Mixing Performance Analysis of Orbitally Shaken Bioreactors
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Power Method for Power Consumption Determination
- The rotary shaking machine (rotating oscillator model: RTO-34) is firmly fixed to a frame [14]. The shaking table power input is regulated using a power meter (Arepu AR78-2, maximum loading power: 2200 w). The sealed vessel filled with a predetermined solution volume (4 L, 6 L, 8 L, 10 L) is placed on the rotary shaken platform. Measurements of the required power and speed data for shaking are recorded and saved on a personal computer [18];
- Operate the closed vessel where the liquid moving is not permitted and then the electric power Pc is determined by multiplying the voltage and current values;
- Operate the open vessel where the liquid can move freely and then the electric power Po is calculated using the same method as step 2;
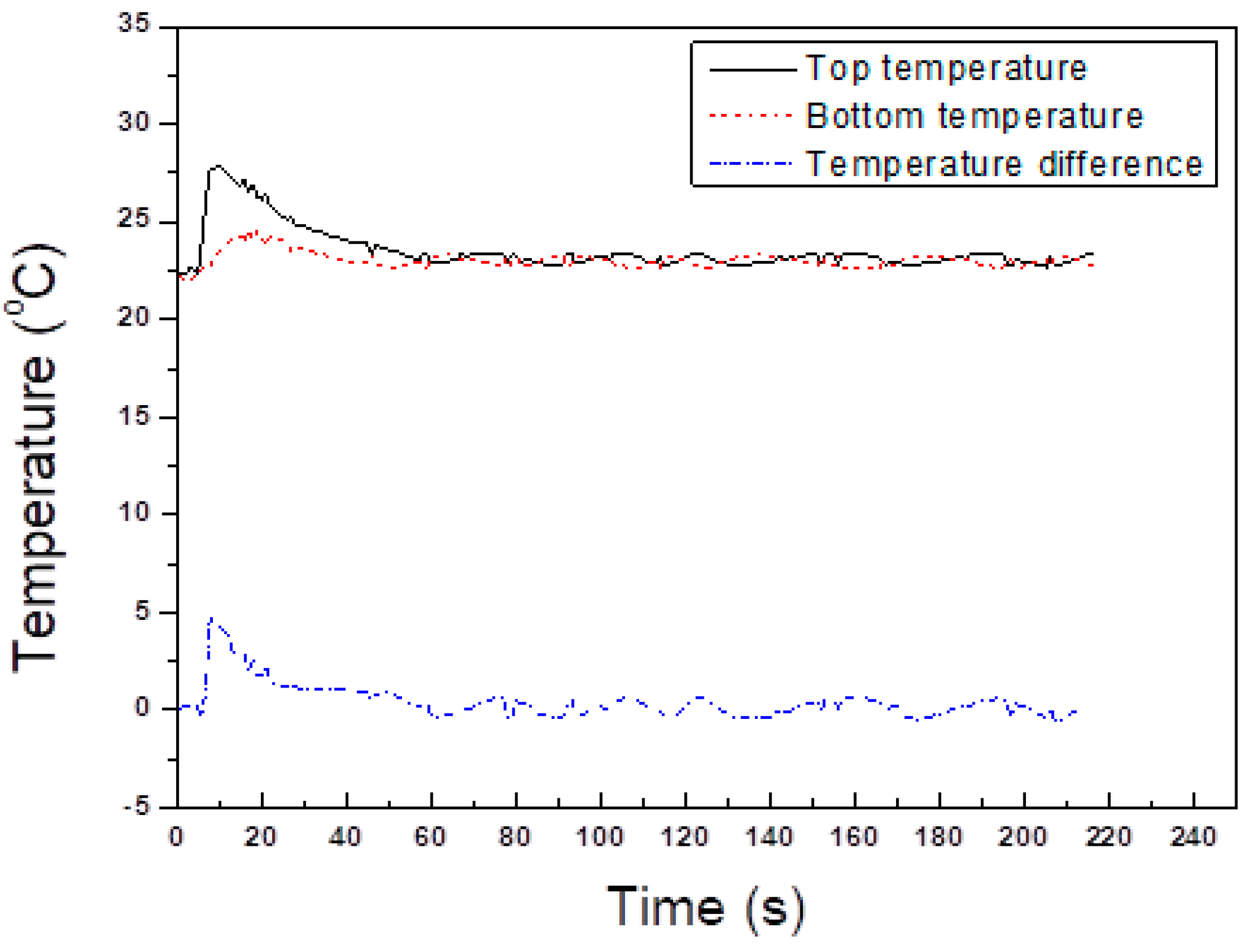
2.3. Temperature Method for Mixing Time Determination
2.4. Theory
3. Results
3.1. Power Input Correlation
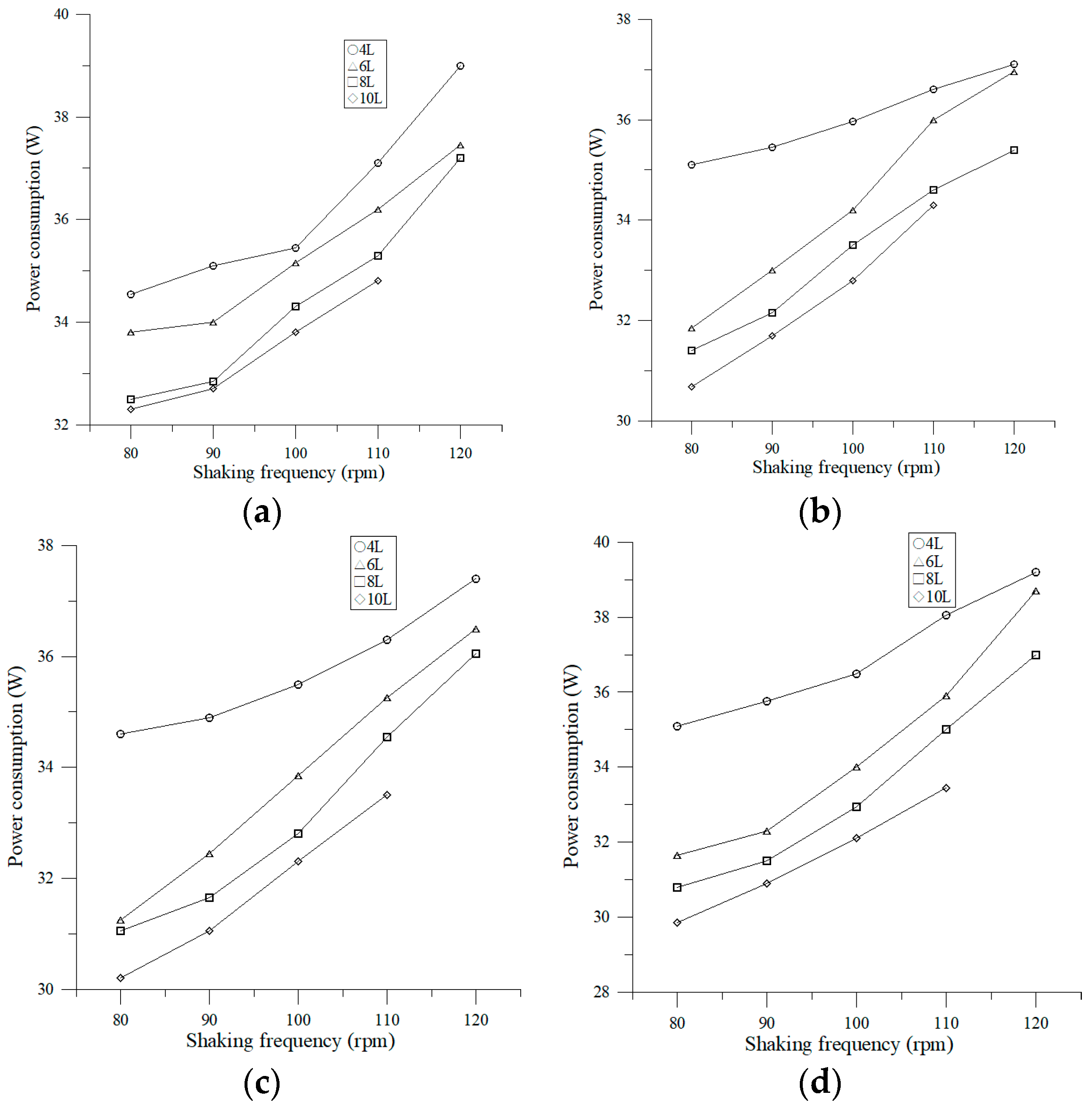
3.1.1. Local Power Consumption
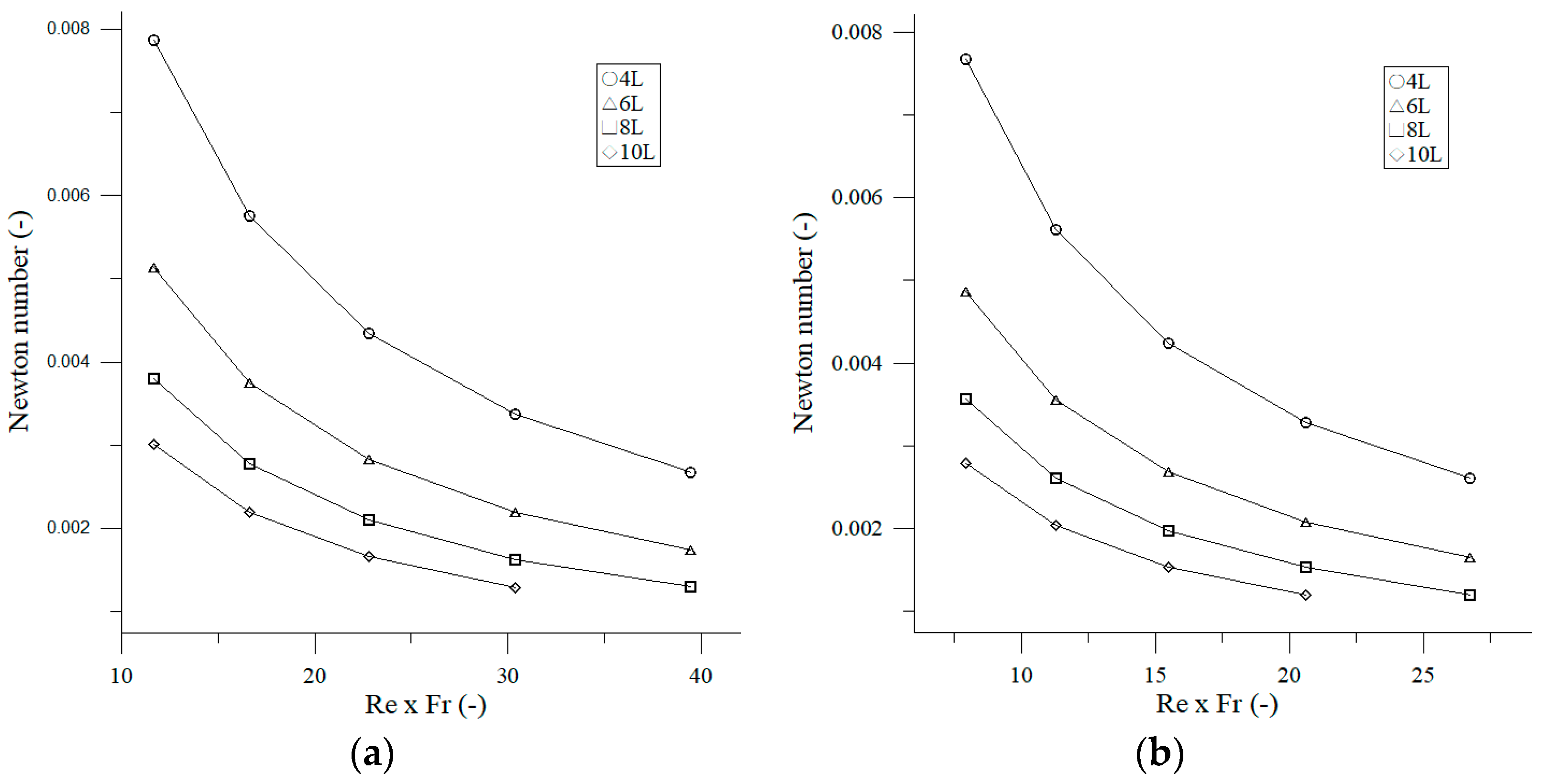
3.1.2. Effect Reynolds Number on Newton Number
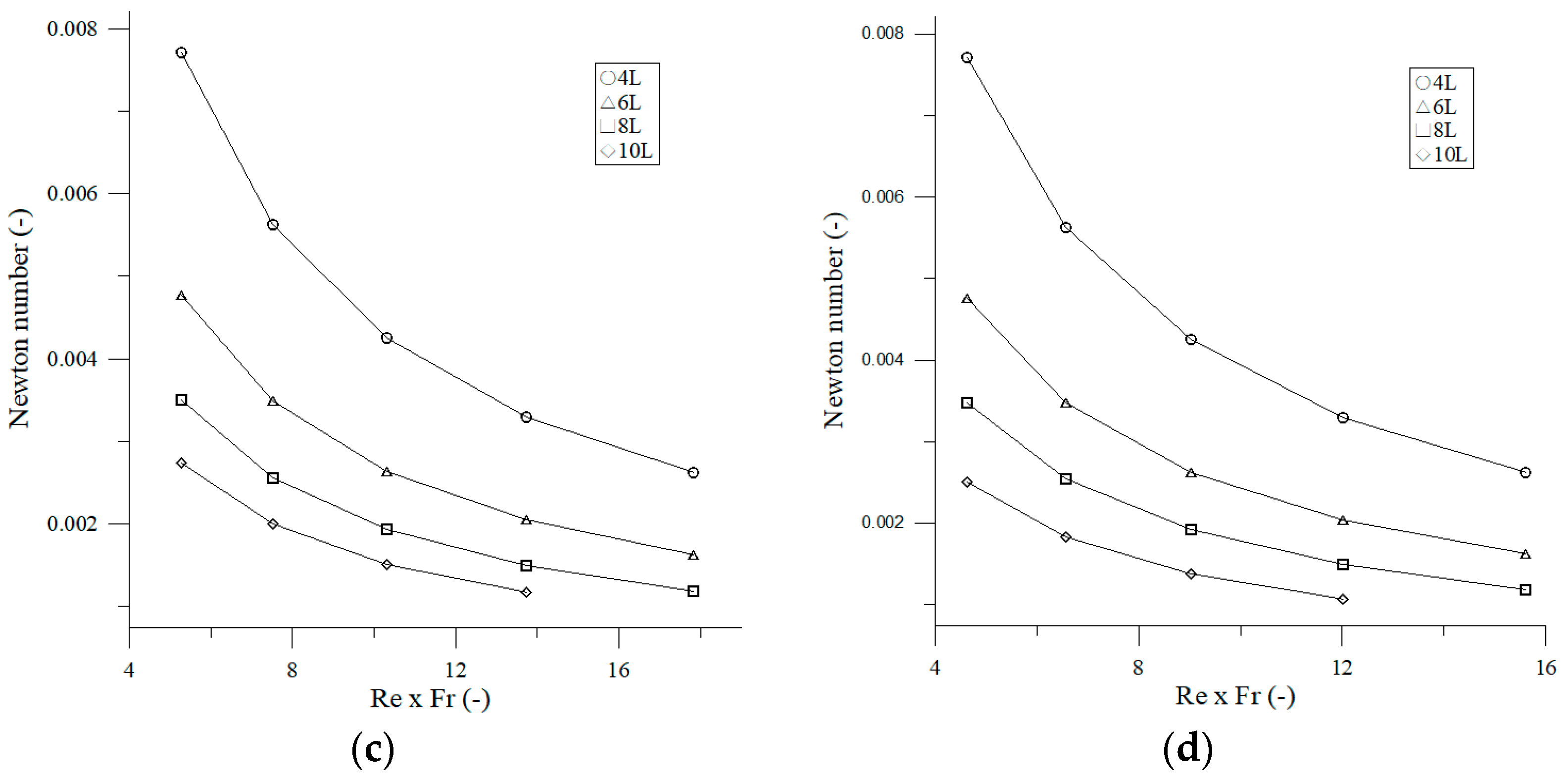
3.1.3. Effect Reynolds Number and Froude Number on Newton Number
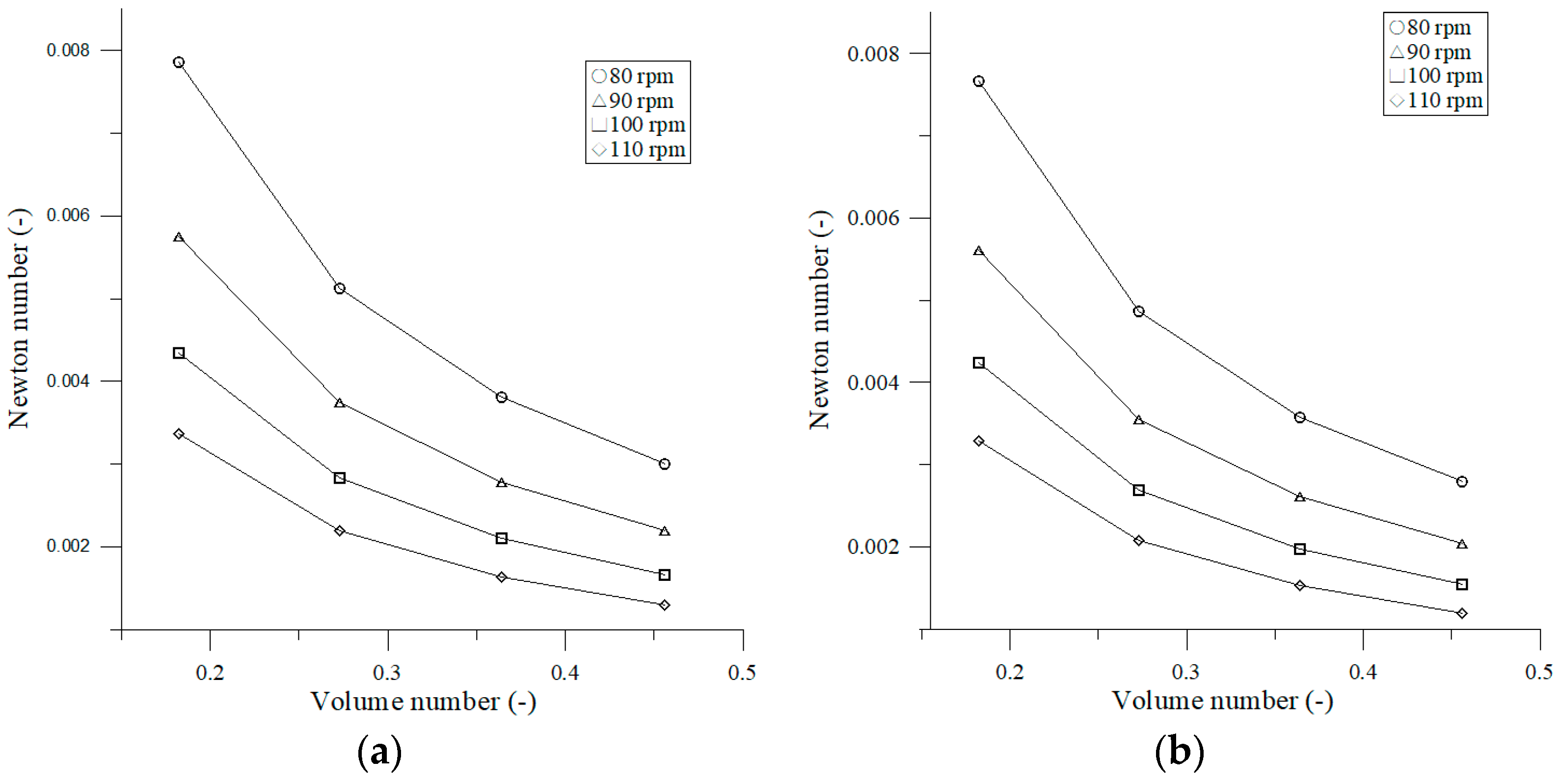
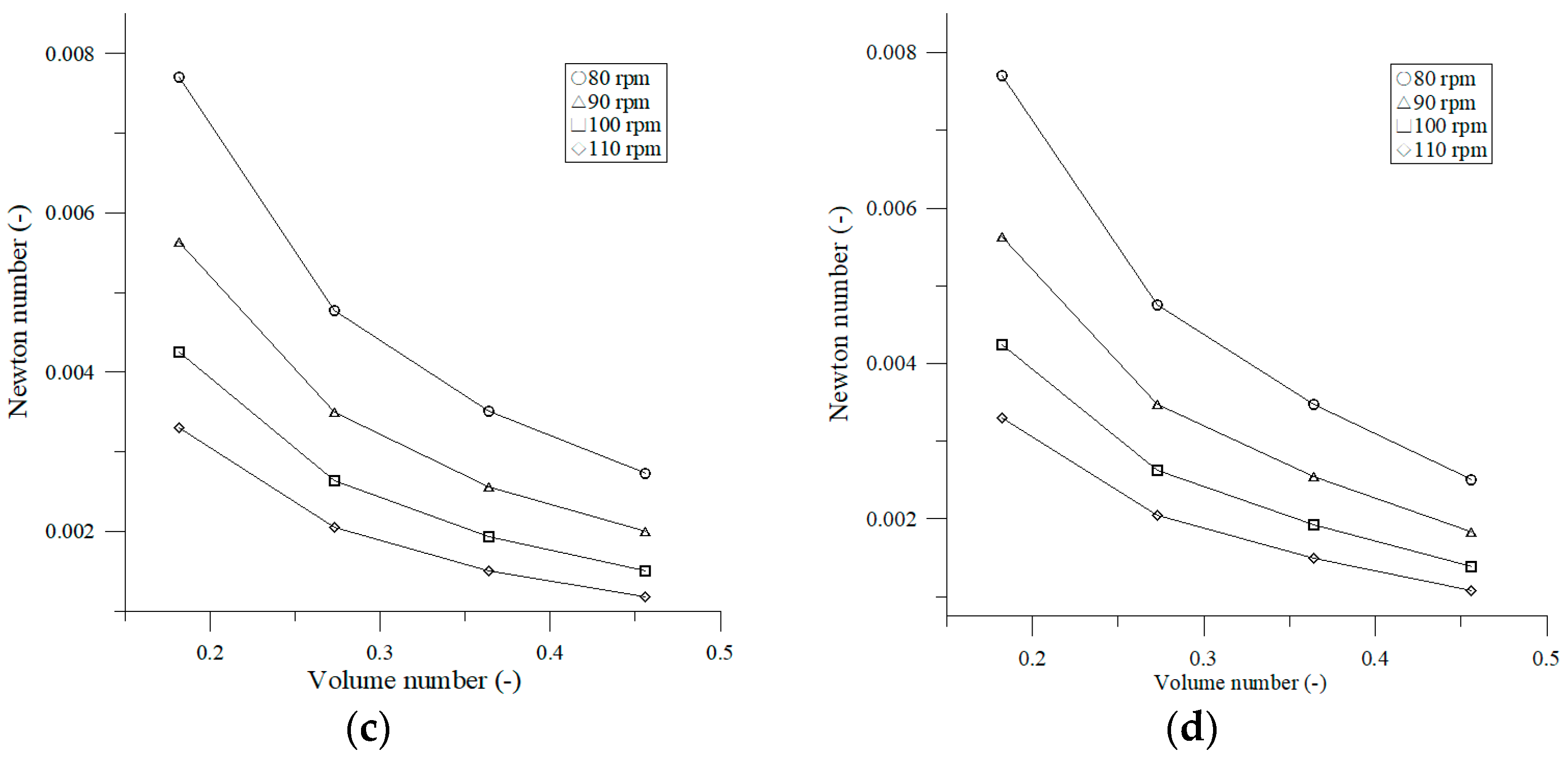
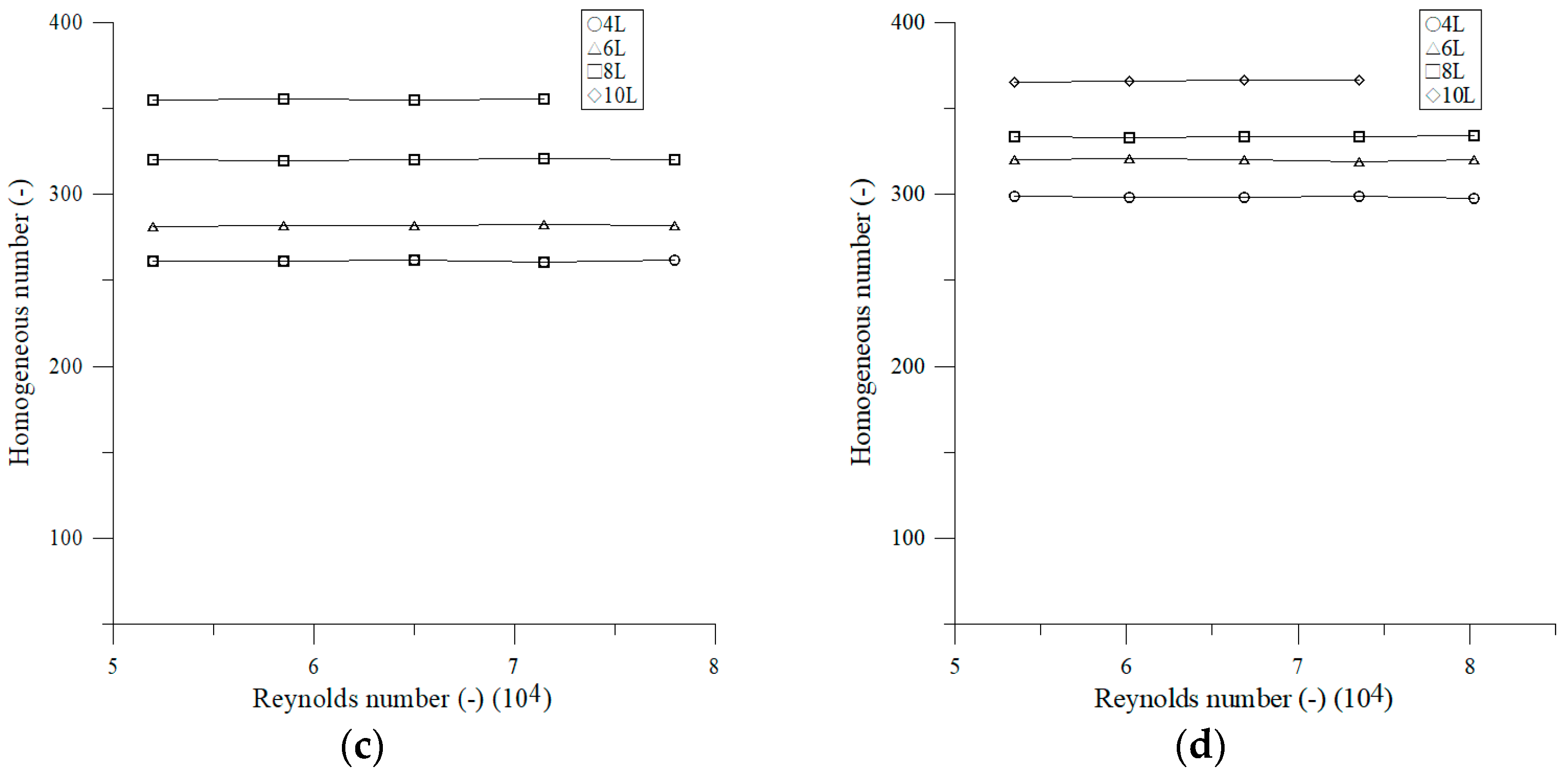
3.1.4. Effect of Volume Number on Newton Number
3.1.5. Validation and Application of Newton Number Models
3.2. Mixing Time
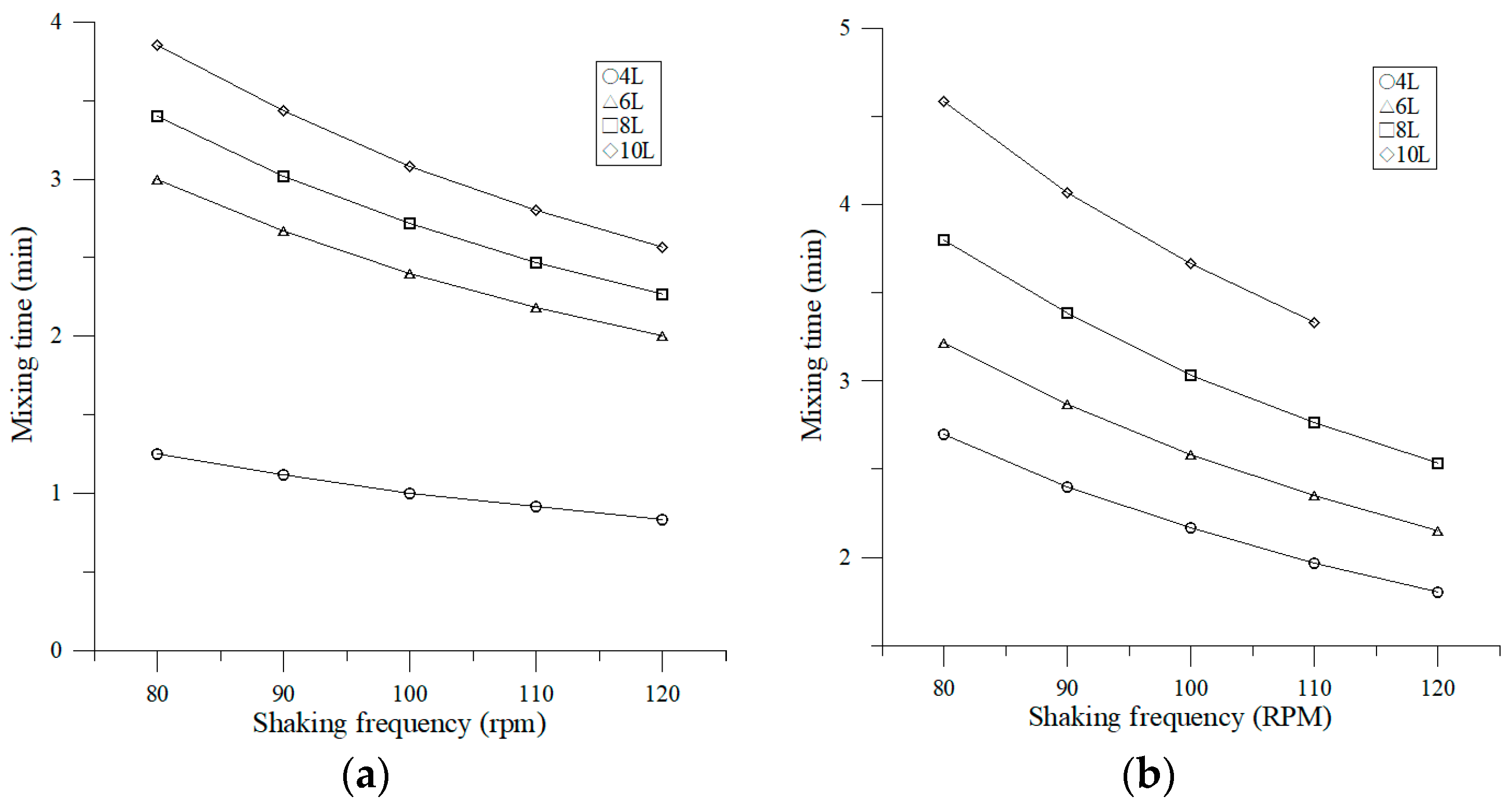
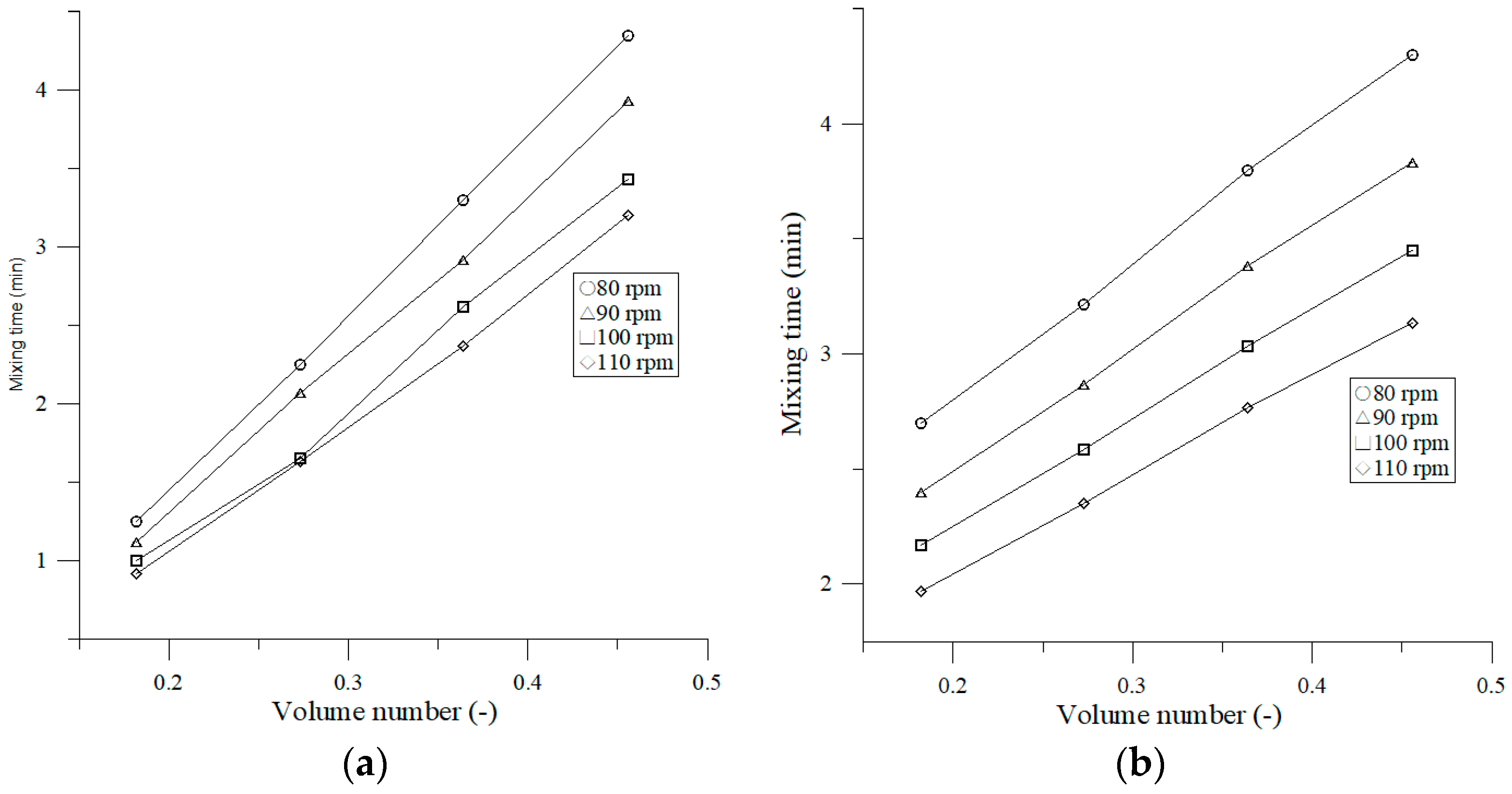
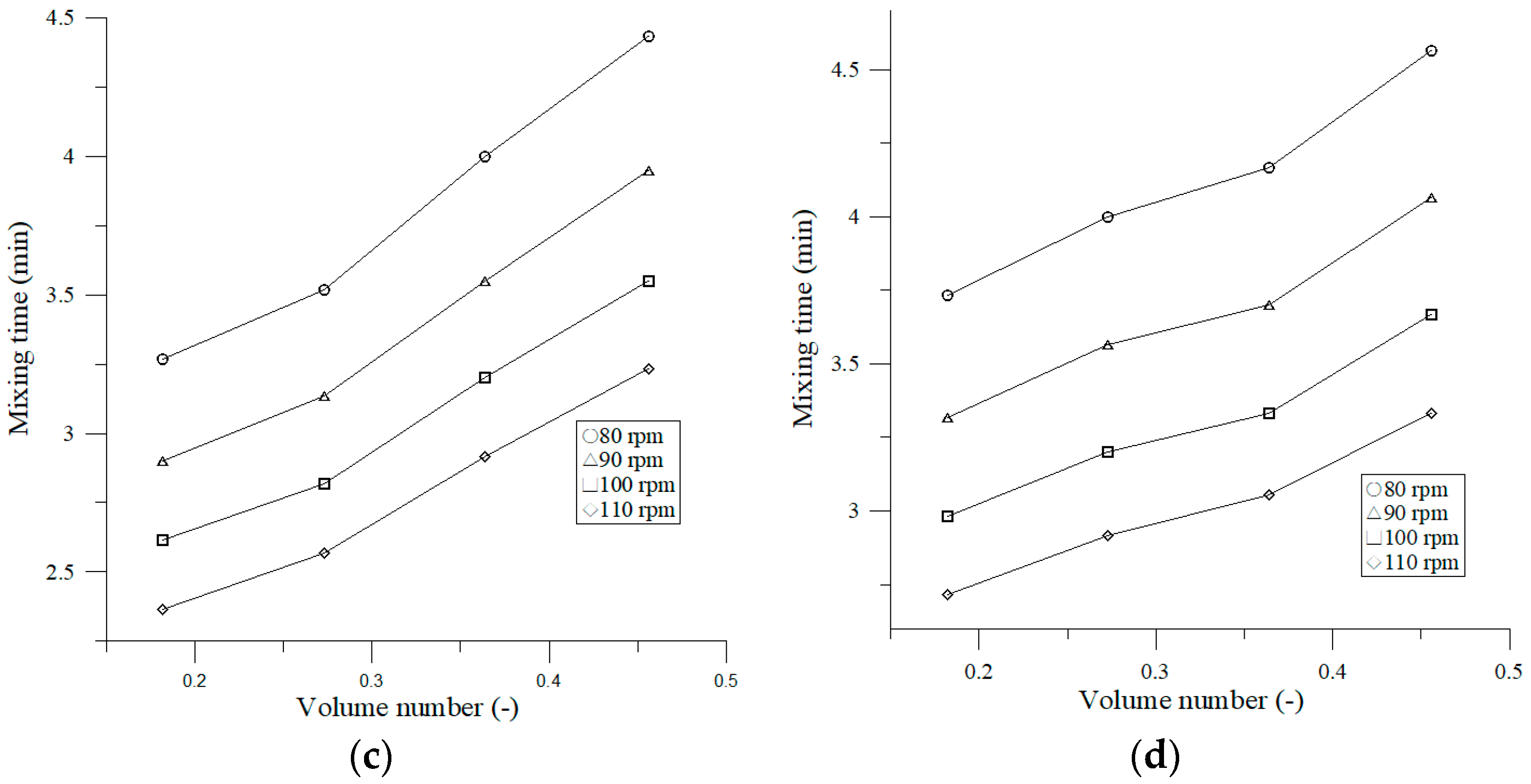
3.2.1. Effect of Shaking Frequency on Mixing Time
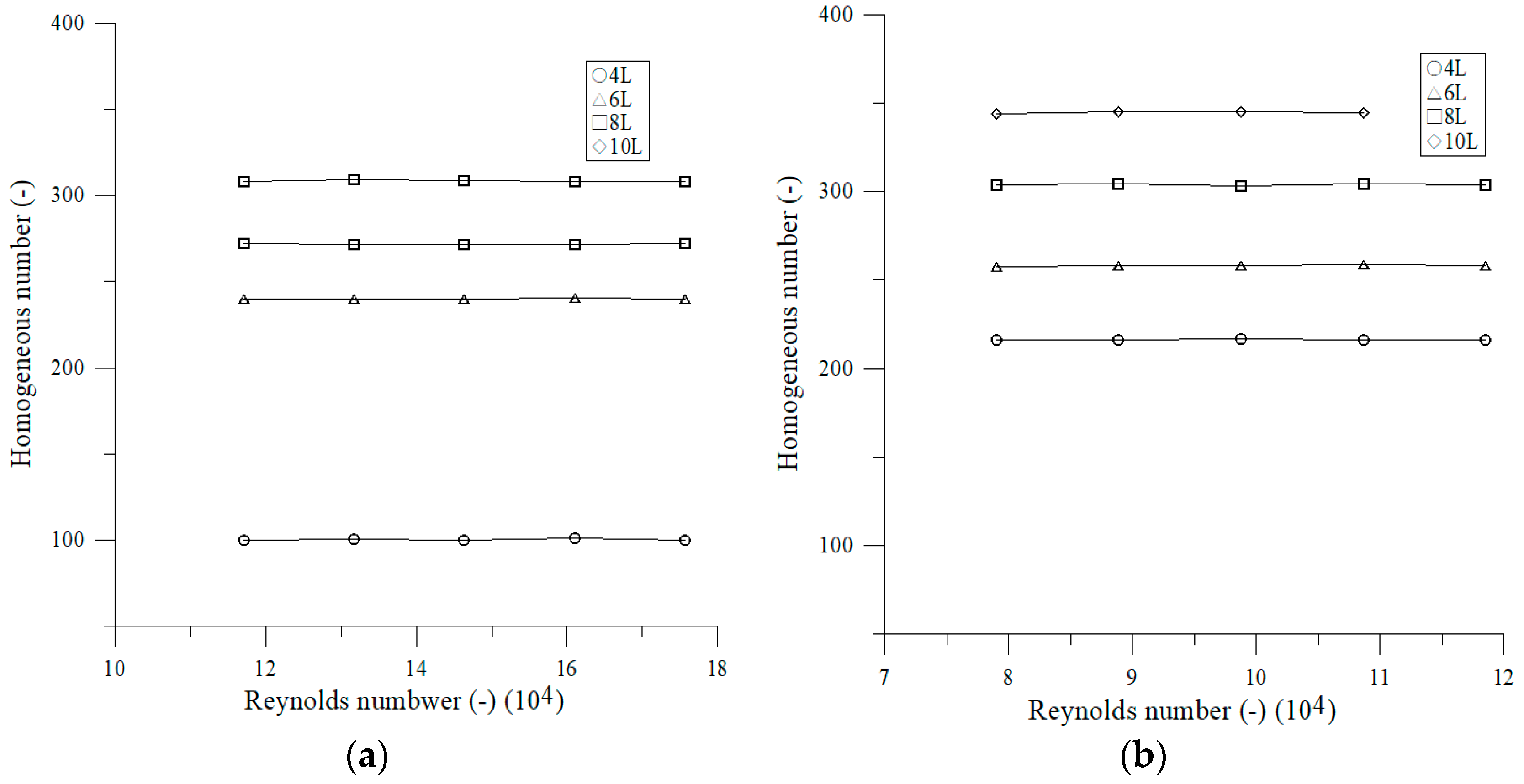
3.2.2. Effect of Reynolds Number on Mixing Number
3.2.3. Effect of Volume Number on Mixing Time
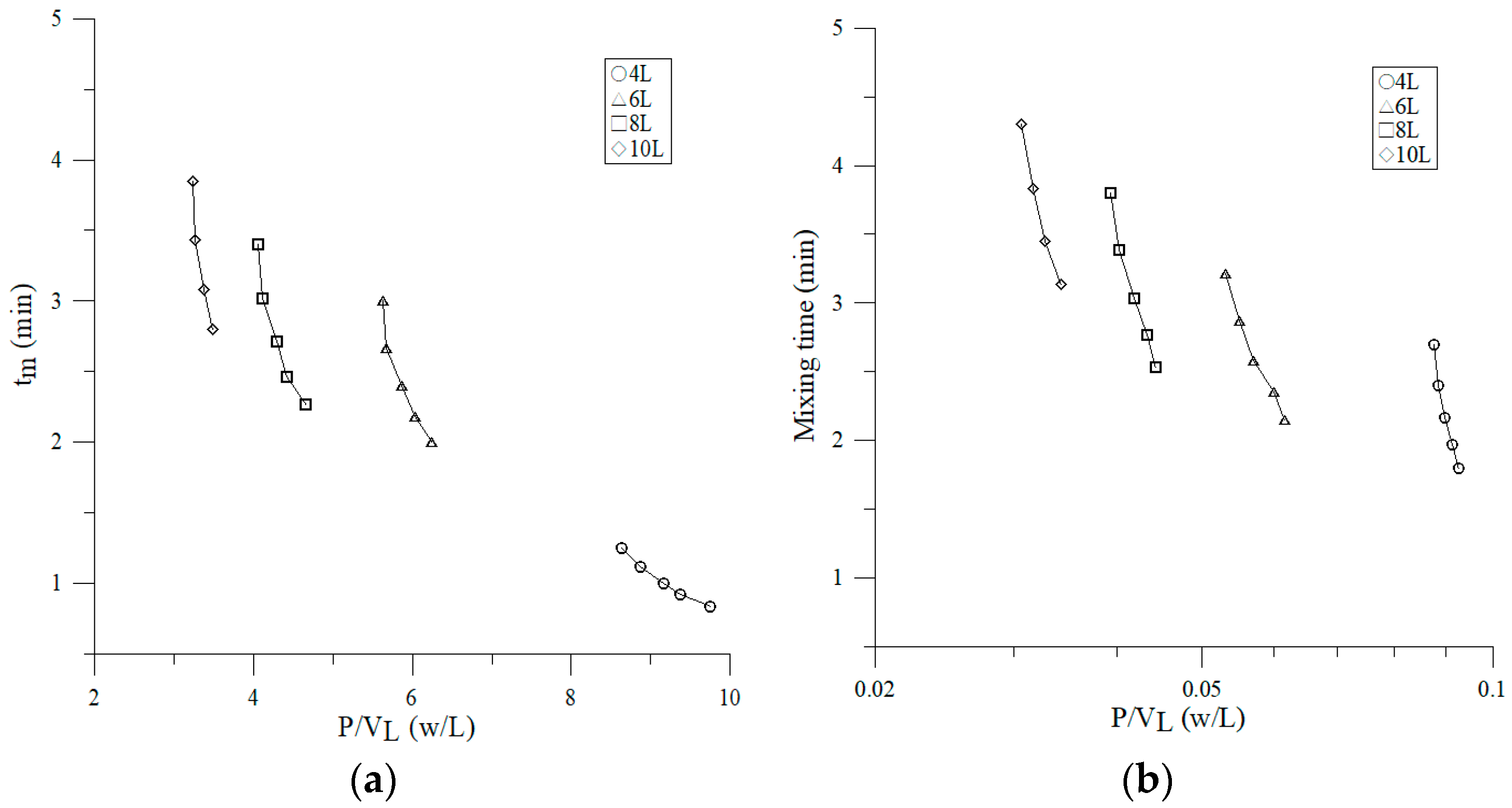
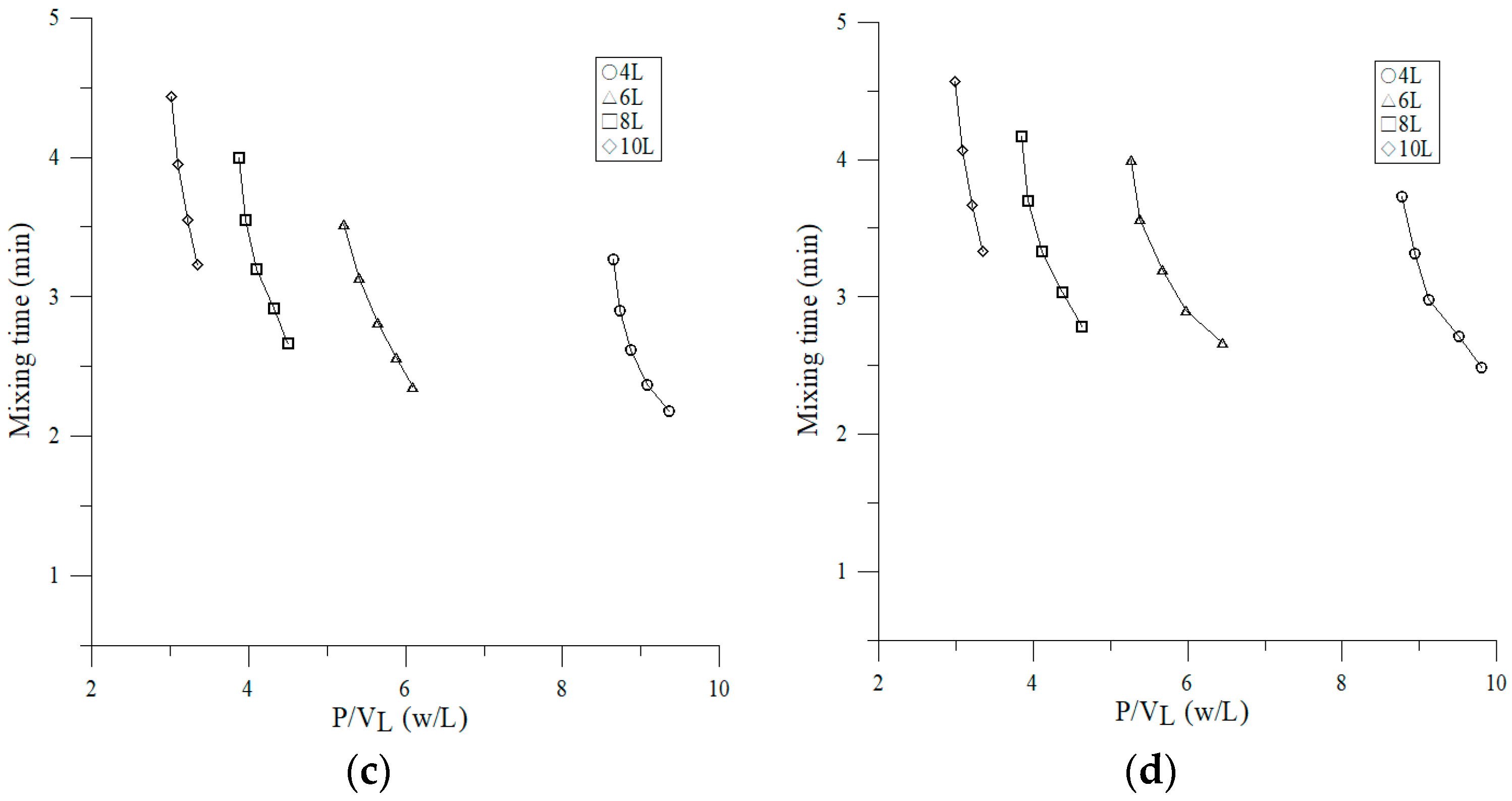
3.2.4. Effect of Volumetric Power Consumption on Mixing Time
3.2.5. Validation and Application of Mixing Time Models
3.3. Mixing Efficiency
4. Discussion
4.1. Effect of Operating Parameters on Newton Number
4.2. Effect of Operating Parameters on Mixing Time and Mixing Number
4.3. Effect of Operating Parameters on Mixing Efficiency
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Ce | computational mixing efficiency | w m−3 min |
| D | vessel diameter | m |
| Fr | Froude number | – |
| g | gravitational acceleration | m s−2 |
| N | shaking frequency | rpm |
| Ne | Newton number | – |
| P | power consumption | w |
| Pv | power consumption of unit volume | w m−3 |
| Re | Reynolds number | – |
| t | shaking time | min |
| VL | filling volume | m3 |
| Greek Symbols | ||
| ρ | liquid density | kg m−3 |
| η | dynamic viscosity of fluid | Pa·s |
References
- Büchs, J. Introduction to advantages and problems of shake cultures. Biochem. Eng. J. 2001, 7, 91–98. [Google Scholar] [CrossRef]
- Suresh, S.; Srivastava, V.C.; Mishra, I.M. Critical analysis of engineering aspects of shaken flask bioreactors. Critical Reviews in Biotechn. Crit. Rev. Biotechnol. 2009, 29, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Platas, O.B.; Sandig, V.; Pörtner, R.; Zeng, A.P. Evaluation of process parameters in shake flasks for mammalian cell culture. BMC Proc. 2013, 7 (Suppl. 6), 17. [Google Scholar] [CrossRef]
- Betts, J.I.; Baganz, F. Miniature bioreactors: Current practices and future opportunities. Microb. Cell Factories 2006, 5, 21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doig, S.D.; Baganz, F.; Lye, G.J. Chapter 12—High-throughput screening and process optimization. In Basic Biotechnology, 3rd ed.; Ratledge, C., Kristiansen, B., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 289–306. [Google Scholar]
- Klöckner, W.; Büchs, J. Advances in shaking technologies. Trends Biotechnol. 2012, 30, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Lara, A.R.; Galindo, E.; Ramı´rez, O.T.; Palomares, L.A. Living with heterogeneities in bioreactors. Mol. Biotechnol. 2006, 34, 355–381. [Google Scholar] [CrossRef]
- Klöckner, W.; Riad Gacem, R.; Anderlei, T.; Raven, N.; Schillberg, S.; Lattermann, C.; Büchs, J. Correlation between mass transfer coefficient kLa and relevant operating parameters in cylindrical disposable shaken bioreactors on a bench-to-pilot scale. J. Biol. Eng. 2013, 7, 28. [Google Scholar] [CrossRef]
- Zhang, X.; Burki, C.; Stettler, M.; Sanctis, D.D.; Perrone, M.; Parolini, N.; Discacciati, M.; Jesus, M.D.; Hacker, D.; Quarteroni, A.; et al. Efficient oxygen transfer by surface aeration in shaken cylindrical containers for mammalian cell cultivation at volumetric scales up to 1000 L. Biochem. Eng. J. 2009, 45, 41–47. [Google Scholar] [CrossRef]
- Zhang, X.; Stettler, M.; Sanctis, D.D.; Perrone, M.; Parolini, N.; Discacciati, M.; Jesus, M.D.; Hacker, D.; Quarteroni, A.; Wurm, F. Use of orbital shaken disposable bioreactors for mammalian cell cultures from the milliliter-scale to the 1,000-liter scale. Adv. Biochem. Engin./Biotechnol. 2009, 115, 33–53. [Google Scholar]
- Dürauer, A.; Hobiger, S.; Walther, C.; Jungbauer, A. Mixing at the microscale: Power input in shaken microtiter plates. Biotechnol. J. 2016, 11, 1539–1549. [Google Scholar] [CrossRef]
- Gamboa-Suasnavart, R.A.; Marı´n-Palacio, L.D.; Martı´nez-Sotelo, J.A. Scale-up from shake flasks to bioreactor, based on power input and Streptomyces lividans morphology, for the production of recombinant APA (45/47 kDa protein) from Mycobacterium tuberculosis. World J. Microbiol. Biotechnol. 2013, 29, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Ducci, A.; Weheliye, W.H. Orbitally shaken bioreactors—Viscosity effects on flow characteristics. AIChE J. 2014, 60, 3951–3968. [Google Scholar] [CrossRef]
- Büchs, J.; Maier, U.; Milbradt, C.; Zoels, B. Power consumption in shaking flasks on rotary shaking machines: I. Power consumption measurement in unbaffled flasks at low liquid viscosity. Biotechnol. Bioeng. 2000, 68, 589–593. [Google Scholar] [CrossRef]
- Klöckner, W.; Diederichs, S.; Büchs, J. Orbitally Shaken Single-Use Bioreactors. Adv. Biochem. Eng. Biotechnol. 2014, 138, 45–60. [Google Scholar] [CrossRef]
- Büchs, J.; Maier, U.; Milbradt, C.; Zoels, B. Power consumption in shaking flasks on rotary shaking machines: II. Nondimensional description of specific power consumption and flow regimes in unbaffled flasks at elevated liquid viscosity. Biotechnol. Bioeng. 2000, 68, 594–601. [Google Scholar] [CrossRef]
- Née Meissner, L.R.; Arndt, J.; Palmen, T.G.; Jestel, T.; Mitsunaga, H.; Fukusaki, E.C.; Büchs, J. Investigation of poly(γ-glutamic acid) production via online determination of viscosity and oxygen transfer rate in shake flasks. J. Biol. Eng. 2017, 11, 23. [Google Scholar] [CrossRef]
- Klöckner, W.; Tissot, S.; Wurmb, F.; Büchs, J. Power input correlation to characterize the hydrodynamics of cylindrical orbitally shaken bioreactors. Biochem. Eng. J. 2012, 65, 63–69. [Google Scholar] [CrossRef]
- Micheletti, M.; Barrett, T.; Doig, S.D.; Baganz, F.; Levy, M.S.; Woodley, J.M.; Lye, G.J. Fluid mixing in shaken bioreactors: Implications for scale-up predictions from microlitre-scale microbial and mammalian cell cultures. Chem. Eng. Sci. 2006, 61, 2939–2949. [Google Scholar] [CrossRef]
- Zlokarnik, M. Stirring, in: Ullmann’s Encyclopedia of Industrial Chemistry, Electronic Release, 7th ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Weheliye, W.; Rodriguez, G.; Anderlei, T.; Micheletti, M.; Yianneskis, M.; Ducci, A. Appraisal of shaken bioreactor mixing efficiency for different operating conditions. In Proceedings of the 14th European Conference on Mixing Warszawa, Warsaw, Poland, 10–13 September 2012; pp. 503–509. [Google Scholar]
- Hiby, J.W. Definition and measurement of the degree of mixing in liquid mixtures. Int. Chem. Eng. 1981, 21, 197–204. [Google Scholar]
- Kasat, G.R.; Pandit, A.B. Mixing time studies in multiple impeller agitated reactors. Can. J. Chem. Eng. 2004, 82, 892–904. [Google Scholar] [CrossRef]
- Li, Y.; Ducci, A.; Micheletti, M. Study on mixing characteristics in shaken microwell systems. Biochem. Eng. J. 2020, 153, 107392. [Google Scholar] [CrossRef]
- Hoogendoorn, C.J.; Den Hartog, A.P. Model studies on mixers in the viscous flow region. Chem. Eng. Sci. 1967, 22, 1689–1699. [Google Scholar] [CrossRef]
- Tan, R.K.; Eberhard, W.; Büchs, J. Measurement and characterization of mixing time in shake flasks. Chem. Eng. Sci. 2011, 66, 440–447. [Google Scholar] [CrossRef]
- Tissot, S.; Farhat, M.; Hacker, D.L.; Anderlei, T.; Kühner, M.; Comninellis, C.; Wurm, F. Determination of a scale-up factor from mixing time studies in orbitally shaken bioreactors. Biochem. Eng. J. 2010, 52, 181–186. [Google Scholar] [CrossRef]
- Rodriguez, G.; Weheliye, W.; Anderlei, T.; Micheletti, M.; Yianneskis, M.; Ducci, A. Mixing time and kinetic energy measurements in a shaken cylindrical bioreactor. Chem. Eng. Res. Des. 2013, 91, 2084–2097. [Google Scholar] [CrossRef]
- Rodrigueza, G.; Anderleib, T.; Michelettia, M.; Yianneskisc, M.; Ducci, A. On the measurement and scaling of mixing time in orbitally shaken bioreactors. Biochem. Eng. J. 2016, 82, 10–21. [Google Scholar] [CrossRef]
- Weheliye, W.; Yianneskis, M.; Ducci, A. On the fluid dynamics of shaken bioreactors—Flow characterization and transition. AIChE J. 2013, 59, 334–344. [Google Scholar] [CrossRef]
- Havas, G.; Sawinsky, J.; Deak, A.; Fekete, A. Investigation of the homogenization efficiency of various propeller agitator type. Period. Polytech. Chem. Eng. 1978, 22, 331–344. [Google Scholar]
- Shiue, S.J.; Wong, C.W. Studies on Homogenization Efficiency of Various Agitators in Liquid Blending. Can. J. Chem. Eng. 1984, 62, 602–609. [Google Scholar] [CrossRef]
- Havas, G.; Sawinsky, J.; Deak, A.; Fekete, A. Investigation of the homogenization efficiency of the screw agitator, helical ribbon agitator, gate type anchor and the multiple-paddle agitator in the mixing of high-viscosity Newtonian liquids. Period. Polytech. Chem. Eng. 1978, 22, 317–330. [Google Scholar]
- Verma, M.; Brar, S.K.; Sreekrishnan, T.R.; Tyagi, R.D.; Surampalli, R.Y. Dimensionless groups as scale-up parameter for wastewater and wastewater sludge treatment in a stirred tank reactor. J. Residuals Sci. Technol. 2007, 4, 35–43. [Google Scholar]
- Yamaguchi, H. Engineering Fluid Mechanics; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Rushton, J. Applications of fluid mechanics and similitude to scale-up problems. Part 1. Chem. Eng. Prog. 1952, 48, 33–38. [Google Scholar]
- Giese, H.; Klockner, W.; Pena, C.; Galindo, E.; Lotter, S.; Wetzel, K.; Meissner, L.; Peter, C.P.; Büchs, J. Effective shear rates in shake flasks. Chem. Eng. Sci. 2014, 118, 102–113. [Google Scholar] [CrossRef]
- Büchs, J.; Zoels, B. Evaluation of maximum to specific power consumption ratio in shaking bioreactors. J. Chem. Eng. Jpn. 2001, 34, 647–653. [Google Scholar] [CrossRef]
- Peter, C.P.; Suzuki, Y.; Buchs, J. Hydromechanical stress in shake flasks: Correlation for the maximum local energy dissipation rate. Biotechnol. Bioeng. 2006, 93, 1164–1176. [Google Scholar] [CrossRef]
- Khorshid, F.A. The Effect of the Medium Viscosity on the Cells Morphology in Reaction of Cells to Topography—I. In Proceedings of the 2nd Saudi Sci. Conl, Fac. Sci., King AbdulAziz University, Jeddah, Saudi Arabia, 15–17 March 2004; Part I. pp. 67–98. [Google Scholar]
- Monteila, D.T.; Tontodonatia, G.; Ghimirea, S.; Baldia, L.; Hackera, D.L.; Burkib, C.A.; Wurm, F.M. Disposable 600-mL orbitally shaken bioreactor for mammalian cell cultivation in suspension. Biochem. Eng. J. 2013, 76, 6–12. [Google Scholar] [CrossRef]
- Mayr, B.; Horvat, P.; Moser, A. Engineering approach to mixing quantification in bioreactors. Bioprocess Eng. 1992, 8, 137–143. [Google Scholar] [CrossRef]
- Espinosa-Solares, T.; Brito-De La Fuente, E.; Tecante, A.; Medina-Torres, L.; Tanguy, P.A. Mixing Time in Rheologically Evolving Model Fluids by Hybrid Dual Mixing Systems. Chem. Eng. Res. Des. 2002, 80, 817–823. [Google Scholar] [CrossRef]
- Harnby, N.; Edwards, M. Mixing in the Process Industries; Butterworth Publishers: Stoneham, MA, USA, 1992. [Google Scholar]
- Tan, R.K. Mixing Time in Shaking Bioreactors. Ph.D. Thesis, Aachen/Publikationsserver der RWTH Aachen University, Aachen, Germany, 2012. [Google Scholar]
- Zhang, P.; Chen, G.H.; Duan, J.H.; Wang, W.W. Mixing characteristics in a vessel equipped with cylindrical stirrer. Res. Phys. 2018, 10, 699–705. [Google Scholar] [CrossRef]
- Raval, K.; Büchs, J. Extended Method to Evaluate Power Consumption in Large Disposable Shaking Bioreactors. J. Chem. Eng. Jpn. 2008, 41, 1075–1082. [Google Scholar] [CrossRef]
- Lotter, S.; Büchs, J. Utilization of specific power input measurements for optimization of culture conditions in shaking flasks. Biochem. Eng. J. 2004, 17, 195–203. [Google Scholar] [CrossRef]
- Sundaramurthy, S. Mixing in Shake Flask Bioreactor. In Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology; Flickinger, M.C., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 1–17. [Google Scholar]
- Peter, C.P.; Suzuki, Y.; Rachinskiy, K.; Lotter, S.; Büchs, J. Volumetric power consumption in baffled shake flasks. Chem. Eng. Sci. 2006, 61, 3771–3779. [Google Scholar] [CrossRef]
- Kato, Y.; Peter, C.P.; Akgün, A.; Büchs, J. Power consumption and heat transfer resistance in large rotary shaking vessels. Biochem. Eng. J. 2004, 21, 83–91. [Google Scholar] [CrossRef]
- Klöckner, W.; Büchs, J. 2.24.2 Specific Power Input in Shake Flasks. In Comprehensive Biotechnology, 3rd ed.; 2019; pp. 307–309. [Google Scholar]
- Sumino, Y.; Akiyama, S.I.; Fukuda, H. Performance of the shaking flask (I) power consumption. J. Ferment. Technol. 1972, 50, 203–208. [Google Scholar]
- Raval, K.; Kato, Y.; Büchs, J. Comparison of torque method and temperature method for determination of power consumption in disposable shaken bioreactors. Biochem. Eng. J. 2007, 34, 224–227. [Google Scholar] [CrossRef]
- Pena, C.; Peter, P.C.; Buchs, J.; Galindo, E. Evolution of the specific power consumption and oxygen transfer rate in alginate-producing cultures of Azotobacter vinelandii conducted in shake flasks. Biochem. Eng. J. 2007, 36, 73–80. [Google Scholar] [CrossRef]
- Kato, Y.; Hiraoka, S.; Tada, Y.; Koh, S.T.; Lee, Y.S. Mixing time and power consumption for a liquid in a vertical cylindrical vessel, shaken in a horizontal circle. Chem. Eng. Res. Des. 1996, 74, 451–455. [Google Scholar]
- Buckingham, E. The principle of similitude. Nature 1915, 96, 396–397. [Google Scholar] [CrossRef]
- Büchs, J.; Lotter, S.; Milbradt, C. Out-of-phase operating conditions, a hitherto unknown phenomenon in shaking bioreactors. Biochem. Eng. J. 2001, 7, 135–141. [Google Scholar] [CrossRef]
- Scargiali, F.; Busciglio, A.; Grisafi, F.; Tamburini, A.; Micale, G.; Brucato, A. Power consumption in uncovered-unbaffled stirred tanks: Influence of viscosity and flow regime. Ind. Eng. Chem. Res. 2013, 52, 14998–15005. [Google Scholar] [CrossRef]
- Scargiali, F.; Tamburini, A.; Caputo, G.; Micale, G. On the assessment of power consumption and critical impeller speed in vortexing unbaffled stirred tanks. Chem. Eng. Res. Des. 2017, 123, 99–110. [Google Scholar] [CrossRef]
- Nienow, A. On impeller circulation and mixing effectiveness in the turbulent flow regime. Chem. Eng. Sci. 1997, 52, 2557–2565. [Google Scholar] [CrossRef]




| Type | Volume | Typical Experimental | Level of Monitoring | Cost of Operation |
|---|---|---|---|---|
| throughput | and control | |||
| Microtiter plates | 100 μL–3 mL | Very high: thousands | Low: T, biomass, | Medium: capital, increased |
| per technician | product | use of disposables | ||
| Small-scale stirred vessels | 10 mL–100 mL | Low/medium: 20 per | Low: T, biomass, | Low |
| technician | product | |||
| Miniaturized stirred | 25 mL–6 L | Medium: 50 max. per | High: pH, oxygen, T, | Medium: capital, |
| bioreactor | technician | biomass and product | labor | |
| Shaken vessels | 2 L–50 L | Low: 1–5 technicians | High: pH, oxygen, T, | High: capital, raw |
| biomass and product | materials, labor | |||
| Conventional stirred-tank | 1 L–100 L | Low: 1–5 technicians | High: pH, oxygen, T, | High: capital, raw |
| bioreactor | biomass and product | materials, labor |
| Liquid | Mimicking Culture Medium | Density * | Viscosity * |
|---|---|---|---|
| (kg/m3) | (Pa·s)(10−4) | ||
| water | low 4% dextran 9000 [37] | 997 | 8.94 |
| glycerin 5 volume% | 0.5% polyvinylpyrrolidone 360 k [37] | 1022.75 | 13.53 |
| glycerin 10 volume% | 1.7% dextran 2,000,000 [37] | 1036 | 20.56 |
| glycerin 20 volume% | 2% dextran 2,000,000 [37] | 1051.95 | 23.87 |
| Bioreactor Type | Shaking Frequency | Shaking Diameter | Correlation | Liquid | Literature |
|---|---|---|---|---|---|
| 20-L orbitally shaken bioreactor | 100 rpm to 300 rpm | 50 mm | P~N2.5 | Tap water | Kato et al. [48] |
| 100- to 2000-mL flask | 80 rpm to 380 rpm | 2.5 cm and 5 cm | P~N2.8 | demineralized water | Büchs et al. [14] |
| 20-L orbitally shaken bioreactor | 300 rpm | 2.5 cm | P~N5.75 | Tap water | Kato et al. [51] |
| 20-L orbitally shaken bioreactor | 80 to 120 rpm | 3.2 cm | P~N0.34 | 4.6 × 104 to 1.75 × 105 | This study |
| Bioreactor Type | Shaking Frequency | Shaking Diameter | Correlation | Reynolds Number | Literature |
|---|---|---|---|---|---|
| 100- to 2000-mL flask | 80 to 380 min−1 L/min | 2.5 and 5 cm | Ne’ = 70 Re−1 + 25 Re−0.6 + 1.5 Re−0.2 | 90 to 9 × 104 | Büchs et al. [16] |
| 250- and 500-mL flask | 160 to 340 rpm | 50 mm | Ne = 12.3 Re−0.386 | 90 to 9 × 104 | Meissner et al. [17] |
| 20-L orbitally shaken bioreactor | 100 rpm | 2.5 cm | Ne = 0.65 Re−0.17 | 2.5 × 103 to 3.5 × 104 | Klöckner et al. [18] |
| 120 rpm | 2.5 cm | Ne = 0.75 Re−0.17 | 3 x 103 to 5 × 104 | Klöckner et al. [18] | |
| 160 rpm | 5 cm | Ne = 1.1 Re−0.17 | 1.8 × 104 to 3 × 105 | Klöckner et al. [18] | |
| 20-L orbitally shaken bioreactor | 80 to 120 rpm | 3.2 cm | Ne = C1·Re−2.66 | 4.6 × 104 to 1.75 × 105 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiue, A.; Chen, S.-C.; Jeng, J.-C.; Zhu, L.; Leggett, G. Mixing Performance Analysis of Orbitally Shaken Bioreactors. Appl. Sci. 2020, 10, 5597. https://doi.org/10.3390/app10165597
Shiue A, Chen S-C, Jeng J-C, Zhu L, Leggett G. Mixing Performance Analysis of Orbitally Shaken Bioreactors. Applied Sciences. 2020; 10(16):5597. https://doi.org/10.3390/app10165597
Chicago/Turabian StyleShiue, Angus, Shih-Chieh Chen, Jyh-Cheng Jeng, Likuan Zhu, and Graham Leggett. 2020. "Mixing Performance Analysis of Orbitally Shaken Bioreactors" Applied Sciences 10, no. 16: 5597. https://doi.org/10.3390/app10165597
APA StyleShiue, A., Chen, S.-C., Jeng, J.-C., Zhu, L., & Leggett, G. (2020). Mixing Performance Analysis of Orbitally Shaken Bioreactors. Applied Sciences, 10(16), 5597. https://doi.org/10.3390/app10165597






