Selection of Tanned-Leather Waste in Recovering Novel Raw Material for Manufacturing Rubber Artifacts: Towards a Zero-Waste Condition
Abstract
1. Introduction
2. Materials And Methods
2.1. Materials
2.2. Preparation of Rubber Compounds
Reinforced Rubber Compounds
3. Mechanical Tests
3.1. Static Mechanical Properties
3.1.1. Tensile Strength
3.1.2. Tear Resistance
3.2. Dynamic Mechanical Properties
3.2.1. Elastic Resilience
3.2.2. Yerzley Resilience
3.2.3. Abrasion Resistance
3.2.4. Shore Hardness Testing
3.2.5. Plasticity Measurements
3.2.6. Vulcanization Kinetics
4. Morphology Analysis
4.1. Optical Microscopy
4.2. Scanning Electron Microscope
5. Results And Discussion
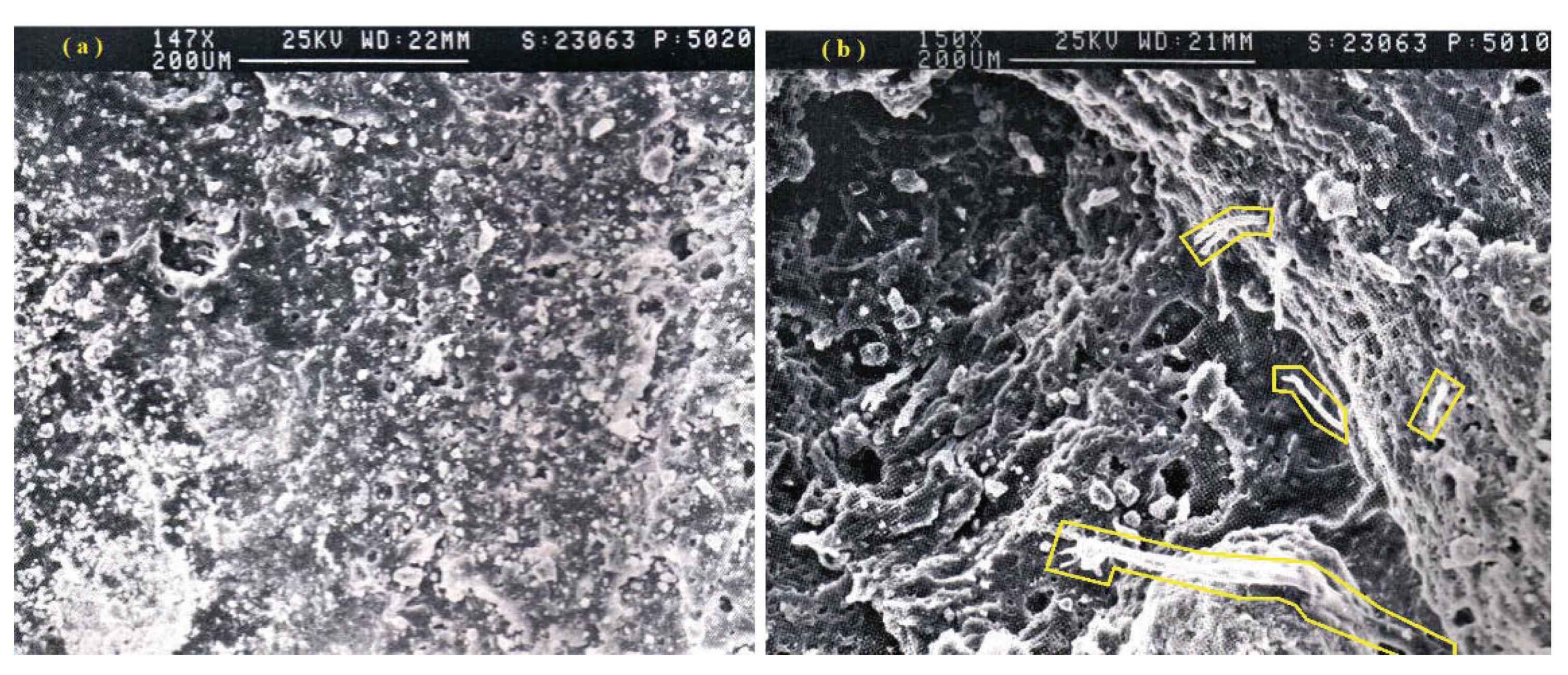
5.1. Optical Microscopy Investigation
5.2. Vulcanization Characteristics
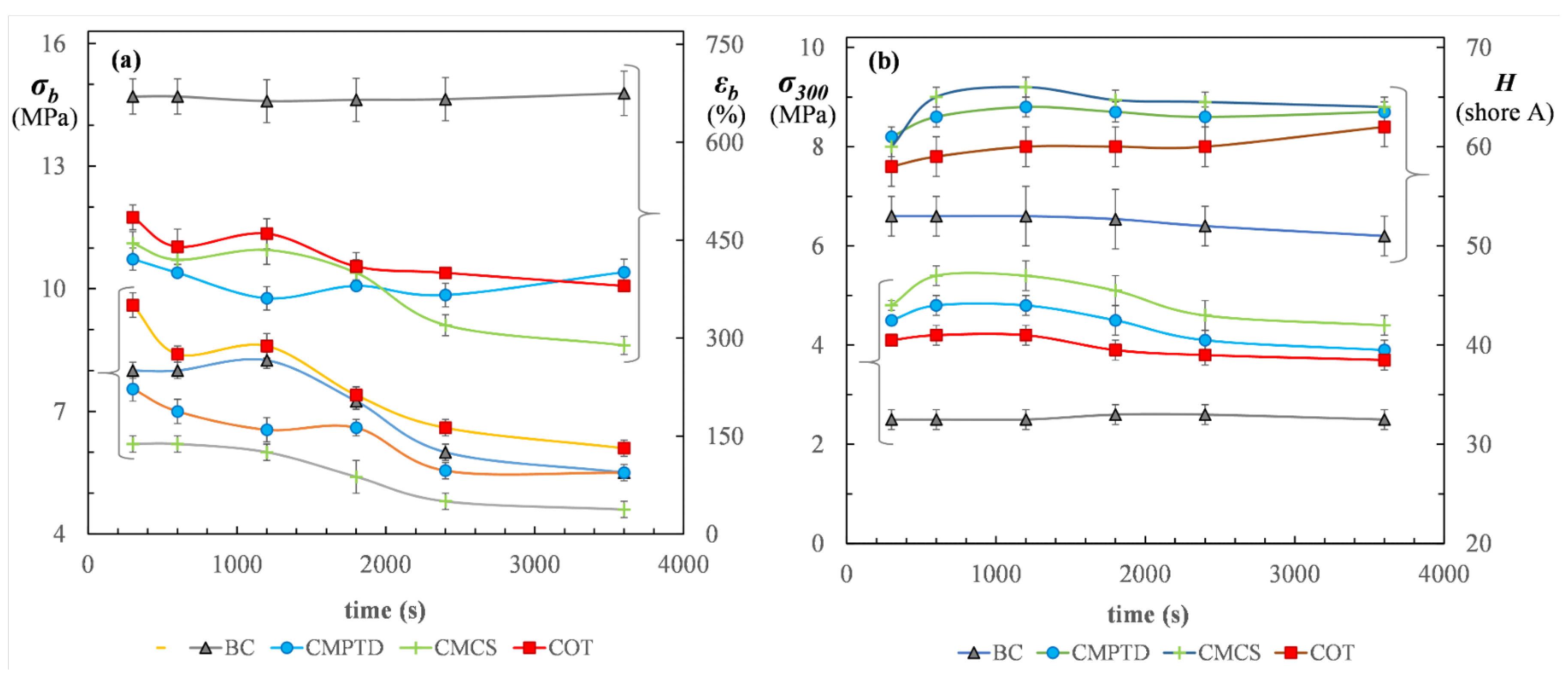
5.3. Static Mechanical Properties
5.4. Reinforced Materials
6. Manufacturing of Rubber Artifacts Based on Chromium-Tanned Waste and Monitoring of Chromium Release
6.1. Artifacts Resistant to Static Stresses
6.2. Artifacts Resistant to Dynamic Stresses
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.; Guo, R.; Lu, W.; Zhu, D. Research progress on resource utilization of leather solid waste. J. Leather Sci. Eng. 2019, 1, 6. [Google Scholar] [CrossRef]
- Knutsen, H.M. Environmental practice in the commodity chain: The dyestuff and tanning industries compared. Rev. Int. Political Econ. 2000, 7, 254–288. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, J.; Han, W. The status and developments of leather solid waste treatment: A mini-review. Waste Manag. Res. 2016, 34, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Sizeland, K.H.; Edmonds, R.L.; Basil-Jones, M.M.; Kirby, N.; Hawley, A.; Mudie, S.; Haverkamp, R.G. Changes to collagen structure during leather processing. J. Agric. Food Chem. 2015, 63, 2499–2505. [Google Scholar] [CrossRef] [PubMed]
- Covington, A.D. Tanning Chemistry: The Science of Leather; Royal Society of Chemistry: London, UK, 2009. [Google Scholar]
- Velusamy, M.; Chakali, B.; Ganesan, S.; Tinwala, F.; Venkatachalam, S.S. Investigation on pyrolysis and incineration of chrome-tanned solid waste from tanneries for effective treatment and disposal: An experimental study. Environ. Sci. Pollut. Res. 2019, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Resende, J.E.; Gonçalves, M.A.; Oliveira, L.C.; da Cunha, E.F.; Ramalho, T.C. Use of ethylenediaminetetraacetic acid as a scavenger for chromium from “wet blue” leather waste: Thermodynamic and kinetics parameters. J. Chem. 2014, 2014, 754526. [Google Scholar] [CrossRef]
- Li, K.; Yu, R.; Zhu, R.; Liang, R.; Liu, G.; Peng, B. pH-Sensitive and Chromium-Loaded Mineralized Nanoparticles as a Tanning Agent for Cleaner Leather Production. ACS Chem. Eng. 2019, 7, 8660–8669. [Google Scholar] [CrossRef]
- Bao, Y.; Ma, J.; Yang, Z. Preparation and application of poly (methacrylic acid)/montmorillonite nanocomposites. Mater. Manuf. Process. 2011, 26, 604–608. [Google Scholar] [CrossRef]
- Jianzhong, M.; Dangge, G.; Bin, L.; Yun, C.; Jianfang, D. Study on PVP/C-MMT nanocomposite material via polymer solution-intercalation method. Mater. Manuf. Process. 2007, 22, 715–720. [Google Scholar] [CrossRef]
- Dixit, S.; Yadav, A.; Dwivedi, P.D.; Das, M. Toxic hazards of leather industry and technologies to combat threat: A review. J. Clean. Prod. 2015, 87, 39–49. [Google Scholar] [CrossRef]
- Venditti, F.; Bufalo, G.; Lopez, F.; Ambrosone, L. Pollutants adsorption from aqueous solutions: The role of the mean lifetime. Chem. Eng. Sci. 2011, 66, 5922–5929. [Google Scholar] [CrossRef]
- Berhe, S.; Leta, S. Anaerobic co-digestion of tannery waste water and tannery solid waste using two-stage anaerobic sequencing batch reactor: Focus on performances of methanogenic step. J. Mater. Cycles Waste Manag. 2018, 20, 1468–1482. [Google Scholar] [CrossRef]
- Marcilla, A.; León, M.; Garcia, A.N.; Bañón, E.; Martínez, P. Upgrading of tannery wastes under fast and slow pyrolysis conditions. Ind. Eng. Chem. Res. 2012, 51, 3246–3255. [Google Scholar] [CrossRef]
- Wells, H.C.; Sizeland, K.H.; Edmonds, R.L.; Aitkenhead, W.; Kappen, P.; Glover, C.; Johannessen, B.; Haverkamp, R.G. Stabilizing chromium from leather waste in biochar. ACS Sustain. Chem. Eng. 2014, 2, 1864–1870. [Google Scholar] [CrossRef]
- Ferreira, S.D.; Junges, J.; Scopel, B.; Manera, C.; Osório, E.; Lazzarotto, I.P.; Godinho, M. Steam Gasification of Biochar Derived from the Pyrolysis of Chrome-Tanned Leather Shavings. Chem. Eng. Technol. 2019, 42, 2530–2538. [Google Scholar] [CrossRef]
- Gomes, C.S.; Repke, J.U.; Meyer, M. The effect of various pre-treatment methods of chromium leather shavings in continuous biogas production. Eng. Life Sci. 2020, 20, 79–89. [Google Scholar] [CrossRef]
- Dagne, H.; Karthikeyan, R.; Feleke, S. Waste to Energy: Response surface methodology for optimization of biodiesel production from leather fleshing waste. J. Energy 2019, 2019, 7329269. [Google Scholar] [CrossRef]
- Dang, X.; Yang, M.; Zhang, B.; Chen, H.; Wang, Y. Recovery and utilization of collagen protein powder extracted from chromium leather scrap waste. Environ. Sci. Pollut. Res. 2019, 26, 7277–7283. [Google Scholar] [CrossRef]
- Şaşmaz, S.; Karaağaç, B.; Uyanık, N. Utilization of chrome-tanned leather wastes in natural rubber and styrene-butadiene rubber blends. J. Mater. Cycles Waste Manag. 2019, 21, 166–175. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Li, L. Reuse of leather shavings as a reinforcing filler for poly (vinyl alcohol). J. Thermoplast. Compos. Mater. 2016, 29, 327–343. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Xu, W.; Shi, B. Natural Rubber-based Elastomer Reinforced by Chemically Modified Multiscale Leather Collagen Fibers with Excellent Toughness. ACS Sustain. Chem. Eng. 2020, 8, 5091–5099. [Google Scholar] [CrossRef]
- Urrego Yepes, W.; Cardona, N.; Velasquez, S.M.; Giraldo Vásquez, D.H.; Posada, J.C. Mechanical and rheometric properties of natural rubber composites filled with untreated and chemically treated leather wastes. J. Compos. Mater. 2019, 53, 1475–1487. [Google Scholar] [CrossRef]
- Romano, G.; Rapposelli, A.; Marrucci, L. Improving waste production and recycling through zero-waste strategy and privatization: An empirical investigation. Resour. Conserv. Recycl. 2019, 146, 256–263. [Google Scholar] [CrossRef]
- Bufalo, G.; Florio, C.; Lopez, F.; Cuomo, F.; Cinelli, G.; Ambrosone, L. Principles of minimal wrecking and maximum separation of solid waste to innovate tanning industries and reduce their environmental impact: The case of paperboard manufacture. J. Clean. Prod. 2018, 174, 224–232. [Google Scholar] [CrossRef]
- Brown, R.P. Physical Testing of Rubber; Springer-Science: Berlin, Germany, 1996. [Google Scholar]
- Sahu, B.; Alla, J.P.; Jayakumar, G.C.; Janardhanan, K. Optimization of Chamois Oxidation Process of Leather using Benzoyl per Oxide as Oxidizing Agent. Available online: https://slub.qucosa.de/api/qucosa%3A34255/attachment/ATT-0/ (accessed on 2 June 2020).
- Ruiz, M.R.; Cabreira, P.L.S.; Budemberg, E.R.; dos Reis, E.A.P.; Bellucci, F.S.; Job, A.E. Chemical evaluation of composites natural rubber/carbon black/leather tannery projected to antistatic flooring. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Abdelsalam, A.A.; Araby, S.; El-Sabbagh, S.H.; Abdelmoneim, A.; Hassan, M.A. Effect of carbon black loading on mechanical and rheological properties of natural rubber/styrene-butadiene rubber/nitrile butadiene rubber blends. J. Thermoplast. Compos. Mater. 2019. [Google Scholar] [CrossRef]
- Jovanović, V.; Budinski-Simendić, J.; Samaržija-Jovanović, S.; Marković, G.; Marinović-Cincović, M. The influence of carbon black on curing kinetics and thermal aging of acrylonitrile-butadiene rubber. Chem. Ind. Chem. Eng. Q. 2009, 15, 283–289. [Google Scholar] [CrossRef]
- Ruiz, M.R.; Budemberg, E.R.; da Cunha, G.P.; Bellucci, F.S.; da Cunha, H.N.; Job, A.E. An innovative material based on natural rubber and leather tannery waste to be applied as antistatic flooring. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- European Council. Directive 2018/75/EC of the European Parliament and of the Council on waste and Repealing Certain Directives; European Council: Brussels, Belgium, 2018. [Google Scholar]
- Mosca, M.; Cuomo, F.; Lopez, F.; Palumbo, G.; Bufalo, G.; Ambrosone, L. Adsorbent properties of olive mill wastes for chromate removal. Desalin. Water Treatment 2015, 54, 275–283. [Google Scholar] [CrossRef]
- Venditti, F.; Ceglie, A.; Palazzo, G.; Colafemmina, G.; Lopez, F. Removal of chromate from water by a new CTAB-silica gelatin composite. J. Coll. Interf. Sci. 2007, 310, 353–361. [Google Scholar] [CrossRef]
- Cavalcante, D.G.; Gomes, A.S.; Dos Reis, E.A.; Danna, C.S.; Kerche-Silva, L.E.; Yoshihara, E.; Job, A.E. In vitro cytotoxicity and genotoxicity of composite mixtures of natural rubber and leather residues used for textile applications. Toxicol. Ind. Health 2017, 33, 478–486. [Google Scholar] [CrossRef] [PubMed]




| Functions | Ingredients | Basic Additives (% w/w) | |||
| Rubber | Natural Rubber (NR) | 62.0 | |||
| Additives | Zinc Oxide | 3.1 | |||
| Calcium Oxide | 1.8 | ||||
| Stearic Acid | 1.2 | ||||
| Sulfenamide | 0.9 | ||||
| Calcium Carbonate | 31.0 | ||||
| Total | 100.0 | ||||
| BC (phr *) | CMPTD (phr) | CMCS (phr) | COT (phr) | ||
| Basic Compounds | Basic additives | 100.0 | 100.0 | 100.0 | 100.0 |
| Vulcanizing | Sulfur | 2.0 | 2.0 | 2.0 | 2.0 |
| Leather waste | MPTD | 0.0 | 13 | 0.0 | 0.0 |
| MCS | 0.0 | 0.0 | 13 | 0.0 | |
| OT | 0.0 | 0.0 | 0.0 | 13 |
| Function | Ingredient | RBC (phr *) | RMPTD (phr *) | RMPTD (phr *) | RMPTD (phr *) | RMPTD1 (phr *) | RFPTD (phr *) | ROT (phr *) |
|---|---|---|---|---|---|---|---|---|
| Basic additives | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Vulcanizating | Sulfur | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Fillers | MPTD | 0 | 6 | 13 | 28 | 0 | 0 | 0 |
| MPTD1 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | |
| FPTD | 0 | 0 | 0 | 0 | 0 | 13 | 0 | |
| OT | 0 | 0 | 0 | 0 | 0 | 0 | 13 | |
| Carbon black | 13 | 13 | 13 | 13 | 13 | 13 | 13 |
| Compounds | (s) | (s) | (Nm) | (s) | kgm | (%) |
|---|---|---|---|---|---|---|
| BC | 131 ± 12 | 678 | 3.3 | 0.18 | 1260 | 80 |
| CMPTD | 115 | 504 | 3.7 | 0.26 | 1260 | 74 |
| CMCS | 122 | 534 | 3.8 | 0.24 | 1270 | 70 |
| COT | 106 | 492 | 2.8 | 0.27 | 1250 | 72 |
| Specimen | Viscosity | |||||||
|---|---|---|---|---|---|---|---|---|
| ML(1+4)100 | (MPa) | (%) | (MPa) | (%) | (kNm) | (%) | kg | |
| RBC | 62 | 18.0 | 510 | 8.0 | 74 | 144 | 360 | 139 |
| RFPTD | 70 | 15.0 | 505 | 5.0 | 52 | 49.0 | 220 | 252 |
| RMPTD | 68 | 10.0 | 305 | 9.8 | 61 | 99 | 210 | 181 |
| RMPTD | 73 | 9.5 | 305 | 9.3 | 57 | 92 | 200 | 225 |
| RMPTD1 | 66 | 10.5 | 330 | 9.6 | 61 | 107 | 220 | 178 |
| RMPTD | 62 | 14.0 | 390 | 9.5 | 64 | 178 | 240 | 162 |
| ROT | 57 | 26.5 | 570 | 8.4 | 62 | 109 | 300 | 184 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bufalo, G.; Molino, B.; Ambrosone, L. Selection of Tanned-Leather Waste in Recovering Novel Raw Material for Manufacturing Rubber Artifacts: Towards a Zero-Waste Condition. Appl. Sci. 2020, 10, 5374. https://doi.org/10.3390/app10155374
Bufalo G, Molino B, Ambrosone L. Selection of Tanned-Leather Waste in Recovering Novel Raw Material for Manufacturing Rubber Artifacts: Towards a Zero-Waste Condition. Applied Sciences. 2020; 10(15):5374. https://doi.org/10.3390/app10155374
Chicago/Turabian StyleBufalo, Gennaro, Bruno Molino, and Luigi Ambrosone. 2020. "Selection of Tanned-Leather Waste in Recovering Novel Raw Material for Manufacturing Rubber Artifacts: Towards a Zero-Waste Condition" Applied Sciences 10, no. 15: 5374. https://doi.org/10.3390/app10155374
APA StyleBufalo, G., Molino, B., & Ambrosone, L. (2020). Selection of Tanned-Leather Waste in Recovering Novel Raw Material for Manufacturing Rubber Artifacts: Towards a Zero-Waste Condition. Applied Sciences, 10(15), 5374. https://doi.org/10.3390/app10155374





