The Effect of Undersized Drilling on the Coronal Surface Roughness of Microthreaded Implants: An In Vitro Study
Abstract
1. Introduction
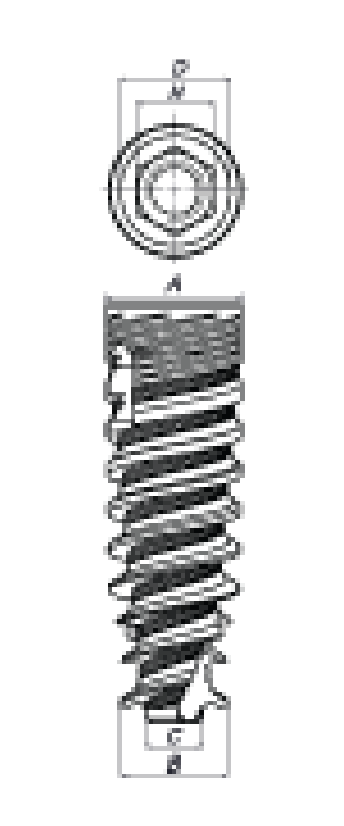
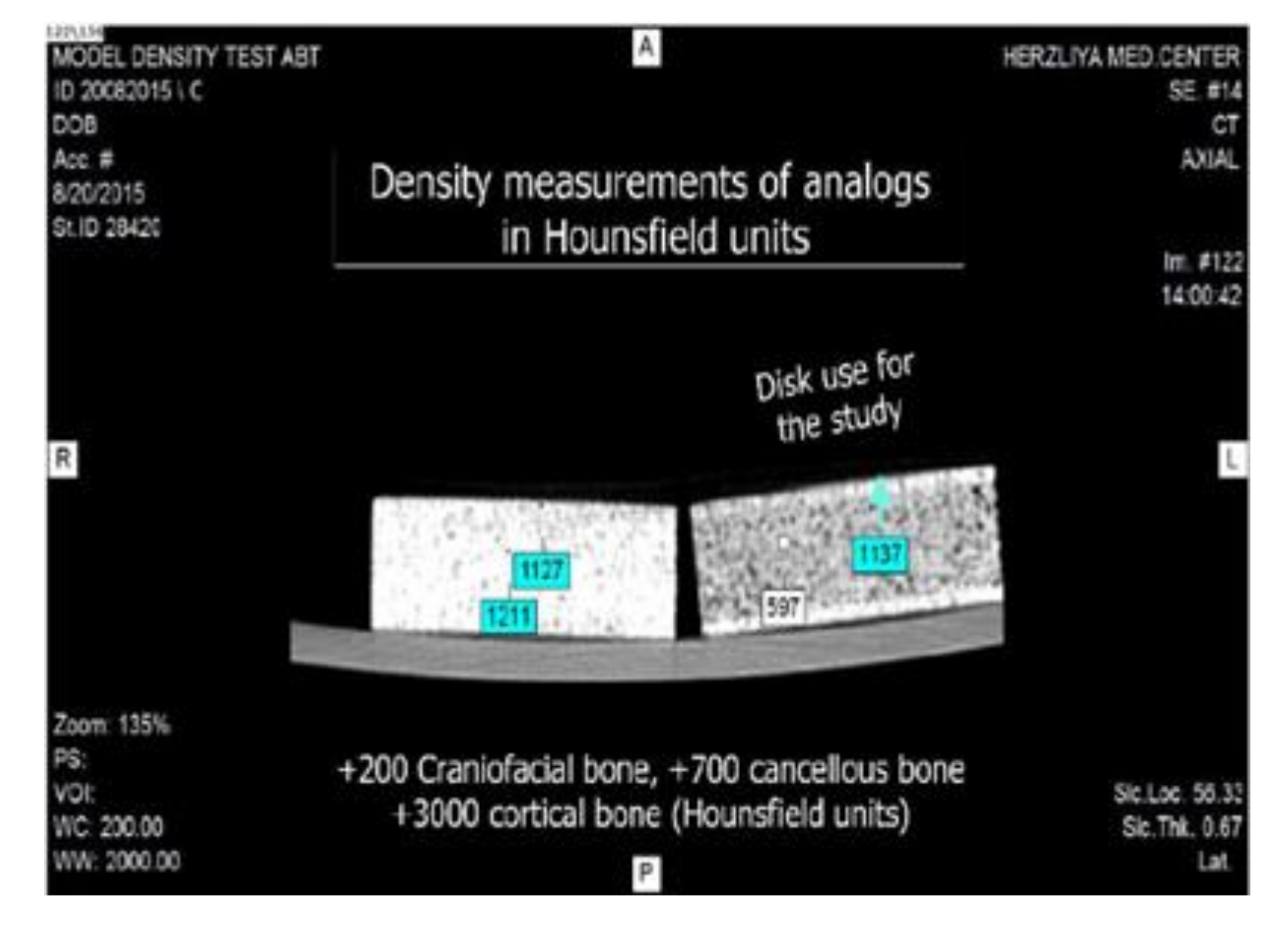
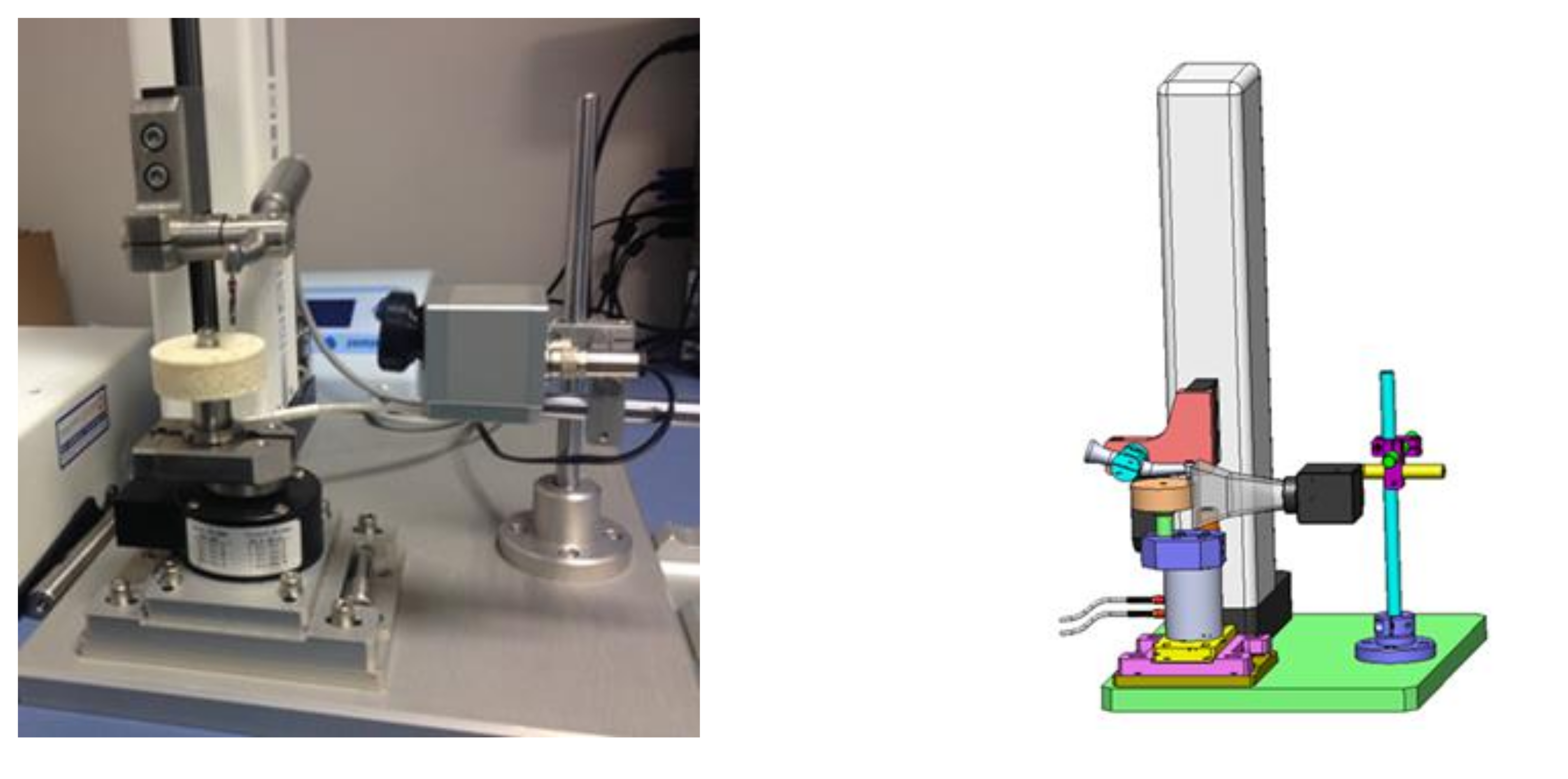
2. Materials and Methods
Statistical Analysis
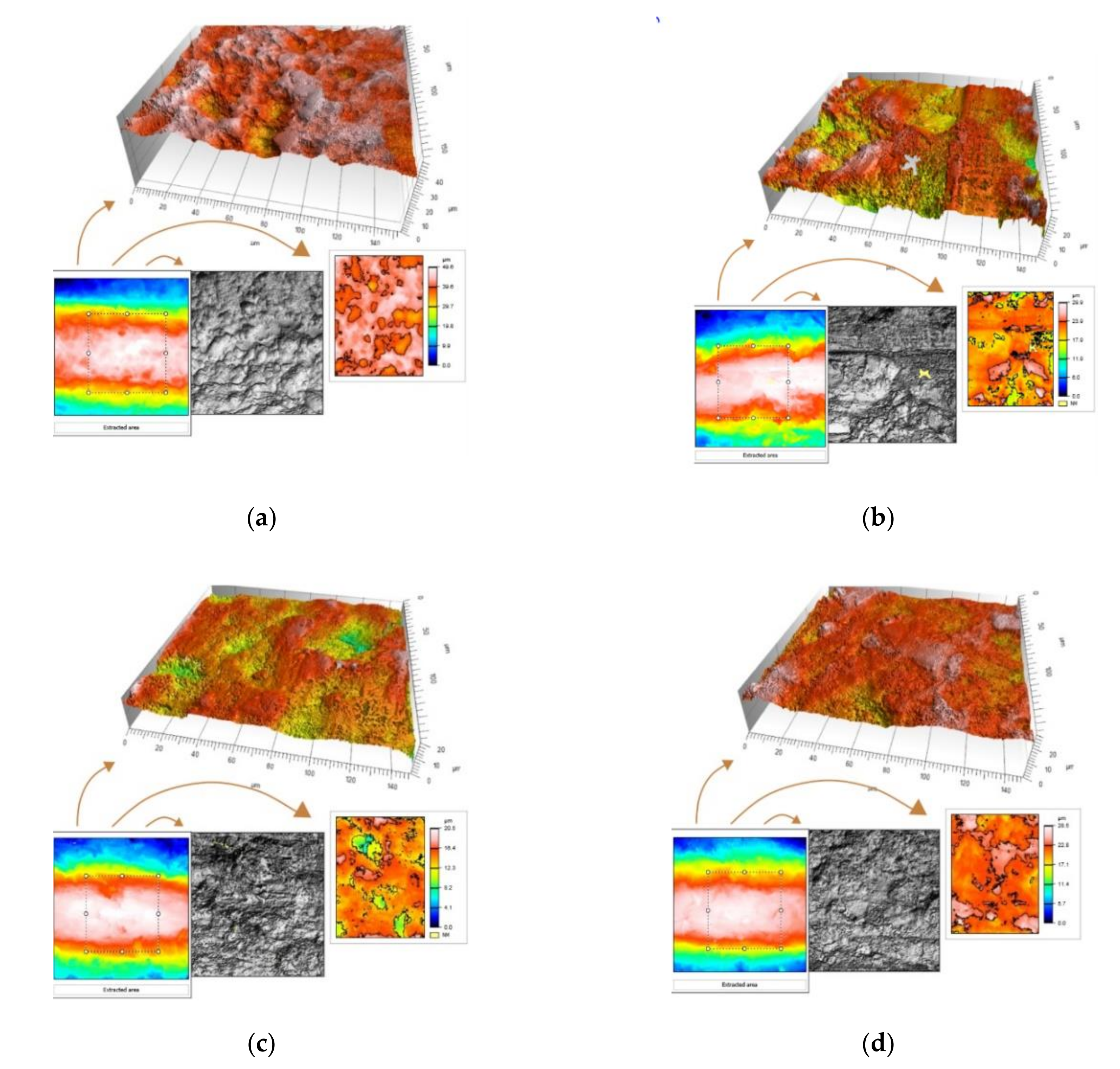
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abrahamsson, I.; Linder, E.; Lang, N.P. Implant stability in relation to osseointegration: An experimental study in the Labrador dog. Clin. Oral Implants Res. 2009, 20, 313–318. [Google Scholar] [CrossRef]
- Martinez, H.; Davarpanah, M.; Missika, P.; Celletti, R.; Lazzara, R. Optimal implant stabilization in low density bone. Clin. Oral Implants Res. 2001, 12, 423–432. [Google Scholar] [CrossRef]
- Abuhussein, H.; Pagni, G.; Rebaudi, A.; Wang, H.L. The effect of thread pattern upon implant osseointegration. Clin. Oral Implants Res. 2010, 21, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Orsini, E.; Giavaresi, G.; Trirè, A.; Ottani, V.; Salgarello, S. Dental implant thread pitch and its influence on the osseointegration process: An in vivo comparison study. Int. J. Oral Maxillofac. Implants 2012, 27, 383–392. [Google Scholar]
- Misch, C.E.; Strong, T.; Bidez, M.W. Scientific rationale for dental implant design. In Contemporary Implant Dentistry; Misch, C.E., Ed.; Mosby: St. Louis, MO, USA, 2008; pp. 200–229. [Google Scholar]
- Shalabi, M.M.; Wolke, J.G.; Jansen, J.A. The effects of implant surface roughness and surgical technique on implant fixation in an in vitro model. Clin. Oral Implants Res. 2006, 17, 172–178. [Google Scholar] [CrossRef]
- Campos, F.E.; Gomes, J.B.; Marin, C.; Teixeira, H.S.; Suzuki, M.; Witek, L.; Zanetta-Barbosa, D.; Coelho, P.G. Effect of drilling dimension on implant placement torque and early osseointegration stages: An experimental study in dogs. J. Oral Maxillofac. Surg. 2012, 70, e43–e50. [Google Scholar] [CrossRef]
- Shalabi, M.M.; Wolke, J.G.; de Ruijter, A.J.; Jansen, J.A. Histological evaluation of oral implants inserted with different surgical techniques into the trabecular bone of goats. Clin. Oral Implants Res. 2007, 18, 489–495. [Google Scholar] [CrossRef]
- Tabassum, A.; Meijer, G.J.; Wolke, J.G.; Jansen, J.A. Influence of the surgical technique and surface roughness on the primary stability of an implant in artificial bone with a density equivalent to maxillary bone: A laboratory study. Clin. Oral Implants Res. 2009, 20, 327–332. [Google Scholar] [CrossRef]
- Cohen, O.; Ormianer, Z.; Tal, H.; Rothamel, D.; Weinreb, M.; Moses, O. Differences in crestal bone-to-implant contact following an under-drilling compared to an over-drilling protocol. A study in the rabbit tibia. Clin. Oral Investig. 2016, 20, 2475–2480. [Google Scholar] [CrossRef]
- Scarano, A.; Carinci, F.; Lorusso, F.; Festa, F.; Bevilacqua, L.; Santos de Oliveira, P.; Maglione, M. Ultrasonic vs Drill Implant Site Preparation: Post-Operative Pain Measurement Through VAS, Swelling and Crestal Bone Remodeling: A Randomized Clinical Study. Materials 2018, 11, 2516. [Google Scholar] [CrossRef]
- Herrero-Climent, M.; Ferreira Lemos, B.; Herrero-Climent, F.; Falcao, C.; Oliveira, H.; Herrera, M.; Javier Gil, F.; Ríos-Carrasco, B.; Ríos-Santos, J.V. Influence of Implant Design and Under-Preparation of the Implant Site on Implant Primary Stability. An In Vitro Study. Int. J. Environ. Res. Public Health 2020, 17, 4436. [Google Scholar] [CrossRef]
- Skalak, R.; Zhao, Y. Interaction of force-fitting and surface roughness of implants. Clin. Implant Dent. Relat. Res. 2000, 2, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Bratu, E.A.; Tandlich, M.; Shapira, L. A rough surface implant neck with microthreads reduces the amount of marginal bone loss: A prospective clinical study. Clin. Oral Implants Res. 2009, 20, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Himmlová, L.; Dostálová, T.; Kácovský, A.; Konvicková, S. Influence of implant length and diameter on stress distribution: A finite element analysis. J. Prosthet. Dent. 2004, 91, 20–25. [Google Scholar] [CrossRef]
- Hansson, S. The implant neck: Smooth or provided with retention elements. A biomechanical approach. Clin. Oral Implants Res. 1999, 10, 394–405. [Google Scholar] [CrossRef]
- Abrahamsson, I.; Berglundh, T. Tissue characteristics at microthreaded implants: An experimental study in dogs. Clin. Implant Dent. Relat. Res. 2006, 8, 107–113. [Google Scholar] [CrossRef]
- Schrotenboer, J.; Tsao, Y.P.; Kinariwala, V.; Wang, H.L. Effect of microthreads and platform switching on crestal bone stress levels: A finite element analysis. J. Periodontol. 2008, 79, 2166–2172. [Google Scholar] [CrossRef]
- Hudieb, M.I.; Wakabayashi, N.; Kasugai, S. Magnitude and direction of mechanical stress at the osseointegrated interface of the microthread implant. J. Periodontol. 2011, 82, 1061–1070. [Google Scholar] [CrossRef]
- Trisi, P.; De Benedittis, S.; Perfetti, G.; Berardi, D. Primary stability, insertion torque and bone density of cylindric implant ad modum Branemark: Is there a relationship? An in vitro study. Clin. Oral Implants Res. 2011, 22, 567–570. [Google Scholar] [CrossRef]
- Streckbein, P.; Wilbrand, J.F.; Kähling, C.; Pons-Kühnemann, J.; Rehmann, P.; Wöstmann, B.; Howaldt, H.P.; Möhlhenrich, S.C. Evaluation of the surface damage of dental implants caused by different surgical protocols: An in vitro study. Int. J. Oral Maxillofac. Surg. 2019, 48, 971–981. [Google Scholar] [CrossRef]
- Cochran, D.L. A comparison of endosseous dental implant surfaces. J. Periodontol. 1999, 70, 1523–1539. [Google Scholar] [CrossRef]
- Golmohammadi, S.; Eskandari, A.; Movahhedy, M.R.; Shirmohammadi, A.; Amid, R. The effect of microthread design on magnitude and distribution of stresses in bone: A three-dimensional finite element analysis. Dent. Res. J. 2018, 15, 347–353. [Google Scholar] [CrossRef]
- Aldahlawi, S.; Demeter, A.; Irinakis, T. The effect of implant placement torque on crestal bone remodeling after 1 year of loading. Clin. Cosmet. Investig. Dent. 2018, 10, 203–209. [Google Scholar] [CrossRef]
- Khayat, P.G.; Arnal, H.M.; Tourbah, B.I.; Sennerby, L. Clinical outcome of dental implants placed with high insertion torques (up to 176Ncm). Clin. Implant Dent. Relat. Res. 2013, 15, 227–233. [Google Scholar] [CrossRef]
- Makary, C.; Rebaudi, A.; Mokbel, N.; Naaman, N. Peak insertion torque correlated to histologically and clinically evaluated bone density. Implant Dent. 2011, 20, 182–191. [Google Scholar] [CrossRef]
- Brown, A.D.; Walters, J.B.; Zhang, Y.X.; Saadatfar, M.; Escobedo-Diaz, J.P.; Hazell, P.J. The mechanical response of commercially available bone simulants for quasi-static and dynamic loading. J. Mech. Behav. Biomed. Mater. 2019, 90, 404–416. [Google Scholar] [CrossRef]
- Li, S.; Chien, S.; Brånemark, P.I. Heat shock-induced necrosis and apoptosis in osteoblasts. J. Orthop. Res. 1999, 17, 891–899. [Google Scholar] [CrossRef]
- Eriksson, A.R.; Albrektsson, T. Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J. Prosthet. Dent. 1983, 50, 101–107. [Google Scholar] [CrossRef]
- Mishra, S.K.; Chowdhary, R. Heat generated by dental implant drills during osteotomy—A review: Heat generated by dental implant drills. J. Indian Prosthodont. Soc. 2014, 14, 131–143. [Google Scholar] [CrossRef]
- Stocchero, M.; Jinno, Y.; Toia, M.; Ahmad, M.; Papia, E.; Yamaguchi, S.; Becktor, J.P. Intraosseous temperature change during installation of dental implants with two different surfaces and different drilling protocols: An in vivo study in sheep. J. Clin. Med. 2019, 8, 1198. [Google Scholar] [CrossRef]

| Drill 1 Pilot | Drill 2 | Drill 3 | Drill 4 | Drill 5 | |
|---|---|---|---|---|---|
| D3.65 | 2 mm | 2.5 mm | 2.8 mm | 3.2 mm | 3.65 mm final |
| D3.2 | 2 mm | 2.5 mm | 2.8 mm | 3.2 mm final | |
| D2.8 | 2 mm | 2.5 mm | 2.8 mm final |
| D2.80 | D3.20 | D3.65 | CONTROL | |
|---|---|---|---|---|
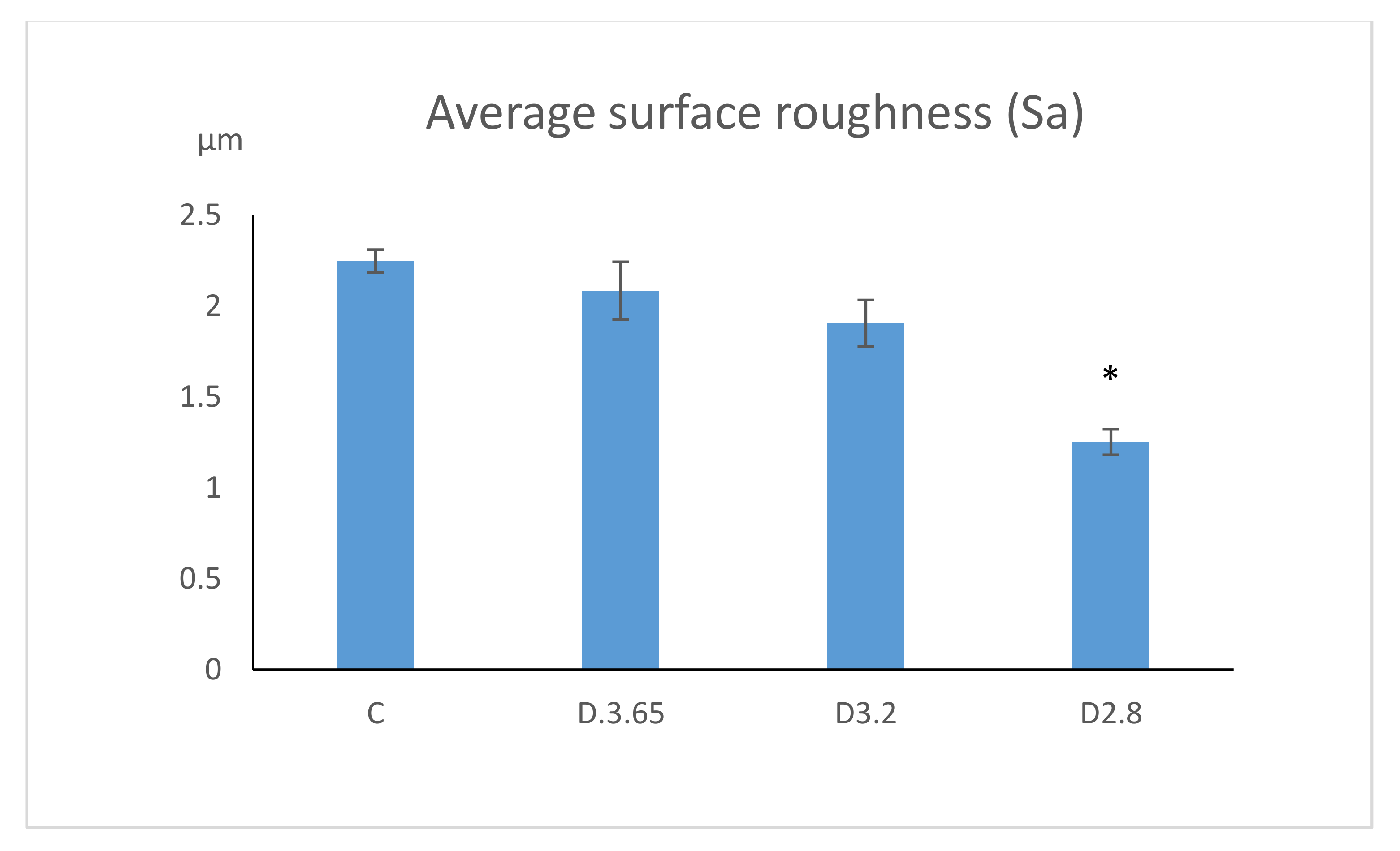
| Surface roughness (Sa) | 1.25 ± 0.07 µm | 1.90 ± 0.13 µm | 2.08 ± 0.16 µm | 2.24 ± 0.06 µm |
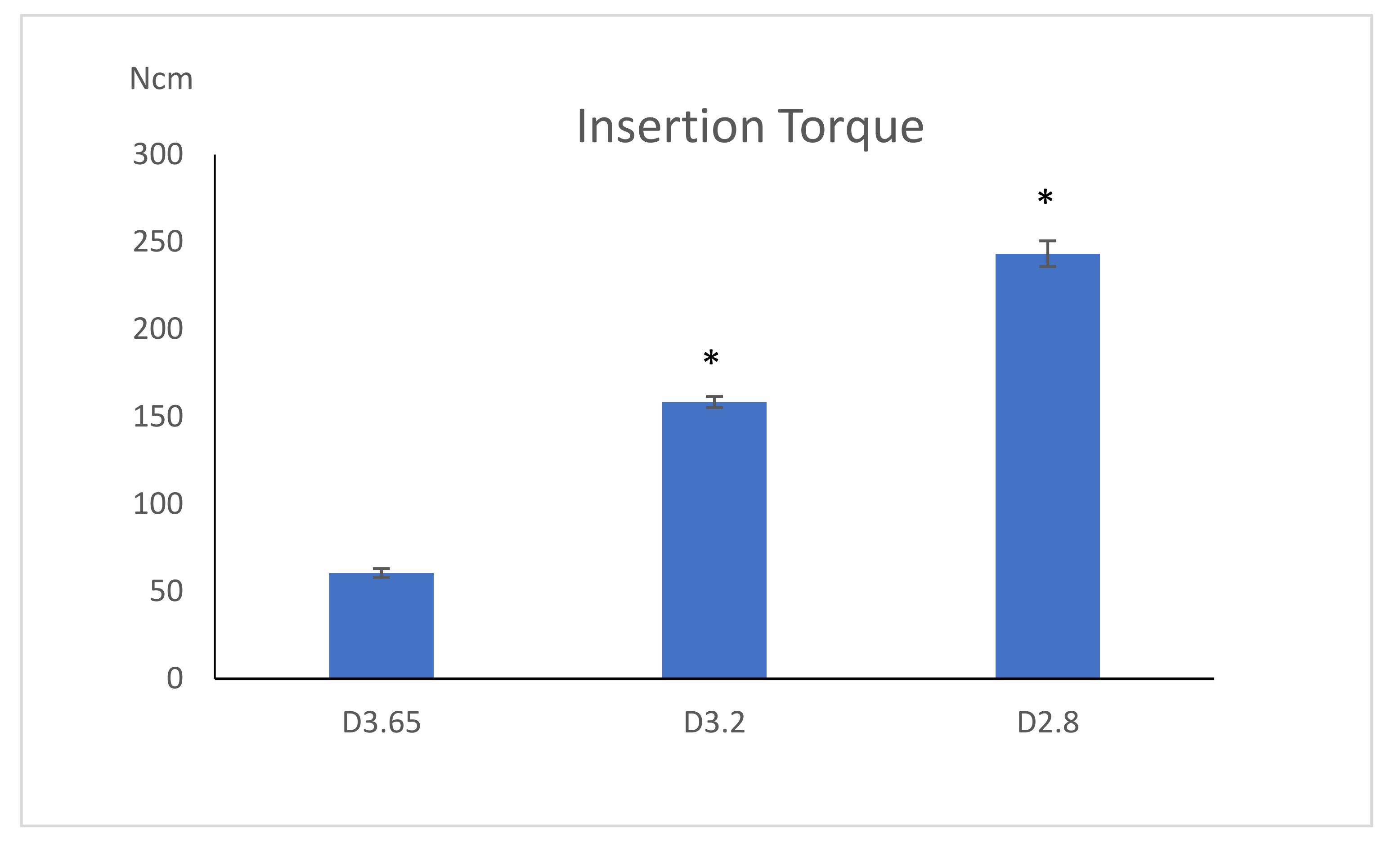
| Insertion torque | 243.26 ± 7.36 Ncm | 158.40 ± 3.17 Ncm | 60.54 ± 2.25 Ncm | |
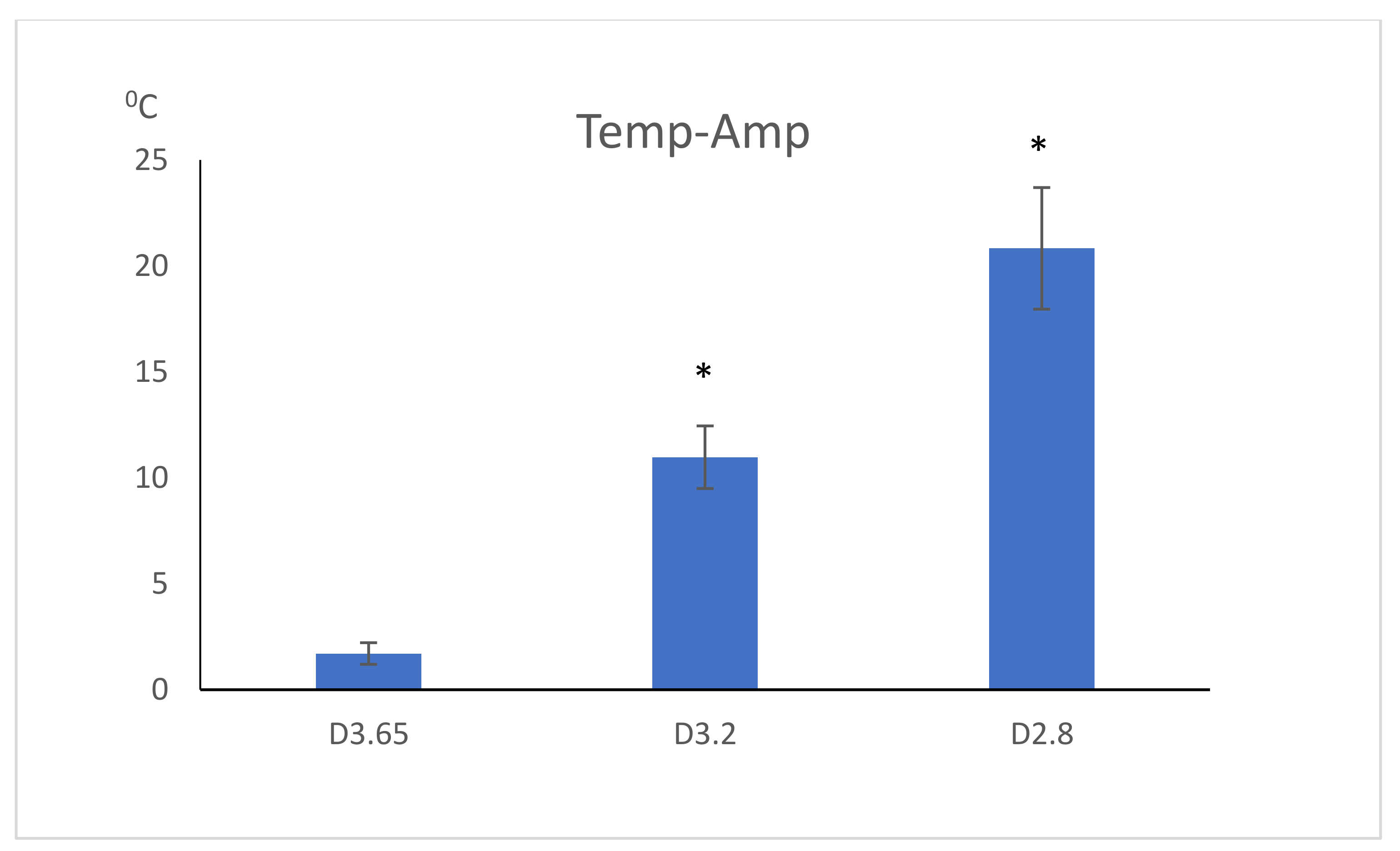
| Temperature | 44.82 ± 2.87 °C | 34.97 ± 1.48 °C | 25.70 ± 0.51 °C |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, O.; Moses, O.; Gurevich, T.; Kolerman, R.; Becker, A.; Ormianer, Z. The Effect of Undersized Drilling on the Coronal Surface Roughness of Microthreaded Implants: An In Vitro Study. Appl. Sci. 2020, 10, 5231. https://doi.org/10.3390/app10155231
Cohen O, Moses O, Gurevich T, Kolerman R, Becker A, Ormianer Z. The Effect of Undersized Drilling on the Coronal Surface Roughness of Microthreaded Implants: An In Vitro Study. Applied Sciences. 2020; 10(15):5231. https://doi.org/10.3390/app10155231
Chicago/Turabian StyleCohen, Omer, Ofer Moses, Talia Gurevich, Roni Kolerman, Alina Becker, and Zeev Ormianer. 2020. "The Effect of Undersized Drilling on the Coronal Surface Roughness of Microthreaded Implants: An In Vitro Study" Applied Sciences 10, no. 15: 5231. https://doi.org/10.3390/app10155231
APA StyleCohen, O., Moses, O., Gurevich, T., Kolerman, R., Becker, A., & Ormianer, Z. (2020). The Effect of Undersized Drilling on the Coronal Surface Roughness of Microthreaded Implants: An In Vitro Study. Applied Sciences, 10(15), 5231. https://doi.org/10.3390/app10155231






