe3(Ni0.3Co0.7)2 magnet (see Section 4).
According to our calculations, the YFe3(Ni0.3Co0.7)2 magnet has a Curie temperature Tc ~900 K that is relatively close to the calculated Curie temperature of the YCo5 magnet, Tc ≈ 892 K. In addition, the maximum energy product of the YFe3(Ni0.3Co0.7)2 magnet is significantly improved compared to the YCo5 magnet (~57% larger).
The calculated intrinsic properties of the magnets suggested in ref. [3,4] and in this paper are reported in Table 5 in conjunction with the experimental data of Nd2Fe14B, SmCo5, and YCo5 magnets for comparison. All four suggested permanent magnets have a Curie temperature significantly higher than that of the Neomax (Nd2Fe14B), Tc ≈ 588 K, spanning from 785 K to 1103 K. In addition, their maximum energy products are significantly higher than that of the commercially used SmCo5 and YCo5 magnets (231 kJ/m3 and 224 kJ/m3, correspondingly), reaching a maximum value of 365 kJ/m3 for YFe3Co2. Our calculated (Table 4, LDA) MAEs for YFe3CoNi magnet is not much smaller than that of YCo5 magnet (10.6 MJ/m3 and 13.5 MJ/m3, correspondingly).

Table 5.
Intrinsic magnetic properties of Nd2Fe14B, SmCo5, and YCo5 (experiment [6,7,10,11]); and SmFe3CoNi [3,4], YFe3CoNi, (K1, LDA), YFe3(Ni0.3Co0.7)2, and YFe3Co2 (theory) permanent magnets.
Although the cobalt price plunged from more than 630 RMB/kg (May 2018) to 282 RMB/kg (June 2020) during the last two years, it is unlikely it will reach its record low value of approximately 157 RMB/kg (June 2016) as the global electric car market (production) continues to grow at an accelerated pace. Indeed, cobalt remains a necessary component in electric vehicle lithium-ion batteries, e.g., LiCoO2, and cobalt costs will probably rise as a result of anticipated demands for lithium-ion batteries, which consume more than 70% of total cobalt mining. Considering SmFe3CoNi and YFe3CoNi magnets comprise 80% less Co than their SmCo5 and YCo5 precursors, maturing of these magnets becomes even more captivating from the current economic viewpoint.
In conclusion, we showed that replacing part of cobalt with iron in SmCo5 and YCo5 magnets stabilized with a small portion of nickel results in novel permanent magnets that we consider to be conceivably synthesized. They are anticipated to have outstanding magnetic properties, a big maximum energy product, a strong magnetic anisotropy, and an exceptionally high Curie temperature. However, it is very important to emphasize that, although these three intrinsic parameters are necessary to be large for an efficient permanent magnet, this is not a sufficient condition. It is also necessary to optimize the microstructure of the alloys in order to optimize the extrinsic magnetic properties.
Author Contributions
Conceptualization, A.L.; methodology, A.L., P.S., A.P., E.E.M.; writing-review and editing, A.L., P.S., A.P., E.E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the Critical Materials Institute, an Energy Innovation Hub funded by the US Department of Energy, Office of Energy Efficiency and Renewable Energy, Advanced Manufacturing Office.
Acknowledgments
The work is performed under the auspices of the US Department of Energy by the Lawrence Livermore National Laboratory under Contract No. DE-AC52-07NA27344. A.L. thanks A. Ruban, O. Peil, P. Korzhavyi, and L. Vitos for technical support. A.P. and A.L. thank the dedication, responsiveness, and expertise of the staff of the LLNL Research Library.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buschow, K.H.J. New developments in hard magnetic materials. Rep. Prog. Phys. 1991, 54, 1123–1214. [Google Scholar] [CrossRef]
- Buschow, K.H.J.; de Boer, F.R. Physics of Magnetism and Magnetic Materials; Kluwer Academic/Plenum: New York, NY, USA, 2004. [Google Scholar]
- Söderlind, P.; Landa, A.; Locht, I.L.M.; Åberg, D.; Kvashnin, Y.; Pereiro, M.; Däne, M.; Turchi, P.E.A.; Antropov, V.P.; Eriksson, O. Prediction of the new efficient permanent magnet SmCoNiFe3. Phys. Rev. B 2017, 96, 100404-1–100404-5(R). [Google Scholar] [CrossRef]
- Landa, A.; Söderlind, P.; Parker, D.; Åberg, D.; Lordi, V.; Perron, A.; Turchi, P.E.A.; Chouhan, R.K.; Paudyal, D.; Lograsso, T.A. Thermodynamics of SmCo5 compound doped with Fe and Ni: An ab initio study. J. Alloy. Compd. 2018, 765, 659–663. [Google Scholar] [CrossRef]
- Zhang, D.-T.; Cai, N.-X.; Zhu, R.-C.; Liu, W.-Q.; Yue, M. Low-cost Sm0.7Y0.3Co5 sintered magnet produced by traditional powder metallurgical techniques. Rare Met. 2019, 39, 421–428. [Google Scholar] [CrossRef]
- Coey, J.M.D. Permanent magnets: Plugging the gap. Scr. Mater. 2012, 67, 524–529. [Google Scholar] [CrossRef]
- Coey, J.M.D. Hard magnetic materials: A Perspective. IEEE Trans. Magn. 2011, 47, 4671–4681. [Google Scholar] [CrossRef]
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic materials, and devices for the 21st century: Stronger, lighter, and more energy efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef]
- Kramer, M.J.; McCallum, R.W.; Anderson, I.A.; Constantinides, S. Prospects for non-rare earth permanent magnets for traction motors and generators. JOM 2012, 64, 752–763. [Google Scholar] [CrossRef]
- Hoffer, G.; Strnat, K. Magnetocrystalline anisotropy of YCo5 and Y2Co17. IEEE Trans. Magn. 1966, 2, 487–489. [Google Scholar] [CrossRef]
- Strnat, K.; Hoffer, G.; Olson, J.; Ostertag, W. A Family of new cobalt-base permanent magnet materials. J. Appl. Phys. 1967, 38, 1001–1002. [Google Scholar] [CrossRef]
- Strnat, K.J. The recent development of permanent magnet materials containing rare earth metals. IEEE Trans. Magn. 1970, 6, 182–189. [Google Scholar] [CrossRef]
- Buschow, K.H. Composition and stability of CaCu5-type compounds of yttrium with iron and cobalt. J. Less-Common Met. 1973, 31, 359–364. [Google Scholar] [CrossRef]
- Laforest, J.; Shan, J.S. Neutron diffraction study of the Th(Co1-xFex)5 alloys. IEEE Trans. Magn. 1973, 9, 217–220. [Google Scholar] [CrossRef]
- Klein, H.P.; Menth, A.; Perkins, R.S. Magnetocrystalline anisotropy of light rare-earth cobalt compounds. Physica B+C 1975, 80, 153–163. [Google Scholar] [CrossRef]
- Heidemann, A.; Richter, R.; Buschow, K.H.J. Investigation of the hyperfine fields in the compounds LaCo13, LaCo5, YCo5 and ThCo5 by means of inelastic neutron scattering. Eur. Phys. J. B 1975, 22, 367–372. [Google Scholar] [CrossRef]
- Ermolenko, A.S. Magnetocrystalline anisotropy of rare earth intermetallics. IEEE Trans. Magn. 1976, 12, 992–996. [Google Scholar] [CrossRef]
- Déportes, J.; Givord, D.; Lemaire, R.; Nagai, H.; Yang, Y.T. Influence of substitutional pairs of cobalt atoms on the magnetocrystalline anisotropy of cobalt-rich rare-earth compounds. J. Less-Common Met. 1976, 44, 273–279. [Google Scholar] [CrossRef]
- Déportes, J.; Givord, D.; Scweizer, J.; Tasset, F. Different contributions of the two cobalt sites to the magnetocrystalline anisotropy of YCo5 and related compounds. IEEE Trans. Magn. 1976, 12, 1000–1002. [Google Scholar] [CrossRef]
- Buschow, K.H.J. Intermetallic compounds of rare-earth and 3d transition metals. Rep. Prog. Phys. 1977, 40, 1179–1256. [Google Scholar] [CrossRef]
- Malik, S.K.; Arlinghaus, F.A.; Wallace, W.E. Spin-polarized energy-band structure of YCo5, SmCo5, and GdCo5. Phys. Rev. B 1977, 16, 1242–1248. [Google Scholar] [CrossRef]
- Kirchmayar, H.R.; Poldy, C.A. Magnetism in rare earth—3d intermetallics. J. Magn. Magn. Mater. 1978, 8, 1–42. [Google Scholar] [CrossRef]
- Streever, R.L. NMR investigation of Co magnetic anisotropy in RCo5 compounds. Phys. Lett. A 1978, 65, 360–362. [Google Scholar] [CrossRef]
- Alameda, J.M.; Déportes, J.; Givord, D.; Lemaire, R.; Lu, Q. Large magnetization anisotropy in uniaxial YCo5 intermetallic. J. Magn. Magn. Mater. 1980, 15–18, 1257–1258. [Google Scholar] [CrossRef]
- Streever, R.L. Individual Co site contributions to the magnetic anisotropy of RCo5 compounds and related structures. Phys. Rev. B 1979, 19, 2704–2711. [Google Scholar] [CrossRef]
- Wohlfarth, E.P. First and second order transitions in some metallic ferromagnets. J. Appl. Phys. 1979, 50, 7542–7544. [Google Scholar] [CrossRef]
- Wohlfarth, E.P. The Curie temperatures of compounds of the heavy rare earths and yttrium with cobalt. J. Phys. F Met. Phys. 1979, 9, L123–L128. [Google Scholar] [CrossRef]
- Schweitzer, J.; Tasset, F. Polarized neutron study of the RCo5 intermetallic compounds. I. The cobalt magnetization in YCo5. J. Phys. F Met. Phys. 1980, 10, 2799–2817. [Google Scholar] [CrossRef]
- Alameda, J.M.; Givord, D.; Lemaire, R.; Lu, Q. Co energy and magnetization anisotropies in RCo5 intermetallics between 4.2 K and 300 K. J. Appl. Phys. 1981, 52, 2079–2081. [Google Scholar] [CrossRef]
- Inomata, K. Individual Co site contributions to the magnetic anisotropy and NMR investigation of Y2(Co1−xMx)17 (M=Cu,Al). Phys. Rev. B 1981, 23, 2076–2081. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Wu, C.H.; Chang, Y.C. Structure and magnetic properties of Y(Co1−xMx)5 compounds. J. Less Common Met. 1982, 84, 201–213. [Google Scholar] [CrossRef]
- Pasturel, A.; Colinet, C.; Allibert, C.; Hicter, P.; Percheron-Guegan, A.; Achard, J.C. A Theoretical and Experimental Study of the Enthalpies of Formation of LaNi5-Type Compounds. Phys. Status Solidi B 1984, 125, 101–106. [Google Scholar] [CrossRef]
- Meyer-Liautaud, F.; Derkaout, S.; Allibert, C.H.; Castanet, R. Structural and thermodynamic data on the pseudobinary phases R(Co1−xCux)5 WITH R ≡ Sm, Y, Ce. J. Less Common Met. 1987, 127, 231–242. [Google Scholar] [CrossRef]
- Franse, J.J.M.; Thuy, N.P.; Hong, N.M. Individual site magnetic anisotropy of the iron and cobalt ions in rare earth-iron and rare earth-cobalt intermetallic compounds. J. Magn. Magn. Mater. 1988, 72, 361–366. [Google Scholar] [CrossRef]
- Strnat, K.J.; Strnat, R.M.W. Rare earth-cobalt permanent magnets. J. Magn. Magn. Mater. 1991, 100, 38–56. [Google Scholar] [CrossRef]
- Zhao, T.-S.; Jin, H.-M.; Gua, G.-H.; Han, X.-F.; Chen, H. Magnetic properties of R ions in RCo5 compounds (R=Pr, Nd, Sm, Gd, Tb, Dy, Ho, and Er). Phys. Rev. B 1991, 43, 8593–8598. [Google Scholar] [CrossRef]
- Nordström, L.; Brooks, M.S.S.; Johansson, B. Calculation of orbital magnetism and magnetocrystalline anisotropy energy in YCo5. J. Phys. Condens. Matter 1992, 4, 3261–3272. [Google Scholar] [CrossRef]
- Coehoorn, R.; Daalderop, G.H.O. Magnetocrystalline anisotropy in new magnetic materials. J. Magn. Magn. Mater. 1992, 104–107, 1081–1085. [Google Scholar] [CrossRef]
- Daalderop, G.H.O.; Kelly, P.J.; Schuurmans, M.F.H. Magnetocrystalline anisotropy of RECo5 compounds. J. Magn. Magn. Mater. 1992, 104–107, 737–738. [Google Scholar] [CrossRef]
- Trygg, J.; Nordström, L.; Johansson, B. First-principles calculations of the mgnetocrystalline anisotropy energy for the pseudobinary compound. In Physics of Transition Metals; Oppeneer, P.M., Kübler, J., Eds.; Publisher Word Scientific: Singapore, 1993; pp. 745–748. [Google Scholar]
- Radwanski, R.J.; Franse, J.J.M. Magnetic properties of the 3d sublattice in RnTm intermetallics. Int. J. Mod. Phys. 1993, 7, 782–785. [Google Scholar] [CrossRef]
- Paoluzi, A.; Pareti, L.; Solzi, M.; Albertini, F. Study of the iron contribution to the 3d-sublattice anisotropy in some uniaxial YCoFe structures derived from the CaCu5 unit cell. J. Magn. Magn. Mater. 1994, 132, 185–190. [Google Scholar] [CrossRef]
- Colinet, C. The thermodynamic properties of rare earth metallic systems. J. Alloy. Compd. 1995, 225, 409–422. [Google Scholar] [CrossRef]
- Crisan, V.; Popescu, V.; Vernes, A.; Andreica, D.; Burda, I.; Cristea, S. On the electronic properties of YCo5−xNix. J. Alloy. Compd. 1995, 223, 147–150. [Google Scholar] [CrossRef]
- Daalderop, G.H.O.; Kelly, P.J.; Schuurmans, M.F.H. Magnetocrystalline anisotropy of YCo5 and related RECo5 compounds. Phys. Rev. B 1996, 53, 14415–14433. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Asano, S. First-principles calculation of the 3d magnetocrystalline anisotropy energy of YCo5. J. Appl. Phys. 1996, 79, 5952–5954. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Asano, S. First-principles calculations of the 3d magnetocrystalline anisotropy energy of YCo5, Y2Co7, YCo3, and Y2Co17. J. Magn. Magn. Mater. 1997, 168, 161–168. [Google Scholar] [CrossRef]
- Kitagawa, I.; Terao, K.; Aoki, M.; Yamada, H. Electronic structure, and magnetism of YCo5, YNi5, and YCo3Ni2. J. Phys. Condens. Matter 1997, 9, 231–239. [Google Scholar] [CrossRef]
- Zhang, G.W.; Feng, Y.P.; Ong, C.K. Electronic and magnetic properties of intermetallic compound YCo5. J. Magn. Magn. Mater. 1998, 184, 215–221. [Google Scholar] [CrossRef]
- Gratz, E.; Lindbaum, A.; Markosyan, A.S.; Milnera, M. Magnetoresistance in Y(Ni1−xCox)5 around the critical concentration for the onset of ferromagnetism. J. Magn. Magn. Mater. 1998, 184, 372–374. [Google Scholar] [CrossRef]
- Yamada, H.; Terao, K.; Ishikawa, F.; Yamaguchi, Y.; Mitamura, H.; Goto, T. Itinerant-electron metamagnetism of Y(Co,Ni)5. J. Phys. Condens. Matter 1999, 11, 483–492. [Google Scholar] [CrossRef]
- Maruyama, F.; Nagai, H.; Amako, Y.; Yoshie, H.; Adachi, K. Magnetic properties of the hypothetical compound YFe5. Physica B 1999, 266, 356–360. [Google Scholar] [CrossRef]
- Téllez-Blanko, J.C.; Grössinger, R.; Sato Turtelli, R.; Estévez-Rams, E. Magnetic and Structural Properties of YCo5xCux. IEEE Trans. Magn. 2000, 36, 3333–3335. [Google Scholar] [CrossRef]
- Al-Omari, I.A.; Skomski, R.; Thomas, R.A.; Lieslie-Pelesky, D.; Sellmyer, D.J. High-temperature magnetic properties of mechanically alloyed SmCo5 and YCo5 magnets. IEEE Trans. Magn. 2001, 37, 2534–2536. [Google Scholar] [CrossRef]
- Steinbeck, L.; Richter, M.; Eschrig, H. Magnetocrystalline anisotropy of RCo5 intermetallics: Itinerant-electron contribution. J. Magn. Magn. Mater. 2001, 226–230, 1011–1013. [Google Scholar] [CrossRef]
- Steinbeck, L.; Richter, M.; Eschrig, H. Itinerant-electron magnetocrystalline anisotropy energy of YCo5 and related compounds. Phys. Rev. B 2001, 63, 184431-1–184431-14. [Google Scholar] [CrossRef]
- Kashyap, A.; Skomski, R.; Sabiryanov, R.R.F.; Jaswal, S.S.; Sellmyer, D.J. Exchange interactions and Curie temperature of Y-Co compounds. IEEE Trans. Magn. 2003, 39, 2908–2910. [Google Scholar] [CrossRef]
- Larson, P.; Mazin, I.I. Calculation of magnetic anisotropy energy in YCo5. J. Magn. Magn. Mater. 2003, 264, 7–13. [Google Scholar] [CrossRef]
- Larson, P.; Mazin, I.I. Magnetic properties of SmCo5 and YCo5. J. Appl. Phys. 2003, 93, 6888–6890. [Google Scholar] [CrossRef]
- Larson, P.; Mazin, I.I.; Papaconstantopoulos, D.A. Effects of doping on the magnetic anisotropy energy in SmCo5−xFex and YCo5−xFex. Phys. Rev. B 2004, 69, 134408-1–134408-7. [Google Scholar] [CrossRef]
- Larson, P.; Mazin, I.I. Effect of impurities on magnetic properties of Y(Co5−xCux) and Y2(Co7−xNix). J. Magn. Magn. Mater. 2004, 269, 176–183. [Google Scholar] [CrossRef]
- Rosner, N.; Koudela, D.; Schwarz, U.; Handstein, A.; Hafland, M.; Opahle, I.; Koepernik, K.; Kuz’min, M.D.; Müller, K.-H.; Mydosh, J.A.; et al. Magneto-elastic lattice collapse in YCo5. Nat. Phys. 2006, 2, 469–472. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, Z.; Xu, G.; Lu, X.; Zhong, K.; Huang, Z. Effects of doping on magnetic properties of YCo5-xFex and YCo5-xAgx—First Principles Calculation. J. Rare Earths 2006, 24 (Suppl. 1), 293–297. [Google Scholar] [CrossRef]
- Koudela, D.; Schwarz, U.; Rosner, H.; Burkhardt, U.; Handstein, A.; Hanfland, M.; Kuz’min, M.D.; Opahle, I.; Koepernik, K.; Müller, K.-H.; et al. Magnetic and elastic properties of YCo5 and LaCo5 under pressure. Phys. Rev. B 2008, 77, 024411-1–024411-7. [Google Scholar] [CrossRef]
- Castillo, M.C.G.; Aquino, J.A.M. Magnetic and electronic properties of the compound Y(Co,Fe)5 calculated by the augmented spherical wave method. Adv. Mater. Res. 2009, 68, 145–151. [Google Scholar]
- Colin, C.V.; Isnard, O.; Guillot, M. High magnetic field study of the anisotropy and neutron diffraction investigation of the crystal and magnetic structure of YCo4. 5Ge0.5. J. Alloy. Compd. 2010, 505, 11–16. [Google Scholar] [CrossRef]
- Liu, X.B.; Altounian, Z.; Yue, M. Effect of Fe partial substitution for Co on the magnetic properties of Y(Co,Fe)5 from first-principles. J. Appl. Phys. 2010, 107, 09A718-1–09A718-3. [Google Scholar] [CrossRef]
- Liu, X.B.; Altounian, Z. Magnetic moments and exchange interaction in Sm(Co, Fe)5 from first-principles. Comput. Mater. Sci. 2011, 50, 841–846. [Google Scholar] [CrossRef]
- Ohashi, K. Present and future of Sm2Co17 magnets. J. Jpn. Inst. Met. 2012, 76, 96–106. [Google Scholar] [CrossRef]
- Zhu, J.-X.; Janoscheck, M.; Rosenberg, R.; Ronning, F.; Thomson, J.D.; Torrez, M.; Bauer, E.D.; Bastia, C.D. LDA+DMFT approach to magnetocrystalline anisotropy of strong magnets. Phys. Rev. X 2014, 4, 021027-1–021027-7. [Google Scholar] [CrossRef]
- Matsumoto, M.; Banerjee, R.; Staunton, J.B. Improvement of magnetic hardness at finite temperatures: Ab initio disordered local-moment approach for YCo5. Phys. Rev. B 2014, 90, 054421-1–054421-11. [Google Scholar] [CrossRef]
- Tozman, P.; Vinkatesan, M.; Zickle, G.A.; Fidler, J.; Coey, J.M.D. Enhanced energy product in Y-Co-Fe magnets intermediate between Nd-Fe-B and ferrite. Appl. Phys. Lett. 2015, 107, 032405-1–032405-4. [Google Scholar] [CrossRef]
- Tozman, P.; Vinkatesan, M.; Coey, J.M.D. Optimization of the magnetic properties of nanostructured Y-Co-Fe alloys for permanent magnets. AIP Adv. 2016, 6, 056016-1–056016-5. [Google Scholar] [CrossRef]
- Patrick, C.E.; Kumar, S.; Balakrishnan, G.; Edwards, R.S.; Lees, M.R.; Mendive-Tapia, E.; Petit, L.; Staunton, J.B. Rare-earth/transition-metal magnetic interactions in pristine and (Ni,Fe)-doped YCo5 and GdCo5. Phys. Rev. Mater. 2017, 1, 024411-1–024411-13. [Google Scholar] [CrossRef]
- Patrick, C.E.; Staunton, J.B. Rare-earth/transition-metal magnets at finite temperature: Self-interaction-corrected relativistic density functional theory in the disordered local moment picture. Phys. Rev. B 2018, 97, 224415-1–224415-15. [Google Scholar] [CrossRef]
- Patrick, C.E.; Kumar, S.; Balakrishnan, G.; Edwards, R.S.; Lees, M.R.; Petit, L.; Staunton, J.B. Calculating the Magnetic Anisotropy of Rare-Earth–Transition-Metal Ferrimagnets. Phys. Rev. Lett. 2018, 120, 097202. [Google Scholar] [CrossRef] [PubMed]
- Burzo, E.; Vlaic, P.; Kozlenko, D.P.; Golosova, N.O.; Kichanov, S.E.; Savenko, B.N.; Östlin, A.; Chionsel, L. Structure and magnetic properties of YCo5 compound at high pressures. JMST 2020, 42, 106–112. [Google Scholar] [CrossRef]
- Sakurai, M.; Wu, S.; Zhao, X.; Nguyen, M.C.; Wang, C.-Z.; Ho, K.-M.; Chelikowsky, J.R. Magnetocrystalline anisotropy in YCo5 and ZrCo5 compounds from first-principles real-space pseudopotentials calculations. Phys. Rev. Mater. 2018, 2, 084410-1–084410-5. [Google Scholar] [CrossRef]
- Nguyen, M.C.; Yao, Y.; Wang, C.-Z.; Ho, K.-M.; Andropov, V.P. Magnetocrystalline anisotropy in cobalt based magnets: A choice of correlation parameters and the relativistic effects. J. Phys. Condens. Matter 2018, 30, 195801-1–195801-8. [Google Scholar] [CrossRef]
- Soderžnik, M.; Korent, M.; Žagar Soderžnik, K.; Dubois, J.-M.; Tozman, P.; Venkatesan, M.; Coey, J.M.D.; Kobe, S. Hot-compaction of YCo4.8Fe0.2 nanocrystals for metal-bonded magnets. J. Magn. Magn. Mater. 2018, 460, 401–408. [Google Scholar] [CrossRef]
- Patrick, C.E.; Matsumoto, M.; Staunton, J.B. First-principles calculations of the magnetocrystalline anisotropy of the prototype 2:17 cell boundary phase. J. Magn. Magn. Mater. 2019, 477, 147–155. [Google Scholar] [CrossRef]
- Patrick, C.E.; Staunton, J.B. Temperature-dependent magnetocrystalline anisotropy of rare earth/transition metal permanent magnets from first principles: The light RCo5 (R=Y, La-Gd) intermetallics. Phys. Rev. Mater. 2019, 3, 101401-1–101401-7. [Google Scholar] [CrossRef]
- Asali, A.; Fidler, J.; Suess, D. Influence of changes in electronic structure on magnetocrystalline anisotropy of YCo5 and related compounds. J. Magn. Magn. Mater. 2019, 485, 61–68. [Google Scholar] [CrossRef]
- Ucar, H.; Choudhary, R.; Paudyal, D. Substitutional and interstitial doping in LaCo5 system for the development of hard magnetic properties: A first principles study. J. Magn. Magn. Mater. 2020, 496, 165902-1–165902-8. [Google Scholar] [CrossRef]
- Subramanian, P.R.; Smith, J.F. Thermodynamics of formation of Y-Co alloys. Metall. Trans. A 1985, 16, 1195–1201. [Google Scholar] [CrossRef]
- Wu, C.H.; Chuang, Y.C. The Co-Y (Cobalt-Yttrium) system. J. Phase Equilibria Diffus. 1991, 12, 587–592. [Google Scholar] [CrossRef]
- Shevchenko, M.A.; Ivanov, M.I.; Berezutski, V.V.; Kudin, V.G.; Sudavtsova, V.S. Thermodynamic properties of alloys of the Co-Sc and Co-Y systems. Russ. J. Phys. Chem. A 2015, 89, 931–940. [Google Scholar] [CrossRef]
- Du, Z.; Zhang, W.; Zhao, L. Thermodynamic Assessment of Co-Y System. Rare Metals 1996, 15, 185–190. [Google Scholar]
- Du, Z.; Lü, D. Thermodynamic modelling of the Co–Y system. J. Alloy. Compd. 2004, 373, 171–178. [Google Scholar] [CrossRef]
- Golumbfskie, W.; Liu, Z.-K. CALPHAD/first-principles re-modeling of the Co–Y binary system. J. Alloy. Compd. 2006, 407, 193–200. [Google Scholar] [CrossRef]
- Gupta, K.G. The Co-Ni-Y (Cobalt-Nickel-Yttrium) system. J. Phase Equilibria Diffus. 2010, 31, 389–394. [Google Scholar] [CrossRef]
- Du, Z.; Lü, D. Thermodynamic modeling of the Co–Ni–Y system. Intermetallics 2005, 13, 586–595. [Google Scholar] [CrossRef]
- Golumbfskie, W.J.; Prins, S.N.; Eden, T.J.; Liu, Z.-K. Predictions of the Al-rich region of the Al–Co–Ni–Y system based upon first-principles and experimental data. Calphad 2009, 33, 124–135. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Wu, C.H.; Chang, Y.C. Study of the 1050 °C isothermal section of the ternary system Y-Co-Fe. J. Less-Common Met. 1986, 118, 7–20. [Google Scholar] [CrossRef]
- Kharchenko, O.I.; Bodak, O.I.; Gladyshevskii, E.I. Interaction of Yttrium with metals of the iron family. Russ. Metall. 1977, 1, 170–176. [Google Scholar]
- Söderlind, P.; Turchi, P.E.A.; Landa, A.; Lordi, V. Ground-state properties of rare-earth metals: An evaluation of density-functional theory. J. Phys. Condens. Matter 2014, 26, 416001-1–416001-8. [Google Scholar] [CrossRef]
- Landa, A.; Söderlind, P.; Parker, D.; Åberg, D.; Lordi, V.; Perron, A.; Turchi, P.E.A.; Chouhan, R.K.; Paudyal, D.; Lograsso, T.A. Thermodynamics of the SmCo5 compound doped with Fe and Ni: An ab initio study. J. Alloys Compd. 2018, 765, 659–663. [Google Scholar] [CrossRef]
- Vitos, L. Total-energy method based on the exact muffin-tin orbital theory. Phys. Rev. B 2001, 64, 014107-1–014107-11. [Google Scholar] [CrossRef]
- Vitos, L. Computational Quantum Mechanics for Materials Engineers: The EMTO Method and Application; Springer: London, UK, 2007. [Google Scholar]
- Kollar, J.; Vitos, L.; Skriver, H.L. From ASA towards the Full Potential. In Lecture Notes in Physics; Dreyssé, H., Ed.; Springer: Berlin, Germany, 2000; pp. 85–113. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Chadi, D.J.; Cohen, M.L. Special Points in the Brillouin Zone. Phys. Rev. B 1973, 8, 5747–5753. [Google Scholar] [CrossRef]
- Pourovskii, L.V.; Ruban, A.V.; Vitos, L.; Ebert, H.; Johansson, B.; Abrikosov, I.A. Fully relativistic spin-polarized exact muffin-tin-orbital method. Phys. Rev. B 2005, 71. [Google Scholar] [CrossRef]
- Faulkner, J.S. The modern theory of alloys. Prog. Mater. Sci. 1982, 27, 1–187. [Google Scholar] [CrossRef]
- Vitos, L.; Abrikosov, I.A.; Johansson, B. Anisotropic lattice distortions in random alloys from first-principles theory. Phys. Rev. Lett. 2001, 87. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V.; Skriver, H.L. Screened Coulomb interaction in metalic alloys. I. Univesal screening in the atomic sphere approximations. Phys. Rev. B 2002, 66, 66. [Google Scholar] [CrossRef]
- Ruban, A.V.; Simak, S.I.; Korzhavyi, P.A.; Skriver, H.L. Screened Coulomb interaction in metalic alloys. II. Screening beyond the single-site and atomic-sphere approximations. Phys. Rev. B 2002, 66, 024202-1–024202-12. [Google Scholar] [CrossRef]
- Ruban, A.V.; Simak, S.I.; Shallcross, S.; Skriver, H.L. Local Lattice relaxations in random metallic alloys: Effective tetrahedron model and supercell approach. Phys. Rev. B 2003, 67, 214302-1–214302-12. [Google Scholar] [CrossRef]
- Staunton, J.; Gyorffy, B.; Pindor, A.; Stocks, G.M.; Winter, H. The “disordered local moment” picture of itinerant magnetism at finite temperatures. J. Magn. Magn. Mater. 1984, 45, 15–22. [Google Scholar] [CrossRef]
- Gyorffy, B.L.; Pindor, A.; Staunton, J.; Stocks, G.M.; Winter, H. A first-principles theory of ferromagnetic phase transitions in metals. J. Phys. F Met. Phys. 1985, 15, 1337–1386. [Google Scholar] [CrossRef]
- Murnaghan, F.D. The compressibility of media under extreme pressures. Proc. Natl. Acad. Sci. USA 1944, 30, 244–247. [Google Scholar] [CrossRef]
- Andersen, O.K. Linear methods in band theory. Phys. Rev. B 1975, 12, 3060–3083. [Google Scholar] [CrossRef]
- Söderlind, P. Delocalization and phase transitions in Pr: Theory. Phys. Rev. B 2002, 65, 115105-1–115105-5. [Google Scholar] [CrossRef]
- Wills, J.M.; Alouani, M.; Andersson, P.; Delin, A.; Eriksson, O.; Grechnyev, O. Full-Potential Electronic Structure Method; Springer Series in Solid-State Science; Springer: Berlin/Heidelberg, Germany, 2010; Volume 167. [Google Scholar]
- von Barth, U.; Hedin, L. A local exchange-correlation potential for the spin polarized case. i. J. Phys. C 1972, 5, 1629–1642. [Google Scholar] [CrossRef]
- Kaufman, L.; Bernstein, H. Computer Calculation of Phase Diagrams with Special Reference to Refractory Metals; Academic Press: New York, NY, USA, 1970. [Google Scholar]
- Saunders, N.; Miodownik, A. CALPHAD Calculation of Phase Diagrams: A Comprehensive Guide; Elsevier Science: New York, NY, USA, 1998. [Google Scholar]
- Lukas, H.; Fries, S.; Sundman, B. Computational Thermodynamics: The CALPHAD Method; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Sundman, B.; Jansson, B.; Andersson, J.-O. The Thermo-Calc databank system. Calphad 1985, 9, 153–190. [Google Scholar] [CrossRef]
- Andersson, J.-O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Du, Z.; Zhang, W.; Zhuang, Y. Thermodynamic Assessment of the Fe-Y System. Rare Metals 1997, 16, 52–58. [Google Scholar]
- Lü, D.; Guo, C.; Li, C.; Du, Z. Thermodynamic description of Fe−Y and Fe−Ni−Y systems. Phys. Procedia 2013, 50, 383–387. [Google Scholar] [CrossRef]
- Konar, B.; Kim, J.; Jung, I.-H. Critical systematic evaluation, and thermodynamic optimization of the Fe-RE system: RE = Gd, Tb, Dy, Ho, Er, Tm, Lu, and Y. J. Phase Equilibria Diffus. 2017, 38, 509–542. [Google Scholar] [CrossRef]
- Kardellass, S.; Servant, C.; Selhaoui, N.; Iddaoudi, A.; Ait Amar, M.; Bouirden, L. Thermodynamic assessments of the Fe-Y and Ni-Sc systems. MATEC Web Conf. 2013, 3, 01008-1–01008-6. [Google Scholar] [CrossRef]
- Kardellass, S.; Servant, C.; Selhaoui, N.; Iddaoudi, A.; Ait Amar, M.; Bouirden, L. A thermodynamic assessment of the iron–yttrium system. J. Alloy. Compd. 2014, 583, 598–606. [Google Scholar] [CrossRef]
- Saenko, I.; Fabrichnaya, O.; Udovsky, A. new thermodynamic assessment of the Fe-Y system. J. Phase Equilibria Diffus. 2017, 38, 684–699. [Google Scholar] [CrossRef]
- Du, Z.; Zhang, W. Thermodynamic assessment of the Ni-Y system. J. Alloy. Compd. 1996, 245, 164–167. [Google Scholar] [CrossRef]
- Mattern, N.; Zinkevich, M.; Löser, W.; Behr, G.; Acker, J. Experimental and Thermodynamic Assessment of the Nb-Ni-Y System. J. Phase Equilibria Diffus. 2008, 29, 141–155. [Google Scholar] [CrossRef]
- Huang, J.; Yang, B.; Chen, H.; Wang, H. Thermodynamic optimization of the Ni-Al-Y ternary system. J. Phase Equilibria Diffus. 2015, 36, 357–365. [Google Scholar] [CrossRef]
- Mezbahul-Islam, M.; Medraj, M. A critical thermodynamic assessment of the Mg–Ni, Ni–Y binary and Mg–Ni–Y ternary systems. Calphad 2009, 33, 478–486. [Google Scholar] [CrossRef]
- Subramanian, P.R.; Smith, J.F. Thermodynamics of formation of Y-Ni alloys. Metall. Trans. B 1985, 16, 577–584. [Google Scholar] [CrossRef]
- Okamoto, H. Supplemental Literature Review of Binary Phase Diagrams: Cd-Se, Cu-Hg, Cu-Ho, Eu-Mg, H-Sr, Hf-Si, La-Mn, Mn-Nd, Nb-Y, Ni-Y, Pb-Se, and Sc-Sr. J. Phase Equilibria Diffus. 2013, 34, 430–436. [Google Scholar] [CrossRef]
- Sato, K.; Katayama-Yoshida, H.; Dederichs, P. Dilute magnetic semiconductors. Newsletter 2005, 70, 93–110. [Google Scholar]
- Ma, D.; Grabowski, B.; Körmann, F.; Neugebauer, J.; Raabe, D. Ab initio thermodynamics of the CoCrFeMnNi high entropy alloy: Importance of entropy contributions beyond the configurational one. Acta Mater. 2015, 100, 90–97. [Google Scholar] [CrossRef]
- Coey, M.D. Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2010; p. 471. [Google Scholar]
- Ekholm, M.; Gambino, D.; Jönsson, H.J.M.; Tasnádi, F.; Alling, B.; Abrikosov, I.A. Assessing the SCAN functional for itinerant electron ferromagnets. Phys. Rev. B 2018, 98, 094413-1–094413-7. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).