Finite Element Analysis of the Stress Field in Peri-Implant Bone: A Parametric Study of Influencing Parameters and Their Interactions for Multi-Objective Optimization
Abstract
1. Introduction
2. Material and Methods
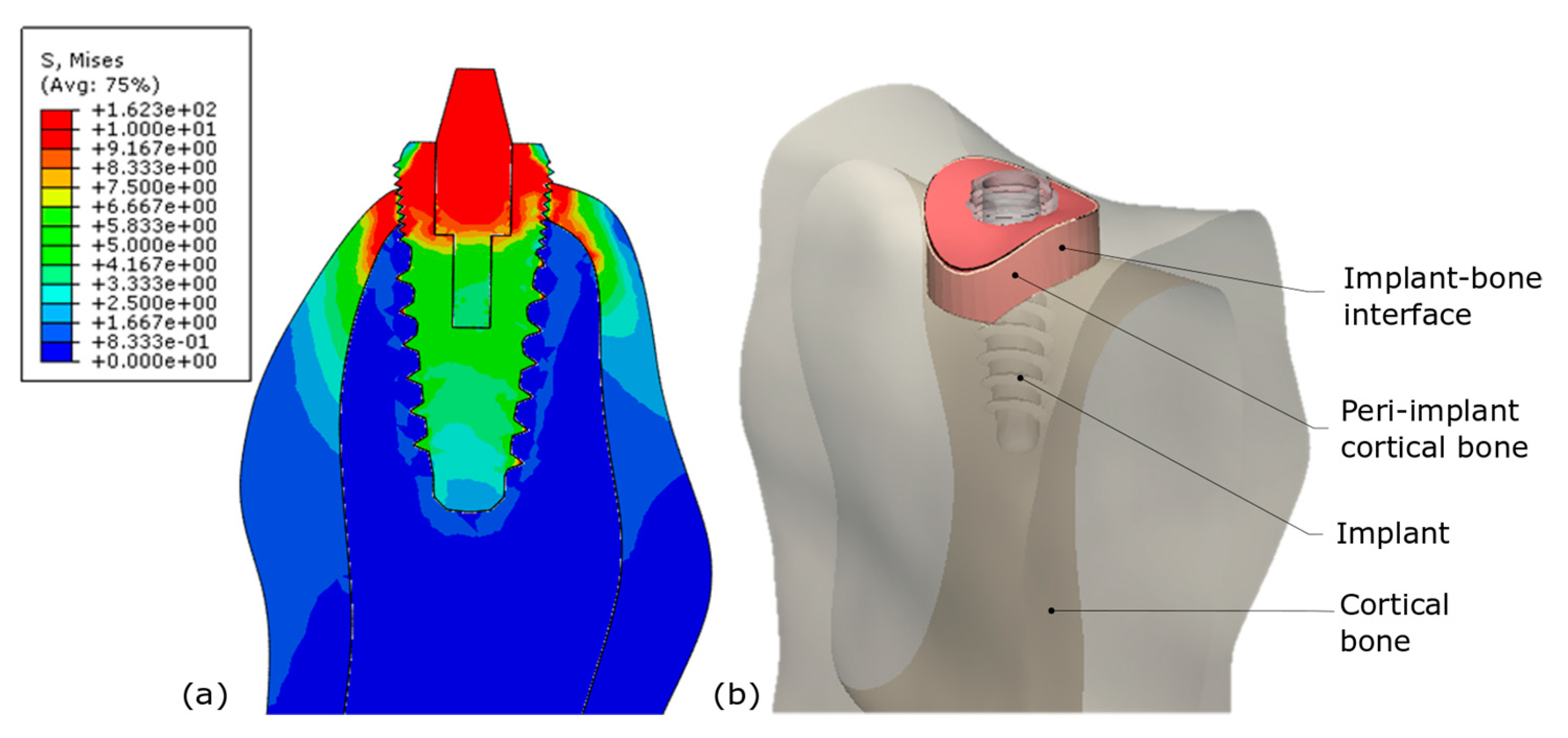
2.1. Presentation of the Finite Element Parametrized Model
2.2. Full Factorial Design
2.3. Two Investigated Biomechanical Responses for Model Classification
2.4. Case Study: Optimization of Implant Placement in Type IV Bone
3. Results
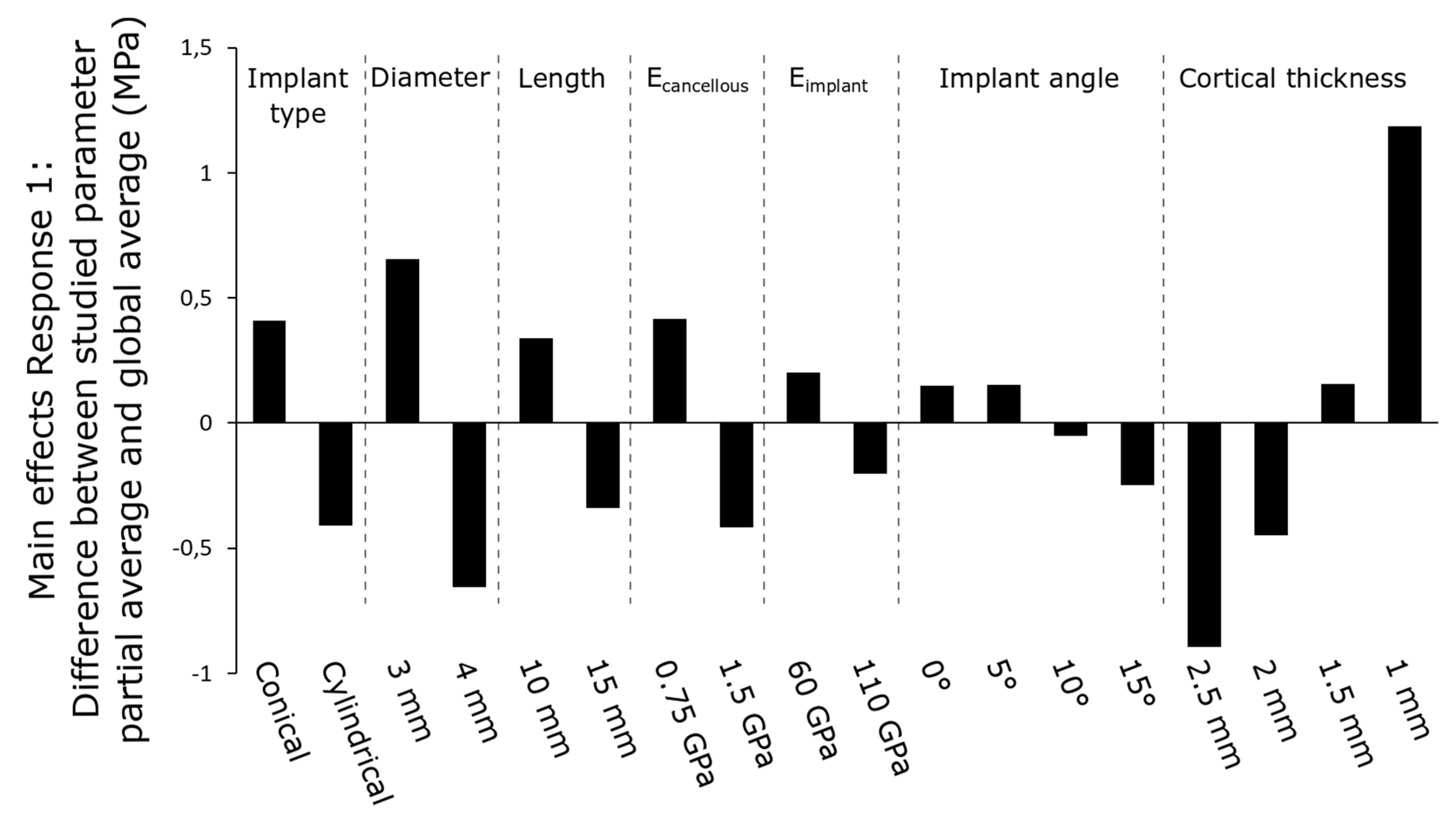
3.1. Main Effects of Parameters
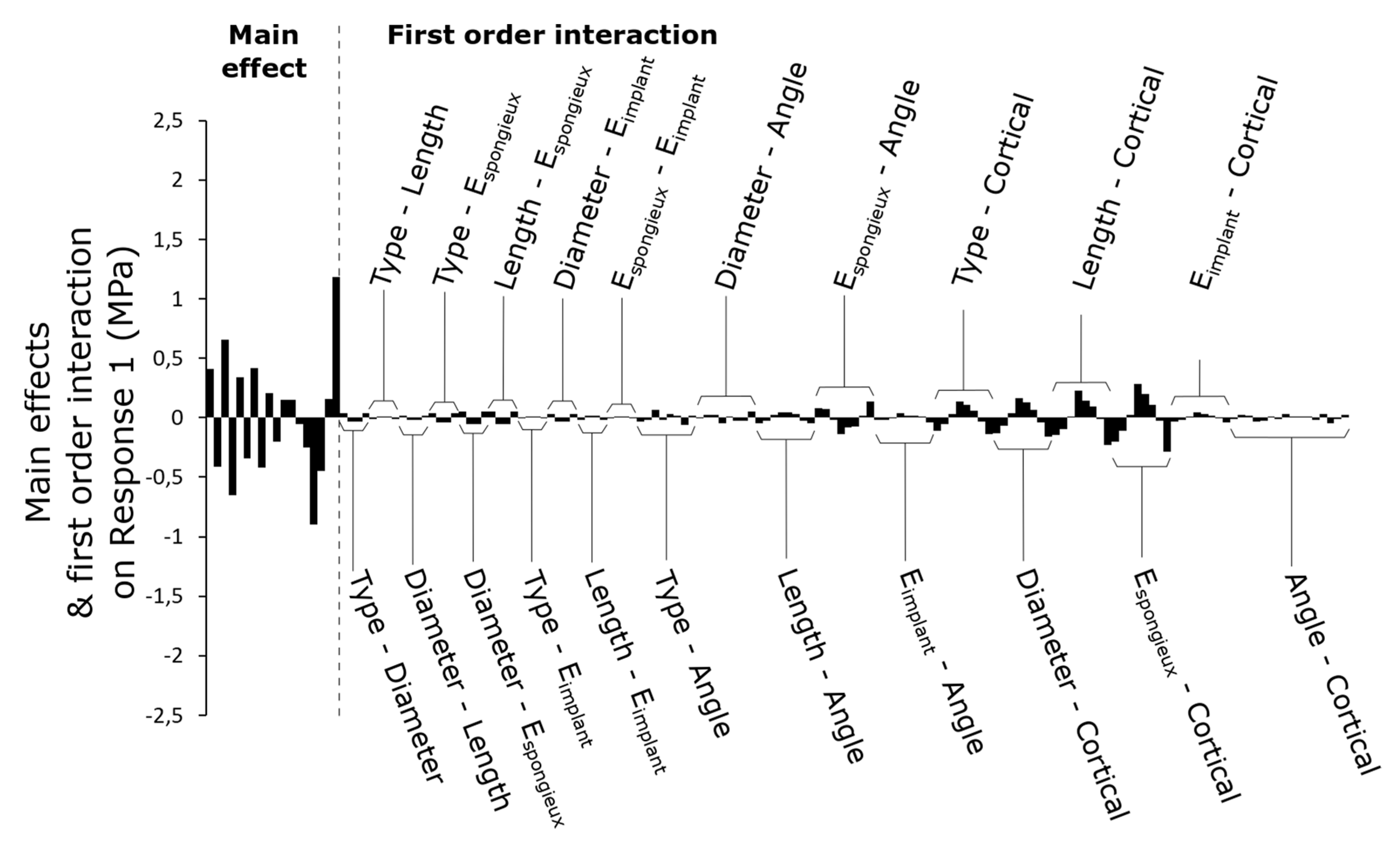
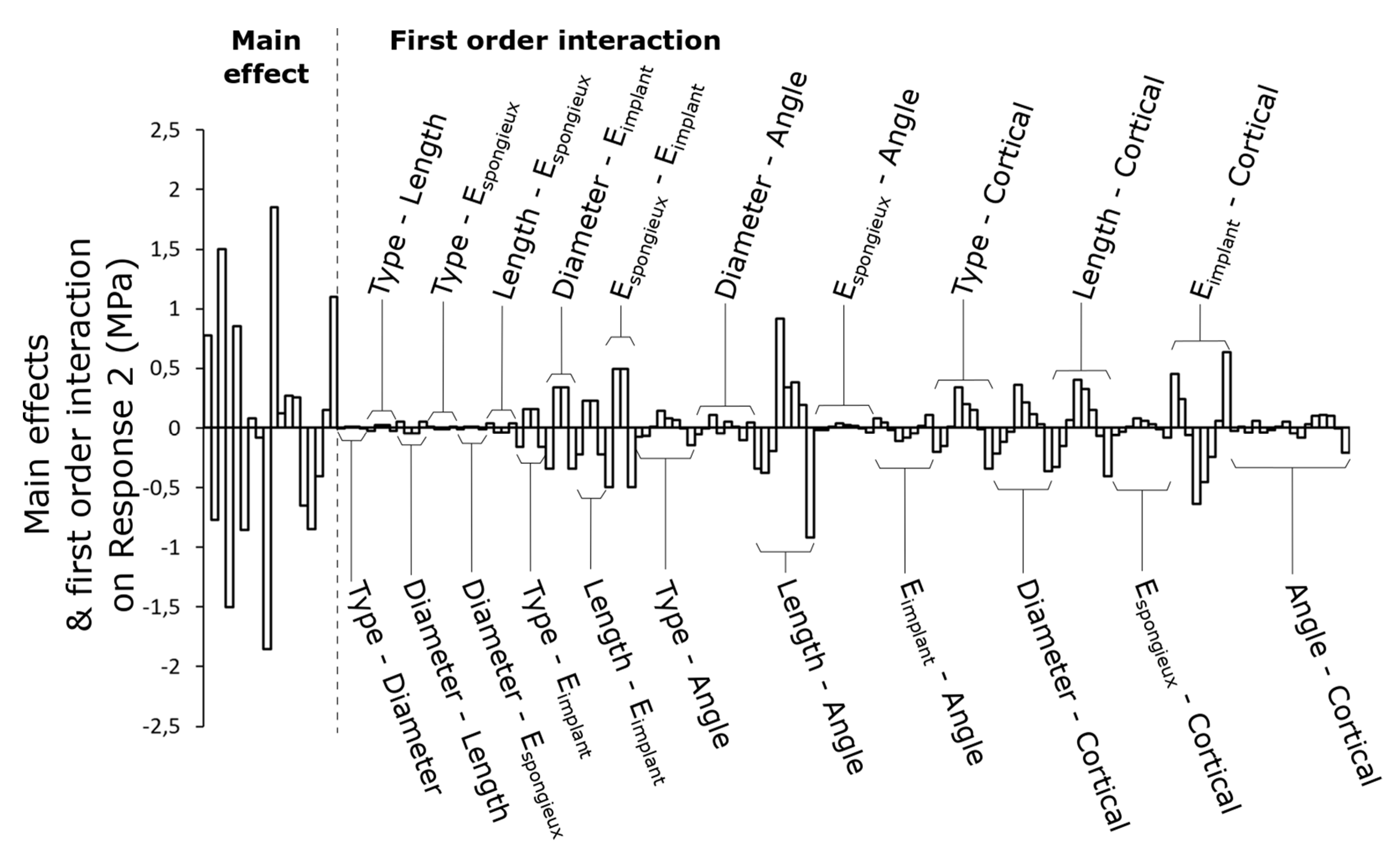
3.2. First-Order Interactions between Parameters
3.3. Case Study: Optimization of the Placement of a Dental Implant in Type IV Bone
4. Discussion
4.1. Full Factorial Design
4.2. Case Study Optimization
4.3. Limitations and Future Developments
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brånemark, P.-I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. 1977, 16, 1–132. [Google Scholar]
- Moraschini, V.; Poubel, L.A.D.C.; Ferreira, V.F.; Barboza, E.D.S.P. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Jeng, M.; Lin, Y.; Lin, C. Biomechanical evaluation of the effects of implant neck wall thickness and abutment screw size: A 3D nonlinear finite element analysis. Appl. Sci. 2020, 10, 3471. [Google Scholar] [CrossRef]
- Petrie, C.S.; Williams, J.L. Comparative evaluation of implant designs: Influence of diameter, length, and taper on strains in the alveolar crest. Clin. Oral Implant. Res. 2005, 16, 486–494. [Google Scholar] [CrossRef]
- Huang, H.; Chang, C.; Hsu, J.; Fallgatter, A.M. Comparison of implant body designs and threaded designs of dental implants: A 3-dimensional finite element analysis. Int. J. Oral Maxillofac. Implant. 2007, 22, 551–562. [Google Scholar]
- Lee, C.; Lin, S.; Kang, M.; Wu, S.; Fu, P. Effects of implant threads on the contact area and stress distribution of marginal bone. J. Dent. Sci. 2010, 5, 156–165. [Google Scholar] [CrossRef]
- Ueda, N.; Takayama, Y.; Yokoyama, A. Minimization of dental implant diameter and length according to bone quality determined by finite element analysis and optimized calculation. J. Prosthodont. Res. 2016, 61, 324–332. [Google Scholar] [CrossRef]
- Chou, H.; Müftü, S. Combined effects of implant insertion depth and alveolar bone quality on periimplant bone strain induced by a wide-diameter, short implant and a narrow-diameter, long implant. J. Prosthet. Dent. 2010, 104, 293–300. [Google Scholar] [CrossRef]
- Tian, K.; Chen, J.; Han, L.; Yang, J.; Huang, W.; Wu, D. Angled abutments result in increased or decreased stress on surrounding bone of single-unit dental implants: A finite element analysis. Med. Eng. Phys. 2012, 34, 1526–1531. [Google Scholar] [CrossRef]
- Butnaru-Moldoveanu, S.A.; Munteanu, F.; Forna, N.C. Virtual bone augmentation in atrophic mandible to assess optimal implant-prosthetic rehabilitation-A finite element study. Appl. Sci. 2020, 10, 401. [Google Scholar] [CrossRef]
- Marcián, P.; Borák, L.; Valášek, J.; Kaiser, J.; Florian, Z.; Wolff, J. Finite element analysis of dental implant loading on atrophic and non-atrophic cancellous and cortical mandibular bone—A feasibility study. J. Biomech. 2014, 47, 3830–3836. [Google Scholar] [CrossRef] [PubMed]
- Akca, K.; Cehreli, M.C. Biomechanical consequences of progressive marginal bone loss around oral implants: A finite element stress analysis. Med. Biol. Eng. Comput. 2006, 44, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Premnath, K.; Sridevi, J.; Kalavathy, N.; Nagaranjani, P.; Sharmila, M.R. Evaluation of stress distribution in bone of different densities using different implant designs: A three-dimensional finite element analysis. J. Indian Prosthodont. Soc. 2013, 13, 555–559. [Google Scholar] [CrossRef]
- De la Rosa Castolo, G.; Guevara Perez, S.V.; Arnoux, P.J.; Badih, L.; Bonnet, F.; Behr, M. Mechanical strength and fracture point of a dental implant under certification conditions: A numerical approach by finite element analysis. J. Prosthet. Dent. 2017, 119, 611–619. [Google Scholar] [CrossRef]
- Çağlar, A.; Bal, B.T.; Aydin, C.; Yilmaz, H. Evaluation of stresses occurring on three different zirconia dental implants: Three-dimensional finite element analysis. Int. J. Oral Maxillofac. Implant. 2010, 25, 95–103. [Google Scholar]
- Bahrami, B.; Shahrbaf, S.; Mirzakouchaki, B.; Ghalichi, F.; Ashtiani, M.; Martin, N. Effect of surface treatment on stress distribution in immediately loaded dental implants—A 3D finite element analysis. Dent. Mater. 2014, 30, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Stegaroiu, R.; Kitamura, E.; Kurokawa, K.; Nomura, S. Influence of maxillary cortical bone thickness, implant design and implant diameter on stress around implants: A three-dimensional finite element analysis. J. Prosthodont. Res. 2010, 54, 133–142. [Google Scholar] [CrossRef]
- El-Anwar, M.I.; El-Zawahry, M.M.; Ibraheem, E.; Nassani, M.; ElGabry, H. New dental implant selection criterion based on implant design. Eur. J. Dent. 2017, 11, 186. [Google Scholar] [CrossRef][Green Version]
- El-Anwar, M.I.; El-Zawahry, M.M. A three dimensional finite element study on dental implant design. J. Genet. Eng. Biotechnol. 2011, 9, 77–82. [Google Scholar] [CrossRef]
- Ohyama, T.; Yasuda, H.; Shibuya, N.; Tadokoro, S.; Nakabayashi, S.; Namaki, S.; Hara, Y.; Ogawa, T.; Ishigami, T. Three-dimensional finite element analysis of the effects of implant diameter and photofunctionalization on peri-implant stress. J. Oral Sci. 2017, 59, 273–278. [Google Scholar] [CrossRef]
- Hasan, I.; Heinemann, F.; Bourauel, C. Biomechanical finite element analysis of self-tapping implants with different dimensions inserted in two bone qualities. Biomed. Eng. 2014, 59, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Thulasingam, C.; Nasser, K.S.G.A.; Balaji, V.; Rajakumar, M.; Rupkumar, P. Evaluation of design parameters of dental implant shape, diameter and length on stress distribution: A finite element analysis. J. Indian Prosthodont. Soc. 2011, 11, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Vairo, G.; Sannino, G. Comparative evaluation of osseointegrated dental implants based on platform-switching concept: Influence of diameter, length, thread shape, and in-bone positioning depth on stress-based performance. Comput. Math. Methods Med. 2013, 2013, 250929. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Todo, M.; Matsushita, Y.; Koyano, D.D.S.K. Effects of implant diameter, insertion depth, and loading angle on stress/strain fields in effects of implant diameter, insertion depth, and loading angle on stress/strain fields in implant/jawbone systems: Finite element analysis. Int. J. Oral Maxillofac. Implant. 2009, 24, 877–886. [Google Scholar]
- Kong, L.; Hu, K.; Li, D.; Song, Y.; Yang, J.; Wu, Z.; Liu, B. Evaluation of the cylinder implant thread height and width: A 3-dimensional finite element analysis. Int. J. Oral Maxillofac. Implant. 2008, 23, 65–74. [Google Scholar]
- Kong, L. Biomechanical optimization of implant diameter and length for immediate loading: Biomechanical optimization of implant diameter and length for immediate loading: A nonlinear finite element analysis. Int. J. Prosthodont. 2009, 22, 607–615. [Google Scholar]
- Li, T.; Hu, K.; Cheng, L.; Ding, Y.; Ding, Y.; Shao, J.; Kong, L. Optimum selection of the dental implant diameter and length in the posterior mandible with poor bone quality—A 3D finite element analysis. Appl. Math. Model. 2011, 35, 446–456. [Google Scholar] [CrossRef]
- Ao, J.; Li, T.; Liu, Y.; Ding, Y.; Wu, G.; Hu, K.; Kong, L. Optimal design of thread height and width on an immediately loaded cylinder implant: A finite element analysis. Comput. Biol. Med. 2010, 40, 681–686. [Google Scholar] [CrossRef]
- Baggi, L.; Cappelloni, I.; Girolamo, D.; Maceri, F.; Vairo, G. The influence of implant diameter and length on stress distribution of osseointegrated implants related to crestal bone geometry: A three- dimensional finite element analysis. J. Prosthet. Dent. 2008, 100, 422–431. [Google Scholar] [CrossRef]
- Roy, S.; Das, M.; Chakraborty, P.; Biswas, J.K.; Chatterjee, S.; Khutia, N.; Saha, S.; Chowdhury, A.R. Optimal selection of dental implant for different bone conditions based on the mechanical response. Acta Bioeng. Biomech. 2017, 19, 11–20. [Google Scholar]
- Holmgren, E.P.; Seckinger, R.J.; Kilgren, L.M.; Mante, F. Evaluating parameters of esseointegrated dental implants using finite element analysis—A two-dimensional comparative study examining the effects of implant diameter, implant shape, and load direction. J. Oral Implantol. 1998, 24, 80–88. [Google Scholar] [CrossRef]
- Himmlova, L.; Dostalova, T.; Kacovsky, A.; Konvickova, S. Influence of implant length and diameter on stress distribution: A finite element analysis. J. Prosthet. Dent. 2004, 91, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Kuo, Y.-C.; Lin, T.-S. Effects of dental implant length and bone quality on biomechanical responses in bone around implants: A 3-D non-linear finite element analysis. Biomed. Eng. Appl. Basis Commun. 2005, 17, 44–49. [Google Scholar] [CrossRef]
- Chun, H.J.; Cheong, S.Y.; Han, J.H.; Heo, S.J.; Chung, J.P.; Rhyu, I.C.; Choi, Y.C.; Baik, H.K.; Ku, Y.; Kim, M.H. Evaluation of design parameters of osseointegrated dental implants using finite element analysis. J. Oral Rehabil. 2002, 29, 565–574. [Google Scholar] [CrossRef]
- Bevilacqua, M.; Tealdo, T.; Pera, F.; Mossolov, A.; Drago, C.; Pera, P. The influence of cantilever length and implant inclination on stress distribution in maxillary implant- supported fixed dentures. J. Prosthet. Dent. 2011, 105, 5–13. [Google Scholar] [CrossRef]
- Macedo, J.P.; Pereira, J.; Faria, J.; Pereira, C.A.; Alves, J.L.; Henriques, B.; Souza, J.C.M.; López-López, J. Finite element analysis of stress extent at peri-implant bone surrounding external hexagon or Morse taper implants. J. Mech. Behav. Biomed. Mater. 2017, 71, 441–447. [Google Scholar] [CrossRef]
- Wolff, J.; Narra, N.; Antalainen, A.-K.; Valášek, J.; Kaiser, J.; Sándor, G.K.; Marcián, P. Finite element analysis of bone loss around failing implants. Mater. Des. 2014, 61, 177–184. [Google Scholar] [CrossRef]
- Quaresma, S.E.T.; Cury, P.R.; Sendyk, W.R.; Sendyk, C. A Finite Element Analysis of 2 diffenrent dental implants: Stress distribution in the prosthesis, abutment, implant and supporting bone. J. Oral Implantol. 2008, 34, 1–6. [Google Scholar] [CrossRef]
- Chang, H.; Chen, Y.; Hsieh, Y. Stress distribution of two commercial dental implant systems: A three-dimensional finite element analysis. J. Dent. Sci. 2013, 8, 261–271. [Google Scholar] [CrossRef]
- Tepper, G.; Haas, R.; Watzek, W.; Wolfgang, Z.; Georg, K. Three-dimensional finite element analysis of implant stability in the atrophic posterior maxilla A mathematical study of the sinus floor. Clin. Oral Implants Res. 1999, 13, 657–665. [Google Scholar] [CrossRef]
- Bozkaya, D.; Müftü, S.; Müftü, A. Evaluation of load transfer characteristics of five different implants in compact bone at different load levels by finite element analysis. J. Prosthet. Dent. 2003, 92, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Aunmeungtong, W.; Khongkhunthian, P.; Rungsiyakull, P. Stress and strain distribution in three different mini dental implant designs using in implant retained overdenture: A finite element analysis study. Oral Implantol. 2016, 9, 202–212. [Google Scholar]
- van Staden, R.C.; Li, X.; Guan, H.; Johnson, N.W.; Reher, P.; Loo, Y.-C. A finite element study of short dental implants. Int. J. Oral Maxillofac. Implants 2014, 29, 147–154. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, H.; Chen, X.; Wang, W.; Chen, J.; Liang, J.; Zhang, P. A three-dimensional finite element study on the biomechanical simulation of various structured dental implants and their surrounding bone tissues. Int. J. Dent. 2016, 2016, 4867402. [Google Scholar] [CrossRef] [PubMed]
- Oswal, M.M.; Amasi, U.N.; Oswal, M.S.; Bhagat, A.S. Influence of three different implant thread designs on stress distribution: A three-dimensional finite element analysis. J. Indian Prosthodont. Soc. 2016, 16, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Zarei, I.; Khajehpour, S.; Sabouri, A.; Haghnegahdar, A.Z.; Jafari, K. Assessing the effect of dental implants thread design on distribution of stress in impact loadings using three dimensional finite element method. J. Dent. Biomater. 2016, 3, 233–240. [Google Scholar]
- Hansson, S.; Werke, M. The implant thread as a retention element in cortical bone: The effect of thread size and thread profile: A finite element study. J. Biomech. 2003, 36, 1247–1258. [Google Scholar] [CrossRef]
- Chun, H.; Shin, H.; Han, C.-H.; Lee, S.-H. Influence of implant abutment type on stress distribution in bone under various loading conditions using finite element analysis. Int. J. Oral Maxillofac. Implant. 2005, 21, 195–202. [Google Scholar]
- Kitamura, E.; Stegaroiu, R.; Nomura, S.; Miyakawa, O. Influence of marginal bone resorption on stress around an implant—A three-dimensional finite element analysis. J. Oral Rehabil. 2005, 32, 279–286. [Google Scholar] [CrossRef]
- Yoon, K.; Kim, S.; Lee, J.; Suh, S. 3D finite element analysis of changes in stress levels and distributions for an osseointegrated implant after vertical. Implant Dent. 2011, 20, 354–359. [Google Scholar] [CrossRef]
- Wang, K.; Geng, J.; Jones, D.; Xu, W. Comparison of the fracture resistance of dental implants with different abutment taper angles. Mater. Sci. Eng. C 2016, 63, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wang, J.; Ramp, M.S.L.C.; Liu, P. Biomechanical response of implant systems placed in the maxillary posterior region under various conditions of angulation, bone density, and loading. Int. J. Oral Maxillofac. Implant. 2008, 23, 57–64. [Google Scholar]
- Sugiura, T.; Yamamoto, K.; Horita, S.; Murakami, K.; Tsutsumi, S.; Kirita, T. The effects of bone density and crestal cortical bone thickness on micromotion and peri-implant bone strain distribution in an immediately loaded implant: A nonlinear finite element analysis. J. Periodontal Implant Sci. 2016, 46, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, D.; Nor, F.M.; Lee, H.Y.; Finite, J.Y.L. Finite element analysis of bone-implant biomechanics: Refinement through featuring various osseointegration conditions. Int. J. Oral Maxillofac. Surg. 2012, 41, 1090–1096. [Google Scholar] [CrossRef]
- Natali, A.N.; Pavan, P.G.; Ruggero, A.L. Analysis of bone-implant interaction phenomena by using a numerical approach. Clin. Oral Implants Res. 2003, 17, 67–74. [Google Scholar] [CrossRef]
- Kitagawa, T.; Tanimoto, Y.; Nemoto, K.; Aida, M. Influence of cortical implant bone quality on stress distribution in bone around dental. Dent. Mater. J. 2005, 24, 219–224. [Google Scholar] [CrossRef]
- Tada, S.; Stegaroiu, R.; Kitamura, E.; Miyakawa, O. Influence of implant design and bone quality on stress/strain distribution in bone around implants: A 3-dimensional finite element analysis. Int. J. Oral Maxillofac. Implants 2003, 18, 357–368. [Google Scholar]
- Linetskiy, I.; Demenko, V.; Linetska, L.; Yefremov, O. Impact of annual bone loss and different bone quality on dental implant success—A finite element study. Comput. Biol. Med. 2017, 91, 318–325. [Google Scholar] [CrossRef]
- Sevimay, M.; Turhan, F.; Kilic, M.A.; Eskitascioglu, G. Three-dimensional finite element analysis of the effect of different bone quality on stress distribution in an implant-supported crown. J. Prosthet. Dent. 2005, 93, 227–234. [Google Scholar] [CrossRef]
- Savadi, R.C.; Agarwal, J. Influence of implant surface topography and loading condition on stress distribution in bone around implants: A comparative 3D FEA. J. Indian Prosthodont. Soc. 2011, 11, 221–231. [Google Scholar] [CrossRef]
- Shi, M.; Li, H.; Liu, X. Multidisciplinary design optimization of dental implant based on finite element method and surrogate models. J. Mech. Sci. Technol. 2017, 31, 5067–5073. [Google Scholar] [CrossRef]
- Huang, H.; Hsu, J.; Fuh, L.; Lin, D.; Chen, M.Y.C. Biomechanical simulation of various surface roughnesses and geometric designs on an immediately loaded dental implant. Comput. Biol. Med. 2010, 40, 525–532. [Google Scholar] [CrossRef]
- Rand, A.; Stiesch, M.; Eisenburger, M.; Greuling, A. The effect of direct and indirect force transmission on peri-implant bone stress–a contact finite element analysis. Comput. Methods Biomech. Biomed. Engin. 2017, 20, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Korabi, R.; Shemtov-yona, K.; Rittel, D. On stress/strain shielding and the material stiffness paradigm for dental implants. Clin. Implant Dent. Relat. Res. 2017, 19, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Sakka, S.; Baroudi, K.; Nassani, M.Z. Factors associated with early and late failure of dental implants. J. Investig. Clin. Dent. 2012, 3, 258–261. [Google Scholar] [CrossRef] [PubMed]
- De Santis, R.; Mollica, F.; Zarone, F.; Ambrosio, L.; Nicolais, L. Biomechanical effects of titanium implants with full arch bridge rehabilitation on a synthetic model of the human jaw. Acta Biomater. 2007, 3, 121–126. [Google Scholar] [CrossRef]
- Lekholm, U.; van Steenberghe, D.; Herrmann, I.; Bolender, C.; Folmer, T.; Gunne, J.; Henry, P.; Higuchi, K.; Laney, W.R.; Lindén, U. Osseointegrated Implants in the Treatment of Partially Edentulous Jaws: A Prospective 5-Year Multicenter. Int. J. Oral Maxillofac. Implants 1994, 9. [Google Scholar]
- Piotrowski, B.; Baptista, A.A.; Patoor, E.; Bravetti, P.; Eberhardt, A.; Laheurte, P. Interaction of bone-dental implant with new ultra low modulus alloy using a numerical approach. Mater. Sci. Eng. C 2014, 38, 151–160. [Google Scholar] [CrossRef]
- Wang, C.; Fu, G.; Deng, F. Difference of natural teeth and implant-supported restoration: A comparison of bone remodeling simulations. J. Dent. Sci. 2015, 10, 190–200. [Google Scholar] [CrossRef][Green Version]
- Dep, K. Multi-Objective Optimization Using Evolutionary Algorithms; John Wiley: New York, NY, USA, 2001; ISBN 047187339X. [Google Scholar]
- Lofaj, F.; Kucera, J.; Nemeth, D.; Kvetkova, L. Finite element analysis of stress distributions in mono- and bi-cortical dental implants. Mater. Sci. Eng. C 2015, 50, 85–96. [Google Scholar] [CrossRef]
- Frost, H.M. A 2003 update of bone physiology and Wolff s law for clinicians. Angle Orthod. 2004, 74, 3–15. [Google Scholar] [PubMed]
- Ceranka, J.; Verga, S.; Kvasnytsia, M.; Lecouvet, F.; Michoux, N.; de Mey, J.; Raeymaekers, H.; Metens, T.; Absil, J.; Vandemeulebroucke, J. Multi-atlas segmentation of the skeleton from whole-body MRI—Impact of iterative background masking. Magn. Reson. Med. 2020, 83, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Uchida, T.; Ishigami, T.; Ohyama, T.; Shibuya, N.; Nakabayashi, S. High bone-implant contact achieved by photofunctionalization to reduce periimplant stress. Implant Dent. 2013, 22, 102–108. [Google Scholar]


| Parameter | Author Reference |
|---|---|
| Implant geometry | |
| Diameter and length | N. Ueda et al. [7], N. Okumura et al. [17], M. I. El-Anwar et al. [18,19], T. Ohyama et al. [20], I. Hasan et al. [21], C. S. Petrie et al. [4], M. M. Ebrahim et al. [22], G. Vairo et al. [23], L. Qian et al. [24], L. Kong et al. [25,26], T. Li et al. [27], J. Ao et al. [28], L. Baggi et al. [29], S. Roy et al. [30], E. P. Holmgren et al. [31], L. Himmlova et al. [32], S. Tada et al., C.-L. Lin et al. [33], H.-J. Chun et al., [34], M. Bevilacqua et al. [35] |
| Type or taper | P. Marcián et al. [11], T. Ohyama et al. [20], J. P. Macedo et al. [36], J. Wolff et al. [37], S. Quaresma et al. [38], H.-S. Chang et al. [39], G. Tepper et al. [40], D. Bozkaya et al. [41], W. Aunmeungtong et al. [42], R. C. Van Staden et al. [43] |
| Thread | C.-C. Lee et al. [6], K. Premnath et al. [13], M. I. El-Anwar et al. [18], G. Vairo et al. [23], L. Kong et al. [25], J. Ao et al. [28], G. Zhang et al. [44], M. M. Oswal et al. [45], I. Zarei et al. [46], S. Hansson et al. [47], H.-J. Chun et al. [48] |
| Depth of insertion | H.-Y. Chou et al. [8], G. de la Rosa Castolo et al. [14], L. Qian et al. [24], E. Kitamura et al. [49], K.-H. Yoon et al. [50] |
| Angle of inclination | K. Tian et al. [9], M. Bevilacqua et al. [35] G. Zhang et al. [44], K. Wang et al. [51], C.-L. Lin et al. [52], |
| Bone geometry | |
| Cortical thickness | N. Ueda et al. [7], P. Marcián et al. [11], K. Akca et al. [12], N. Okumura et al. [17], I. Hasan et al. [21], C.-L. Lin et al. [33], J. Wolff et al. [37], H.-S. Chang et al. [39], T. Sugiura et al. [53], D. Kurniawan et al. [54], A. N. Natali et al. [55], T. Kitagawa et al. [56] |
| Materials | |
| Bone parts | N. Ueda et al. [7], H.-Y. Chou et al. [8], K. Premnath et al. [13], C.-L. Lin et al. [33], H.-S. Chang et al. [39], T. Sugiura et al. [53], D. Kurniawan et al. [54], T. Kitagawa et al. [56], S. Tada et al. [57], I. Linetskiy et al. [58], M. Sevimay et al. [59], |
| Implant parts | G. de la Rosa Castolo et al. [14], A. Çağlar [15] |
| Others | |
| Interaction and contact | P. Marcián et al. [11], G. de la Rosa Castolo et al. [14], B. Bahrami et al. [16], T. Ohyama et al. [20], D. Kurniawan et al. [54], R. C. Savadi et al. [60], M. Shi et al. [61], H.-L. Huang et al. [62] |
| Loading conditions | N. Okumura et al. [17], T. Ohyama et al. [20], L. Qian et al. [24], L. Kong et al. [25], E. P. Holmgren et al. [31], J. P. Macedo et al. [36], H.-S. Chang et al. [39], W. Aunmeungtong et al. [42], E. Kitamura et al. [56], R. C. Savadi et al. [60], A. Rand et al. [63], R. Korabi et al. [64] |
| Material | Young’s Modulus (GPa) | Poisson Ratio |
|---|---|---|
| Ti-6Al-4V alloy | 110 | 0.3 |
| Cortical bone | 15 | 0.3 |
| Cancellous bone | 1.5 | 0.3 |
| Parameters | Range in the Literature | Investigated in the Study |
|---|---|---|
| Diameter | (3; 6 mm) | 3 mm and 4 mm |
| Length | (5; 20 mm) | 10 mm and 15 mm |
| General shape and taper | Various shapes and tapers | Cylindrical and conical |
| Angle of inclination | [0; 20°] | 0°, 5°, 10° and 15° |
| Cortical thickness | Various shapes and thicknesses | 1 mm, 1.5 mm, 2.0 mm and 2.5 mm |
| Cancellous Young’s modulus (Ecancellous) | (0.1; 9.5 GPa) | 0.75 GPa and 1.5 GPa |
| Implant Young’s modulus (Eimplant) | Ti-6Al-4V (110 GPa) Zirconia (200 GPa) | Ti-6Al-4V (110 GPa) Ti-Nb (60 GPa) |
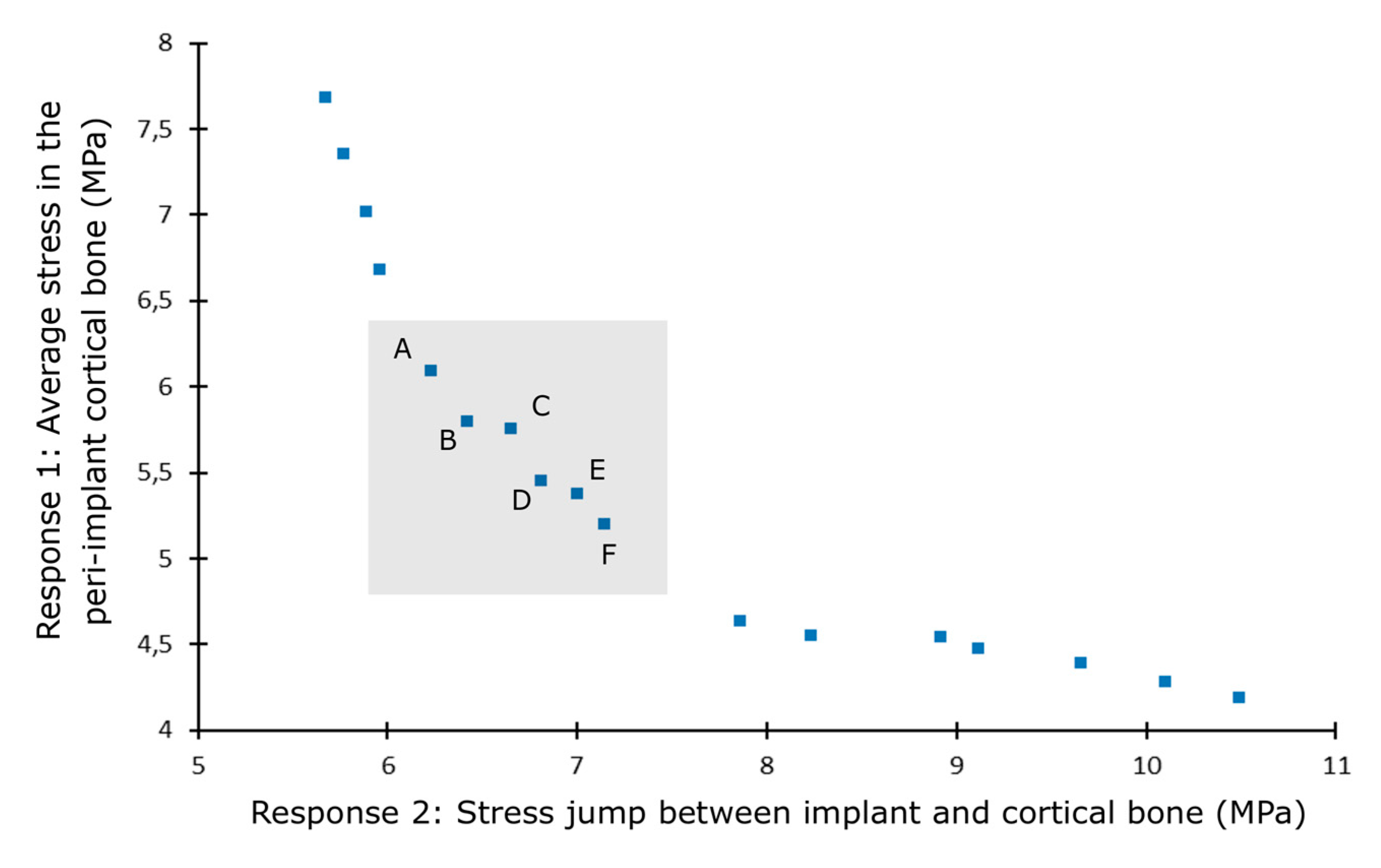
| Solution | Stress Jump (MPa) | Average Stress (MPa) | Taper (°) | Diameter (mm) | Length (mm) | Angle (°) | Implant Young’s Modulus (GPa) |
|---|---|---|---|---|---|---|---|
| A | 6.23 | 6.09 | 0.35 | 3.79 | 14.99 | 0.07 | 60.34 |
| B | 6.42 | 5.80 | 0.75 | 3.90 | 14.98 | 0.41 | 60.50 |
| C | 6.65 | 5.75 | 1.2 | 3.86 | 14.98 | 0.94 | 62.22 |
| D | 6.81 | 5.45 | 1.7 | 3.95 | 14.97 | 1.14 | 61.68 |
| E | 7.00 | 5.38 | 2.45 | 3.89 | 14.98 | 0.64 | 60.85 |
| F | 7.14 | 5.20 | 2.55 | 3.97 | 14.96 | 1.25 | 62.57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Didier, P.; Piotrowski, B.; Le Coz, G.; Joseph, D.; Bravetti, P.; Laheurte, P. Finite Element Analysis of the Stress Field in Peri-Implant Bone: A Parametric Study of Influencing Parameters and Their Interactions for Multi-Objective Optimization. Appl. Sci. 2020, 10, 5973. https://doi.org/10.3390/app10175973
Didier P, Piotrowski B, Le Coz G, Joseph D, Bravetti P, Laheurte P. Finite Element Analysis of the Stress Field in Peri-Implant Bone: A Parametric Study of Influencing Parameters and Their Interactions for Multi-Objective Optimization. Applied Sciences. 2020; 10(17):5973. https://doi.org/10.3390/app10175973
Chicago/Turabian StyleDidier, Paul, Boris Piotrowski, Gael Le Coz, David Joseph, Pierre Bravetti, and Pascal Laheurte. 2020. "Finite Element Analysis of the Stress Field in Peri-Implant Bone: A Parametric Study of Influencing Parameters and Their Interactions for Multi-Objective Optimization" Applied Sciences 10, no. 17: 5973. https://doi.org/10.3390/app10175973
APA StyleDidier, P., Piotrowski, B., Le Coz, G., Joseph, D., Bravetti, P., & Laheurte, P. (2020). Finite Element Analysis of the Stress Field in Peri-Implant Bone: A Parametric Study of Influencing Parameters and Their Interactions for Multi-Objective Optimization. Applied Sciences, 10(17), 5973. https://doi.org/10.3390/app10175973





