1. Introduction
Tea is currently one of the most popular beverages in the world [
1,
2]. Every year, the consumption of teas, especially fruit teas, is increasing as a result of raised public awareness of their health-promoting properties. Fruit teas are a mixture of dried fruit, flowers, or leaves. Contrary to popular belief, these products do not contain black tea leaves. The addition of fruit juice concentrates and acidity regulators (e.g., citric acid) improves the taste and aroma of fruit teas [
3]. Indeed, fruit tea infusions are successfully replacing sweetened drinks and juices. Interestingly, studies performed in the USA in the years 2011–2016 demonstrated that the highest tea consumption was observed in elderly people aged 51 to 70 years, non-Latin Asians, white people, and people with higher education and income [
2]. This is not surprising, because tea has a number of health-promoting properties. One of them is strong antioxidant activity manifested by scavenging reactive oxygen species (ROS).
Oxygen free radicals are atoms or molecules with one unpaired electron or more. They are most often formed under the influence of various physical factors, such as temperature, UV, and ionizing radiation, as well as xenobiotics (e.g., medicines, cigarette smoke, and air pollution) [
4,
5,
6]. The situation in which the production of free radicals exceeds the body’s ability to neutralize them is called oxidative stress (OS). It leads to oxidative damage to proteins, lipids, and DNA, which in turn disturbs cell metabolism and entails the development of cardiovascular [
7], immunological [
8], neurodegenerative [
9,
10], and metabolic diseases [
11,
12], as well as cancer [
13,
14].
The human body is equipped with specialized antioxidant mechanisms (antioxidant enzymes: catalase, superoxide dismutase, and glutathione peroxidase; low molecular weight antioxidants: uric acid, glutathione, and albumin; and antioxidant vitamins: ascorbic acid and α-Tocopherol) [
5,
14]. In states with impaired antioxidant barrier and/or overproduction of ROS, the use of extrinsic antioxidants is recommended [
8,
15]. Exogenous antioxidant compounds are widespread in plant material, animal tissues, and microorganisms. The most important sources of antioxidants are flavonoids, carotenoids, phytosterols, phenolic compounds, and vitamins C and E [
16,
17,
18,
19]. They occur in particularly large quantities in black, red, and green teas, as well as herbal teas [
3,
17,
20]. The antioxidant abilities of teas depend mainly on the composition of a given tea [
3,
17,
20]. The total content of polyphenols and total antioxidant activity are higher in bearberry tea than in cranberry, mint, chamomile, and even green and black teas [
21]. It is assumed that the antioxidant properties of teas may also be determined by the origin of tea ingredients [
22]. Moreover, the antioxidant potential of teas of the same composition but obtained from another manufacturer may vary. This is most likely due to different cultivation methods, harvesting period, differences in agronomic procedures, fermentation time, and other treatments typical of the tea-making process of a given manufacturer [
22,
23,
24]. Interestingly, antioxidant properties of teas also depend on the brewing method [
16,
25]. It has been shown that the brewing temperature and time are extremely important in the process of phenolic component extraction [
17,
23,
25]. Some studies have confirmed that elevated levels of polyphenolic compounds of black, green, and white tea can be reached by extending the brewing time to more than ten minutes [
25,
26,
27]. High antioxidant capacity is also demonstrated by herbal tea infusions, and this activity is correlated with the content of polyphenols in a given tea [
22,
27,
28].
While much attention has been paid to traditional leaf teas, there have been few studies assessing the antioxidant properties of infusions made from dried fruit [
20]. Moreover, there have been no studies comparing the antioxidant potential of fruit teas with respect to brewing temperature and time. Bearing in mind that the processes of fermentation, drying, sorting, and storage of raw material may affect the quality and properties of teas, we were the first to compare the antioxidant potential of fruit teas produced under the same conditions by one manufacturer. We also evaluated the effect of selected extracts on albumin glycooxidation in vitro.
4. Discussion
The tea industry has been developing rapidly over the last two decades. More and more interest in a healthy lifestyle as well as the discovery of beneficial properties of teas have contributed to increased consumption of these beverages [
2,
25,
38]. Indeed, teas are classified as functional food, i.e., products with documented health-promoting effect [
39]. Although fruit teas are very popular, still little is known about their beneficial effects on the human body.
The antioxidant properties of fruit teas depend on the type and quality of the ingredients used in the process of tea production, location of the crops, and manner of the raw material processing. Interestingly, technological processes used in the production of fruit teas as well as conditions of raw material storage significantly affect the quality and health-promoting properties of teas [
17,
25,
28,
29]. Numerous studies have demonstrated that during the drying procedures (due to intensive aeration), plant raw material loses even 50% of its initial antioxidant capacity [
17,
40]. The raw material containing volatile substances (e.g., essential oils) and vitamins (mainly vitamin C) are particularly sensitive to processing [
18,
40]. Therefore, comparing antioxidant potential of fruit teas from different manufacturers (i.e., produced under different conditions) is of little and questionable value.
Tea brewing conditions (e.g., temperature and time of brewing) also significantly influence the quality of the infusion. Extraction is the simplest method of obtaining individual plant substances from a mixture of solids or liquids. Extracted compounds pass to a properly selected solvent that determines the value of the partition coefficient [
40,
41]. In case of fruit teas, the only solvent used is water. Therefore, to increase the efficiency of extraction, the temperature of water is raised and/or the brewing time is prolonged. However, a large part of biologically active compounds (mainly polyphenols) contained in fruit teas is susceptible to oxidation, thus high temperature and alkaline environment may be responsible for their degradation [
18,
40,
41]. Consequently, suitable conditions of raw material extraction are crucial for the quality of the obtained infusion [
23,
41]. Our study is the first to compare the effect of the brewing temperature and time on the antioxidant properties of fruit teas produced and stored under the same conditions.
Based on literature analysis, we chose the most frequently used brewing times (3, 5, and 10 min) and temperatures (70 °C and 100 °C) [
16,
17,
25,
26,
29]. We demonstrated that infusions with the longest brewing time have the highest antiradical activity.
A number of parameters are used to assess antioxidant properties, starting from the evaluation of individual antioxidants. However, much more information is provided by the resultant free radical scavenging capacity of a given sample, taking into account the synergistic effect of interactions between antioxidants [
30,
42]. In order to obtain a reliable picture of the antioxidant activity (considering the strengths and weaknesses of a given method and its applicability), it is recommended to apply at least two different methods [
30,
42]. In our study we evaluated TAC, DPPH, and FRAP. Although individual infusions were characterized by varied antiradical activity, the highest values of TAC, DPPH, and FRAP were observed for the longest time (10 min) and the highest temperature (100 °C) of brewing.
According to the manufacturer’s recommendations, the average brewing time for dried tea should be between 3 and 5 min. Our research revealed, however, that this time is not sufficient to obtain an infusion with maximum antioxidant potential. Therefore, we recommend extending the brewing time to ~10 min. Longer extraction time enables more antioxidants to pass to the infusion, which results in better health-promoting properties (an increase in TAC, DPPH, and FRAP and a decrease in TOS).
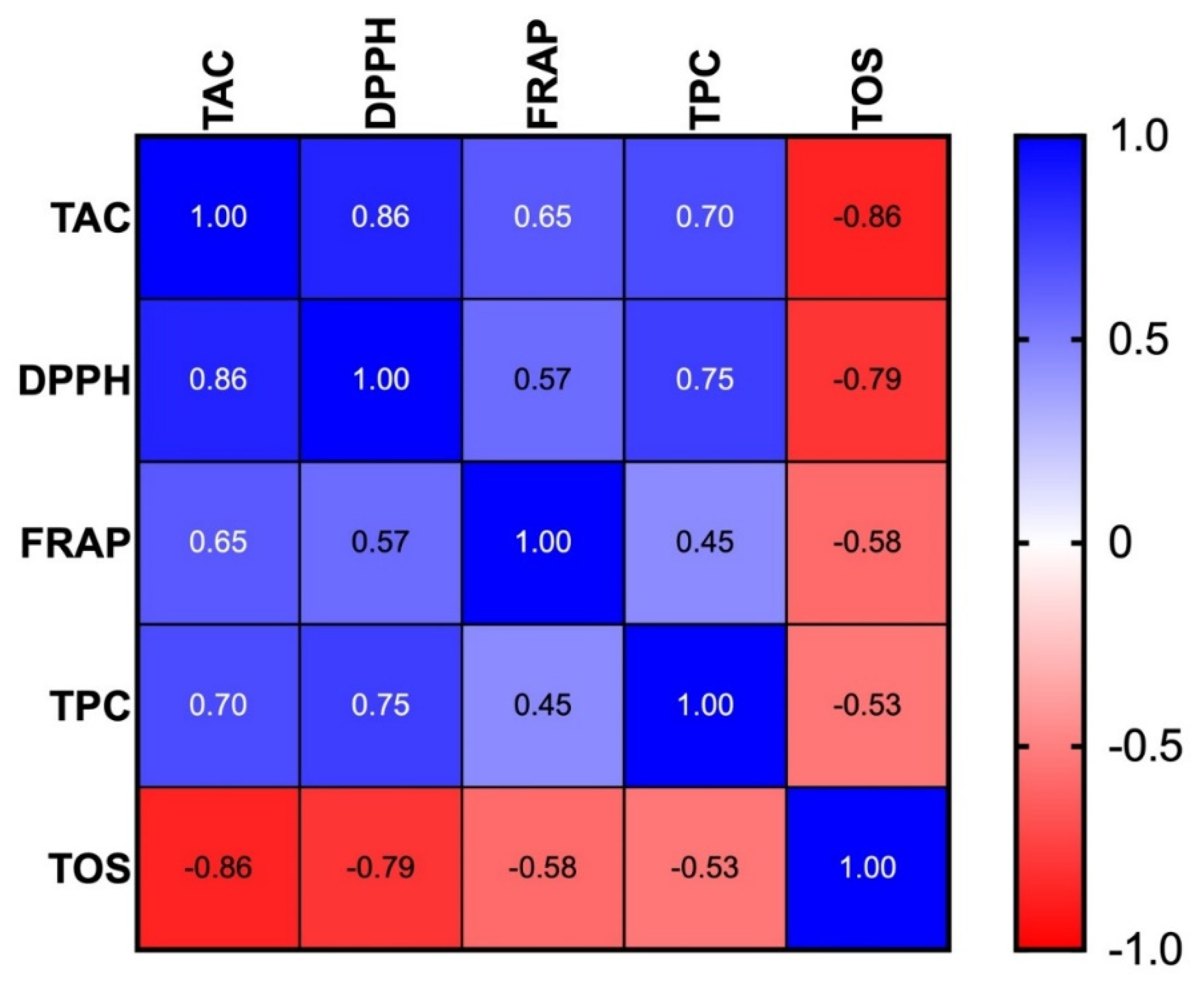
The content of TAC, DPPH, and FRAP correlated positively with total phenolic content (TPC). Thus, increased antioxidant activity of infusions may result from high level of polyphenolic compounds. Indeed, it is believed that polyphenols are the main source of antioxidants in fruit teas. These include flavonoids, tannins, phenolic acids, stilbenoids, lignans, and others [
18,
40,
43]. They present not only antiradical, but also anti-inflammatory or even anticancer effects [
18,
38]. Therefore, it is not surprising that polyphenols are used in the treatment of some systemic diseases [
18,
44]. The usage of polyphenols is also indicated in prevention of civilization diseases [
18,
44]. It is suggested that the health-promoting effects of fruit teas depend on the total phenolic content [
44]. Although the scope of TPC in the analyzed infusions is quite wide, it coincides with the data available in literature [
3,
16,
27]. However, the comparison of the obtained results is hindered by the fact that different solvents (water, ethanol, and methanol) were used in the various studies available in literature and the manner of presenting the results is also varied (gallic acid/catechin equivalents or TPC expressed in dry sample mass/solution volume). In our study, the only applied solvent was water, as it is used to prepare the extract by the consumer.
Although we did not directly evaluate the degree of oxidation/degradation of polyphenolic compounds, TPC was highest in teas with the longest brewing time (10 min) and the highest brewing temperature (100 °C). Therefore, it can be assumed that neither an increase in extraction time nor higher temperature of water cause degradation of polyphenols in the analyzed teas. However, it cannot be excluded that polyphenols may undergo partial oxidation during the brewing process. Interestingly, partially oxidized polyphenols are characterized by a boosted ability to bind free radicals compared to their non-oxidized precursors. These changes were observed, inter alia, in catechin subjected to enzymatic oxidation [
18,
40,
45,
46]. Indeed, the improved antiradical properties of partially oxidized polyphenols can be explained by an increased ability to release hydrogen atoms of the hydroxyl group at the aromatic ring. Moreover, they may result from keeping the unpaired electrons in the aromatic ring by relocating them in the π coating [
18,
40,
45,
46].
In addition to ionizing/ultraviolet radiation and xenobiotics, high temperature is one of the most important boosters of free radicals [
4,
5]. For that reason, in our study we evaluated the total oxidant status (TOS) determining the total oxidant content of the analyzed sample. This parameter is particularly useful for assessing the oxidation/degradation rate of food ingredients [
33]. We observed the highest TOS values at the highest brewing temperature and the shortest brewing time, while the lowest TOS values were noted at 100 °C after 10 min of brewing. It is therefore very likely that the total oxidant status decreases under the influence of antioxidants passing to infusions during the extraction process. Our hypothesis was confirmed by the negative correlation between TOS concentration and the analyzed antioxidant parameters (TAC, DPPH, and FRAP).
There are no available studies comparing the antioxidant properties of fruit tea infusions depending on the brewing temperature and time. With respect to black and green teas, it has been demonstrated that TPC is significantly higher in teas brewed in hot water, as confirmed by the results of our study [
29]. On the other hand, Shannon E. et al. [
27] proved that the optimum brewing time for black, green, white, and chamomile teas as well as for the blend of berry and hibiscus tea is 5 min. Extending the brewing time to 10 min did not increase the antioxidant properties [
27]. Therefore, it is probable that the antiradical activity of an infusion is largely dependent on the qualitative composition of the blend (black/herbal/fruit tea).
The composition of fruit teas is not limited to one raw ingredient. The presence of particular ingredients in tea blends is determined, on the one hand, by the price of the raw material, and on the other hand, by the sensory properties responsible for the flavor, aroma, and color of the obtained infusions [
2,
27]. The most common ingredients of fruit teas are hibiscus flower and fruits of chokeberry, black currant, rosehip, and raspberry [
47]. Although they mainly determine the color and taste of the infusions, these raw materials are a rich source of both organic acids (citric, malic, tartaric, and oxalic) and also anthocyanins and polyphenolic compounds such as protocatechuic acid [
48,
49]. Therefore, they may be responsible for the antioxidant properties of fruit teas. However, despite numerous studies on the antioxidant properties of green, black, and red teas, still little is known about teas made from dried fruit. Pękal et al. [
3] have shown that the antioxidant activity of studied teas increases in the following order; fruit tea, flavored black tea, and premium black tea. Indeed, the antioxidant properties of a particular infusion are strongly influenced by the percentage of individual raw materials in the mass of the entire product, which translates into a different content of biologically active compounds. In our study, the highest antioxidant activity (highest TAC, DPPH, and TPC) was found in the mixture of lemon balm and pear. In this product, 50% of the dry matter is constituted by the lemon balm herb, which is basically an oil-bearing raw material. However, apart from the essential oil, this raw material also has other biologically active compounds, mainly phenolic acids: rosemarinic, caffeic, chlorogenic, and ferulic acids, which are both esters and glycosides. This raw material also contains triterpenic acids, flavonoids, and minerals [
50]. On the other hand, pear fruit has a high content of vitamin C, anthocyanins, and other antioxidants, which may affect health-promoting properties of the tea made from this material [
51,
52]. Indeed, it is believed that the main source of antioxidants are fruits, among which berries are particularly rich in anthocyanins and tannins [
53]. Unfortunately, the available literature lacks data on the total antioxidant potential of the raw material. In our study, infusions of forest fruits, cranberry with pomegranate, raspberry and raspberry with linden also showed high antioxidant activity. Indeed, many studies have shown that these raw materials are rich sources of flavonoids (mainly quercetin, kaempferol, and acacetin derivatives) as well as essential oil [
16,
53,
54,
55]. Interestingly, linden, apart from flavonoids (rutin, hyperoside, quercetin, astragalin, and thiroside) and essential oil, also contains mucous compounds, amino acids or tocopherol, which also determines antioxidant properties [
56,
57]. The addition of these ingredients may intensify the antiradical effect in comparison to tea with rasberry alone. Sahin et al. [
16] demonstrated that pomegranate fruit has the highest antioxidant capacity among the evaluated fruit teas. In our study, the cranberry with pomegranate tea also reached high values of TAC, DPPH, FRAP, and TPC, while the cranberry infusion was already characterized by low antioxidant activity. It can therefore be assumed that it is the pomegranate fruit that is responsible for the health benefits of the cranberry-pomegranate tea. Sahin et al. [
16] also showed a significant antioxidant capacity of berry teas (blueberry and blackberry), which was also covered by our study. We showed that forest fruit tea is one of the five teas with the best antioxidant properties.
An important part of our study was also the assessment of the effect of fruit tea extracts on the glycoxidative properties of albumin. Albumin is one of the best-known proteins of the human body involved in the maintenance of oncotic pressure, regulation of the acid–base and oxidative–antioxidative balance as well as transport of many endogenous and exogenous substances [
58]. The most sensitive to oxidation are alkaline, aromatic, and sulfur-containing amino acids [
59]. In the present study, we evaluated the effect of extracts with the best antioxidant properties on albumin glycooxidation in vitro. We have shown that the analyzed fruit teas reduce oxidation and glycation of albumin observed as a decrease in fluorescence of aromatic amino acids (dityrosine, kynurenine, and
N-formylkynurenine), reduced AGE and AOPP levels as well as an increase in tryptophan content. However, the ability to counteract glycation and protein oxidation has been demonstrated mainly in tea extracts with the longest extraction time and the highest temperature. This confirms our previous results on the relationship between antioxidant properties and brewing temperature/time. It is well known that the products of protein oxidation and glycation interact with other proteins, disturbing their structure and function. AGE and AOPP bind to specific receptors and activate many signaling pathways (e.g., NF-κB, NJK, and p21 RAS) that intensify the secretion of proinflammatory cytokines and pro-thrombotic factors [
60,
61]. AGE and AOPP can also accumulate in tissues, which disrupts the functioning of many organs [
60,
61]. Thus, fruit tea could improve the redox balance in the course of diseases with a proven etiology of oxidative stress. They can also be used to prevent cancer, inflammation, neurodegenerative diseases, or metabolic diseases, where increased protein glycooxidation occurs. Therefore, our study is a starting point for further research on the therapy of reducing protein oxidation/glycation in living systems.
In conclusion, we proved that brewing time has a significant impact on the antioxidant properties of fruit teas. This may be related to a better release of the biologically active substances contained in the dried fruit and their easier transfer to the infusion at a longer extraction time (10 min). Although fruit teas are a rich source of numerous natural antioxidants, it should be remembered that their proper preparation is crucial. Further studies should aim at developing the optimum qualitative–quantitative composition of teas in order to obtain maximum antioxidant properties at acceptable sensory qualities. The evaluation of antioxidant properties of fruit teas may contribute to the consumer’s conscious choice of the final product which, apart from its flavor, will offer valuable health-promoting properties. It is also necessary to assess the effect of fruit tea extracts on antioxidant properties in vivo.
Our study also had certain limitations. First of all, we did not assess individual phytochemicals separately, so we do not know which compounds are responsible for the observed antioxidant properties. Moreover, the extraction of dried fruit was performed in only one solvent, which is also a limitation of our work. As teas with the best antioxidant effect are produced by berries, it is possible that phytochemicals such as anthocyanins have the most antiradical activity. Therefore, obtaining a phytochemical/antioxidant profile of the raw material should be the next step in research. Interestingly, Atoui et al. [
62] identified over 60 different flavonoids, phenolic acids and their derivatives in leaf tea and herbal infusions.