Post-Effect on the Centre of Feet Pressure during Stance by Continuous Asymmetric Mediolateral Translations of a Supporting Platform—A Preliminary Study in Healthy Young Adults
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Task and Procedures
2.2. Data Acquisition and Treatment
2.3. Statistical Analysis
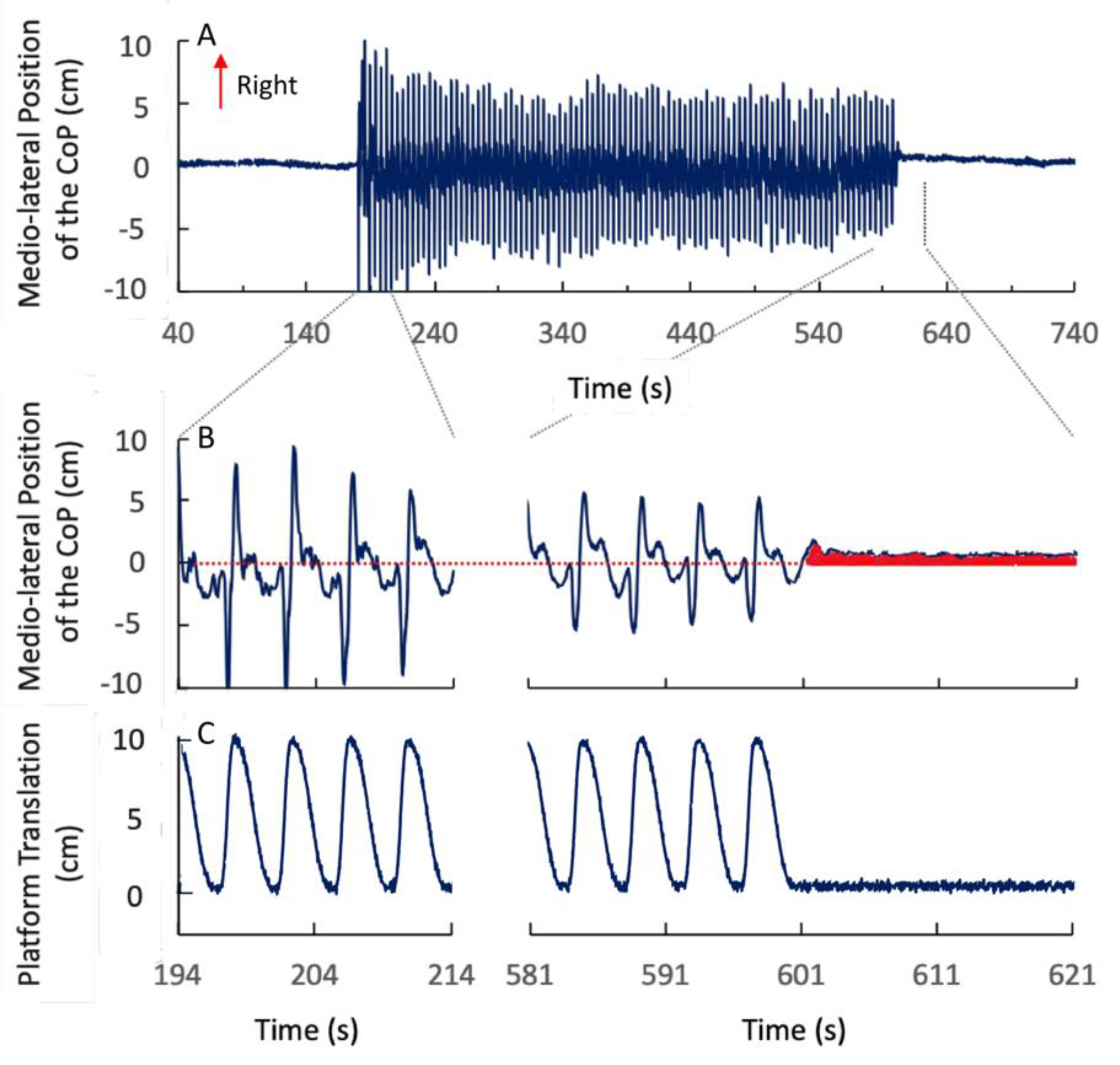
3. Results
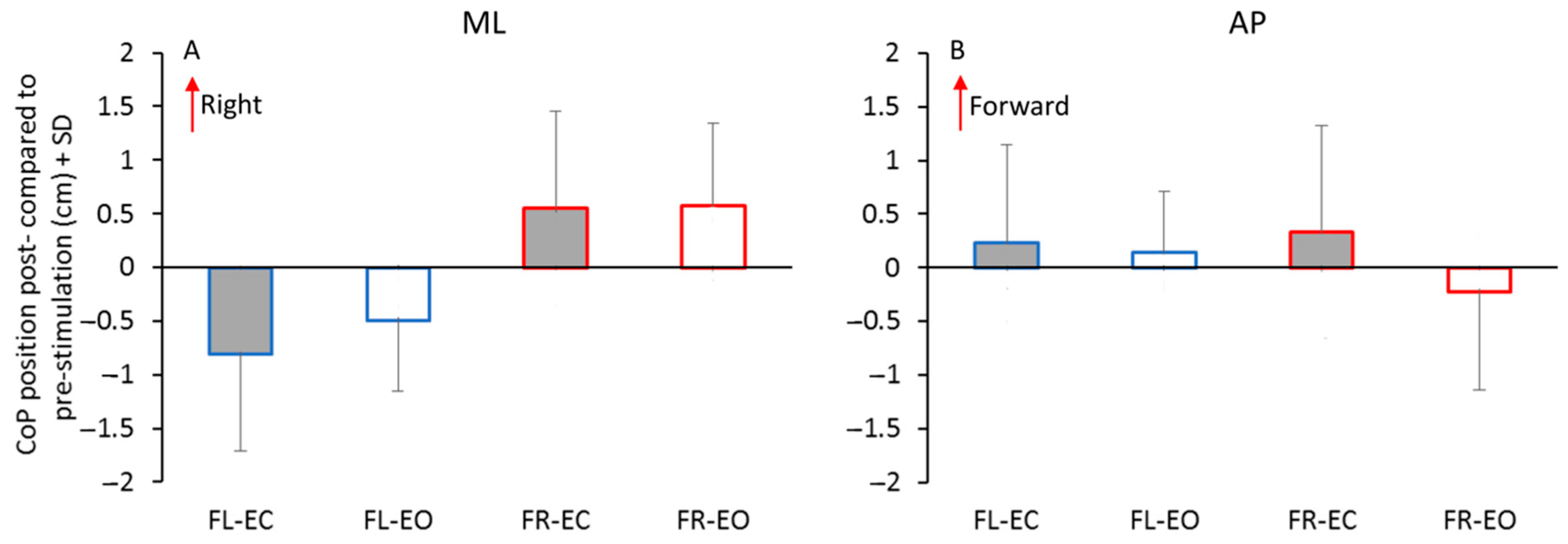
3.1. The CoP Position Pre- and Post-Stimulation
3.2. Antero–Posterior Position of the CoP
3.3. Body Sway around the Mean CoP Position
3.4. Closing the Eyes at the End of the Stimulation with EO did not Modify the Post-Effect on the CoP Position
3.5. Symmetric Perturbation Cycles
3.6. Duration of the Post-Effect
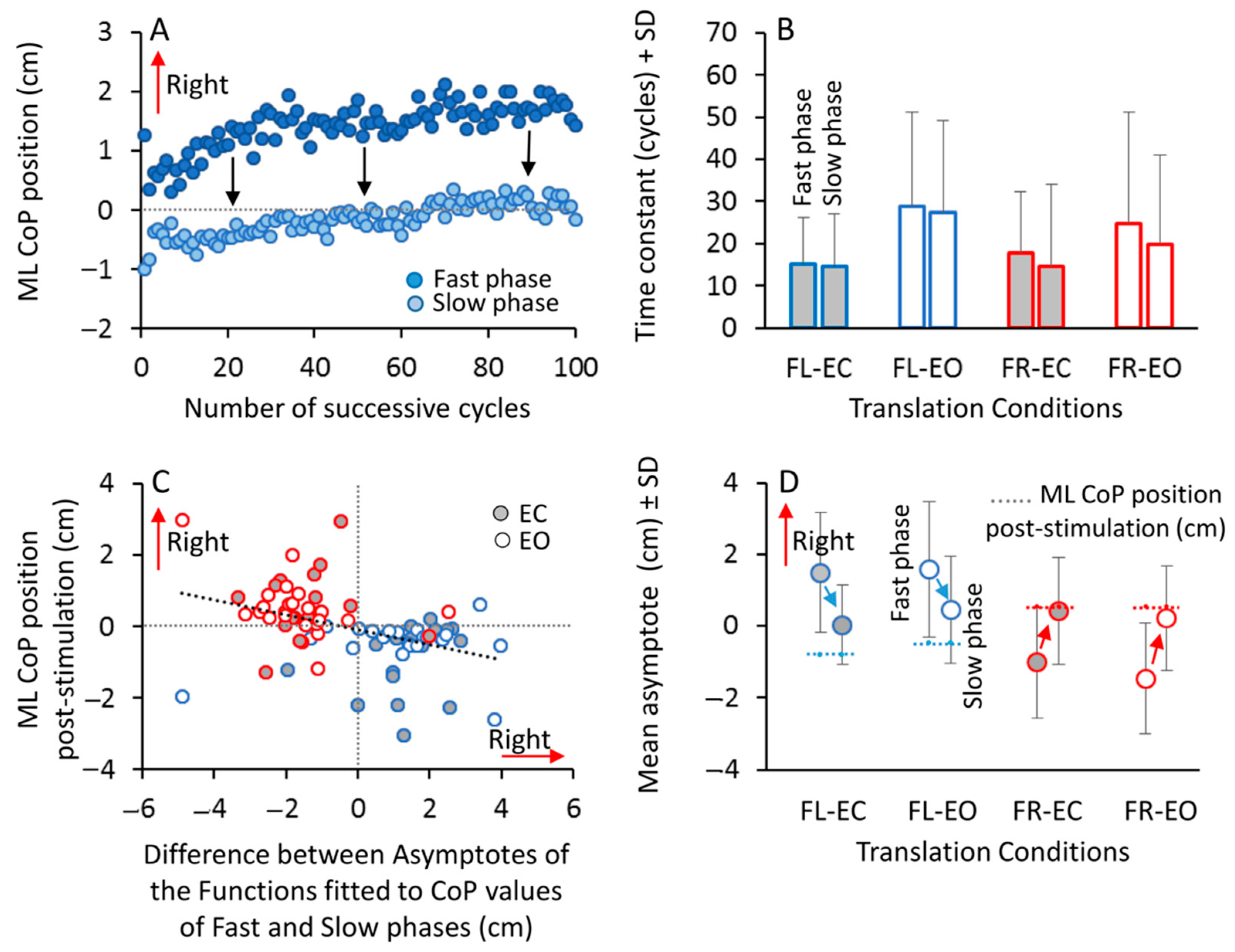
3.7. Habituation to the Series of Perturbations
4. Discussion
4.1. The CoP Displacement after the Sequence of Asymmetric Perturbations
4.2. Relationship between the CoP Motion during the Platform Perturbation Cycles and the Post-Effect
4.3. The Effect of Vision in the Post-Stimulation Period
4.4. Limitations
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Schieppati, M.; Hugon, M.; Grasso, M.; Nardone, A.; Galante, M. The limits of equilibrium in young and elderly normal subjects and in parkinsonians. Electroencephalogr. Clin. Neurophysiol. 1994, 93, 286–298. [Google Scholar] [CrossRef]
- Morasso, P.G.; Schieppati, M. Can muscle stiffness alone stabilize upright standing? J. Neurophysiol. 1999, 82, 1622–1626. [Google Scholar] [CrossRef]
- Masani, K.; Popovic, M.R.; Nakazawa, K.; Kouzaki, M.; Nozaki, D. Importance of body sway velocity information in controlling ankle extensor activities during quiet stance. J. Neurophysiol. 2003, 90, 3774–3782. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Nashner, L.M. Central programming of postural movements: Adaptation to altered support-surface configurations. J. Neurophysiol. 1986, 55, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Nardone, A.; Schieppati, M. Human Balance in Response to Continuous, Predictable Translations of the Support Base: Integration of Sensory Information, Adaptation to Perturbations, and the Effect of Age, Neuropathy and Parkinson’s Disease. Appl. Sci. 2019, 9, 5310. [Google Scholar] [CrossRef]
- Winter, D.A.; Prince, F.; Frank, J.S.; Powell, C.; Zabjek, K.F. Unified theory regarding A/P and M/L balance in quiet stance. J. Neurophysiol. 1996, 75, 2334–2343. [Google Scholar] [CrossRef]
- Soames, R.W.; Atha, J. The role of the antigravity musculature during quiet standing in man. Eur. J. Appl. Physiol. Occup. Physiol. 1981, 47, 159–167. [Google Scholar] [CrossRef]
- Ivanenko, Y.; Gurfinkel, V.S. Human Postural Control. Front. Neurosci. 2018, 12, 171. [Google Scholar] [CrossRef]
- Sozzi, S.; Honeine, J.L.; Do, M.C.; Schieppati, M. Leg muscle activity during tandem stance and the control of body balance in the frontal plane. Clin. Neurophysiol. 2013, 124, 1175–1186. [Google Scholar] [CrossRef]
- Rietdyk, S.; Patla, A.E.; Winter, D.A.; Ishac, M.G.; Little, C.E. NACOB presentation CSB New Investigator Award. Balance recovery from medio-lateral perturbations of the upper body during standing. North American Congress on Biomechanics. J. Biomech. 1999, 32, 1149–1158. [Google Scholar] [CrossRef]
- Bingham, J.T.; Choi, J.T.; Ting, L.H. Stability in a frontal plane model of balance requires coupled changes to postural configuration and neural feedback control. J. Neurophysiol. 2011, 106, 437–448. [Google Scholar] [CrossRef]
- Henry, S.M.; Fung, J.; Horak, F.B. Control of stance during lateral and anterior/posterior surface translations. IEEE Trans. Rehabil. Eng. 1998, 6, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Slobounov, S.; Hallett, M.; Cao, C.; Newell, K. Modulation of cortical activity as a result of voluntary postural sway direction: An EEG study. Neurosci. Lett. 2008, 442, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Nandi, T.; Fisher, B.E.; Hortobágyi, T.; Salem, G.J. Increasing mediolateral standing sway is associated with increasing corticospinal excitability, and decreasing M1 inhibition and facilitation. Gait Posture 2018, 60, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Turcato, A.M.; Godi, M.; Giordano, A.; Schieppati, M.; Nardone, A. The generation of centripetal force when walking in a circle: Insight from the distribution of ground reaction forces recorded by plantar insoles. J. Neuroeng. Rehabil. 2015, 12, 4. [Google Scholar] [CrossRef]
- Honeine, J.L.; Schieppati, M.; Crisafulli, O.; Do, M.C. The Neuro-Mechanical Processes That Underlie Goal-Directed Medio-Lateral APA during Gait Initiation. Front. Hum. Neurosci. 2016, 10, 445. [Google Scholar] [CrossRef]
- Schieppati, M.; Grasso, M.; Siliotto, R.; Nardone, A. Effect of Age, Chronic Diseases and Parkinsonism on Postural Control. In Sensorimotor Impairment in the Elderly. NATO ASI Series; Stelmach, G.E., Hömberg, V., Eds.; Series D: Behavioural and Social Sciences; Springer: Dordrecht, The Netherlands, 1993; Volume 75, pp. 355–373. [Google Scholar] [CrossRef]
- Hilliard, M.J.; Martinez, K.M.; Janssen, I.; Edwards, B.; Mille, M.L.; Zhang, Y.; Rogers, M.W. Lateral balance factors predict future falls in community-living older adults. Arch. Phys. Med. Rehabil. 2008, 89, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Piirtola, M.; Era, P. Force platform measurements as predictors of falls among older people—A review. Gerontology 2006, 52, 1–16. [Google Scholar] [CrossRef]
- Inacio, M.; Creath, R.; Rogers, M.W. Low-dose hip abductor-adductor power training improves neuromechanical weight-transfer control during lateral balance recovery in older adults. Clin. Biomech. 2018, 60, 127–133. [Google Scholar] [CrossRef]
- Nardone, A.; Schieppati, M. Group II spindle fibres and afferent control of stance. Clues from diabetic neuropathy. Clin. Neurophysiol. 2004, 115, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Bensoussan, L.; Viton, J.M.; Schieppati, M.; Collado, H.; Milhe de Bovis, V.; Mesure, S.; Delarque, A. Changes in postural control in hemiplegic patients after stroke performing a dual task. Arch. Phys. Med. Rehabil. 2007, 88, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Corriveau, H.; Hébert, R.; Raîche, M.; Dubois, M.F.; Prince, F. Postural stability in the elderly: Empirical confirmation of a theoretical model. Arch. Gerontol. Geriatr. 2004, 39, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Eklund, G. General features of vibration-induced effects on balance. Ups. J. Med. Sci. 1972, 77, 112–124. [Google Scholar] [CrossRef]
- De Nunzio, A.M.; Nardone, A.; Picco, D.; Nilsson, J.; Schieppati, M. Alternate trains of postural muscle vibration promote cyclic body displacement in standing parkinsonian patients. Mov. Disord. 2008, 23, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, M.M.; Gilhodes, J.C.; Roll, J.P. Vibration-induced postural posteffects. J. Neurophysiol. 1998, 79, 143–150. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Wright, W.G.; Gurfinkel, V.S.; Horak, F.; Cordo, P. Interaction of involuntary post-contraction activity with locomotor movements. Exp. Brain Res. 2006, 169, 255–260. [Google Scholar] [CrossRef][Green Version]
- Pettorossi, V.E.; Panichi, R.; Botti, F.M.; Kyriakareli, A.; Ferraresi, A.; Faralli, M.; Schieppati, M.; Bronstein, A.M. Prolonged asymmetric vestibular stimulation induces opposite, long-term effects on self-motion perception and ocular responses. J. Physiol. 2013, 591, 1907–1920. [Google Scholar] [CrossRef]
- Weber, K.D.; Fletcher, W.A.; Gordon, C.R.; Melvill Jones, G.; Block, E.W. Motor learning in the “podokinetic” system and its role in spatial orientation during locomotion. Exp. Brain. Res. 1998, 120, 377–385. [Google Scholar] [CrossRef]
- Sozzi, S.; Schieppati, M. Stepping in Place While Voluntarily Turning Around Produces a Long-Lasting Posteffect Consisting in Inadvertent Turning While Stepping Eyes Closed. Neural. Plast. 2016, 2016, 7123609. [Google Scholar] [CrossRef]
- Zanetti, C.; Schieppati, M. Quiet stance control is affected by prior treadmill but not overground locomotion. Eur. J. Appl. Physiol. 2007, 100, 331–339. [Google Scholar] [CrossRef]
- Hirjaková, Z.; Bizovská, L.; Bzdúšková, D.; Hlavačka, F.; Janura, M. Postural stability after treadmill and overground walking in young and elderly. Gait Posture 2020, 80, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Gurfinkel, V.S.; Ivanenko, Y.U.P.; Levik, Y.U.S.; Babakova, I.A. Kinesthetic reference for human orthograde posture. Neuroscience 1995, 68, 229–243. [Google Scholar] [CrossRef]
- Cappa, P.; Patanè, F.; Rossi, S.; Petrarca, M.; Castelli, E.; Berthoz, A. Effect of changing visual condition and frequency of horizontal oscillations on postural balance of standing healthy subjects. Gait Posture 2008, 28, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Assländer, L.; Hettich, G.; Mergner, T. Visual contribution to human standing balance during support surface tilts. Hum. Mov. Sci. 2015, 41, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, S.; Nardone, A.; Schieppati, M. Orientation in space during quiet stance is affected by prolonged asymmetric translations of the supporting platform. In Proceedings of the RIN, 1st Annual Meeting, Milan, Italy, 24–25 March 2020; p. 183. [Google Scholar]
- Bonnet, C.T.; Davin, T.; Hoang, J.Y.; Baudry, S. Relations between Eye Movement, Postural Sway and Cognitive Involvement in Unprecise and Precise Visual Tasks. Neuroscience 2019, 416, 177–189. [Google Scholar] [CrossRef]
- Campbell, A.D.; Squair, J.W.; Chua, R.; Inglis, J.T.; Carpenter, M.G. First trial and StartReact effects induced by balance perturbations to upright stance. J. Neurophysiol. 2013, 110, 2236–2245. [Google Scholar] [CrossRef][Green Version]
- Sozzi, S.; Monti, A.; De Nunzio, A.M.; Do, M.C.; Schieppati, M. Sensori-motor integration during stance: Time adaptation of control mechanisms on adding or removing vision. Hum. Mov. Sci. 2011, 30, 172–189. [Google Scholar] [CrossRef]
- Schmid, M.; Bottaro, A.; Sozzi, S.; Schieppati, M. Adaptation to continuous perturbation of balance: Progressive reduction of postural muscle activity with invariant or increasing oscillations of the center of mass depending on perturbation frequency and vision conditions. Hum. Mov. Sci. 2011, 30, 262–278. [Google Scholar] [CrossRef]
- Sozzi, S.; Do, M.C.; Monti, A.; Schieppati, M. Sensorimotor integration during stance: Processing time of active or passive addition or withdrawal of visual or haptic information. Neuroscience 2012, 212, 59–76. [Google Scholar] [CrossRef]
- O’Keefe, D.J. Brief Report: Post Hoc Power, Observed Power, A Priori Power, Retrospective Power, Prospective Power, Achieved Power: Sorting Out Appropriate Uses of Statistical Power Analyses. Commun. Methods Meas. 2007, 1, 291–299. [Google Scholar] [CrossRef]
- De Haart, M.; Geurts, A.C.; Dault, M.C.; Nienhuis, B.; Duysens, J. Restoration of weight-shifting capacity in patients with postacute stroke: A rehabilitation cohort study. Arch. Phys. Med. Rehabil. 2005, 86, 755–762. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, A.; Zucchella, C.; Spicciato, F.; Tortola, P.; Vecchione, C.; Pierelli, F.; Bartolo, M. Biofeedback rehabilitation of posture and weightbearing distribution in stroke: A center of foot pressure analysis. Funct. Neurol. 2014, 29, 127–134. [Google Scholar] [PubMed]
- Genthon, N.; Gissot, A.S.; Froger, J.; Rougier, P.; Pérennou, D. Posturography in patients with stroke: Estimating the percentage of body weight on each foot from a single force platform. Stroke 2008, 39, 489. [Google Scholar] [CrossRef]
- Anker, L.C.; Weerdesteyn, V.; van Nes, I.J.; Nienhuis, B.; Straatman, H.; Geurts, A.C. The relation between postural stability and weight distribution in healthy subjects. Gait Posture 2008, 27, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Raymakers, J.A.; Samson, M.M.; Verhaar, H.J. The assessment of body sway and the choice of the stability parameter(s). Gait Posture 2005, 21, 48–58. [Google Scholar] [CrossRef]
- Benvenuti, F.; Mecacci, R.; Gineprari, I.; Bandinelli, S.; Benvenuti, E.; Ferrucci, L.; Baroni, A.; Rabuffetti, M.; Hallett, M.; Dambrosia, J.M.; et al. Kinematic characteristics of standing disequilibrium: Reliability and validity of a posturographic protocol. Arch. Phys. Med. Rehabil. 1999, 80, 278–287. [Google Scholar] [CrossRef]
- Paillard, T.; Noé, F. Techniques and Methods for Testing the Postural Function in Healthy and Pathological Subjects. Biomed. Res. Int. 2015, 2015, 891390. [Google Scholar] [CrossRef]
- Jacono, M.; Casadio, M.; Morasso, P.G.; Sanguineti, V. The sway-density curve and the underlying postural stabilization process. Motor Control 2004, 8, 292–311. [Google Scholar] [CrossRef]
- Yamamoto, T.; Smith, C.E.; Suzuki, Y.; Kiyono, K.; Tanahashi, T.; Sakoda, S.; Morasso, P.; Nomura, T. Universal and individual characteristics of postural sway during quiet standing in healthy young adults. Physiol. Rep. 2015, 3, e12329. [Google Scholar] [CrossRef]
- Lee, K.H.; Baksh, A.; Bryant, A.; McGowan, M.; McMillan, R.; Chong, R.K. Two Mechanisms of Sensorimotor Set Adaptation to Inclined Stance. Front. Hum. Neurosci. 2017, 11, 480. [Google Scholar] [CrossRef]
- Lee, Y.J.; Liang, J.N.; Chen, B.; Aruin, A.S. Characteristics of medial-lateral postural control while exposed to the external perturbation in step initiation. Sci. Rep. 2019, 9, 16817. [Google Scholar] [CrossRef] [PubMed]
- Hof, A.L.; Duysens, J. Responses of human hip abductor muscles to lateral balance perturbations during walking. Exp. Brain Res. 2013, 230, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Mühlbeier, A.; Puta, C.; Boström, K.J.; Wagner, H. Monosynaptic Stretch Reflex Fails to Explain the Initial Postural Response to Sudden Lateral Perturbations. Front. Hum. Neurosci. 2017, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Aruin, A.S. The effect of asymmetry of posture on anticipatory postural adjustments. Neurosci. Lett. 2006, 401, 150–153. [Google Scholar] [CrossRef]
- Bouisset, S.; Do, M.C. Posture, dynamic stability, and voluntary movement. Neurophysiol. Clin. 2008, 38, 345–362. [Google Scholar] [CrossRef]
- Goodworth, A.D.; Peterka, R.J. Influence of stance width on frontal plane postural dynamics and coordination in human balance control. J. Neurophysiol. 2010, 104, 1103–1118. [Google Scholar] [CrossRef]
- Sozzi, S.; Nardone, A.; Schieppati, M. Adaptation of balancing behaviour during continuous perturbations of stance. Supra-postural visual tasks and platform translation frequency modulate adaptation rate. PLoS ONE 2020, 15, e0236702. [Google Scholar] [CrossRef]
- Fransson, P.A.; Hafström, A.; Karlberg, M.; Magnusson, M.; Tjäder, A.; Johansson, R. Postural control adaptation during galvanic vestibular and vibratory proprioceptive stimulation. IEEE Trans. Biomed. Eng. 2003, 50, 1310–1319. [Google Scholar] [CrossRef]
- Schieppati, M.; Giordano, A.; Nardone, A. Variability in a dynamic postural task attests ample flexibility in balance control mechanisms. Exp. Brain Res. 2002, 144, 200–210. [Google Scholar] [CrossRef]
- Berencsi, A.; Ishihara, M.; Imanaka, K. The functional role of central and peripheral vision in the control of posture. Hum. Mov. Sci. 2005, 24, 689–709. [Google Scholar] [CrossRef]
- Albertsen, I.M.; Ghédira, M.; Gracies, J.M.; Hutin, É. Postural stability in young healthy subjects—Impact of reduced base of support, visual deprivation, dual tasking. J. Electromyogr. Kinesiol. 2017, 33, 27–33. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, A.M.; Zanetti, C.; Schieppati, M. Post-effect of forward and backward locomotion on body orientation in space during quiet stance. Eur. J. Appl. Physiol. 2009, 105, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Cofré Lizama, L.E.; Pijnappels, M.; Reeves, N.P.; Verschueren, S.M.; van Dieën, J.H. Can explicit visual feedback of postural sway efface the effects of sensory manipulations on mediolateral balance performance? J. Neurophysiol. 2016, 115, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Falvo, M.J.; Schmidt, H.E.; Horak, F.B.; Earhart, G.M. Influence of visual and haptic cues on podokinetic after-rotation. J. Mot. Behav. 2009, 41, 553–560. [Google Scholar] [CrossRef]
- Kluzik, J.; Horak, F.B.; Peterka, R.J. Differences in preferred reference frames for postural orientation shown by after-effects of stance on an inclined surface. Exp. Brain Res. 2005, 162, 474–489. [Google Scholar] [CrossRef]
- Earhart, G.M.; Henckens, J.M.; Carlson-Kuhta, P.; Horak, F.B. Influence of vision on adaptive postural responses following standing on an incline. Exp. Brain Res. 2010, 203, 221–226. [Google Scholar] [CrossRef]
- Corna, S.; Tarantola, J.; Nardone, A.; Giordano, A.; Schieppati, M. Standing on a continuously moving platform: Is body inertia counteracted or exploited? Exp. Brain Res. 1999, 124, 331–341. [Google Scholar] [CrossRef]
- Nardone, A.; Grasso, M.; Tarantola, J.; Corna, S.; Schieppati, M. Postural coordination in elderly subjects standing on a periodically moving platform. Arch. Phys. Med. Rehabil. 2000, 81, 1217–1223. [Google Scholar] [CrossRef]
- De Nunzio, A.M.; Schieppati, M. Time to reconfigure balancing behaviour in man: Changing visual condition while riding a continuously moving platform. Exp. Brain Res. 2007, 178, 18–36. [Google Scholar] [CrossRef]
- Sozzi, S.; Nardone, A.; Schieppati, M. Vision Does Not Necessarily Stabilize the Head in Space during Continuous Postural Perturbations. Front. Neurol. 2019, 10, 748. [Google Scholar] [CrossRef]
- O’Connor, S.M.; Kuo, A.D. Direction-dependent control of balance during walking and standing. J. Neurophysiol. 2009, 102, 1411–1419. [Google Scholar] [CrossRef]
- Hatzitaki, V.; Amiridis, I.G.; Nikodelis, T.; Spiliopoulou, S. Direction-induced effects of visually guided weight-shifting training on standing balance in the elderly. Gerontology 2009, 55, 145–152. [Google Scholar] [CrossRef]
- Rasouli, O.; Solnik, S.; Furmanek, M.P.; Piscitelli, D.; Falaki, A.; Latash, M.L. Unintentional drifts during quiet stance and voluntary body sway. Exp. Brain Res. 2017, 235, 2301–2316. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, S.; Nardone, A.; Schieppati, M. Calibration of the Leg Muscle Responses Elicited by Predictable Perturbations of Stance and the Effect of Vision. Front. Hum. Neurosci. 2016, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Duclos, C.; Roll, R.; Kavounoudias, A.; Roll, J.P. Cerebral correlates of the “Kohnstamm phenomenon”: An fMRI study. Neuroimage 2007, 34, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.P.; Beyer, K.B.; Williams, L.; Miyasike-daSilva, V.; McIlroy, W.E. Standing still: Is there a role for the cortex? Neurosci. Lett. 2015, 590, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Massa, R.E.; Rosso, A.; Metti, A.L.; Sparto, P.J.; Aizenstein, H.; Ferrucci, L.; Divecha, A.; Rosano, C. Health ABC Study. Neuroimaging correlates of lateral postural control in older ambulatory adults. Aging Clin. Exp. Res. 2019, 31, 611–619. [Google Scholar] [CrossRef]
- Sozzi, S.; Nardone, A.; Crisafulli, O.; Schieppati, M. Podokinetic After-Rotation Is Transiently Enhanced or Reversed by Unilateral Axial Muscle Proprioceptive Stimulation. Neural. Plast. 2019, 2019, 7129279. [Google Scholar] [CrossRef]
- Pettorossi, V.E.; Schieppati, M. Neck proprioception shapes body orientation and perception of motion. Front. Hum. Neurosci. 2014, 8, 895. [Google Scholar] [CrossRef]
- Day, B.L.; Steiger, M.J.; Thompson, P.D.; Marsden, C.D. Effect of vision and stance width on human body motion when standing: Implications for afferent control of lateral sway. J. Physiol. 1993, 469, 479–499. [Google Scholar] [CrossRef]
- Bonnet, C.T.; Cherraf, S.; Szaffarczyk, S.; Rougier, P.R. The contribution of body weight distribution and center of pressure location in the control of mediolateral stance. J. Biomech. 2014, 47, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Peterka, R.J.; Loughlin, P.J. Dynamic regulation of sensorimotor integration in human postural control. J Neurophysiol. 2004, 91, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M.; Lang, C.E.; Zeiler, S.; Byblow, W.D. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020, 19, 348–360. [Google Scholar] [CrossRef]
- Barra, J.; Oujamaa, L.; Chauvineau, V.; Rougier, P.; Pérennou, D. Asymmetric standing posture after stroke is related to a biased egocentric coordinate system. Neurology 2009, 72, 1582–1587. [Google Scholar] [CrossRef]
- Hsiao, H.; Gray, V.L.; Creath, R.A.; Binder-Macleod, S.A.; Rogers, M.W. Control of lateral weight transfer is associated with walking speed in individuals post-stroke. J. Biomech. 2017, 60, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Curuk, E.; Lee, Y.; Aruin, A.S. Individuals with stroke improve anticipatory postural adjustments after a single session of targeted exercises. Hum. Mov. Sci. 2020, 69, 102559. [Google Scholar] [CrossRef]
- Aman, J.E.; Elangovan, N.; Yeh, I.L.; Konczak, J. The effectiveness of proprioceptive training for improving motor function: A systematic review. Front. Hum. Neurosci. 2015, 8, 1075. [Google Scholar] [CrossRef]
- Frame, H.B.; Finetto, C.; Dean, J.C.; Neptune, R.R. The influence of lateral stabilization on walking performance and balance control in neurologically-intact and post-stroke individuals. Clin. Biomech. 2020, 73, 172–180. [Google Scholar] [CrossRef]
- Ribeiro, F.; Oliveira, J. Aging effects on joint proprioception: The role of physical activity in proprioception preservation. Eur. Rev. Aging Phys. Act. 2007, 4, 71–76. [Google Scholar] [CrossRef]
- Bonan, I.V.; Yelnik, A.P.; Colle, F.M.; Michaud, C.; Normand, E.; Panigot, B.; Roth, P.; Guichard, J.P.; Vicaut, E. Reliance on visual information after stroke. Part II: Effectiveness of a balance rehabilitation program with visual cue deprivation after stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2004, 85, 274–278. [Google Scholar] [CrossRef]

| ML | AP | |||||
|---|---|---|---|---|---|---|
| Pre-Stimulation | Post-Stimulation | Pre-Stimulation | Post-Stimulation | |||
| FL–EC | 0.20 cm ± 0.13 | 0.37 cm ± 0.32 | p < 0.01 | 0.39 cm ± 0.12 | 0.48 cm ± 0.15 | p < 0.01 |
| FL–EO | 0.22 cm ± 0.15 | 0.30 cm ± 0.17 | p < 0.05 | 0.37 cm ± 0.13 | 0.39 cm ± 0.13 | p = 0.42 |
| FR–EC | 0.22 cm ± 0.12 | 0.33 cm ± 0.15 | p < 0.01 | 0.42 cm ± 0.14 | 0.53 cm ± 0.18 | p < 0.01 |
| FR–EO | 0.23 cm ± 0.15 | 0.31 cm ± 0.19 | p < 0.05 | 0.39 cm ± 0.14 | 0.45 cm ± 0.18 | p = 0.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sozzi, S.; Nardone, A.; Corna, S.; Schieppati, M. Post-Effect on the Centre of Feet Pressure during Stance by Continuous Asymmetric Mediolateral Translations of a Supporting Platform—A Preliminary Study in Healthy Young Adults. Appl. Sci. 2020, 10, 5969. https://doi.org/10.3390/app10175969
Sozzi S, Nardone A, Corna S, Schieppati M. Post-Effect on the Centre of Feet Pressure during Stance by Continuous Asymmetric Mediolateral Translations of a Supporting Platform—A Preliminary Study in Healthy Young Adults. Applied Sciences. 2020; 10(17):5969. https://doi.org/10.3390/app10175969
Chicago/Turabian StyleSozzi, Stefania, Antonio Nardone, Stefano Corna, and Marco Schieppati. 2020. "Post-Effect on the Centre of Feet Pressure during Stance by Continuous Asymmetric Mediolateral Translations of a Supporting Platform—A Preliminary Study in Healthy Young Adults" Applied Sciences 10, no. 17: 5969. https://doi.org/10.3390/app10175969
APA StyleSozzi, S., Nardone, A., Corna, S., & Schieppati, M. (2020). Post-Effect on the Centre of Feet Pressure during Stance by Continuous Asymmetric Mediolateral Translations of a Supporting Platform—A Preliminary Study in Healthy Young Adults. Applied Sciences, 10(17), 5969. https://doi.org/10.3390/app10175969






