Synthesis, Characterization and Mechanical Properties of Nanocomposites Based on Novel Carbon Nanowires and Polystyrene
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Synthesis of Hard Template MCM-41
2.3. Synthesis of Carbon Nanowires (CNWs)
2.4. Chemical Oxidation and Surface Modification of CNWs
2.5. Preparation of the Nanocomposites
2.6. Characterization
3. Results
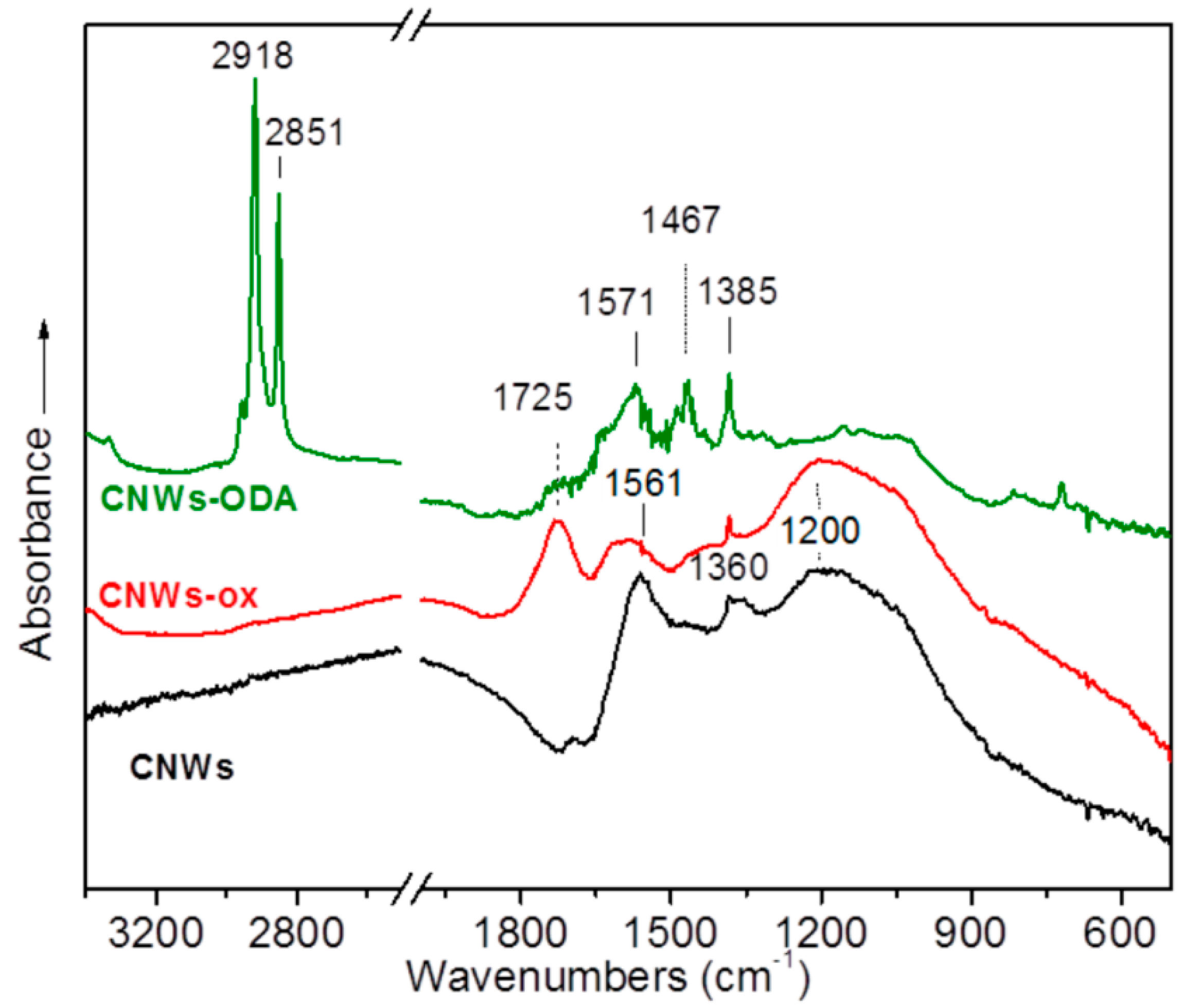
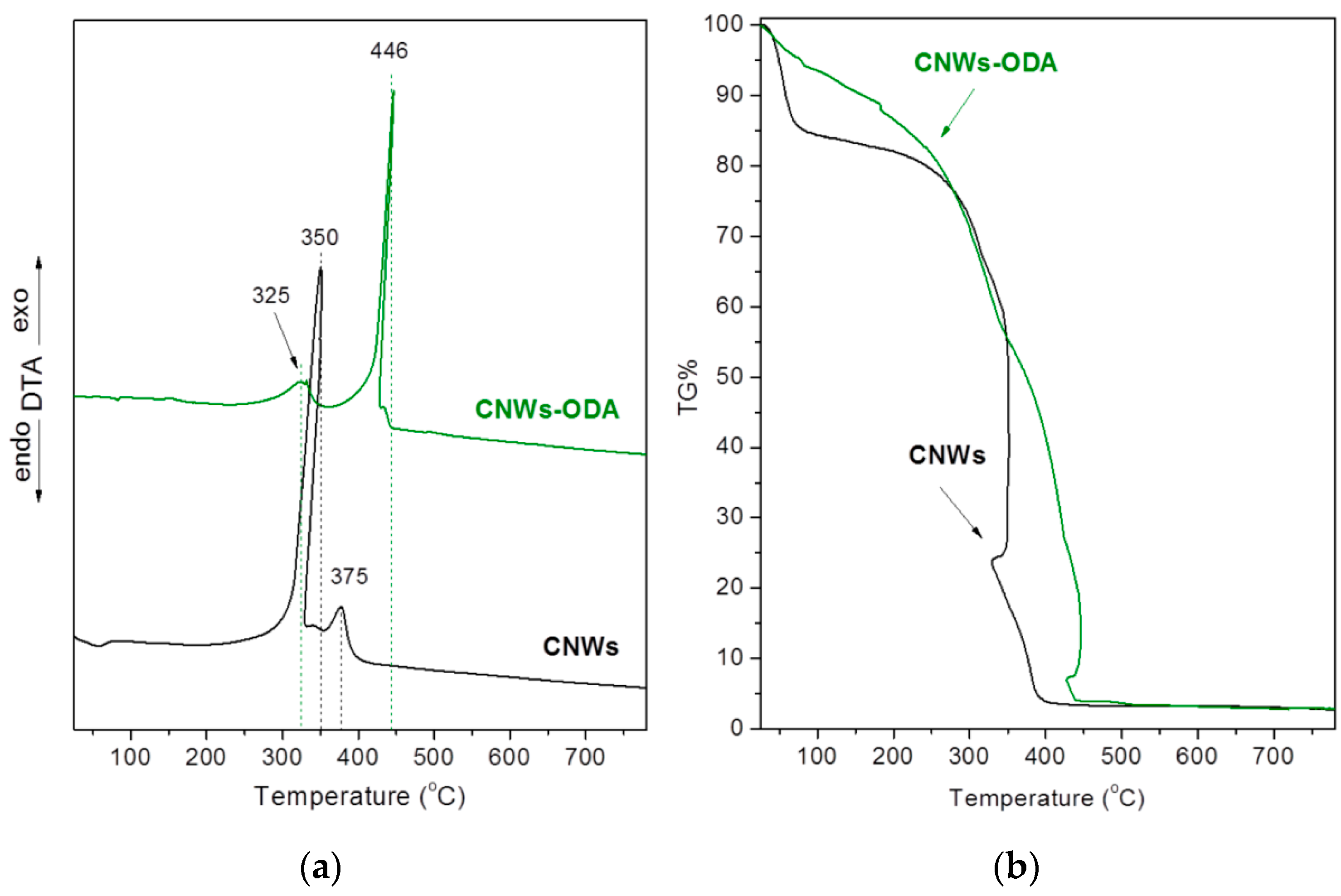
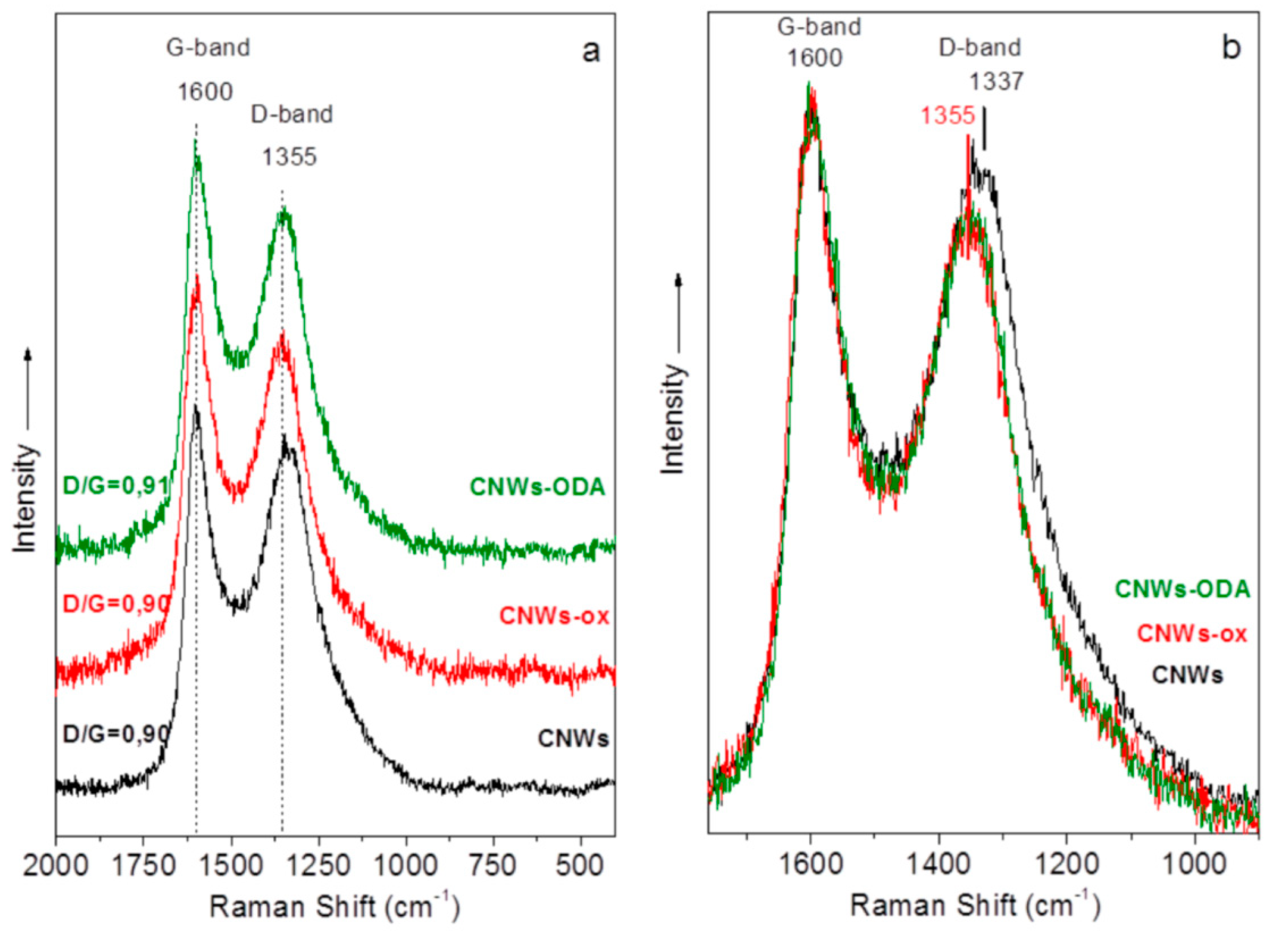
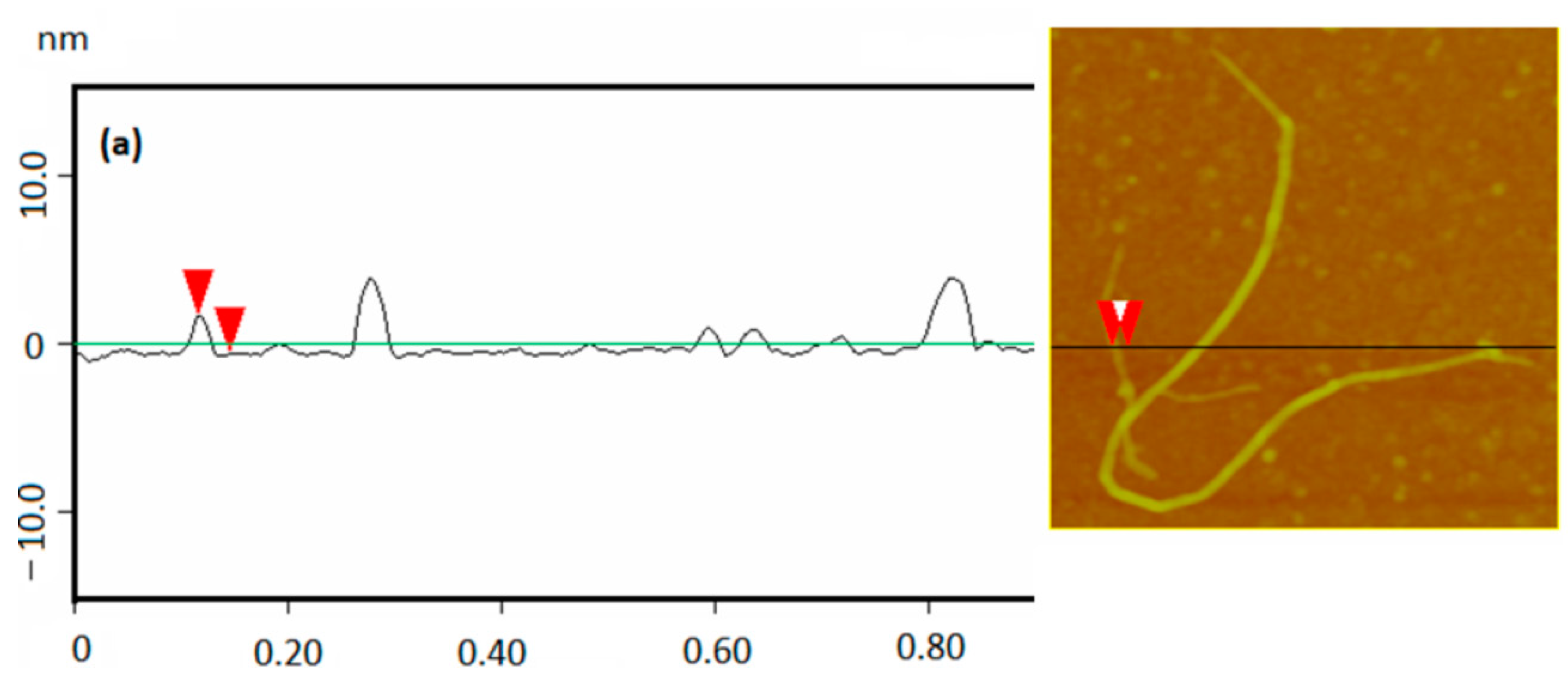
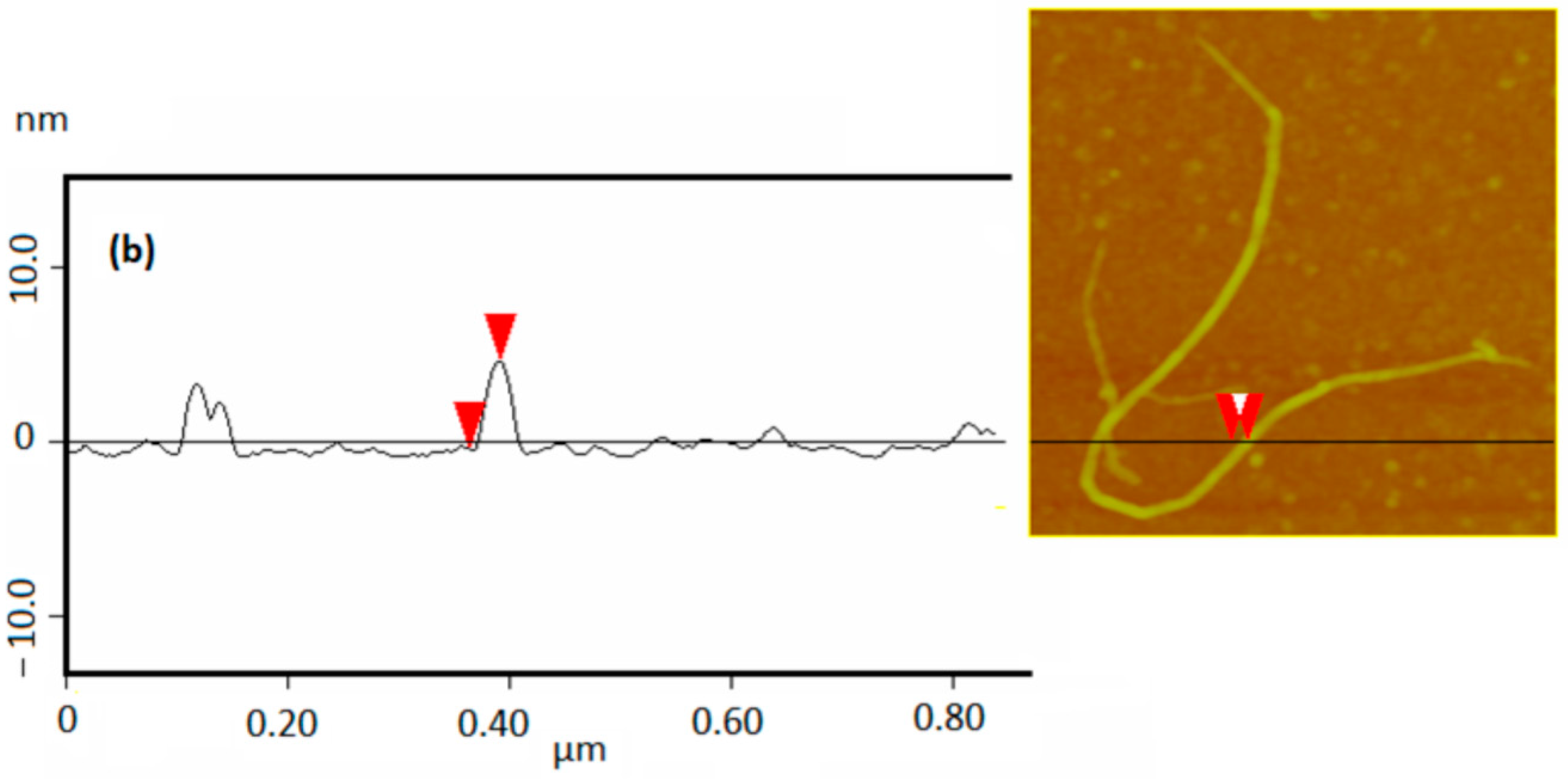
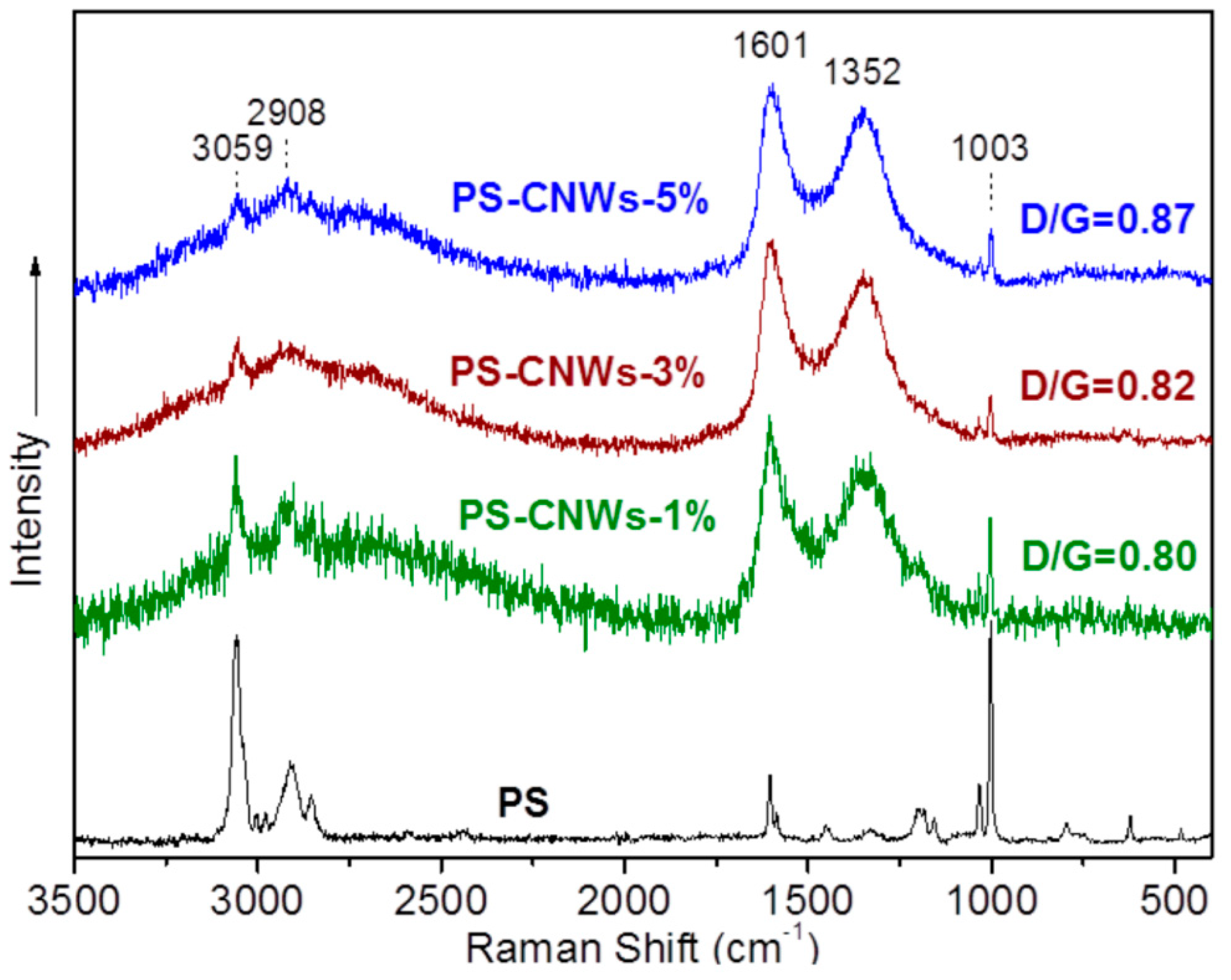
3.1. Structural Characterization and Material Properties of the Nanofillers
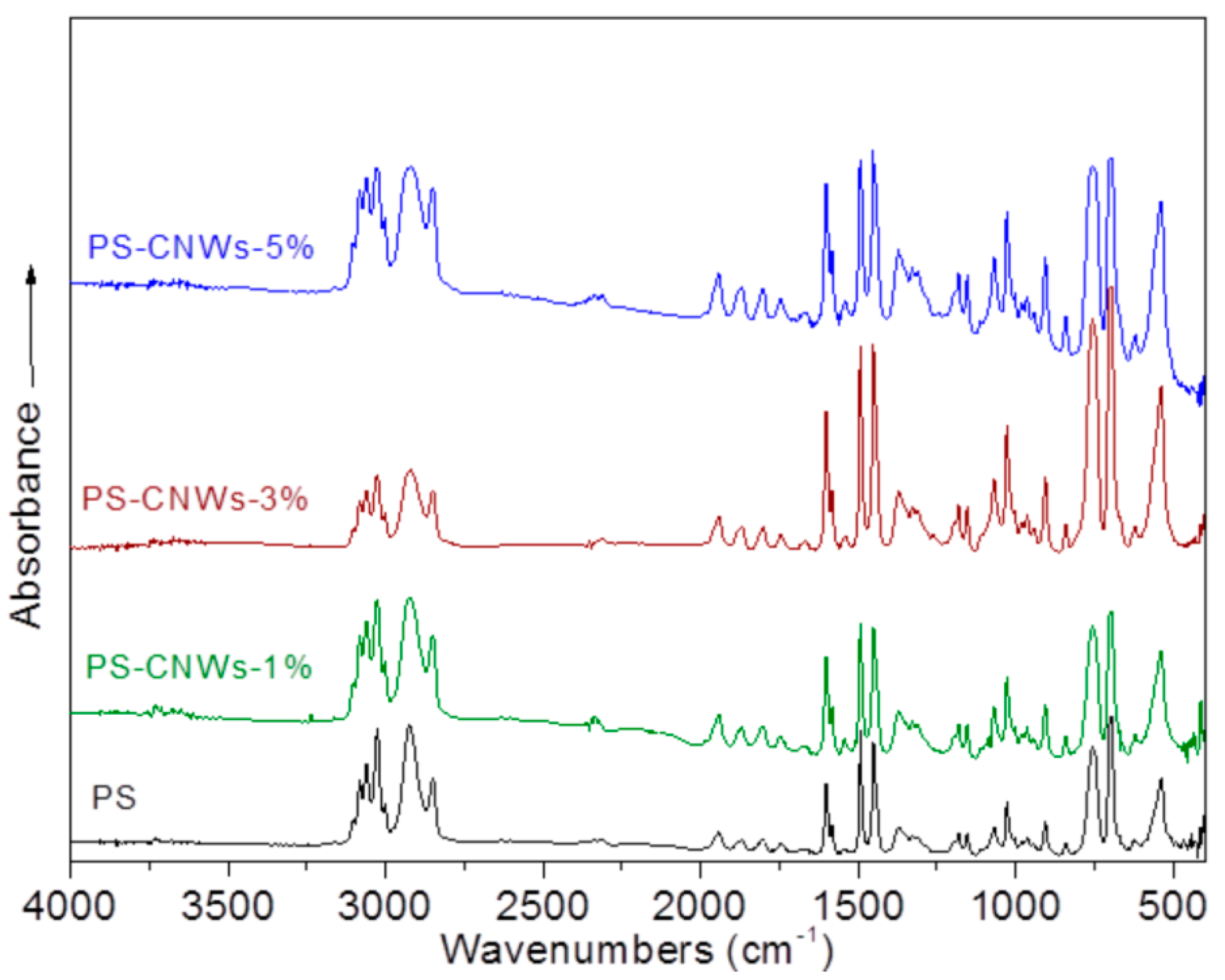
3.2. Structural Characterization and Material Properties of the Nanocomposites
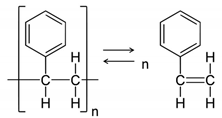
3.3. Mechanical Strength of PS-CNWs Nanocomposites
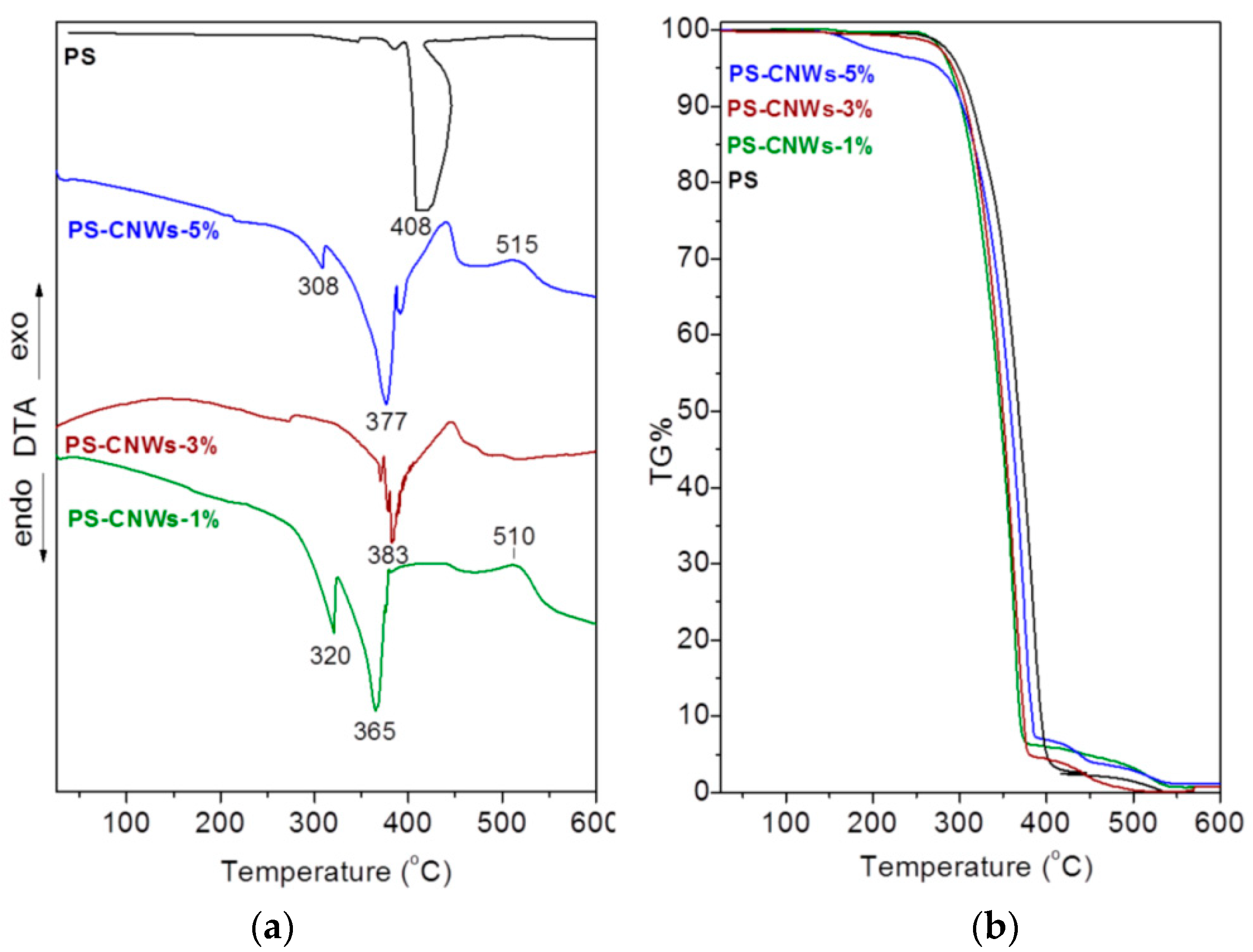
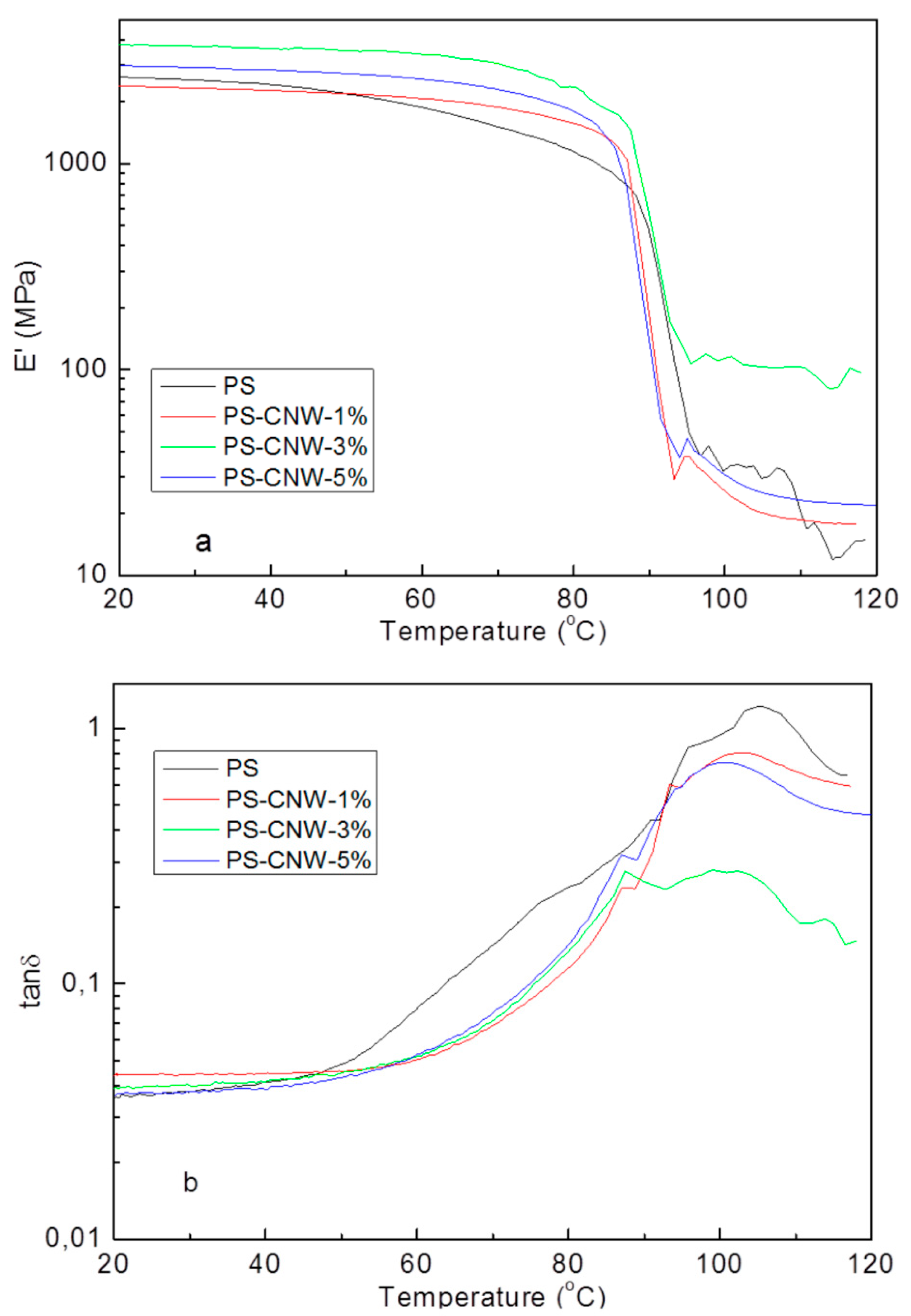
3.4. Thermomechanical Measurements on PS-CNWs Nanocomposites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Crosby, A.J.; Lee, J.Y. Polymer Nanocomposites: The “Nano” Effect on Mechanical Properties. Polym. Rev. 2007, 47, 217–229. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, M.C.; Dudley, K.L.; Lawrence, R.W. Novel Carbon Nanotube−Polystyrene Foam Composites for Electromagnetic Interference Shielding. Nano Lett. 2005, 5, 2131–2134. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Fu, Y.Q.; Ahmad, M.; Luo, J.K.; Huang, W.M.; Kraft, A.; Reuben, R.; Pei, Y.T.; Chen, Z.G.; De Hosson, J.T.M. Thermo-mechanical properties of polystyrene-based shape memory nanocomposites. J. Mater. Chem. 2010, 20, 3442–3448. [Google Scholar] [CrossRef]
- Umek, P.; Huskić, M.; Škapin, A.S.; Florjančič, U.; Zupančič, B.; Emri, I.; Arčon, D. Structural and mechanical properties of polystyrene nanocomposites with 1D titanate nanostructures prepared by an extrusion process. Polym. Compos. 2009, 30, 1318–1325. [Google Scholar] [CrossRef]
- Suresh, K.; Kumar, M.; Pugazhenthi, G.; Uppaluri, R. Enhanced mechanical and thermal properties of polystyrene nanocomposites prepared using organo-functionalized NiAl layered double hydroxide via melt intercalation technique. J. Sci. Adv. Mater. Devices 2017, 2, 245–254. [Google Scholar] [CrossRef]
- Moskalyuk, O.A.; Belashov, A.V.; Beltukov, Y.M.; Ivan’kova, E.M.; Popova, E.N.; Semenova, I.V.; Yelokhovsky, V.Y.; Yudin, V.E. Polystyrene-based nanocomposites with different fillers: Fabrication and mechanical properties. arXiv 2020, arXiv:2005.03360, 1–2. [Google Scholar]
- Thostenson, E.T.; Chou, T.W. On the elastic properties of carbon nanotube-based composites: Modelling and characterization. J. Phys. D Appl. Phys. 2003, 36, 573–582. [Google Scholar] [CrossRef]
- Fragneaud, B.; Masenelli-Varlot, K.; Gonzalez-Montiel, A.; Terrones, M.; Cavaillé, J.Y. Mechanical behavior of polystyrene grafted carbon nanotubes/polystyrene nanocomposites. Compos. Sci. Technol. 2008, 68, 3265–3271. [Google Scholar] [CrossRef][Green Version]
- Poncharal, P.; Wang, Z.L.; Ugarte, D.; De Heer, W.A. Electrostatic deflections and electromechanical resonances of carbon nanotubes. Science 1999, 283, 1513–1516. [Google Scholar] [CrossRef]
- Okamoto, M.; Morita, S.; Taguchi, H.; Kim, Y.H.; Kotaka, T.; Tateyama, H. Synthesis and structure of smectic clay/poly(methyl methacrylate) and clay/polystyrene nanocomposites via in situ intercalative polymerization. Polymer 2000, 41, 3887–3890. [Google Scholar] [CrossRef]
- Haider, S.; Kausar, A.; Muhammad, B. Overview of various sorts of polymer nanocomposite reinforced with layered silicate. Polym. Plast. Technol. Eng. 2016, 55, 723–743. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.G.; Yan, D.X.; Chen, T.; Zeng, B.Q.; Li, Z.M. Improved properties of highly oriented graphene/polymer nanocomposites. J. Appl. Polym. Sci. 2011, 121, 3167–3174. [Google Scholar] [CrossRef]
- Morales-Teyssier, O.; Sánchez-Valdes, S.; Ramos-de Volle, L.F. Effect of carbon nanofiber functionalization on the dispersion and physical and mechanical properties of polystyrene nanocomposites. Macromol. Mater. Eng. 2006, 291, 1547–1555. [Google Scholar] [CrossRef]
- Shi, D.; Lian, J.; He, P.; Wang, L.M.; Xiao, F.; Yang, L.; Schulz, M.J.; Mast, D.B. Plasma coating of carbon nanofibers for enhanced dispersion and interfacial bonding in polymer composites. Appl. Phys. Lett. 2003, 83, 5301–5303. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Dimos, K.; Koutselas, I.B.; Karakassides, M.A. Synthesis and Characterization of ZnS Nanosized Semiconductor Particles within Mesoporous Solids. J. Phys. Chem. B 2006, 110, 22339–22345. [Google Scholar] [CrossRef]
- Baikousi, M.; Bourlinos, A.B.; Douvalis, A.; Bakas, T.; Anagnostopoulos, D.F.; Tuček, J.; Šafářová, K.; Zboril, R.; Karakassides, M.A. Synthesis and Characterization of γ-Fe2O3/Carbon Hybrids and Their Application in Removal of Hexavalent Chromium Ions from Aqueous Solutions. Langmuir 2012, 28, 3918–3930. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Gournis, D.; Petridis, D.; Szabó, T.; Szeri, A.; Dékány, I. Graphite Oxide: Chemical Reduction to Graphite and Surface Modification with Primary Aliphatic Amines and Amino Acids. Langmuir 2003, 19, 6050–6055. [Google Scholar] [CrossRef]
- Enotiadis, A.; Litina, K.; Gournis, D.; Rangou, S.; Avgeropoulos, A.; Xidas, P.; Triantafyllidis, K. Nanocomposites of Polystyrene-b-Poly(isoprene)-b-Polystyrene Triblock Copolymer with Clay–Carbon Nanotube Hybrid Nanoadditives. J. Phys. Chem. B 2013, 117, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric sufactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Karakassides, M.A.; Stathi, P.; Deligiannakis, Y.; Zboril, R.; Dallas, P.; Steriotis, T.A.; Stubos, A.K.; Trapalis, C. Pyrolytic formation of a carbonaceous solid for heavy metal adsorption. J. Mater. Sci. 2011, 46, 975–982. [Google Scholar] [CrossRef]
- Sokoll, R.; Hobert, H. Infrared study of the adsorption of octadecylamine at the MgO/CCl4 interface. A comparison with investigations of the adsorption of octadecylamine on SiO2 and Al2O3. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1986, 82, 1527–1535. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, J.; Ezzeddine, A.; Mahfouz, R.; Al-Shahrani, A.; Alabedi, G.; Khashab, N. Superior Performance Nanocomposites from Uniformly Dispersed Octadecylamine Functionalized Multi-Walled Carbon Nanotubes. C J. Carbon Res. 2015, 1, 58–76. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Wang, S.; Zhang, Y. Preparation and properties of octadecylamine modified graphene oxide/styrene-butadiene rubber composites through an improved melt compounding method. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- McIntosh, G.J.; Metson, J.B. Surface area characteristics of furfuryl-alcohol-derived inverse opal carbons produced from silica inverse opal templates. J. Mater. Sci. 2016, 51, 2573–2584. [Google Scholar] [CrossRef]
- Tan, S.; Zou, W.; Jiang, F.; Tan, S.; Liu, Y.; Yuan, D. Facile fabrication of copper-supported ordered mesoporous carbon for antibacterial behavior. Mater. Lett. 2010, 64, 2163–2166. [Google Scholar] [CrossRef]
- Rinki, K.; Dutta, P.K.; Hunt, A.J.; MacQuarrie, D.J.; Clark, J.H. Chitosan aerogels exhibiting high surface area for biomedical application: Preparation, characterization, and antibacterial study. Int. J. Polym. Mater. Polym. Biomater. 2011, 60, 988–999. [Google Scholar] [CrossRef]
- P’Yanova, L.G.; Baklanova, O.N.; Likholobov, V.A.; Drozdov, V.A.; Salanov, A.N.; Talzi, V.P.; Sedanova, A.V.; Knyazheva, O.A. Studies of the effect of surface modification of carbon sorbents by poly-N-vinylpyrrolidone using a complex of physicochemical and microbiological methods. Prot. Met. Phys. Chem. Surf. 2013, 49, 430–439. [Google Scholar] [CrossRef]
- Kim, T.W.; Park, I.S.; Ryoo, R. A synthetic route to ordered mesoporous carbon materials with graphitic pore walls. Angew. Chem. Int. Ed. 2003, 42, 4375–4379. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.D. ISU MatE453/MSE 553—Lab 3 FTIR. Available online: https://slideplayer.com/slide/4495580/ (accessed on 18 August 2020).
- Grassie, N. Chemistry of High Polymer Degradation Processes. L. Butterworths Publ. 1956, 335. [Google Scholar]
- Still, R.H. Some problems associated with the application of thermal methods to polymers. Br. Polym. J. 1979, 11, 101–114. [Google Scholar] [CrossRef]
- Dauengauer, S.A.; Utkina, O.G.; Popova, G.S.; Sazanov, Y.N. Investigation of thermal degradation of polystyrene with the aid of thermal analysis. J. Therm. Anal. 1987, 32, 311–314. [Google Scholar] [CrossRef]
- Vinu, A.; Srinivasu, P.; Takahashi, M.; Mori, T.; Balasubramanian, V.V.; Ariga, K. Controlling the textural parameters of mesoporous carbon materials. Microporous Mesoporous Mater. 2007, 100, 20–26. [Google Scholar] [CrossRef]
- Panagiotou, K. Science and Technology of Polymers; Pegasus: Thessaloniki, Greece, 2006. [Google Scholar]
- Rieger, J. The glass transition temperature of polystyrene. Results of a round robin test. J. Therm. Anal. 1996, 46, 965–972. [Google Scholar] [CrossRef]
- Amr, I.T.; Al-Amer, A.; Selvin, T.P.; Al-Harthi, M.; Girei, S.A.; Sougrat, R.; Atieh, M.A. Effect of acid treated carbon nanotubes on mechanical, rheological and thermal properties of polystyrene nanocomposites. Compos. Part B Eng. 2011, 42, 1554–1561. [Google Scholar] [CrossRef]


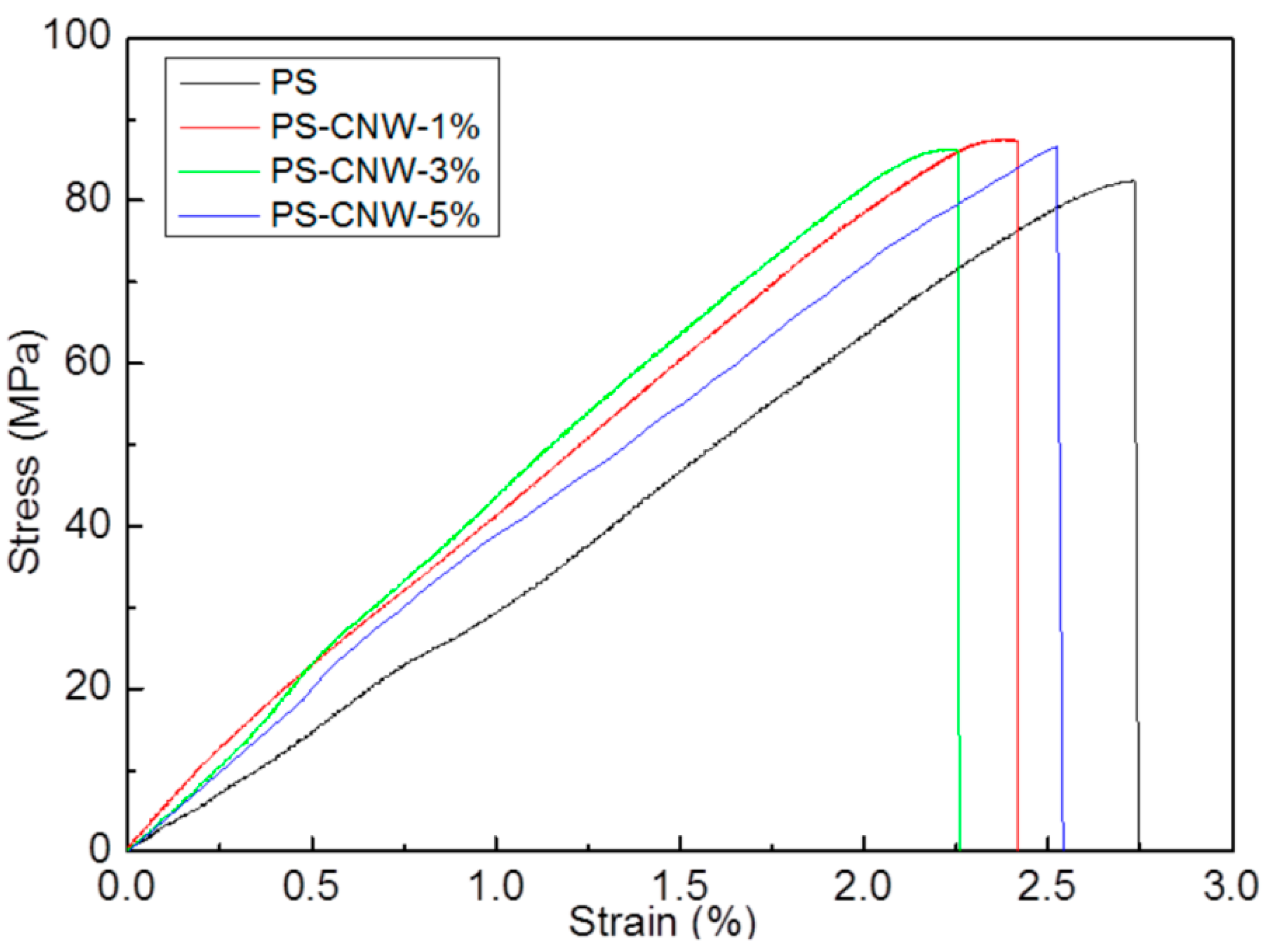
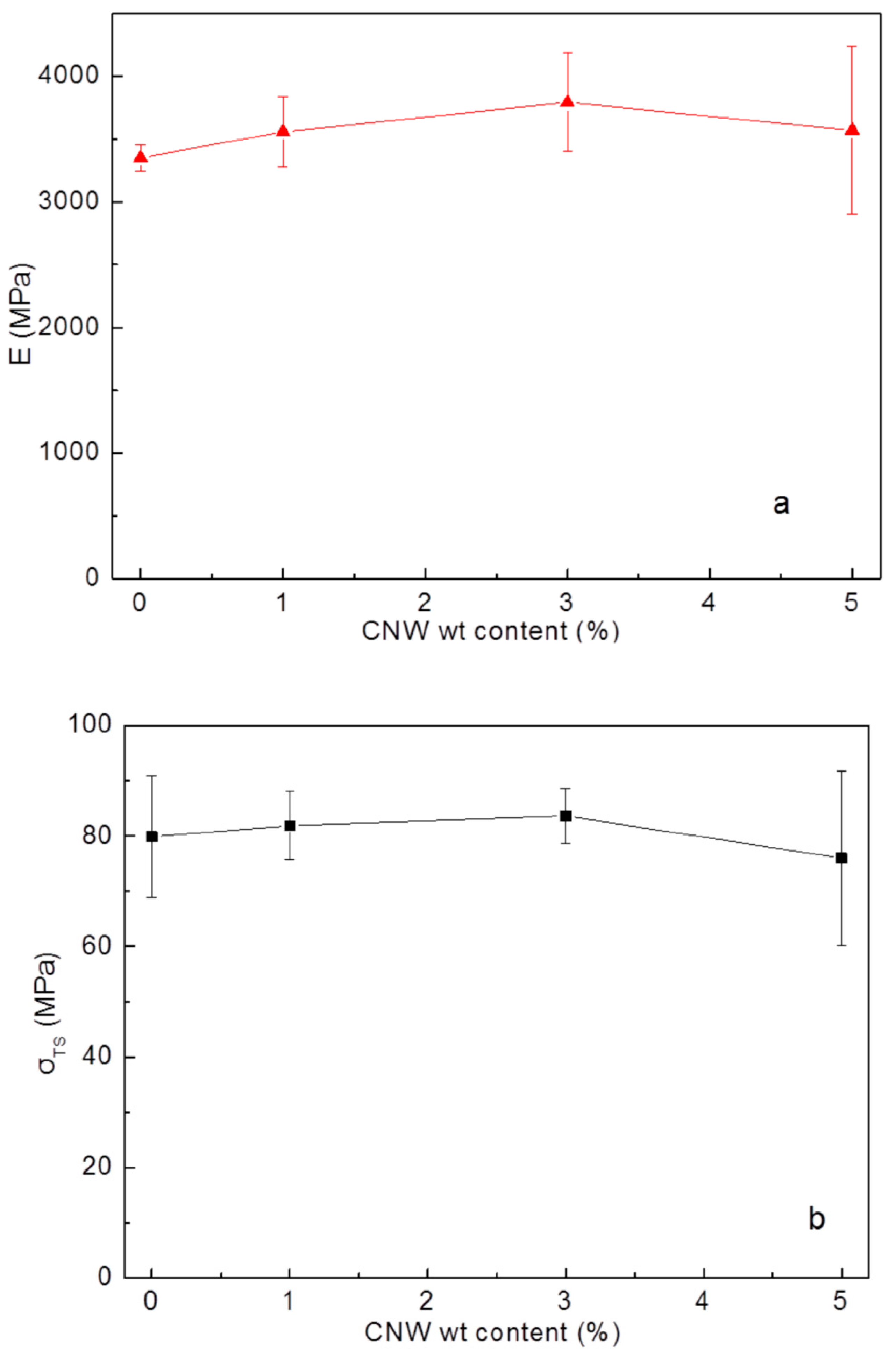
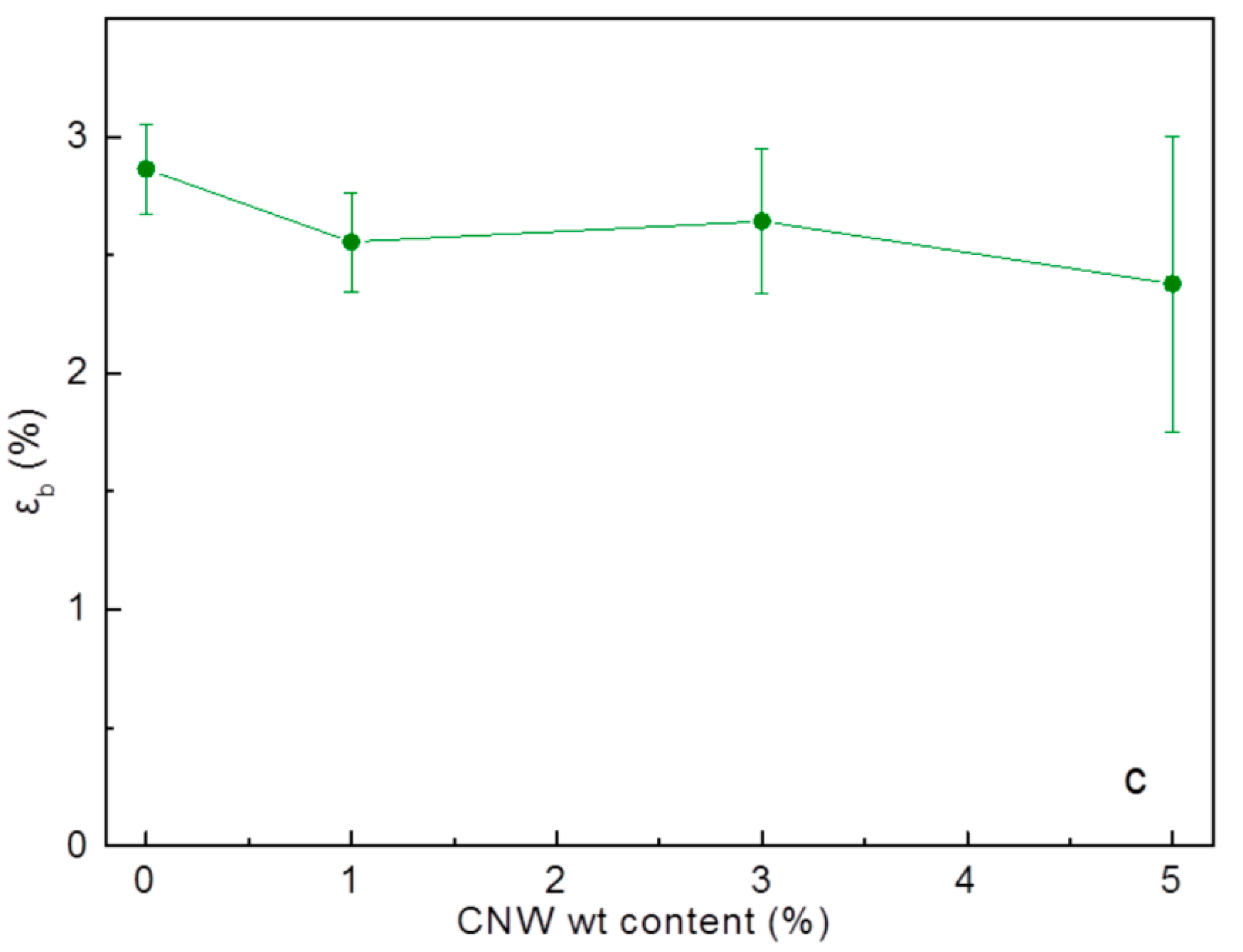
| Sample | Concentration of CNWs (%) | Stiffness (MPa) | Tensile Strength (MPa) | Distortion to Breakage (%) |
|---|---|---|---|---|
| PS | 0 | 3349 ± 107 | 79.87 ± 11.02 | 2.86 ± 0.19 |
| PS-CNWs-1% | 1 | 3557 ± 278 | 81.90 ± 6.23 | 2.56 ± 0.21 |
| PS-CNWs-3% | 3 | 3792 ± 394 | 83.63 ± 5.04 | 2.64 ± 0.31 |
| PS-CNWs-5% | 5 | 3568 ± 669 | 76.00 ± 15.77 | 2.38 ± 0.62 |
| Sample | Concentration of CNWs (%) | Tg (°C) | Storage Modulus @ 40 °C (MPa) | Storage Modulus @ 110 °C (MPa) |
|---|---|---|---|---|
| PS | 0 | 105 | 2418 | 20 |
| PS-CNWs-1% | 1 | 102 | 2261 | 19 |
| PS-CNWs-3% | 3 | 101 | 3608 | 102 |
| PS-CNWs-5% | 5 | 100 | 2848 | 23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostas, V.; Baikousi, M.; Barkoula, N.-M.; Giannakas, A.; Kouloumpis, A.; Avgeropoulos, A.; Gournis, D.; Karakassides, M.A. Synthesis, Characterization and Mechanical Properties of Nanocomposites Based on Novel Carbon Nanowires and Polystyrene. Appl. Sci. 2020, 10, 5737. https://doi.org/10.3390/app10175737
Kostas V, Baikousi M, Barkoula N-M, Giannakas A, Kouloumpis A, Avgeropoulos A, Gournis D, Karakassides MA. Synthesis, Characterization and Mechanical Properties of Nanocomposites Based on Novel Carbon Nanowires and Polystyrene. Applied Sciences. 2020; 10(17):5737. https://doi.org/10.3390/app10175737
Chicago/Turabian StyleKostas, Vasilis, Maria Baikousi, Nektaria-Marianthi Barkoula, Aris Giannakas, Antonios Kouloumpis, Apostolos Avgeropoulos, Dimitrios Gournis, and Michael A. Karakassides. 2020. "Synthesis, Characterization and Mechanical Properties of Nanocomposites Based on Novel Carbon Nanowires and Polystyrene" Applied Sciences 10, no. 17: 5737. https://doi.org/10.3390/app10175737
APA StyleKostas, V., Baikousi, M., Barkoula, N.-M., Giannakas, A., Kouloumpis, A., Avgeropoulos, A., Gournis, D., & Karakassides, M. A. (2020). Synthesis, Characterization and Mechanical Properties of Nanocomposites Based on Novel Carbon Nanowires and Polystyrene. Applied Sciences, 10(17), 5737. https://doi.org/10.3390/app10175737










