The Potential of X-ray Photoelectron Spectroscopy for Determining Interface Dipoles of Self-Assembled Monolayers
Abstract
:1. Introduction
2. Computational Methodology
3. Results and Discussion
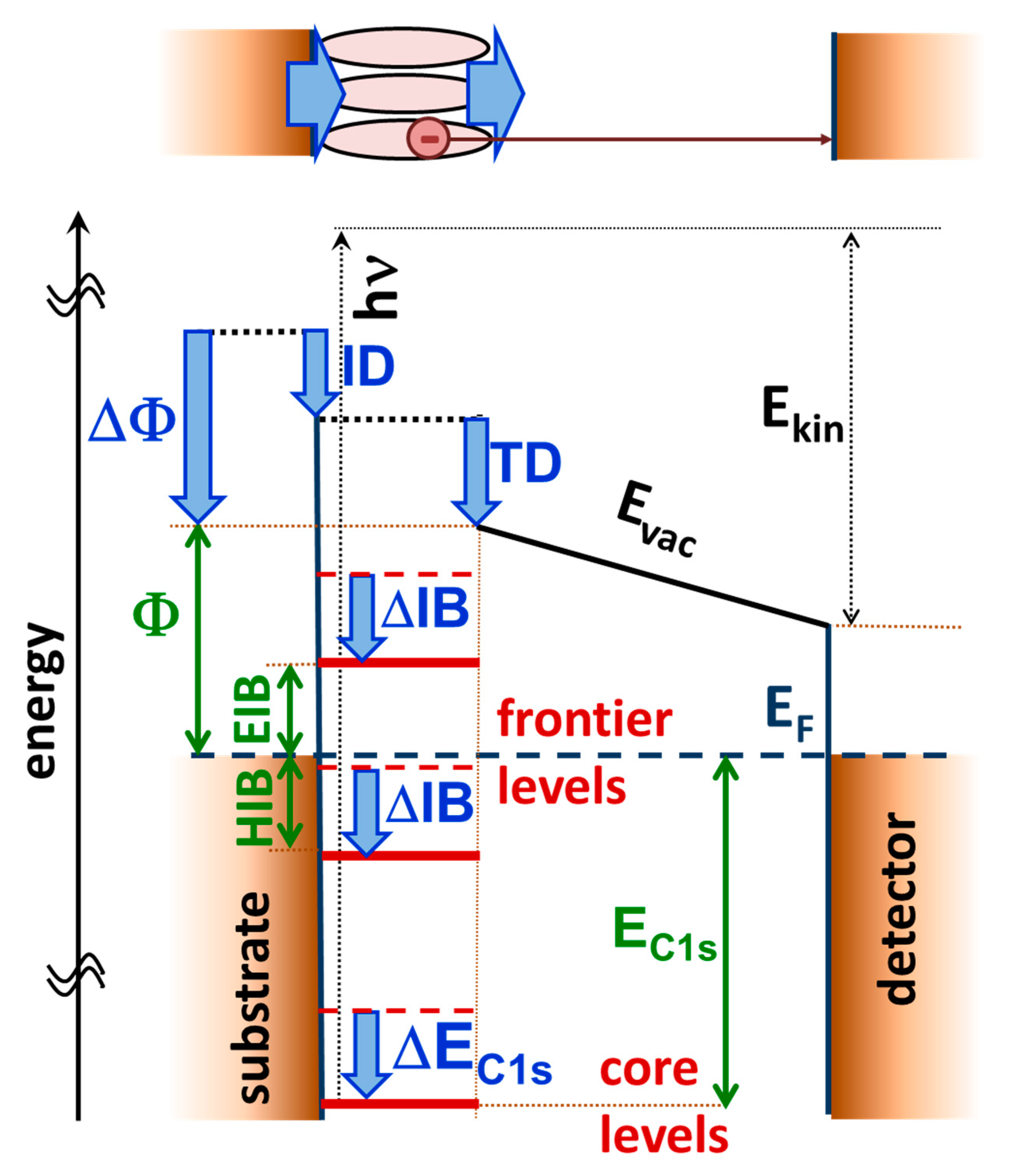
3.1. Energetics of a Metal–SAM Interface
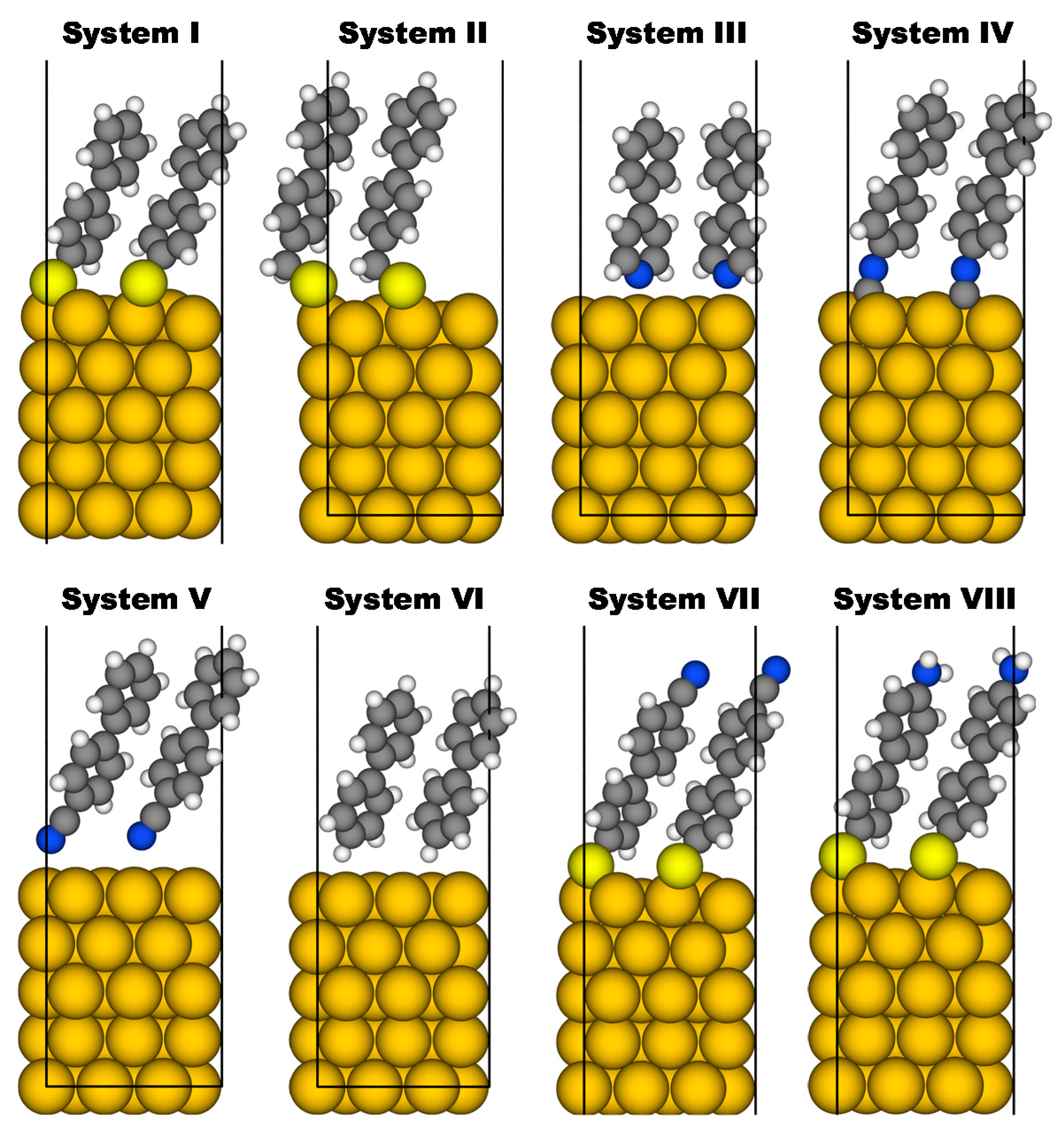
3.2. Investigated Systems
3.3. Calculated Core-Level Binding Energies, XP Spectra, and Work Function Changes
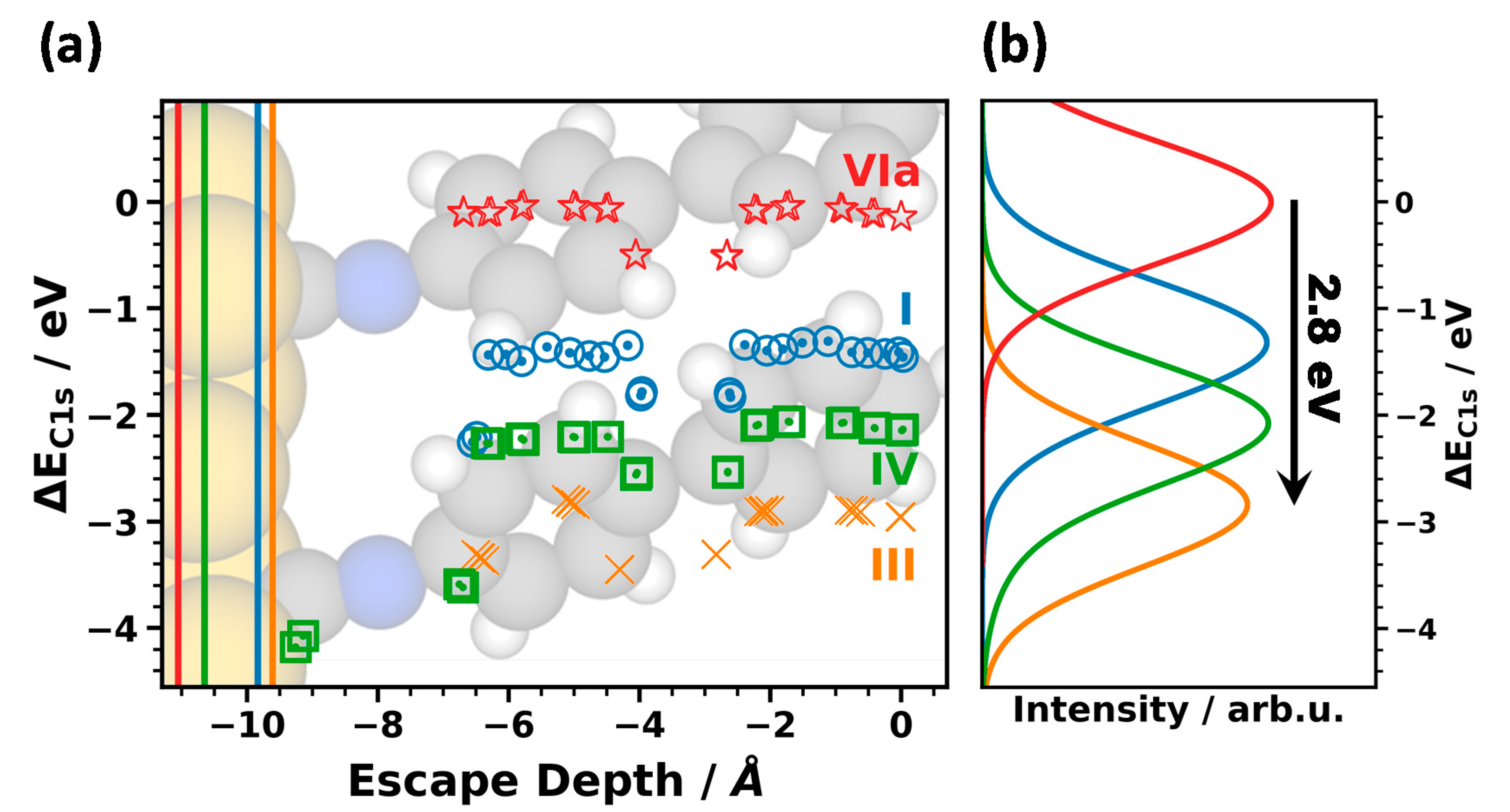
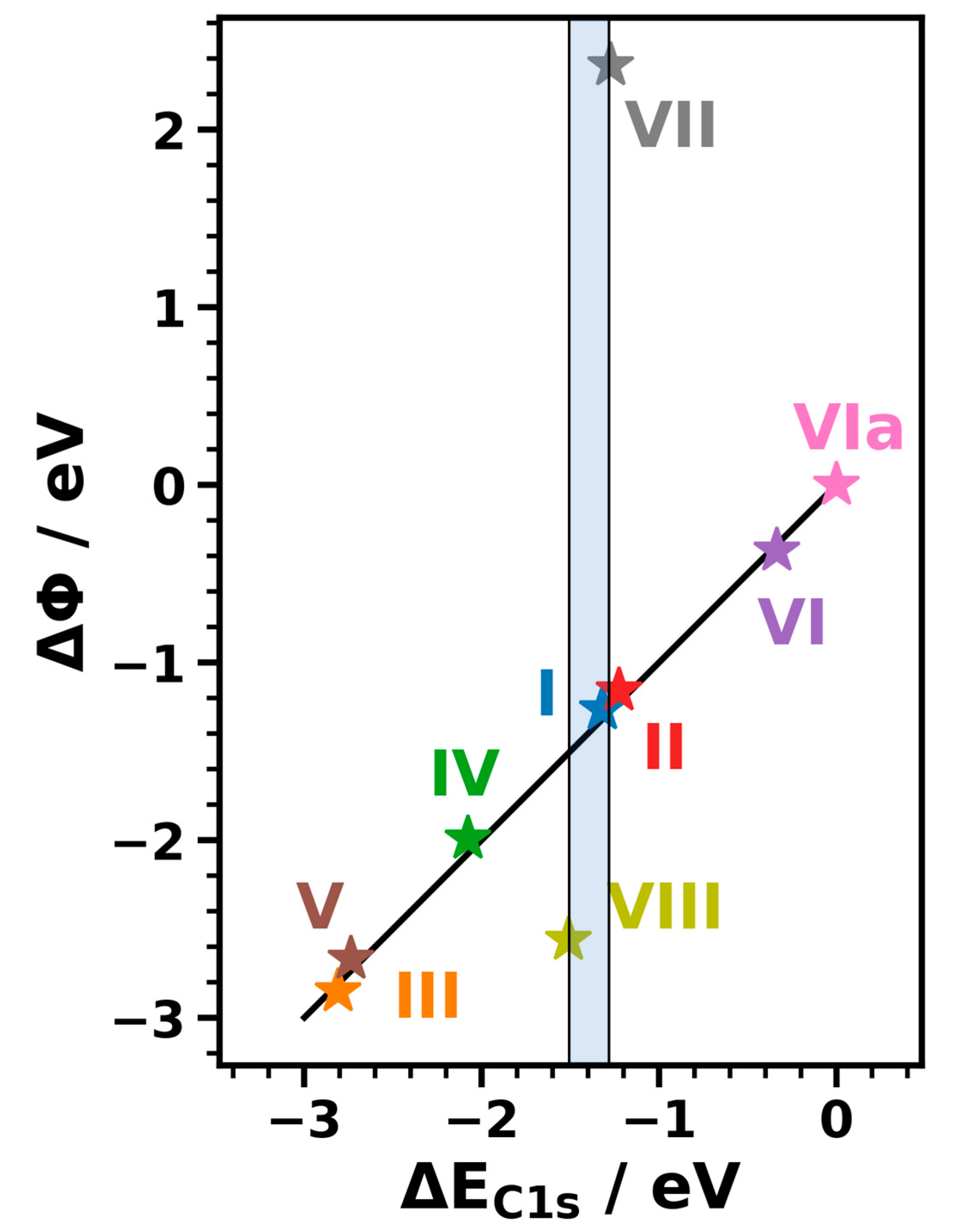
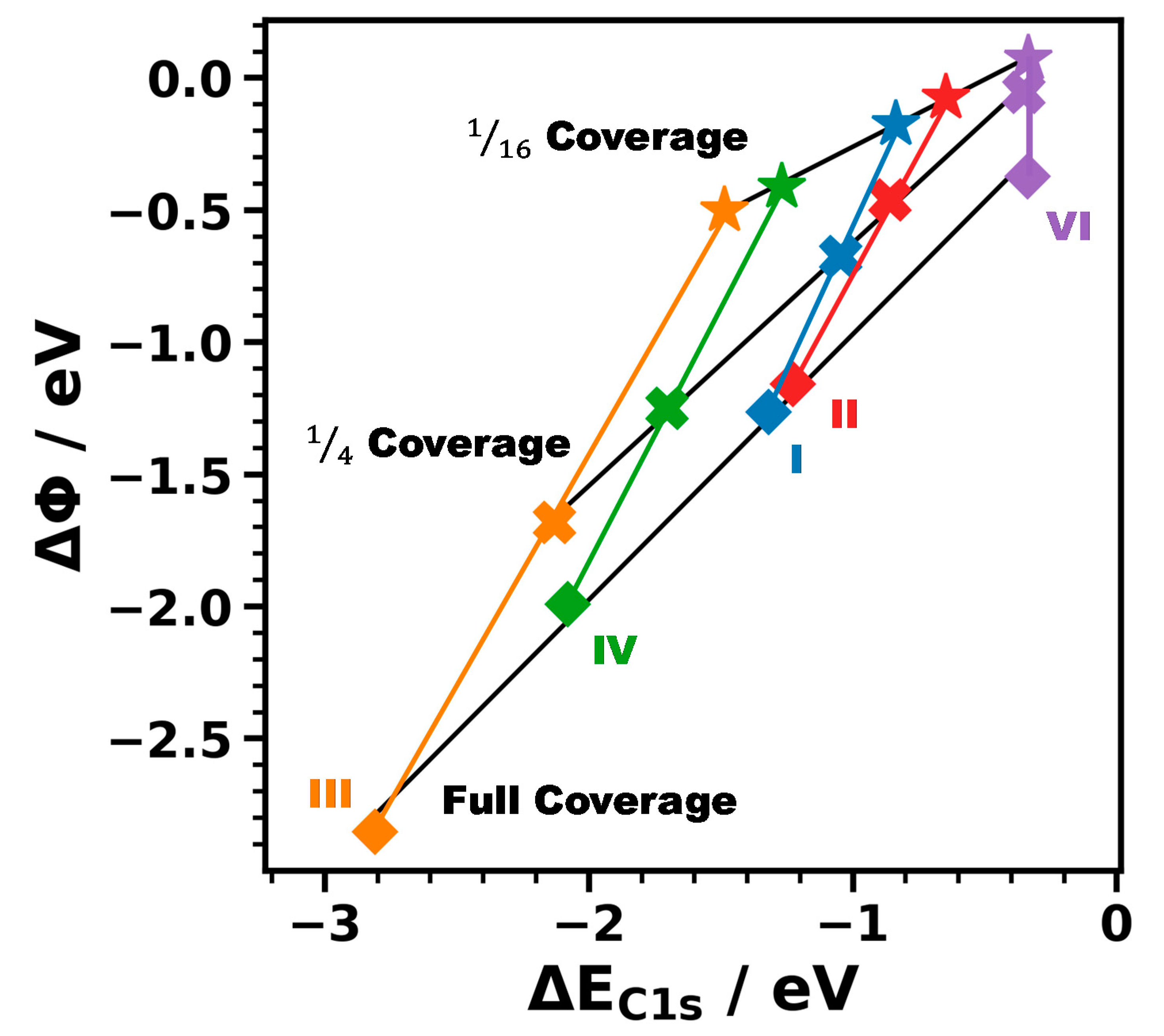
3.3.1. Energetic Shifts Due to Variations in the Interface Dipole
3.3.2. Influence of Different Tail Groups on the XP Spectra
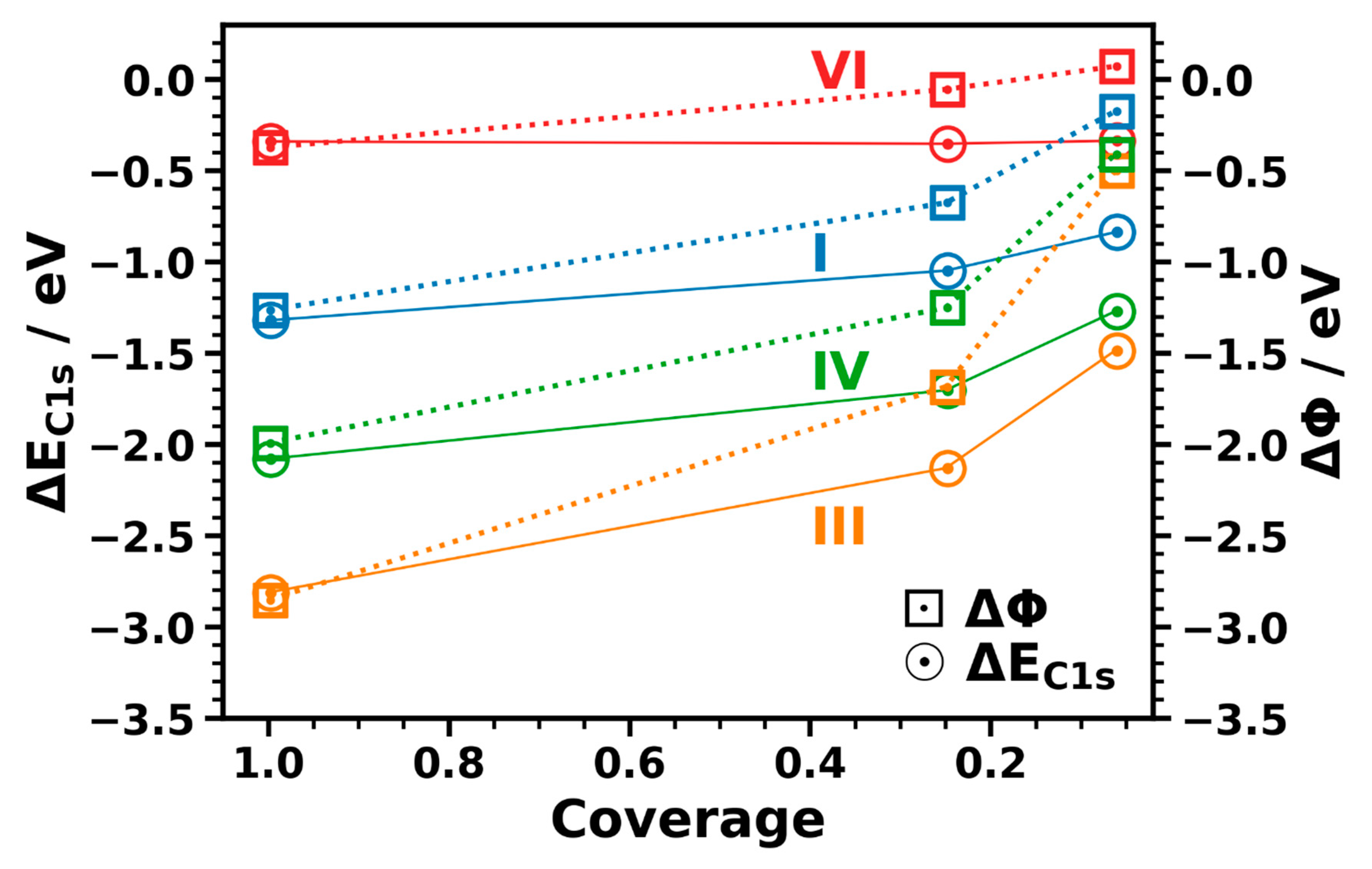
3.3.3. Coverage Dependence
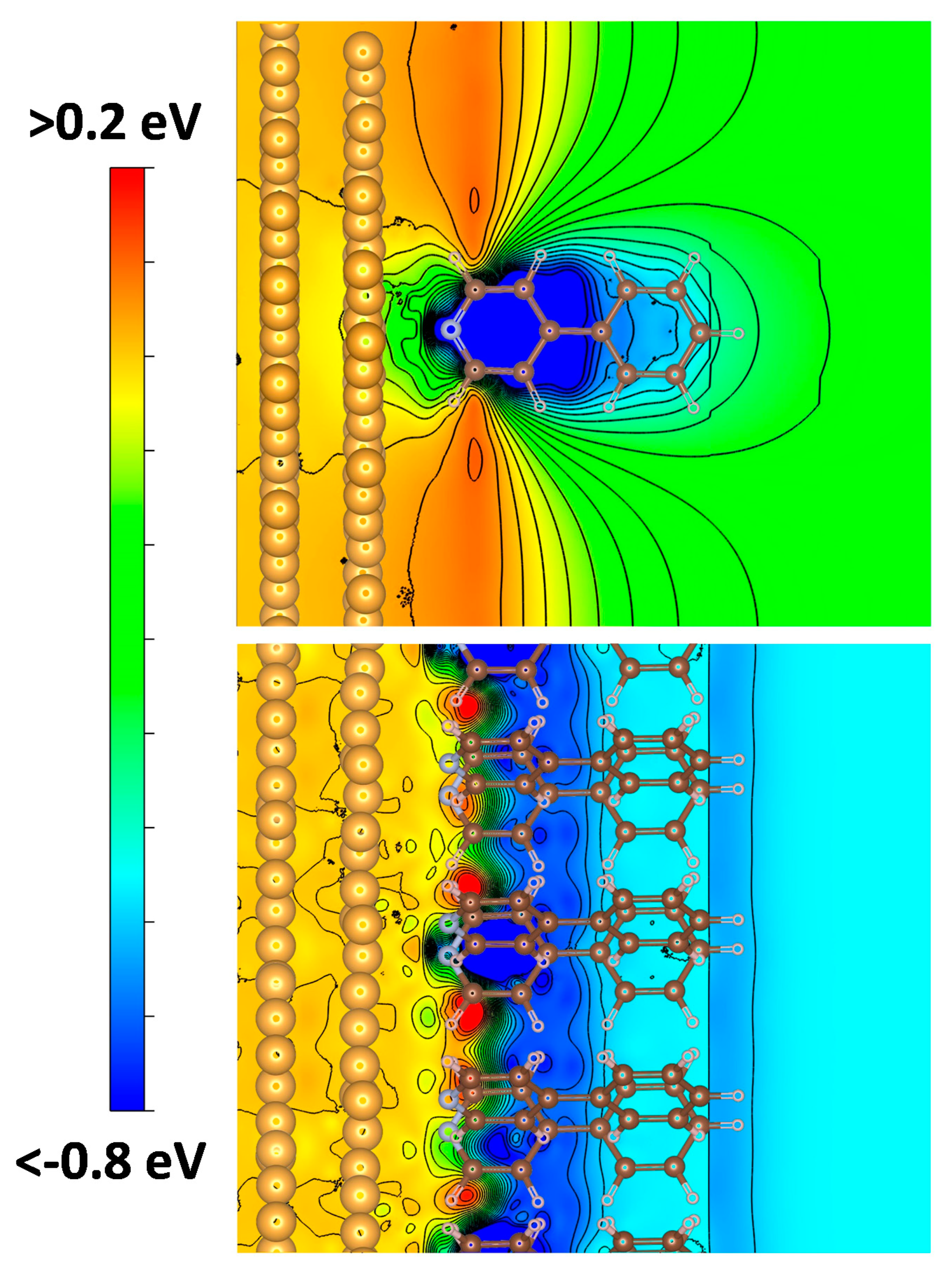
3.3.4. The Role of Structural Imperfections of the Interface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Casalini, S.; Bortolotti, C.A.; Leonardi, F.; Biscarini, F. Self-assembled monolayers in organic electronics. Chem. Soc. Rev. 2017, 46, 40–71. [Google Scholar] [CrossRef] [PubMed]
- Halik, M.; Hirsch, A. The Potential of Molecular Self-Assembled Monolayers in Organic Electronic Devices. Adv. Mater. 2011, 23, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.L.; Zaumseil, J.; Chang, J.F.; Ou, E.C.W.; Ho, P.K.H.; Sirringhaus, H.; Friend, R.H. General observation of n-type field-effect behaviour in organic semiconductors. Nature 2005, 434, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Nishikawa, T.; Takenobu, T.; Mori, S.; Shimoda, T.; Mitani, T.; Shimotani, H.; Yoshimoto, N.; Ogawa, S.; Iwasa, Y. Control of carrier density by self-assembled monolayers in organic field-effect transistors. Nat. Mater. 2004, 3, 317–322. [Google Scholar] [CrossRef]
- Pernstich, K.P.; Haas, S.; Oberhoff, D.; Goldmann, C.; Gundlach, D.J.; Batlogg, B.; Rashid, A.N.; Schitter, G. Threshold voltage shift in organic field effect transistors by dipole monolayers on the gate insulator. J. Appl. Phys. 2004, 96, 6431–6438. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Lex, A.; Proschek, V.; Etschmaier, H.; Tchernychova, E.; Sezen, M.; Scherf, U.; Grogger, W.; Trimmel, G.; Slugovc, C.; et al. Chemical Control of Local Doping in Organic Thin-Film Transistors: From Depletion to Enhancement. Adv. Mater. 2008, 20, 3143–3148. [Google Scholar] [CrossRef]
- Aghamohammadi, M.; Rödel, R.; Zschieschang, U.; Ocal, C.; Boschker, H.; Weitz, R.T.; Barrena, E.; Klauk, H. Threshold-Voltage Shifts in Organic Transistors Due to Self-Assembled Monolayers at the Dielectric: Evidence for Electronic Coupling and Dipolar Effects. ACS Appl. Mater. Interfaces 2015, 7, 22775–22785. [Google Scholar] [CrossRef]
- Campbell, I.H.; Rubin, S.; Zawodzinski, T.A.; Kress, J.D.; Martin, R.L.; Smith, D.L.; Barashkov, N.N.; Ferraris, J.P. Controlling Schottky energy barriers in organic electronic devices using self-assembled monolayers. Phys. Rev. B 1996, 54, R14321–R14324. [Google Scholar] [CrossRef]
- Campbell, I.H.; Kress, J.D.; Martin, R.L.; Smith, D.L.; Barashkov, N.N.; Ferraris, J.P. Controlling charge injection in organic electronic devices using self-assembled monolayers. Appl. Phys. Lett. 1997, 71, 3528–3530. [Google Scholar] [CrossRef]
- Yan, H.; Huang, Q.; Cui, J.; Veinot, J.G.C.; Kern, M.M.; Marks, T.J. High-Brightness Blue Light-Emitting Polymer Diodes via Anode Modification Using a Self-Assembled Monolayer. Adv. Mater. 2003, 15, 835–838. [Google Scholar] [CrossRef]
- De Boer, B.; Hadipour, A.; Mandoc, M.M.; van Woudenbergh, T.; Blom, P.W.M. Tuning of Metal Work Functions with Self-Assembled Monolayers. Adv. Mater. 2005, 17, 621–625. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.; Cho, J.H.; Kim, D.H.; Park, Y.D.; Hwang, M.; Cho, K. Effects of the permanent dipoles of self-assembled monolayer-treated insulator surfaces on the field-effect mobility of a pentacene thin-film transistor. Appl. Phys. Lett. 2007, 90, 132104. [Google Scholar] [CrossRef]
- Kitamura, M.; Kuzumoto, Y.; Kang, W.; Aomori, S.; Arakawa, Y. High conductance bottom-contact pentacene thin-film transistors with gold-nickel adhesion layers. Appl. Phys. Lett. 2010, 97, 033306. [Google Scholar] [CrossRef]
- Schulz, P.; Schäfer, T.; Zangmeister, C.D.; Effertz, C.; Meyer, D.; Mokros, D.; van Zee, R.D.; Mazzarello, R.; Wuttig, M. A New Route to Low Resistance Contacts for Performance-Enhanced Organic Electronic Devices. Adv. Mater. Interfaces 2014, 1, 1300130. [Google Scholar] [CrossRef]
- Kim, J.; Rim, Y.S.; Liu, Y.; Serino, A.C.; Thomas, J.C.; Chen, H.; Yang, Y.; Weiss, P.S. Interface Control in Organic Electronics Using Mixed Monolayers of Carboranethiol Isomers. Nano Lett. 2014, 14, 2946–2951. [Google Scholar] [CrossRef]
- Fenwick, O.; Van Dyck, C.; Murugavel, K.; Cornil, D.; Reinders, F.; Haar, S.; Mayor, M.; Cornil, J.; Samorì, P. Modulating the charge injection in organic field-effect transistors: Fluorinated oligophenyl self-assembled monolayers for high work function electrodes. J. Mater. Chem. C 2015, 3, 3007–3015. [Google Scholar] [CrossRef] [Green Version]
- Petritz, A.; Krammer, M.; Sauter, E.; Gärtner, M.; Nascimbeni, G.; Schrode, B.; Fian, A.; Gold, H.; Cojocaru, A.; Karner-Petritz, E.; et al. Embedded Dipole Self-Assembled Monolayers for Contact Resistance Tuning in p-Type and n-Type Organic Thin Film Transistors and Flexible Electronic Circuits. Adv. Funct. Mater. 2018, 28, 1804462. [Google Scholar] [CrossRef]
- Borchert, J.W.; Peng, B.; Letzkus, F.; Burghartz, J.N.; Chan, P.K.L.; Zojer, K.; Ludwigs, S.; Klauk, H. Small contact resistance and high-frequency operation of flexible low-voltage inverted coplanar organic transistors. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Evans, S.D.; Urankar, E.; Ulman, A.; Ferris, N. Self-assembled monolayers of alkanethiols containing a polar aromatic group: Effects of the dipole position on molecular packing, orientation, and surface wetting properties. J. Am. Chem. Soc. 1991, 113, 4121–4131. [Google Scholar] [CrossRef]
- Cabarcos, O.M.; Shaporenko, A.; Weidner, T.; Uppili, S.; Dake, L.S.; Zharnikov, M.; Allara, D.L. Physical and Electronic Structure Effects of Embedded Dipoles in Self-Assembled Monolayers: Characterization of Mid-Chain Ester Functionalized Alkanethiols on Au{111}. J. Phys. Chem. C 2008, 112, 10842–10854. [Google Scholar] [CrossRef]
- Abu-Husein, T.; Schuster, S.; Egger, D.A.; Kind, M.; Santowski, T.; Wiesner, A.; Chiechi, R.; Zojer, E.; Terfort, A.; Zharnikov, M. The Effects of Embedded Dipoles in Aromatic Self-Assembled Monolayers. Adv. Funct. Mater. 2015, 25, 3943–3957. [Google Scholar] [CrossRef] [Green Version]
- Gärtner, M.; Sauter, E.; Nascimbeni, G.; Wiesner, A.; Kind, M.; Werner, P.; Schuch, C.; Abu-Husein, T.; Asyuda, A.; Bats, J.W.; et al. Self-Assembled Monolayers with Distributed Dipole Moments Originating from Bipyrimidine Units. J. Phys. Chem. C 2020, 124, 504–519. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Belding, L.; Yuan, L.; Park, J.; Al-Sayah, M.H.; Bowers, C.M.; Whitesides, G.M. Dipole-Induced Rectification Across AgTS /SAM//Ga2O3/EGaIn Junctions. J. Am. Chem. Soc. 2019, 141, 8969–8980. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.F.; Ulman, A.; Liao, S.; Jordan, R. Mixed Self-Assembled Monolayers of Highly Polar Rigid Biphenyl Thiols. Langmuir 1999, 15, 2095–2098. [Google Scholar] [CrossRef]
- Zehner, R.W.; Parsons, B.F.; Hsung, R.P.; Sita, L.R. Tuning the Work Function of Gold with Self-Assembled Monolayers Derived from X−[C6H4−C⋮C−]nC6H4−SH (n = 0, 1, 2; X = H, F, CH3, CF3, and OCH3). Langmuir 1999, 15, 1121–1127. [Google Scholar] [CrossRef]
- Ganzorig, C.; Kwak, K.J.; Yagi, K.; Fujihira, M. Fine tuning work function of indium tin oxide by surface molecular design: Enhanced hole injection in organic electroluminescent devices. Appl. Phys. Lett. 2001, 79, 272–274. [Google Scholar] [CrossRef]
- Heimel, G.; Romaner, L.; Brédas, J.-L.; Zojer, E. Interface Energetics and Level Alignment at Covalent Metal-Molecule Junctions: π-Conjugated Thiols on Gold. Phys. Rev. Lett. 2006, 96. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Selloni, A. Interface and Molecular Electronic Structure vs Tunneling Characteristics of CH3- and CF3-Terminated Thiol Monolayers on Au(111). J. Phys. Chem. A 2006, 110, 11396–11400. [Google Scholar] [CrossRef]
- Yip, H.L.; Hau, S.K.; Baek, N.S.; Ma, H.; Jen, A.K.Y. Polymer Solar Cells That Use Self-Assembled-Monolayer-Modified ZnO/Metals as Cathodes. Adv. Mater. 2008, 20, 2376–2382. [Google Scholar] [CrossRef]
- Demirkan, K.; Mathew, A.; Weiland, C.; Yao, Y.; Rawlett, A.M.; Tour, J.M.; Opila, R.L. Energy level alignment at organic semiconductor/metal interfaces: Effect of polar self-assembled monolayers at the interface. J. Chem. Phys. 2008, 128, 074705. [Google Scholar] [CrossRef]
- Heimel, G.; Romaner, L.; Zojer, E.; Bredas, J.L. The Interface Energetics of Self-Assembled Monolayers on Metals. Acc. Chem. Res. 2008, 41, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Malicki, M.; Guan, Z.; Ha, S.D.; Heimel, G.; Barlow, S.; Rumi, M.; Kahn, A.; Marder, S.R. Preparation and Characterization of 4′-Donor Substituted Stilbene-4-thiolate Monolayers and Their Influence on the Work Function of Gold. Langmuir 2009, 25, 7967–7975. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Bowers, C.M.; Baghbanzadeh, M.; Whitesides, G.M. The Rate of Charge Tunneling Is Insensitive to Polar Terminal Groups in Self-Assembled Monolayers in AgTSS(CH2)nM(CH2)mT//Ga2O3/EGaIn Junctions. J. Am. Chem. Soc. 2014, 136, 16–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Jamison, A.C.; Lee, T.R. Surface Dipoles: A Growing Body of Evidence Supports Their Impact and Importance. Acc. Chem. Res. 2015, 48, 3007–3015. [Google Scholar] [CrossRef]
- Vilan, A.; Cahen, D. Chemical Modification of Semiconductor Surfaces for Molecular Electronics. Chem. Rev. 2017, 117, 4624–4666. [Google Scholar] [CrossRef]
- Lindell, L.; Vahlberg, C.; Uvdal, K.; Fahlman, M.; Braun, S. Self-assembled monolayer engineered interfaces: Energy level alignment tuning through chain length and end-group polarity. J. Electron Spectrosc. Relat. Phenom. 2015, 204, 140–144. [Google Scholar] [CrossRef]
- MacLeod, B.A.; Horwitz, N.E.; Ratcliff, E.L.; Jenkins, J.L.; Armstrong, N.R.; Giordano, A.J.; Hotchkiss, P.J.; Marder, S.R.; Campbell, C.T.; Ginger, D.S. Built-In Potential in Conjugated Polymer Diodes with Changing Anode Work Function: Interfacial States and Deviation from the Schottky–Mott Limit. J. Phys. Chem. Lett. 2012, 3, 1202–1207. [Google Scholar] [CrossRef]
- Schmidt, C.; Witt, A.; Witte, G. Tailoring the Cu(100) Work Function by Substituted Benzenethiolate Self-Assembled Monolayers. J. Phys. Chem. A 2011, 115, 7234–7241. [Google Scholar] [CrossRef]
- Heimel, G.; Romaner, L.; Zojer, E.; Brédas, J.L. Toward Control of the Metal−Organic Interfacial Electronic Structure in Molecular Electronics: A First-Principles Study on Self-Assembled Monolayers of π-Conjugated Molecules on Noble Metals. Nano Lett. 2007, 7, 932–940. [Google Scholar] [CrossRef]
- Natan, A.; Kronik, L.; Haick, H.; Tung, R.T. Electrostatic Properties of Ideal and Non-ideal Polar Organic Monolayers: Implications for Electronic Devices. Adv. Mater. 2007, 19, 4103–4117. [Google Scholar] [CrossRef]
- Wang, L.; Rangger, G.M.; Ma, Z.; Li, Q.; Shuai, Z.; Zojer, E.; Heimel, G. Is there a Au–S bond dipole in self-assembled monolayers on gold? Phys. Chem. Chem. Phys. 2010, 12, 4287–4290. [Google Scholar] [CrossRef] [PubMed]
- Zojer, E.; Taucher, T.C.; Hofmann, O.T. The Impact of Dipolar Layers on the Electronic Properties of Organic/Inorganic Hybrid Interfaces. Adv. Mater. Interfaces 2019, 6, 1900581. [Google Scholar] [CrossRef] [Green Version]
- Alloway, D.M.; Hofmann, M.; Smith, D.L.; Gruhn, N.E.; Graham, A.L.; Colorado, R.; Wysocki, V.H.; Lee, T.R.; Lee, P.A.; Armstrong, N.R. Interface Dipoles Arising from Self-Assembled Monolayers on Gold: UV-Photoemission Studies of Alkanethiols and Partially Fluorinated Alkanethiols. J. Phys. Chem. B 2003, 107, 11690–11699. [Google Scholar] [CrossRef]
- Rusu, P.C.; Brocks, G. Surface Dipoles and Work Functions of Alkylthiolates and Fluorinated Alkylthiolates on Au(111). J. Phys. Chem. B 2006, 110, 22628–22634. [Google Scholar] [CrossRef] [Green Version]
- Cahen, D.; Naaman, R.; Vager, Z. The Cooperative Molecular Field Effect. Adv. Funct. Mater. 2005, 15, 1571–1578. [Google Scholar] [CrossRef]
- Monti, O.L.A. Understanding Interfacial Electronic Structure and Charge Transfer: An Electrostatic Perspective. J. Phys. Chem. Lett. 2012, 3, 2342–2351. [Google Scholar] [CrossRef]
- Ishii, H.; Sugiyama, K.; Ito, E.; Seki, K. Energy Level Alignment and Interfacial Electronic Structures at Organic/Metal and Organic/Organic Interfaces. Adv. Mater. 1999, 11, 605–625. [Google Scholar] [CrossRef]
- Hehn, I.; Schuster, S.; Wächter, T.; Abu-Husein, T.; Terfort, A.; Zharnikov, M.; Zojer, E. Employing X-ray Photoelectron Spectroscopy for Determining Layer Homogeneity in Mixed Polar Self-Assembled Monolayers. J. Phys. Chem. Lett. 2016, 7, 2994–3000. [Google Scholar] [CrossRef] [Green Version]
- Gärtner, M.; Sauter, E.; Nascimbeni, G.; Petritz, A.; Wiesner, A.; Kind, M.; Abu-Husein, T.; Bolte, M.; Stadlober, B.; Zojer, E.; et al. Understanding the Properties of Tailor-Made Self-Assembled Monolayers with Embedded Dipole Moments for Interface Engineering. J. Phys. Chem. C 2018, 122, 28757–28774. [Google Scholar] [CrossRef]
- Braun, S.; Salaneck, W.R.; Fahlman, M. Energy-Level Alignment at Organic/Metal and Organic/Organic Interfaces. Adv. Mater. 2009, 21, 1450–1472. [Google Scholar] [CrossRef]
- Venkataraman, N.V.; Zürcher, S.; Rossi, A.; Lee, S.; Naujoks, N.; Spencer, N.D. Spatial Tuning of the Metal Work Function by Means of Alkanethiol and Fluorinated Alkanethiol Gradients. J. Phys. Chem. C 2009, 113, 5620–5628. [Google Scholar] [CrossRef]
- Lange, I.; Reiter, S.; Pätzel, M.; Zykov, A.; Nefedov, A.; Hildebrandt, J.; Hecht, S.; Kowarik, S.; Wöll, C.; Heimel, G.; et al. Tuning the Work Function of Polar Zinc Oxide Surfaces using Modified Phosphonic Acid Self-Assembled Monolayers. Adv. Funct. Mater. 2014, 24, 7014–7024. [Google Scholar] [CrossRef]
- Kim, B.; Choi, S.H.; Zhu, X.-Y.; Frisbie, C.D. Molecular Tunnel Junctions Based on π-Conjugated Oligoacene Thiols and Dithiols between Ag, Au, and Pt Contacts: Effect of Surface Linking Group and Metal Work Function. J. Am. Chem. Soc. 2011, 133, 19864–19877. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Darancet, P.; Widawsky, J.R.; Kotiuga, M.; Quek, S.Y.; Neaton, J.B.; Venkataraman, L. Determination of Energy Level Alignment and Coupling Strength in 4,4′-Bipyridine Single-Molecule Junctions. Nano Lett. 2014, 14, 794–798. [Google Scholar] [CrossRef]
- Egger, D.A.; Rissner, F.; Zojer, E.; Heimel, G. Polarity Switching of Charge Transport and Thermoelectricity in Self-Assembled Monolayer Devices. Adv. Mater. 2012, 24, 4403–4407. [Google Scholar] [CrossRef]
- Egger, D.A.; Liu, Z.F.; Neaton, J.B.; Kronik, L. Reliable Energy Level Alignment at Physisorbed Molecule–Metal Interfaces from Density Functional Theory. Nano Lett. 2015, 15, 2448–2455. [Google Scholar] [CrossRef] [Green Version]
- Obersteiner, V.; Egger, D.A.; Heimel, G.; Zojer, E. Impact of Collective Electrostatic Effects on Charge Transport through Molecular Monolayers. J. Phys. Chem. C 2014, 118, 22395–22401. [Google Scholar] [CrossRef]
- Obersteiner, V.; Huhs, G.; Papior, N.; Zojer, E. Unconventional Current Scaling and Edge Effects for Charge Transport through Molecular Clusters. Nano Lett. 2017, 17, 7350–7357. [Google Scholar] [CrossRef]
- Xie, Z.; Bâldea, I.; Smith, C.E.; Wu, Y.; Frisbie, C.D. Experimental and Theoretical Analysis of Nanotransport in Oligophenylene Dithiol Junctions as a Function of Molecular Length and Contact Work Function. ACS Nano 2015, 9, 8022–8036. [Google Scholar] [CrossRef]
- Xie, Z.; Bâldea, I.; Frisbie, C.D. Determination of Energy-Level Alignment in Molecular Tunnel Junctions by Transport and Spectroscopy: Self-Consistency for the Case of Oligophenylene Thiols and Dithiols on Ag, Au, and Pt Electrodes. J. Am. Chem. Soc. 2019, 141, 3670–3681. [Google Scholar] [CrossRef]
- Xie, Z.; Bâldea, I.; Frisbie, C.D. Energy Level Alignment in Molecular Tunnel Junctions by Transport and Spectroscopy: Self-Consistency for the Case of Alkyl Thiols and Dithiols on Ag, Au, and Pt Electrodes. J. Am. Chem. Soc. 2019, 141, 18182–18192. [Google Scholar] [CrossRef] [PubMed]
- Vilan, A.; Aswal, D.; Cahen, D. Large-Area, Ensemble Molecular Electronics: Motivation and Challenges. Chem. Rev. 2017, 117, 4248–4286. [Google Scholar] [CrossRef] [PubMed]
- Taucher, T.C.; Hehn, I.; Hofmann, O.T.; Zharnikov, M.; Zojer, E. Understanding Chemical versus Electrostatic Shifts in X-ray Photoelectron Spectra of Organic Self-Assembled Monolayers. J. Phys. Chem. C 2016, 120, 3428–3437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabarcos, O.M.; Schuster, S.; Hehn, I.; Zhang, P.P.; Maitani, M.M.; Sullivan, N.; Giguère, J.-B.; Morin, J.-F.; Weiss, P.S.; Zojer, E.; et al. Effects of Embedded Dipole Layers on Electrostatic Properties of Alkanethiolate Self-Assembled Monolayers. J. Phys. Chem. C 2017, 121, 15815–15830. [Google Scholar] [CrossRef]
- El-Sayed, A.; Borghetti, P.; Goiri, E.; Rogero, C.; Floreano, L.; Lovat, G.; Mowbray, D.J.; Cabellos, J.L.; Wakayama, Y.; Rubio, A.; et al. Understanding Energy-Level Alignment in Donor–Acceptor/Metal Interfaces from Core-Level Shifts. ACS Nano 2013, 7, 6914–6920. [Google Scholar] [CrossRef]
- Siegbahn, K. (Ed.) ESCA. Atomic, Molecular and Solid State Structure Studied by Means of Electron Spectroscopy; Nova Acta Regiae Societatis Scientiarum Upsaliensis; Uppsala Almqvist & Wiksell: Stockholm, Sweden, 1967; Volume 20, Ser. 4. [Google Scholar]
- Benesh, G.A.; King, D.A. Core-level shift spectroscopy for adsorbates: Ionic, covalent or metallic bonding? Chem. Phys. Lett. 1992, 191, 315–319. [Google Scholar] [CrossRef]
- Bagus, P.S.; Ilton, E.S.; Nelin, C.J. The interpretation of XPS spectra: Insights into materials properties. Surf. Sci. Rep. 2013, 68, 273–304. [Google Scholar] [CrossRef]
- Bagus, P.S.; Ilton, E.; Nelin, C.J. Extracting Chemical Information from XPS Spectra: A Perspective. Catal. Lett. 2018, 148, 1785–1802. [Google Scholar] [CrossRef]
- Kirchhuebel, T.; Monti, O.L.A.; Munakata, T.; Kera, S.; Forker, R.; Fritz, T. The role of initial and final states in molecular spectroscopies. Phys. Chem. Chem. Phys. 2019, 21, 12730–12747. [Google Scholar] [CrossRef]
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef]
- Schreiber, F. Structure and growth of self-assembling monolayers. Prog. Surf. Sci. 2000, 65, 151–257. [Google Scholar] [CrossRef]
- Heister, K.; Zharnikov, M.; Grunze, M.; Johansson, L.S.O. Adsorption of Alkanethiols and Biphenylthiols on Au and Ag Substrates: A High-Resolution X-ray Photoelectron Spectroscopy Study. J. Phys. Chem. B 2001, 105, 4058–4061. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Shaporenko, A.; Cyganik, P.; Buck, M.; Terfort, A.; Zharnikov, M. Self-Assembled Monolayers of Aromatic Selenolates on Noble Metal Substrates. J. Phys. Chem. B 2005, 109, 13630–13638. [Google Scholar] [CrossRef] [PubMed]
- Käfer, D.; Bashir, A.; Witte, G. Interplay of Anchoring and Ordering in Aromatic Self-Assembled Monolayers. J. Phys. Chem. C 2007, 111, 10546–10551. [Google Scholar] [CrossRef]
- Merzlikin, S.V.; Tolkachev, N.N.; Strunskus, T.; Witte, G.; Glogowski, T.; Wöll, C.; Grünert, W. Resolving the depth coordinate in photoelectron spectroscopy—Comparison of excitation energy variation vs. angular-resolved XPS for the analysis of a self-assembled monolayer model system. Surf. Sci. 2008, 602, 755–767. [Google Scholar] [CrossRef]
- Beccari, M.; Kanjilal, A.; Betti, M.G.; Mariani, C.; Floreano, L.; Cossaro, A.; Di Castro, V. Characterization of benzenethiolate self-assembled monolayer on Cu(100) by XPS and NEXAFS. J. Electron Spectrosc. Relat. Phenom. 2009, 172, 64–68. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiols and dithiols on gold: New challenges for a well-known system. Chem. Soc. Rev. 2010, 39, 1805. [Google Scholar] [CrossRef]
- Zaba, T.; Noworolska, A.; Bowers, C.M.; Breiten, B.; Whitesides, G.M.; Cyganik, P. Formation of Highly Ordered Self-Assembled Monolayers of Alkynes on Au(111) Substrate. J. Am. Chem. Soc. 2014, 136, 11918–11921. [Google Scholar] [CrossRef] [Green Version]
- Euti, E.M.; Vélez Romero, P.; Linarez Pérez, O.; Ruano, G.; Patrito, E.M.; Zampieri, G.; Leiva, E.P.M.; Macagno, V.A.; Cometto, F.P. Electrochemical, HR-XPS and SERS study of the self-assembly of biphenyl 4,4′-dithiol on Au(111) from solution phase. Surf. Sci. 2014, 630, 101–108. [Google Scholar] [CrossRef]
- Ossowski, J.; Nascimbeni, G.; Żaba, T.; Verwüster, E.; Rysz, J.; Terfort, A.; Zharnikov, M.; Zojer, E.; Cyganik, P. Relative Thermal Stability of Thiolate- and Selenolate-Bonded Aromatic Monolayers on the Au(111) Substrate. J. Phys. Chem. C 2017, 121, 28031–28042. [Google Scholar] [CrossRef]
- Kang, J.F.; Liao, S.; Jordan, R.; Ulman, A. Mixed Self-assembled Monolayers of Rigid Biphenyl Thiols: Impact of Solvent and Dipole Moment. J. Am. Chem. Soc. 1998, 120, 9662–9667. [Google Scholar] [CrossRef]
- Rong, H.T.; Frey, S.; Yang, Y.J.; Zharnikov, M.; Buck, M.; Wühn, M.; Wöll, C.; Helmchen, G. On the Importance of the Headgroup Substrate Bond in Thiol Monolayers: A Study of Biphenyl-Based Thiols on Gold and Silver. Langmuir 2001, 17, 1582–1593. [Google Scholar] [CrossRef]
- Azzam, W.; Fuxen, C.; Birkner, A.; Rong, H.T.; Buck, M.; Wöll, C. Coexistence of Different Structural Phases in Thioaromatic Monolayers on Au(111). Langmuir 2003, 19, 4958–4968. [Google Scholar] [CrossRef]
- Shaporenko, A.; Terfort, A.; Grunze, M.; Zharnikov, M. A detailed analysis of the photoemission spectra of basic thioaromatic monolayers on noble metal substrates. J. Electron Spectrosc. Relat. Phenom. 2006, 151, 45–51. [Google Scholar] [CrossRef]
- Cyganik, P.; Buck, M.; Strunskus, T.; Shaporenko, A.; Wilton-Ely, J.D.E.T.; Zharnikov, M.; Wöll, C. Competition as a Design Concept: Polymorphism in Self-Assembled Monolayers of Biphenyl-Based Thiols. J. Am. Chem. Soc. 2006, 128, 13868–13878. [Google Scholar] [CrossRef]
- Stoycheva, S.; Himmelhaus, M.; Fick, J.; Korniakov, A.; Grunze, M.; Ulman, A. Spectroscopic Characterization of ω-Substituted Biphenylthiolates on Gold and Their Use as Substrates for “On-Top” Siloxane SAM Formation. Langmuir 2006, 22, 4170–4178. [Google Scholar] [CrossRef]
- Krzykawska, A.; Ossowski, J.; Żaba, T.; Cyganik, P. Binding groups for highly ordered SAM formation: Carboxylic versus thiol. Chem. Commun. 2017, 53, 5748–5751. [Google Scholar] [CrossRef]
- Sauter, E.; Nascimbeni, G.; Trefz, D.; Ludwigs, S.; Zojer, E.; von Wrochem, F.; Zharnikov, M. A dithiocarbamate anchoring group as a flexible platform for interface engineering. Phys. Chem. Chem. Phys. 2019, 21, 22511–22525. [Google Scholar] [CrossRef] [Green Version]
- Blum, V.; Gehrke, R.; Hanke, F.; Havu, P.; Havu, V.; Ren, X.; Reuter, K.; Scheffler, M. Ab initio molecular simulations with numeric atom-centered orbitals. Comput. Phys. Commun. 2009, 180, 2175–2196. [Google Scholar] [CrossRef] [Green Version]
- Havu, V.; Blum, V.; Havu, P.; Scheffler, M. Efficient integration for all-electron electronic structure calculation using numeric basis functions. J. Comput. Phys. 2009, 228, 8367–8379. [Google Scholar] [CrossRef]
- Marek, A.; Blum, V.; Johanni, R.; Havu, V.; Lang, B.; Auckenthaler, T.; Heinecke, A.; Bungartz, H.-J.; Lederer, H. The ELPA library: Scalable parallel eigenvalue solutions for electronic structure theory and computational science. J. Phys. Condens. Matter 2014, 26, 213201. [Google Scholar] [CrossRef] [PubMed]
- Togo, A.; Tanaka, I. Spglib: A software library for crystal symmetry search. arXiv 2018, arXiv:1808.01590. [Google Scholar]
- Yu, V.W.; Corsetti, F.; García, A.; Huhn, W.P.; Jacquelin, M.; Jia, W.; Lange, B.; Lin, L.; Lu, J.; Mi, W.; et al. ELSI: A unified software interface for Kohn–Sham electronic structure solvers. Comput. Phys. Commun. 2018, 222, 267–285. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. ERRATA: Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, V.G.; Liu, W.; Zojer, E.; Scheffler, M.; Tkatchenko, A. Density-Functional Theory with Screened van der Waals Interactions for the Modeling of Hybrid Inorganic-Organic Systems. Phys. Rev. Lett. 2012, 108. [Google Scholar] [CrossRef] [Green Version]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. Phys. Rev. Lett. 2009, 102. [Google Scholar] [CrossRef] [Green Version]
- Neugebauer, J.; Scheffler, M. Adsorbate-substrate and adsorbate-adsorbate interactions of Na and K adlayers on Al(111). Phys. Rev. B 1992, 46, 16067–16080. [Google Scholar] [CrossRef] [Green Version]
- Freysoldt, C.; Eggert, P.; Rinke, P.; Schindlmayr, A.; Scheffler, M. Screening in two dimensions: G W calculations for surfaces and thin films using the repeated-slab approach. Phys. Rev. B 2008, 77, 235428. [Google Scholar] [CrossRef] [Green Version]
- Verwüster, E.; Hofmann, O.T.; Egger, D.A.; Zojer, E. Electronic Properties of Biphenylthiolates on Au(111): The Impact of Coverage Revisited. J. Phys. Chem. C 2015, 119, 7817–7825. [Google Scholar] [CrossRef]
- Vackář, J.; Hyt’ha, M.; Šimůnek, A. All-electron pseudopotentials. Phys. Rev. B 1998, 58, 12712–12720. [Google Scholar] [CrossRef]
- Methfessel, M.; Fiorentini, V.; Oppo, S. Connection between charge transfer and alloying core-level shifts based on density-functional calculations. Phys. Rev. B 2000, 61, 5229–5236. [Google Scholar] [CrossRef]
- Morikawa, Y.; Hayashi, T.; Liew, C.C.; Nozoye, H. First-principles theoretical study of alkylthiolate adsorption on Au (111). Surf. Sci. 2002, 507, 46–50. [Google Scholar] [CrossRef]
- Heimel, G.; Romaner, L.; Brédas, J.L.; Zojer, E. Organic/metal interfaces in self-assembled monolayers of conjugated thiols: A first-principles benchmark study. Surf. Sci. 2006, 600, 4548–4562. [Google Scholar] [CrossRef]
- Giesbers, M.; Marcelis, A.T.M.; Zuilhof, H. Simulation of XPS C1s Spectra of Organic Monolayers by Quantum Chemical Methods. Langmuir 2013, 29, 4782–4788. [Google Scholar] [CrossRef]
- Pueyo Bellafont, N.; Illas, F.; Bagus, P.S. Validation of Koopmans’ theorem for density functional theory binding energies. Phys. Chem. Chem. Phys. 2015, 17, 4015–4019. [Google Scholar] [CrossRef]
- Ishiwari, F.; Nascimbeni, G.; Sauter, E.; Tago, H.; Shoji, Y.; Fujii, S.; Kiguchi, M.; Tada, T.; Zharnikov, M.; Zojer, E.; et al. Triptycene Tripods for the Formation of Highly Uniform and Densely Packed Self-Assembled Monolayers with Controlled Molecular Orientation. J. Am. Chem. Soc. 2019, 141, 5995–6005. [Google Scholar] [CrossRef] [Green Version]
- Pueyo Bellafont, N.; Bagus, P.S.; Illas, F. Prediction of core level binding energies in density functional theory: Rigorous definition of initial and final state contributions and implications on the physical meaning of Kohn-Sham energies. J. Chem. Phys. 2015, 142, 214102. [Google Scholar] [CrossRef]
- Viñes, F.; Sousa, C.; Illas, F. On the prediction of core level binding energies in molecules, surfaces and solids. Phys. Chem. Chem. Phys. 2018, 20, 8403–8410. [Google Scholar] [CrossRef]
- Taucher, T.C.; Hofmann, O.T.; Zojer, E. Final-State Simulations of Core-Level Binding Energies at Metal-Organic Hybrid Interfaces: Artifacts Caused by Spurious Collective Electrostatic Effects. 2020; Submitted. [Google Scholar]
- Jackson, J.D. Classical Electrodynamics, 3rd ed.; Wiley: New York, NY, USA, 1999; ISBN 978-0-471-30932-1. [Google Scholar]
- Neaton, J.B.; Hybertsen, M.S.; Louie, S.G. Renormalization of Molecular Electronic Levels at Metal-Molecule Interfaces. Phys. Rev. Lett. 2006, 97, 216405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamont, C.L.; Wilkes, J. Attenuation length of electrons in self-assembled monolayers of n-alkanethiols on gold. Langmuir 1999, 15, 2037–2042. [Google Scholar] [CrossRef]
- Van der Walt, S.; Colbert, S.C.; Varoquaux, G. The NumPy Array: A Structure for Efficient Numerical Computation. Comput. Sci. Eng. 2011, 13, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- The GIMP Development Team GIMP 2019. Available online: https://www.gimp.org (accessed on 30 March 2020).
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2007; ISBN 978-0-471-14323-9. [Google Scholar]
- Stähler, J.; Rinke, P. Global and local aspects of the surface potential landscape for energy level alignment at organic-ZnO interfaces. Chem. Phys. 2017, 485-486, 149–165. [Google Scholar] [CrossRef]
- Heimel, G.; Rissner, F.; Zojer, E. Modeling the Electronic Properties of π-Conjugated Self-Assembled Monolayers. Adv. Mater. 2010, 22, 2494–2513. [Google Scholar] [CrossRef]
- Natan, A.; Zidon, Y.; Shapira, Y.; Kronik, L. Cooperative effects and dipole formation at semiconductor and self-assembled-monolayer interfaces. Phys. Rev. B 2006, 73. [Google Scholar] [CrossRef] [Green Version]
- Egelhoff, W.F. Core-level binding-energy shifts at surfaces and in solids. Surf. Sci. Rep. 1987, 6, 253–415. [Google Scholar] [CrossRef]
- Bagus, P.S.; Nelin, C.J.; Zhao, X.; Levchenko, S.V.; Davis, E.; Weng, X.; Späth, F.; Papp, C.; Kuhlenbeck, H.; Freund, H.J. Revisiting surface core-level shifts for ionic compounds. Phys. Rev. B 2019, 100, 115419. [Google Scholar] [CrossRef] [Green Version]
- Vericat, C.; Vela, M.E.; Corthey, G.; Pensa, E.; Cortés, E.; Fonticelli, M.H.; Ibañez, F.; Benitez, G.E.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiolates on metals: A review article on sulfur-metal chemistry and surface structures. RSC Adv. 2014, 4, 27730–27754. [Google Scholar] [CrossRef]
- Ma, Z.; Rissner, F.; Wang, L.; Heimel, G.; Li, Q.; Shuai, Z.; Zojer, E. Electronic structure of pyridine-based SAMs on flat Au(111) surfaces: Extended charge rearrangements and Fermi level pinning. Phys. Chem. Chem. Phys. 2011, 13, 9747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, O.T.; Deinert, J.-C.; Xu, Y.; Rinke, P.; Stähler, J.; Wolf, M.; Scheffler, M. Large work function reduction by adsorption of a molecule with a negative electron affinity: Pyridine on ZnO(101¯0). J. Chem. Phys. 2013, 139, 174701. [Google Scholar] [CrossRef]
- Hébert, P.; Le Rille, A.; Zheng, W.Q.; Tadjeddine, A. Vibrational spectroscopic study of the adsorption of pyridine at the Au(111)-electrolyte interface by in situ difference frequency generation. J. Electroanal. Chem. 1998, 447, 5–9. [Google Scholar] [CrossRef]
- Stadler, R.; Thygesen, K.S.; Jacobsen, K.W. Forces and conductances in a single-molecule bipyridine junction. Phys. Rev. B 2005, 72, 241401. [Google Scholar] [CrossRef] [Green Version]
- Kamenetska, M.; Quek, S.Y.; Whalley, A.C.; Steigerwald, M.L.; Choi, H.J.; Louie, S.G.; Nuckolls, C.; Hybertsen, M.S.; Neaton, J.B.; Venkataraman, L. Conductance and Geometry of Pyridine-Linked Single-Molecule Junctions. J. Am. Chem. Soc. 2010, 132, 6817–6821. [Google Scholar] [CrossRef]
- Obersteiner, V.; Egger, D.A.; Zojer, E. Impact of Anchoring Groups on Ballistic Transport: Single Molecule vs Monolayer Junctions. J. Phys. Chem. C 2015, 119, 21198–21208. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.E.; Xie, Z.; Bâldea, I.; Frisbie, C.D. Work function and temperature dependence of electron tunneling through an N-type perylene diimide molecular junction with isocyanide surface linkers. Nanoscale 2018, 10, 964–975. [Google Scholar] [CrossRef]
- Tsunoi, A.; Lkhamsuren, G.; Mondarte, E.A.Q.; Asatyas, S.; Oguchi, M.; Noh, J.; Hayashi, T. Improvement of the Thermal Stability of Self-Assembled Monolayers of Isocyanide Derivatives on Gold. J. Phys. Chem. C 2019, 123, 13681–13686. [Google Scholar] [CrossRef]
- Bagus, P.S.; Staemmler, V.; Wöll, C. Exchangelike Effects for Closed-Shell Adsorbates: Interface Dipole and Work Function. Phys. Rev. Lett. 2002, 89. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, H.; Koike, H.; Kuroda, K.; Ishida, Y.; Nakayama, M.; Mase, K.; Kondo, T.; Shin, S.; Kanai, K. Effect of physisorption of inert organic molecules on Au(111) surface electronic states. Phys. Chem. Chem. Phys. 2017, 19, 18646–18651. [Google Scholar] [CrossRef] [PubMed]
- Zangmeister, C.D.; Robey, S.W.; van Zee, R.D.; Kushmerick, J.G.; Naciri, J.; Yao, Y.; Tour, J.M.; Varughese, B.; Xu, B.; Reutt-Robey, J.E. Fermi Level Alignment in Self-Assembled Molecular Layers: The Effect of Coupling Chemistry. J. Phys. Chem. B 2006, 110, 17138–17144. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Beebe, J.M.; Jun, Y.; Zhu, X.Y.; Frisbie, C.D. Correlation between HOMO Alignment and Contact Resistance in Molecular Junctions: Aromatic Thiols versus Aromatic Isocyanides. J. Am. Chem. Soc. 2006, 128, 4970–4971. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.R.; Barlow, C.A. Work Function Change on Monolayer Adsorption. J. Chem. Phys. 1963, 39, 412–422. [Google Scholar] [CrossRef]
- Maschhoff, B.L.; Cowin, J.P. Corrected electrostatic model for dipoles adsorbed on a metal surface. J. Chem. Phys. 1994, 101, 8138–8151. [Google Scholar] [CrossRef] [Green Version]
- Gershevitz, O.; Sukenik, C.N.; Ghabboun, J.; Cahen, D. Molecular Monolayer-Mediated Control over Semiconductor Surfaces: Evidence for Molecular Depolarization of Silane Monolayers on Si/SiOx. J. Am. Chem. Soc. 2003, 125, 4730–4731. [Google Scholar] [CrossRef]
- De Renzi, V.; Rousseau, R.; Marchetto, D.; Biagi, R.; Scandolo, S.; del Pennino, U. Metal Work-Function Changes Induced by Organic Adsorbates: A Combined Experimental and Theoretical Study. Phys. Rev. Lett. 2005, 95, 046804. [Google Scholar] [CrossRef] [Green Version]
- Fukagawa, H.; Yamane, H.; Kera, S.; Okudaira, K.K.; Ueno, N. Experimental estimation of the electric dipole moment and polarizability of titanyl phthalocyanine using ultraviolet photoelectron spectroscopy. Phys. Rev. B 2006, 73, 041302. [Google Scholar] [CrossRef]
- Cornil, D.; Olivier, Y.; Geskin, V.; Cornil, J. Depolarization Effects in Self-Assembled Monolayers: A Quantum-Chemical Insight. Adv. Funct. Mater. 2007, 17, 1143–1148. [Google Scholar] [CrossRef]
- Sushko, M.L.; Shluger, A.L. Intramolecular Dipole Coupling and Depolarization in Self-Assembled Monolayers. Adv. Funct. Mater. 2008, 18, 2228–2236. [Google Scholar] [CrossRef]
- Piacenza, M.; D’Agostino, S.; Fabiano, E.; Della Sala, F. Ab initio depolarization in self-assembled molecular monolayers: Beyond conventional density-functional theory. Phys. Rev. B 2009, 80, 153101. [Google Scholar] [CrossRef]
- Blumenfeld, M.L.; Steele, M.P.; Monti, O.L.A. Near- and Far-Field Effects on Molecular Energy Level Alignment at an Organic/Electrode Interface. J. Phys. Chem. Lett. 2010, 1, 145–148. [Google Scholar] [CrossRef]
- Wang, L.; Rangger, G.M.; Romaner, L.; Heimel, G.; Bučko, T.; Ma, Z.; Li, Q.; Shuai, Z.; Zojer, E. Electronic Structure of Self-Assembled Monolayers on Au(111) Surfaces: The Impact of Backbone Polarizability. Adv. Funct. Mater. 2009, 19, 3766–3775. [Google Scholar] [CrossRef]
- Koller, G.; Winter, B.; Oehzelt, M.; Ivanco, J.; Netzer, F.P.; Ramsey, M.G. The electronic band alignment on nanoscopically patterned substrates. Org. Electron. 2007, 8, 63–68. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taucher, T.C.; Zojer, E. The Potential of X-ray Photoelectron Spectroscopy for Determining Interface Dipoles of Self-Assembled Monolayers. Appl. Sci. 2020, 10, 5735. https://doi.org/10.3390/app10175735
Taucher TC, Zojer E. The Potential of X-ray Photoelectron Spectroscopy for Determining Interface Dipoles of Self-Assembled Monolayers. Applied Sciences. 2020; 10(17):5735. https://doi.org/10.3390/app10175735
Chicago/Turabian StyleTaucher, Thomas C., and Egbert Zojer. 2020. "The Potential of X-ray Photoelectron Spectroscopy for Determining Interface Dipoles of Self-Assembled Monolayers" Applied Sciences 10, no. 17: 5735. https://doi.org/10.3390/app10175735
APA StyleTaucher, T. C., & Zojer, E. (2020). The Potential of X-ray Photoelectron Spectroscopy for Determining Interface Dipoles of Self-Assembled Monolayers. Applied Sciences, 10(17), 5735. https://doi.org/10.3390/app10175735




