Abstract
Iron slag samples unearthed from archaeological sites lying on the Eastern limes sector of Roman Dacia (the Brâncoveneşti and Călugăreni auxiliary forts and the Vătava watchtower) were studied in order to assess the probability of local iron working (smelting and smithing) during the 2nd–3rd centuries CE. Structural-mineralogic aspects revealed by PXRD analysis and FTIR spectroscopy indicate different slag types corresponding to different iron production and processing stages allowing the supposition that refining of the bloom and processing of the refined iron took place on the sites. The FTIR absorption bands obtained in the spectral domain 2000–400 cm−1 show that mineralogically the samples are constituted mainly of silicates associated with minor quantities of aluminates and carbonates. The fayalite, haematite, and magnetite phases appearing on both the X-ray diffractograms and the FTIR spectra agree with the redox conditions of the slag formation process which result from the Fe3+/Fe2+ ratio determined using the EPR-method. The bulk macro-elemental PXRF and ICP-MS spectroscopy data support the slag typization proposed on the basis of the probable working conditions; trace-elemental bulk composition suggests that the provenance of the raw materials may be different.
1. Introduction
The iron slag samples investigated in the present study were unearthed at three major archaeologic sites located on the Eastern frontier (limes) of Roman Dacia, in today Mureş County, Romania (Figure 1). At Brâncoveneşti (Hungarian name: Marosvécs) and Călugăreni (Mikháza), the remains of the Roman auxiliary forts and the adjacent military settlements are known since the 18th and 19th century. The fortlet (watchtower) identified at Vătava (Felsőrépa) during a field survey in 2011 is supposed to be closely linked to the Brâncoveneşti fort. Relying on the natural protection offered by the nearby mountains and hills, the defensive structures of the eastern limes controlled the main traffic routes towards the barbaricum. The Brâncoveneşti fort, assisted by watchtowers, monitored the border section towards the upper Mureş Valley (Felső Maros-mente), whilst the Călugăreni fort supervised the upper Niraj Valley (Felső Nyárád-mente). In the 2nd and 3rd centuries centuries AD, both forts were strategically important military locations of the eastern border of the Dacian provinces.
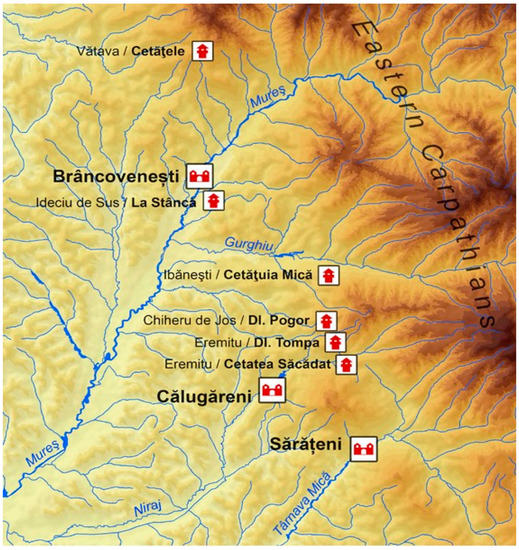
Figure 1.
Roman fortifications on the eastern Dacian limes on today Mureş County (© Szabó Máté).
During the research excavations carried out at Brâncoveneşti and Călugăreni, in the military forts and the adjacent settlements (vici) as well as at the watchtower from Vătava, plenty of Roman material was recovered, mainly ceramic vessels, building materials and animal bones, but also stone, bone, glass, iron and copper alloy artifacts. At each location, rich iron slag deposits have been found; at Vătava, even some blacksmithing tools have been recovered [1,2,3,4]. The multitude of the iron slags indicate the presence of some kind of metallurgical workshops; however, up to now, neither smelter (furnace) nor smithery remains weren’t discovered, so presently the exact whereabouts of the presumably practiced activity is unknown.
Knowledge on the slag finds’ chemical-mineralogical composition and their microstructural analyses could enable to identify the remains as smelting byproducts or primary/secondary smithing debris. The value of the Fe3+/Fe2+ ratio, defined mainly by the redox condition evolved during each particular stage of processing, could allow us to conclude if iron-producing or iron-working took place at either location.
The present study is focused primarily on the elemental analysis of the slag samples. Chemical composition was determined by PXRF (Field Portable X-ray Fluorescence Spectroscopy) and ICP-MS (Inductively Coupled Plasma Mass Spectrometry). In addition, structural-mineralogical investigation of selected samples was carried out by PXRD (Powder X-ray Diffraction) and FTIR (Fourier Transform Infrared) spectroscopy, and the Fe3+/Fe2+ ratio was determined using EPR (Electron Paramagnetic Resonance) spectroscopy measurements. The information acquired facilitate the categorization of the finds and could shed some light on the nature of the metallurgical activity practiced at the archaeological sites in question (iron production, refining or processing), the metallurgical techniques used, and on the closer provenance or more distant sourcing of the raw materials used [5,6,7].
2. Materials and Methods
The study continues the preliminary characterization started on 17 iron slag smallfinds deriving from Călugăreni [8]. The samples investigated in the present phase (Table 1) came from the Călugăreni auxiliary fort principia (headquarter building), from the Călugăreni vicus, the civil settlement evolved next to the fort, from the retentura (“backyard”) of the principia of the Brâncoveneşti auxiliary fort, and one representative find from Vătava, East of the tower location.

Table 1.
The iron slag samples.
Surface macro- and micro-elemental composition of the samples was characterized by PXRF measurements carried out in three different points of the carefully cleaned finds, using an INNOV-X Alpha-6500 spectrometer (Olympus, Woburn, MA USA) (spot size 2 mm2, 35 kV, 15 μA, 3 mm filter, Be window, PIN Si detector, counting time 60 s in two consecutive 30 s runs).
Bulk macro- and micro-elemental compositions were determined, in parallel, by PXRF and ICP-MS measurements.
For the bulk PXRF measurements, the same PXRF spectrometer was used, the analysis being performed on three disc-shaped pellets (d = 1 cm) prepared from each sample by pressing 1.00 ± 0.05 g amounts of the finely pulverized (<63 μm) material grinded in agate mill, after the external (environmentally contaminated and possibly weathered) layer removed.
Despite the typical bias of the acquired data as compared to the results of the usual wet laboratory measurements, PXRF is presently a routine field analytical method for elements with medium to high atomic mass (K to U), in the concentration range of a few mg/kg to a few %. The differences can be attributed to the basically different sample preparation and measurement methodology (point-and-shoot surface measurement vs. bulk measurement on homogenized samples), chemical matrix effects (particularly at high Fe contents), matrix heterogeneity, and spectral interferences. Detection limits vary with sample matrix composition; high abundance of heavier major elements, mainly iron, negatively affects trace element detection [9]. PXRF spectrometry can’t accurately quantify lighter elements (e.g., Na, P), nor Ti, V, Cr, Co, Ni and Ba at their typical concentrations in slag-like matrices, and its reliability is unsatisfactory in the case of the trace elements [10]. Consequently, PXRF generally can reliably provide qualitative (at best semiquantitative) data; gathering of quantitative data is problematic, particularly when the material is heterogeneous in nature. In case of iron slag samples analyzed in parallel with the PXRF method and wet chemistry, the PXRF analytical performance is defined by its <30% error, in samples containing very low or very high quantities of the analyzed element the error reaching >30% [6].
In the present study, the samples of bulk elemental composition determined by PXRF was compared with the data obtained on the same probe by ICP-MS, chosen as the wet chemical analysis method. ICP-MS measurements were performed using an Elan DRC II quadrupole spectrometer (plasma power 1250 W; concentric nebulizer Meinhardt; argon flow 0.86 mL min−1), on three 0.25 ± 0.05 g amounts of the same powdered bulk sample, solubilized following the total acid digestion method earlier presented [11]. For data processing, the TotalQuant semi-quantitative measurement mode of the Elan 3.4 software was used, with multiple point calibration for low, medium, and high masses. The method is less accurate for Na, Mg, Al, Si, K, and Fe; however, the accuracy is generally better than 15%, the detection limit being in the ppt (ng L−1) range, without significant matrix effects above 1 ppm (1 μg L−1) [12]. In this specific case, according to the value given by the instrument, the detection limit, LOD, was 0.02 mg kg−1 overall.
In order to characterize the slags from a mineralogical-structural point of view, FTIR spectroscopy and PXRD analysis were carried out on the pulverized bulk samples presented above; the FTIR spectra were recorded on the samples carefully removed, and also finely powdered external layer.
FTIR determinations were realized using a JASCO 6100 FTIR spectrometer (Manufacturer: JASCO Applied Sciences, Silver Spring, MD USA) (spectral domain: 4000 ¬ 400 cm−1, resolution: 2 cm−1, KBr pellet technique).
PXRD analysis was performed with a Bruker D8 Advance diffractometer (Bruker Corporation, Billerica, MA, USA) working in Bragg-Brentano mode (acquisition conditions: λCuKα1 = 1.5406 A, 40 kV, 40 mA, scan interval 5 to 70 degrees 2θ, step size 0.02 degrees 2θ, count time 2 s); observed peak positions were matched using the ICDD-JCPDS database.
The Fe3+/Fe2+ ratio was determined by EPR spectroscopy at room temperature, using a Bruker ELEXSYS E500 X-band spectrometer (9.46 GHz) following the procedure previously presented [13]. EPR experiments were performed on 20 mg amounts of the finely powdered samples, firstly in absence of thermal conditioning, then after the quantitative oxidation of the total Fe2+ content by 6 h heating at 300 °C in atmospheric conditions. Data acquisition and processing were assured by the Bruker Xepr suite for ELEXSYS spectrometers.
3. Results and Discussion
3.1. Elemental Analysis
Archaeological iron slag is a complex, heterogeneous material with the major constitutive elements Al, Si, K, Ca, Ti, Mn, Fe, Sr, Zr, and Ba. The presence and the concentration level of Al, Si, Ca, Mn, Sr, and Ba are related to the choice of fluxes; K and Ca level is able to indicate the fuel sources used, while Fe, Ti, and Zr levels are indicative of the iron ore source [6].
Surface elemental composition data of the slags are presented in Table 2 (major elements) and Table 3 (trace elements); the measurements were carried out in three different superficial points of the carefully cleaned finds.

Table 2.
PXRF surface analysis–major lithophile elements (mg kg−1).

Table 3.
PXRF surface analysis–trace elements (mg kg−1).
The mean surface elemental concentration values offer a good view on the samples’ mineral-chemical heterogeneity.
The major elemental composition (Table 2) is dominated by Fe. Ca level is rather low, otherwise the slags are also poor in other major lithophile elements (notice that Na, Si, and Al could not be determined). Ti (2500–5500 ppm) was detected in samples 4264, 4009, 4169, 4222 (Călugăreni-vicus, Cal-v), 9487, 9454 (Brâncoveneşti-retentura, Br-r) and Vat (Vătava, East of the tower), while Ba (500–1500 ppm) in 4251, 4264, 4169, 4222 (Cal-v), 9487, 9445, and 9454 (Br-r). Mn (500–5500 ppm) and Sr (120–250 ppm) are overall present.
The slag surfaces are very poor in volatiles; however, in some samples, detectable amounts of As (4169) and Br (4137, 4251, 9445, 9454, Vat) were found.
The surfaces are relatively poor in trace elements too (Table 3). Co (at relatively high level) and Rb are present in all samples; in some cases, Zn (4251, 4264, 4169, 4222, 9487, 9454), Mo, and Pb (4137, 4251, 4009, 4169, 4222, 9487, 9445, 9454, Vat), respectively Bi (4137, 4251, 4009, 4169, 9445, 9454, Vat) were detected in significant amounts. Cu appears in measurable amount in two samples only (4169, 4222). The somewhat surprising presence of gold on the surface of samples 4137, 9454, and Vat was confirmed microscopically [8] (Au globules embedded by “accidental” contamination?). It should be noted that Au doesn’t appear in the PXRF or the ICP-MS bulk results.
PXRF bulk data (Table 4) generally agree with the similarly determined surface data; however, there are some differences.

Table 4.
PXRF bulk analysis—major and trace elements (mg kg−1).
The dominant lithophile element of the bulk is Fe. Ca level is relatively low, the slags being generally poor in lithophiles excepting Fe. On the surface, K is not detected, and even in the bulk appears in sample 4264 only (9500 mg kg−1). Contrary to the surface data where Ti is present in almost all samples, in the bulk samples, PXRF is detected in 4264 and 4009 alone (3500–4500 mg kg−1). Ba is present in all Brâncoveneşti and Călugăreni samples, missing that from Vătava (Vat).
Concerning the trace elements, Cu is present in sample 4264 only; Zn in 4137, 4251, 4264, 4009; and As in 9454. Rb is missing from 9445, Mo from 4264, Pb from 4264, and Bi from 4264 and 4009. Sn appears in samples 4251, 9487, and 9445. Br, originating probably from the local wood fuel used [14,15], is overall present.
ICP-MS data of the samples’ bulk elemental composition are presented in Table 5 (according to the value given by the measuring instrument, LOD is overall 0.02 mg kg−1).

Table 5.
The slags elemental composition, ICP-MS analysis data (mg kg−1).
PXRF and ICP-MS bulk data differ significantly, as both the required sample preparation methodology and the concentration ranges characterizing the analytical methods are different (for example, measuring the same samples by using the more sensitive ICP-MS method, the presence and concentration level of further trace elements—Cr, Ni, Ag, Cd, Sb, I—could be determined, whilst Ca and Br couldn’t be measured). Commonly, it can be stated that, at concentration levels of 50–100 mg kg−1 or more, PXRF data should be considered more reliable, while, below 50 mg kg−1, the ICP-MS values are more creditable [16,17].
However, the general trend of the elemental concentrations measured by PXRF and ICP-MS is running parallel, especially when speaking on the slags’ major elements.
Bulk and surface chemical compositions equally suggest that at least bloom refining and/or the refined iron processing took place on the sites investigated. The slag samples seem to be most of all byproducts of the bloom refining process carried out in pit-furnaces.
Concluding about questions related to the provenance of raw materials (including the raw bloom supposed to be refined) would be very difficult as the quantity of the slag pieces collected was individually far below the recommended minimal amount of 200–300 g recommended for reliable bulk analysis of such highly heterogeneous materials [18,19,20].
3.2. PXRD Analysis
Ancient iron slags are mainly constituted of iron-bearing silicate minerals: olivine, typically fayalite (Fe2SiO4), pyroxene, frequently hedenbergite (CaFe2+Si2O6), and glass, with iron oxide-hydroxide minerals—mostly wüstite (FeO), magnetite (Fe3O4), goethite (α-FeO(OH)), and metallic iron as minor common components. Short range mineralogical variations seen in slags suggest that, during formation, there were relative unstable oxygen and temperature conditions [21].
According to their mineral composition, iron slags can be divided into two main classes [22].
The first type is principally constituted of the typical iron oxide-hydroxide minerals: wüstite, magnetite, and the weathering products goethite and lepidocrocite (γ-FeO(OH)); then, an appreciable amount of quartz (SiO2), glassy phases, and metallic iron. Wüstite is very common in bloomery slag; in smithing slag; where, during the slag formation, the temperature and/or oxygen content are high, instead of wüstite, magnetite occurs instead. Glass forms from the ‘residual melt’ and may vary considerably, depending on which minerals had previously crystallized, the total composition of the slag, and the progress of cooling. Droplets of few micrometers sized metallic iron formed during the reduction process are also common slag inclusions.
The major mineral phases of the more common second type are pyroxenes (hedenbergite), olivines (fayalite), and iron oxides, although olivine minerals and wüstite could be present only in small quantities or absent. The presence of (usually smaller quantities) of other minerals formed by firing at over 900 °C—mullite (Al6Si2O13), cristobalite (SiO2), pyrolusite (MnO2), akermanite (Ca2Mg(Si2O7)), etc.—can be ascribed on the account of furnace or smith’s hearth linings; the silica polymorph cristobalite formed actually suggests a heating temperature of at least 1200 °C.
In case of the iron slag samples being selected for PXRD investigation, only part of the mineral components visible by petrographic microscopy [8] could be confirmed. While on the diffractograms recorded (Figure 2) some of the anterior detected minerals (quartz, cristobalite, goethite, magnetite, pyroxene) can be reliably identified, confirmation of the presence (and evaluation of the quantity) of other phases is difficult. Most of the finely disseminated, microscopically visible silicates crystallized in the glassy matrix (the fayalite and the coexisting hedenbergite) and part of the iron oxides (magnetite, haematite, wustite) could not be reliably evidenced due to the complex superposition of the minerals’ individual PXRD pattern and to the signal degradation caused by the firing caused vitrification in addition to the amorphization occurring due to the burial environmental circumstances [23,24,25].
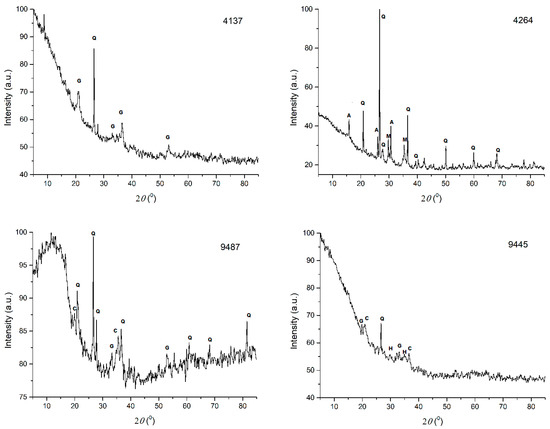
Figure 2.
X-ray diffractograms of selected samples. Samples 4137, 4264 are from the Călugăreni (vicus) site; 9487, 9445, and 9454 from Brâncoveneşti, the retentura; sample Vat is from Vătava, East of the tower location. The mineral components notation: Q—quartz; G—goethite; A—analcime; M—magnetite; C—cristobalite; H—hedenbergite; Cl—clinoclase; D—dolomite, W—wüstite.
The semiquantitatively estimated most important components of the PXRD-analyzed samples are presented in Table 6.

Table 6.
The most abundant PXRD—identified mineral phases of the selected slags.
Except for the Vat (Vătava) sample (very poor in quartz), the dominant crystalline phase is quartz, followed by cristobalite (though in some samples only in traces) and the (most probably weathering product) goethite. A large quantity of amorphous and/or glassy material was also evidenced. Magnetite and hedenbergite were detected in traces in most samples or were absent. Fayalite and wüstite, practically mandatory in iron slags, are not evidenced, except for the Vat sample deriving from the Vătava site (Figure 2). The analcime (NaAlSi2O6·H2O) in the 4264 sample (Călugăreni), as well as the clinoclase (Cu3AsO4(OH)3) and dolomite (CaMg(CO3)2) in the Vat (Vătava) sample, could appear accidentally as they aren’t really confirmed by the elemental analysis results.
The Vat sample, while poor in quartz, contains olivines (fayalite) and wüstite which indicate that this slag originated most probably from smithing operations.
The differences of the PXRD evidenced mineral content, in accordance with the PXRF and ICP-MS determined elemental compositions suggest that the iron-workers operant at the three different archaeological sites probably used different technologies. The majority of the finds seems to be a primary smithing slag [26]. The amount of the quartz present (acting as flux) is indicative that, at the site, bloom refining probably took place. Silica sand rich soils were added to the smithing hearth, possibly associated with other fluxes, in order to reduce the melting point of the slag, making it easier to squeeze out [6]. Removal of the slag became easier, also increasing the smithing hearth temperature; the presence of more considerable quantities of cristobalite in the Brâncoveneşti slags suggests processing (slag formation) temperatures from 900 °C to 1200 °C [27].
The iron oxides are indicators of the redox conditions at the time of the slag solidification. The appreciable amount of goethite, a typical weathering mineral formed by wüstite oxidation during burial, indicates mildly reducing conditions. Presumably initially wüstite was the most frequent oxide phase, with minor amounts of magnetite (partially transformed into haematite) [28,29].
3.3. FTIR Spectroscopy
FTIR absorption spectra were recorded on both the surface layer and the inside bulk material of the samples in the entire mid-IR region (4000–400 cm–1). However, the mineral composition of the slags could be altered during burial, the alteration process depending on the environmental conditions. The altered layer forms starting from the surface and progresses over time deeper and deeper towards the bulk, the possible modifications affecting primarily the 4000–1500 cm–1 (principally OH and CO2 governed) region of the FTIR spectra. Considering that, for the time being, in this specific case, an adequate study of these aspects is not available, FTIR spectral data interpretation is limited to the less affected 2000–400 cm–1 spectral domain (Figure 3).
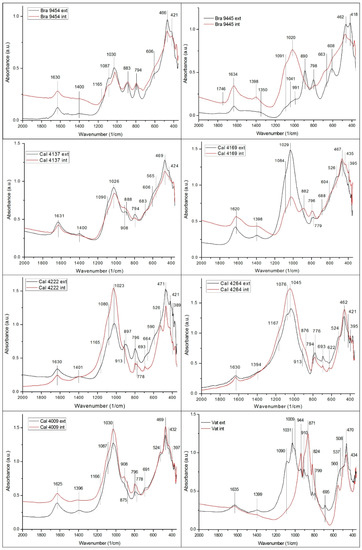
Figure 3.
FTIR spectra recorded on the external layer (black line) and on the bulk (red pattern) of the selected samples (Bra: Brâncoveneşti; Cal: Călugăreni; Vat: Vătava).
Except for a few cases (9445 and Vat), the absorption peaks of the FTIR spectra recorded on the outer layer and the inside bulk of the samples in the 2000–400 cm–1 domain practically coincide, suggesting that, even if some geochemical processes took place during burial, their influence is mostly negligible. The “deviancy” of some findings probably must be ascribed to specific micro-environmental influences; an adequate explanation would necessitate a deeper investigation of the exact location of the unearthing.
The frequency assignment of the main absorption peaks in the 2000–400 cm–1 spectral domain is presented in Table 7; spectral data were interpreted considering literature data published [30,31,32,33]. As the FTIR spectra recorded on the outer layer and the inside bulk of the samples in the 2000–400 cm–1 region (in most cases) are practically superposed, it can be considered that the FTIR behavior is satisfactorily described by the inside (bulk) data.

Table 7.
The main FTIR peaks assignment of selected slag sample (bulky matter).
With minor differences, Table 7 data indicate a close spectral behavior of the samples, independently from the exact location of their uncovering.
According to the absorption peaks appearing in the spectral domain 2000–400 cm–1, the samples are mainly constituted from silicates, aluminosilicates, and aluminates. Carbonates, formed most probably in environmentally induced carbonation processes occurring during burial, are also present. Other additional differences can also be attributed to burial conditions. The CO2 peaks on some spectra appeared probably due to the groundwater or humid soil caused deep carbonation.
All this denotes the relative closeness of the smithery practiced on the different locations where most probably the iron objects were processed starting from pig-iron (bloom) of relatively close provenance, using a similar technology.
3.4. EPR Investigation
Smithing slags, very common on archaeological sites, can be classified in three main types, each related to a kind of metallurgical activity [34]. The first type, dominated by fayalite, with a variable amount of iron oxide (mainly wüstite) and a small amount of interstitial glass, is mainly produced by hot oxidation of the metal with a small input of silica from various sources (hearth lining, charcoal, dust, flux) during forging. The second type, richer in silica and minerals deriving from granitic rocks (granites, sandstones, clays) and with low iron content, is produced during fashioning of iron pieces and processing of steel objects. The third type of slag is richer in iron (as metal, oxide, or oxy-hydroxide) contains fayalite and inclusions of charcoal, and is produced during the work of a poorly compacted metal, or when the smith is working close to the melting point of the metal, for example during welding.
Smithing slags are generally formed under reducing conditions controlled by the CO/CO2 ratio defined by the fluctuating oxygen pressure in the hearth atmosphere. Since formation of the ancient slags is very sensitive to the oxygen content, at the same chemical composition of the melt, it is possible the crystallization of mineral phases with differing Fe2+/Fe3+ ratios. This issue can be described with the quartz, fayalite and magnetite (QFM) buffer equilibrium:
3 Fe2+2SiO4 + O2 ⟷ 2 Fe2+Fe3+2O4 + 3 SiO2
If the oxygen concentration in the gas atmosphere is sufficiently high, magnetite and a silica-rich compound (like pyroxene) crystallize first. If oxygen is low, no magnetite will be formed, but fayalite (or even metallic iron) precipitates [20].
The Fe3+/Fe2+ ratio essentially will be then defined by the raw materials used and the manufacturing technology (the redox conditions which occur during the metallurgical process of iron processing). The knowledge of the Fe3+/Fe2+ ratio could enable the samples’ identification as primary (bloom refining) or secondary (iron bar processing) smithing slags [26].
EPR spectroscopy is widely used in materials research, predominantly in structural investigations [35,36], and also has applications in geological and archaeological dating [37]. As the quantitative analytical method performs only occasionally, mostly in studies carried out on Fe and Mn containing clays, glasses, and ceramics. As the reliable standardization assuring the avoidance of matrix related errors in this case is difficult, EPR spectroscopy only delivers semiquantitative results [38]. However, semiquantitative data permit the adequate determination of the Fe3+/Fe2+ ratio; in addition, the method has the advantage of simple and easy sample preparation, without the need for expensive standards, costly, and/or hazardous chemicals.
Experimental EPR spectra recorded for Fe2O3 at room temperature (a broader line superposed on a narrow line) are typical superparamagnetic resonance spectra. The broad component of the EPR signal shifts left with increasing Fe2O3 concentration; the narrow one is observed at the same field~3500 G (g = 2) regardless of concentration. With the increase in the Fe2O3 concentration, the narrow component also broadens and becomes less visible until it is completely unobservable in the highly concentrated samples [35,36,37,38,39,40].
The integrated intensity I of the EPR signal (area beneath the absorption curve) is proportional to the concentration of the paramagnetic centers in the sample. EPR spectral features (I) recorded at room temperature on the untreated sample specimens (e.g., before any thermal treatment) correspond to the presence of the Fe3+ paramagnetic centers alone, since, above 77 K, the resonance assigned to Fe2+ can’t be detected. IFe(tot) can be obtained from the room temperature spectrum of the same specimen after the quantitative oxidation of the Fe2+ content to Fe3+. This may be achieved keeping the finely pulverized slag at 300 °C in the presence of air atmospheric for 6 h proved to assure the quantitative oxidation of Fe2+ to Fe3+. The ferrous iron quantity will be the difference of the integrated intensities (IFe(tot)–IFe(III)).
The experimental EPR spectra recorded on selected samples are presented in Figure 4.
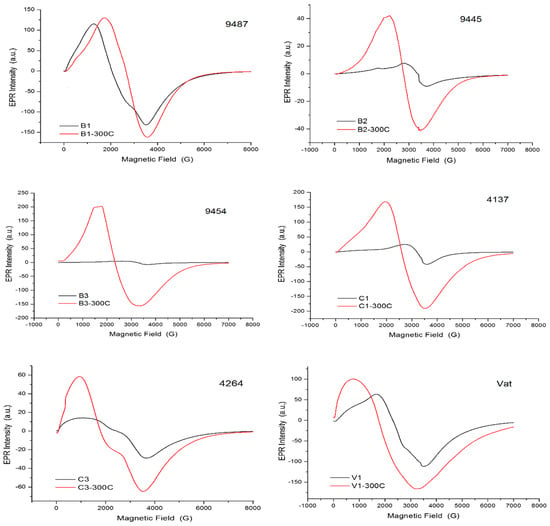
Figure 4.
EPR spectra of selected samples: 9487 (B1), 9445 (B2), 9454 (B3) from Brâncoveneşti; 4137 (C1), 4264 (C3) from Călugăreni; and Vat (V1) from Vătava. Black spectra were recorded on the untreated samples; red spectra after thermal treatment applied.
Table 8 contains the calculated Fe3+/Fe2+ ratio values from experimental results.

Table 8.
Experimental Fe3+/Fe2+ ratio of the investigated samples.
As shown by the experimental spectra presented in Figure 4 and the data from Table 8, the EPR method can be used to characterize the redox conditions of slag formation even if, due to the pronounced heterogeneity of the samples, the (obviously only local) data obtained in this way can’t be extended to the whole of the sample. The Fe3+/Fe2+ ratio values of Table 8 are calculated from EPR measurements realized on probes of amounts of only few milligrams. The “unreliability of global conclusions” results also when the data from Table 8 are compared to those from Table 6.
Still, the differences of the Fe3+/Fe2+ resulting ratios are able to suggest successfully the differing redox ambience during the slag formation caused by the supposedly different working conditions. All finds can be considered smithing slags; however, the iron-workers operant at the three different archaeological sites should use different technologies. The considerable amount of quartz present in the Brâncoveneşti and Călugăreni samples suggests second type smithing slags, the quartz deriving from silica sand rich soils added to the smithing hearth as flux, while the findings from Vătava seem to be third type smithing slags [34].
The iron oxides could be indicators of the redox conditions at the time of the slag solidification. The appreciable amount of goethite, a typical weathering mineral, indicates mildly reducing conditions.
4. Conclusions
Surface screening of the slag samples with a hand-held XRF spectrometer confirms that, compositionally, they are highly heterogeneous. Elemental compositions suggest the different provenance of the raw materials.
Chemical composition as well as structural analyses permit affirming that at least bloom refining and processing of the refined iron took place on the sites. The slag cakes found could be byproducts of the refining process carried out in pit-furnaces [34]. The Fe dominancy in all the samples (even in the Vat sample which is the poorest in iron) can be attributed to the presence of a significant quantity of metallic globules (probably alloyed iron) embedded in the slags and formed during primary and/or secondary smithing.
Mineralogically, archaeological iron slags are a heterogeneous mixture of silicates (olivines like fayalite, pyroxenes like hedenbergite), iron oxides (wüstite, magnetite), amorphous material (mostly glass phases), and alteration products (like goethite). The FTIR spectral data indicate the presence of mineral phases expected in different (primary and/or secondary) smithing slag types. Corresponding to the PXRD data, in accordance with the FTIR results, besides the generally dominant quartz and a relatively great amount of amorphous, mostly glassy material, the main mineral phases of the Brâncoveneşti and Călugăreni samples are the pyroxene hedenbergite, the iron oxyhydroxide goethite and the iron oxide magnetite—while, in the case of the Vătava sample, the same iron oxides and (not seen by XRD) the olivine fayalite.
PXRD and FTIR results suggest that the studied slags can be considered primary or secondary smithing debris (metallurgical waste produced in the last steps of the smithing operations carried out by the blacksmith). Primary smithing slags are generated during the refining of the bloom, and secondary smithing slags are formed during the manufacturing and/or repair of artifacts [26].
The PXRD identified minerals are formed as the result of a complex smithing process supposing different temperature regimes. Hedenbergite, the predominant pyroxene of the slags from Brâncoveneşti and Călugăreni, is segregated at crystallization temperatures ranging from 700 to 900 °C; olivines (the fayalite appearing on the diffractogram recorded on the Vătava sample—see Figure 2) crystallize at 1100–1200 °C; the presence of cristobalite also indicates the achievement of high temperatures during the metal processing. The quartz found in all samples derives most probably from flux addition. The most frequent iron oxide phase, associated with minor amounts of magnetite, is wüstite, practically degraded during burial. The omnipresent weathering mineral goethite is formed in the course of wüstite oxidation [31,34].
The EPR spectra are able to properly prove the presence of Fe3+ ions in the samples, the method allowing the determination of the Fe3+/Fe2+ ratio in fair agreement with the chemical analysis data and the mildly reducing conditions of the slag solidification indicated by the amount of goethite (formed mostly by wüstite oxidation during burial) typically present in the samples.
Author Contributions
Conceptualization, E.B. and S.-P.P.; methodology, E.B., S.-P.P., and E.V.; investigation, data curation I.K., C.T., G.B., D.T. and Z.K.-B.; resources, E.B.; writing—original draft preparation, E.V., I.K., C.T., G.B., and Z.K.-B.; writing—review and editing, E.B. and S.-P.P. All authors have read and agreed to the published version of the manuscript.
Funding
The PXRD: FTIR and EPR measurements were financially supported by the Romanian Ministry of Research and Innovation, Core Program, Projects PN 19 35 02 01 and PN19 35 02 03.
Acknowledgments
The archaeometric (archaeometallurgical) research carried out is part of the Project for Technical and Cultural Heritage Preservation in Transylvania, initiated and supervised by the Research Institute of the Transylvanian Museum Society. The financial support was assured by the 2018–2019/HTMT grant of the Homeland Research Program supervised by the Hungarian Science Abroad Presidential Committee of the Hungarian Scientific Academy. From 2013, the field research from Călugăreni was organized as archaeological field school, involving the participation of students and lecturers of archaeology, geophysics, architecture, conservation, and restoration from the University of Cologne, the University of Pécs, the Budapest University of Technology and Economics, the Eötvös Loránd University, the University of Applied Sciences Erfurt, the Babeş–Bolyai University of Cluj-Napoca, and the Petru Maior University of Târgu Mureş, as part of the international interdisciplinary project The Roman Limes as a European Cultural Landscape coordinated by the Mureş County Museum and the Winckelman Institute of the Humboldt University of Berlin. Since 2016, the research was financed by the Mureş County Council, the Chair of the Roman Provinces at the Archaeological Institute of the University of Cologne, the Erasmus Fund of the Humboldt University of Berlin, and the Romanian Ministry of Culture. The excavation from Brâncoveneşti and Vătava have been organized by the Mureş County Museum and financed by the Mureş County Council.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pánczél, S.P.; Lenkey, L.; Pethe, M.; Laczkó, N. Updating our Knowledge about the Roman Fort from Brâncovenești, Mureș County. Marisia 2012, 32, 105–115. [Google Scholar]
- Pánczél, S.P.; Mustaţă, S.; Dobos, A. The Research at the Roman Auxiliary Fort of Mikháza/Călugăreni. Magy. Régészet/Hung. Archaeology 2018, 2018/1, 13–20. [Google Scholar]
- Dobos, A.; Fiedler, M.; Höpken, C.; Mustaţă, S.; Pánczél, S.P. Militärlager und vicus in Călugăreni/Mikháza (Kreis Mureş, Rumänien) am Dakischen Ostlimes. KuBA 2017, 7, 145–154. [Google Scholar]
- Szabó, M.; Pánczél, S.P.; Cioată, M.D. Római kori lelőhelyek kutatása a Kelemen-havasok lábánál. In Várak, Kastélyok, Templomok Évkönyv; Kósa, P., Ed.; Zöld Infó Média: Pécs, Hungary, 2017; pp. 116–119. [Google Scholar]
- Ingoglia, C.; Triscari, M.; Sabatino, G. Archaeometallurgy in Messina: Iron slag from a dig at block P, laboratory analyses and interpretation. MAA 2008, 8, 49–60. [Google Scholar]
- Scott, R.B.; Eekelers, K.; Degryse, P. Quantitative chemical analysis of archaeological slag material using handheld X-ray fluorescence spectrometry. Appl. Spectrosc. 2016, 70, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Nemet, I.; Rončević, S.; Bugar, A.; Ferri, T.Z.; Pitarević, L. Classification analysis of archaeological findings from early-iron production (Turopolje region, NW Croatia) based on multi-analytical profiling. JAAS 2018, 33, 2053–2061. [Google Scholar] [CrossRef]
- Bitay, E.; Kacsó, I.; Pánczél, S.P.; Veress, E. Comparative Study of Roman Iron Slags Discovered in the Roman Auxiliary Fort and Settlement of Călugăreni. Acta Mat. Transyl. 2018, 1, 65–72. [Google Scholar] [CrossRef]
- Lemiere, B. A review of pXRF (field portable X-ray fluorescence) applications for applied geochemistry. J. Geochem. Explor. 2018, 188, 350–363. [Google Scholar] [CrossRef]
- Hunt, A.M.; Speakman, R.J. Portable XRF analysis of archaeological sediments and ceramics. J.Archaeol. Sci. 2015, 53, 626–638. [Google Scholar] [CrossRef]
- Bačeva, K.; Stafilov, T.; Šajn, R.; Tănăselia, C.; Makreski, P. Distribution of chemical elements in soils and stream sediments in the area of abandoned Sb–As–Tl Allchar mine, Republic of Macedonia. Environ. Res. 2014, 133, 77–89. [Google Scholar] [CrossRef]
- Levei, E.; Frenţiu, T.; Ponta, M.; Tănăselia, C.; Borodi, G. Characterization and assessment of potential environmental risk of tailings stored in seven impoundments in the Aries river basin, Western Romania. Chem. Cent. J. 2013, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Bitay, E.; Kacsó, I.; Toloman, D.; Pánczél, S.P.; Veress, E. EPR spectroscopic determination of the Fe(II)/Fe(III) ratio in roman slags from Brâncoveneşti (Marosvécs), Călugăreni (Mikháza) and Vătava (Felsőrépa), Romania. Papers Tech. Sci. 2017, 7, 103–106. [Google Scholar]
- Misra, M.K.; Ragland, K.W.; Baker, A.J. Wood ash composition as a function of furnace temperature. Biomass Bioenerg. 1993, 4, 103–116. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mishra, M.; Suganya, O.M. The incorporation of wood waste ash as a partial cement replacement material for making structural grade concrete: An overview. Ain Shams Eng. J. 2015, 6, 429–437. [Google Scholar] [CrossRef]
- Griffith, D.A.; Johnson, D.L.; Hunt, A. The geographic distribution of metals in urban soils: The case of Syracuse, NY. GeoJournal 2009, 74, 275–291. [Google Scholar] [CrossRef]
- Al Maliki, A.; Al-lami, A.K.; Hussain, H.M.; Al-Ansari, N. Comparison between inductively coupled plasma and X-ray fluorescence performance for Pb analysis in environmental soil samples. Environ. Earth Sci. 2017, 76, 433. [Google Scholar] [CrossRef]
- Molnár, F. Salakok és fémek archeometriai vizsgálata. In Régészeti kézikönyv; Müller, R., Ed.; Magyar Régész Szövetség: Budapest, Hunagary, 2011; pp. 510–524. [Google Scholar]
- Charlton, M.F.; Blakelock, E.; Martinón-Torres, M.; Young, T. Investigating the production provenance of iron artifacts with multivariate methods. J. Archaeol. Sci. 2012, 39, 2280–2293. [Google Scholar] [CrossRef]
- Hauptmann, A. The investigation of archaeometallurgical slag. In Archaeometallurgy in Global Perspective. Methods and Syntheses; Roberts, B.W., Thornton, C.P., Eds.; Springer: New York, NY, USA, 2014; pp. 91–105. [Google Scholar]
- Andersson, D. Iron-working at Hornlandsudde: Archaeometallurgic analyses: Rogsta Parish, Hälsingland. In GAL Analysrapport 7-2007; Riksantikvarieämbetet, Avdelningen för arkeologiska undersökningar: Stockholm, Sweden, 2007; 12p. [Google Scholar]
- Kramar, S.; Lux, J.; Pristacz, H.; Mirtic, B.; Rogan-Smuc, N. Mineralogical and geochemical characterization of Roman slag from the archaeological site near Mosnje (Slovenia). Mater. Technol. (MTAEC9) 2015, 49, 343–348. [Google Scholar] [CrossRef]
- Mateus, A.; Pinto, A.; Alves, L.C.; Matos, J.X.; Figueiras, J.; Neng, N.R. Roman and modern slag at S. Domingos mine (IPB, Portugal): Compositional features and implications for their long-term stability and potential reuse. IJEWM 2011, 8, 133–159. [Google Scholar] [CrossRef]
- Sheikh, M.R.; Acharya, B.S.; Gartia, R.K. Characterization of iron slag of Kakching, Manipur by X-ray and optical spectroscopy. IJPAP 2010, 48, 632–634. [Google Scholar]
- Mohassab, Y.; Sohn, H.Y. Application of spectroscopic analysis techniques to the determination of slag structures and properties: Effect of water vapor on slag chemistry relevant to a novel flash ironmaking technology. JOM 2013, 65, 1559–1565. [Google Scholar] [CrossRef]
- McDonnell, J.G. A model for the formation of smithing slags. Mater. Archeol. 1991, 26, 23–26. [Google Scholar]
- Gotić, M.; Musić, S. Mössbauer, FT-IR and FE SEM investigation of iron oxides precipitated from FeSO4 solutions. J. Mol. Struct. 2007, 834–836, 445–453. [Google Scholar] [CrossRef]
- Kramar, S.; Lux, J.; Mladenović, A.; Pristacz, H.; Mirtič, B.; Sagadin, M.; Rogan-Šmuc, N. Mineralogical and geochemical characteristics of Roman pottery from an archaeological site near Mošnje (Slovenia). Appl. Clay Sci. 2012, 57, 39–48. [Google Scholar] [CrossRef]
- Di Bella, M.; Aleo Nero, C.; Chiovaro, M.; Italiano, F.; Quartieri, S.; Romano, D.; Leonetti, F.; Marcianò, G.; Sabatino, G. Archaeometric study of the hellenistic metallurgy in Sicily: Mineralogical and chemical characterization of iron slags from punic Panormos (Palermo, Italy). MAA 2018, 18, 127–139. [Google Scholar]
- Merzbacher, C.I.; White, W.B. The structure of alkaline earth aluminosilicate glasses as determined by vibrational spectroscopy. J. Non-Cryst. Solids 1991, 130, 18–34. [Google Scholar] [CrossRef]
- ElBatal, H.A.; Ghoneim, N.A.; Ouis, M.A. Preparation and characterization of glass and glass-ceramics from industrial waste materials including iron slag and cement dust. In Proceedings of the ICCM-17–17th International Conference on Composite Materials, Edinburgh, UK, 27–31 July 2009; Available online: http://www.iccm-central.org/Proceedings/ICCM17proceedings/Themes/Industry/ADV%20COMP%20MATS%20IN%20CONSTRUCTION/INT%20-%20ADV%20COMP%20MATS%20IN%20CONSTR/IA1.2%20Ouis.pdf (accessed on 20 June 2020).
- Olovčić, A.; Memić, M.; Žero, S.; Huremović, J.; Kahrović, E. Chemical analysis of iron slags and metallic artefacts from early iron age. Int. Res. J. Pure Appl. Chem. 2014, 4, 859–870. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Wharton, D.; Hickey, L.; Frost, R.L. Infrared and Raman study of interlayer anions CO32−, NO3−, SO42− and ClO4− in Mg/Al-hydrotalcite. Am. Mineral. 2002, 87, 623–629. [Google Scholar] [CrossRef]
- Serneels, V.; Perret, S. Quantification of smithing activities based on the investigation of slag and other material remains. In Proceedings of the International Conference Archaeometallurgy in Europe, Milano, Italy, 24–26 September 2003; Associazione Italiana di Metallurgia: Milano, Italy, 2003; Volume 1, pp. 469–478. [Google Scholar]
- Prakash, C.; Husain, S.; Singh, R.J.; Mollah, S. Electron paramagnetic resonance of Fe3+ ions in Bi2O3–PbO–Fe2O3 glasses. J. Alloy. Compd. 2001, 326, 47–49. [Google Scholar] [CrossRef]
- Roessler, M.M.; Salvadori, E. Principles and applications of EPR spectroscopy in the chemical sciences. Chem. Soc. Rev. 2018, 47, 2534–2553. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Schmidt, M.P.; Stavitski, E.; Martínez, C.E. Iron speciation in peats: Chemical and spectroscopic evidence for the co-occurrence of ferric and ferrous iron in organic complexes and mineral precipitates. Org. Geochem. 2018, 115, 124–137. [Google Scholar] [CrossRef]
- Hofmeister, A.M.; Rossman, G.R. Determination of Fe3+ and Fe2+ concentrations in feldspar by optical absorption and EPR spectroscopy. Phys. Chem. Miner. 1984, 11, 213–224. [Google Scholar] [CrossRef]
- Koksharov, Y.A.; Pankratov, D.A.; Gubin, S.P.; Kosobudsky, I.D.; Beltran, M.; Khodorkovsky, Y.; Tishin, A.M. Electron paramagnetic resonance of ferrite nanoparticles. J. Appl. Phys. 2001, 89, 2293–2298. [Google Scholar] [CrossRef]
- Noginov, M.M.; Noginova, N.; Amponsah, O.; Bah, R.; Rakhimov, R.; Atsarkin, V.A. Magnetic resonance in iron oxide nanoparticles: Quantum features and effect of size. J. Magn. Magn. Mater. 2008, 320, 2228–2232. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).