Abstract
Neurotoxicity is an obvious adverse effect in Patients encountering a complete course of chemotherapy. The present work is conducted to evaluate the neuroprotective effect of Ginkgo biloba (Ginkgo) against the neurotoxicity induced by Cisplatin (Cis) in rats. Forty male Wistar albino rats were arranged into four groups: (1) Control group, rats were given saline; (2) Cis group, rats were injected by Cis 2 mg/kg body weight i.p., twice a week starting on the fifth day for thirty days; (3) Ginkgo group, rats were administered Ginkgo (50 mg/kg orally) daily for thirty days; and (4) Ginkgo+Cis group, rats received Ginkgo (50 mg/kg orally) daily and on the fifth day, rats were injected with Cis (2 mg/Kg body weight i.p.) twice a week for thirty days. Cis significantly increased Gamma glutamyltransferase (GGT) and Acetyl Cholinesterase (CHE) as compared to the control group and also disturbed cerebral oxidative/antioxidant redox. Co-administration of Ginkgo and Cis reversed the adverse effect of Cis on the brain tissue. Moreover, co-administration of Ginkgo and Cis ameliorated Cis induced brain damage by reducing Amyloid precursor protein (APP), amyloid β (Aβ), P2Y12R and P2X7R mRNA expressions and proteins. Furthermore, Ginkgo regulated XIAP/BDNF expressions with a consequent decrease of caspase-3 and DNA fragmentation%. The current results concluded that concurrent treatment with Ginkgo can mitigate neurotoxicity mediated by Cis in experimental animals through exhibiting antioxidant effect by restoring cerebral oxidative/antioxidant redox and anti-apoptotic effect via regulating cerebral APP/Aβ/P2Y12R/P2X7R and XIAP/BDNF signaling pathways.
Keywords:
cisplatin; neuro toxicity; Ginkgo biloba; XIAP; APP; BDNF; purinergic receptors; caspase-3 1. Introduction
Cisplatin (cis-diamminedichloroplatinum II) (Cis), is a heavy metal compound that comprises a central atom of platinum surrounded by two chloride molecules and two ammonia molecules. Its molecular formula is CL2-H6-N2-Pt [1]. Plasma proteins such as albumin, transferrin and gamma globulin, irreversibly bound to the platinum component of Cis [2].
Cis is considered one of the most essential anti-cancer chemotherapeutic agents which can be concerned with the management of numerous human malignancies in different organs [3]. One of the most important mechanisms of Cis is its ability to bind with purine bases of deoxyribonucleic acid (DNA), hinder the repair mechanisms of DNA and boost DNA damage, in addition to prohibition of cell proliferation, and death of tumor cell [4]. Cis mainly targets DNA and many cytoplasmic components, such as proteins, thiol peptides and RNA [5].
One of the major apparent complications of Cis chemotherapy is the brain toxicity [6]. The cerebral toxicity of the Cis is considered dose-dependent [7]. Cis has a powerful penetrating power into the blood–brain barrier (BBB), and so it can debilitate mature neurons in the brain [8]. Hydrogen peroxide and hydroxyl radicals are examples of reactive oxygen species (ROS) which are formed by Cis. The formed free radicals interact with DNA, proteins and fats, resulting in lipid peroxidation and destruction of DNA [9].
One of greatest key mechanisms of Cis toxicity is the induction of oxidative stress. Under normal conditions, ROS are controlled by cells through harmonizing the production of ROS with their removal by scavenging system. Oxidative stress results in extreme damage of cellular proteins, lipids and DNA, leading to critical cellular injuries. Cis can induce mitochondrial oxidative damage with a disruption of sulfhydryl group of mitochondrial protein, hindering calcium uptake and weakening the mitochondrial membrane potential [10], decreasing the antioxidant defense system and reducing glutathione (GSH) [11].
Indeed, too much ROS production, DNA injury, inflammation, mitochondrial dysfunction, and also programmed cell death in the nervous system are considered the chief mechanisms in which neurotoxicity is provoked by Cis [12]. Many previous studies recorded the toxic mechanism of Cis on the nervous system but there is no satisfactory information to explain the mechanistic pathway of Cis induced-brain apoptosis.
Nowadays, a great attention is given to the amyloid precursor protein (APP) and β amyloid (Aβ) as they have a pivotal role to play in the development of Alzheimer’s disease (AD). Free-radical oxidative stress, neuronal lipids peroxidation, proteins denaturation and DNA fragmentation were detected in AD brain areas in which Aβ is abundant [13]. APP and Aβ are considered sensitive biomarkers in neuronal damage and neuronal cell death [14]. It is reported that accumulation of Aβ directly activates caspase-3 concerned with the death of neuronal cells [15]. Also, it increases the expression of Purinergic receptors especially P2Y12R and P2X7R [16]. These receptors became a target for examination of the sites of chronic inflammation, neuro-degeneration, and neuropsychiatric troubles [17] as they enhance the formation of neuro-immune cells [18] and the release of IL-1β; one of the neuro-degenerative and neuro-inflammatory mediators [19].
On the contrary, there are many protective mechanisms and mediators which can protect against cerebral injuries and apoptosis. One of the most important inhibitor apoptosis proteins (IPAs) is XIAP. It directly binds and inhibits the caspases which carry out the cell-death program [20]. Another important neuro-protective protein is brain-derived neurotrophic factor (BDNF). BDNF belongs to neurotrophin family proteins which can be detected in both the peripheral and central nervous systems [21]. It has an essential function in survival, development, differentiation and plasticity of neurons. It could protect neurons from induced neuronal cell oxidative stress and apoptosis [22]. Many studies have revealed that cleaved caspases and caspase-3 mediate the release of the cytokines interleukin-1β (IL-1β) and IL-18. In addition, inflammatory caspases modulate distinct forms of programmed cell death [23]. Liu et al. stated that both cleaved caspase-3 and caspase-3 are sensitive biomarkers for apoptosis and different tumor stages [24].
The previously mentioned research has provoked the need to find novel, effective and safe neuroprotective agents to protect against the cis-induced cerebral apoptosis. So we aimed to study the anti-apoptotic effect of a well-known antioxidant herbal plant; Ginkgo biloba (Ginkgo) on Cis-induced cerebral apoptosis. Ginkgo is used as a conventional herbal medicine to cure anxiety, headaches, depression, and poor memory in Asia and Europe [25]. Ginkgo is composed chemically of flavonoid glycosides (22−27%), terpenetrilactones (5.4−6.6%), ginkgolides (2.8−3.4%), bilobalide (2.6−3.2%) and trace ginkgolic acid [26,27].
Noticeable cerebral antioxidant, anti-ischemic, anti-apoptotic effects of Ginkgo have been previously reported [28,29]. The antioxidant effect of Ginkgo could be attributed to its ability to reduce cerebral H2O2 and ROS production especially superoxide anion in the brain cell [30,31,32]. These influential properties of Ginkgo could be attributed to the flavonoid contents of Ginkgo [33]. A previous report recorded that Ginkgo has a power anti-apoptotic effect in rat brains with acute cerebral infarction [34]. Added to that, Ginkgo has potent antioxidant and anti-inflammatory effects in the cerebral infarction [35]. No available studies concern the effect of Ginkgo on inhibitor apoptosis protein (IAPs) or BDNF in the brain toxicity induced by Cis.
We aimed to verify whether Ginkgo therapy may eliminate the toxic effect of Cis on the brain tissue. Thus, this study was designed to assess the effect of Ginkgo as a neuroprotective agent on neurotoxicity induced by Cis in the rat model by assessing the antioxidant condition, certain gene expressions and anti-apoptotic specific protein markers in the brain tissue of experimental animals.
2. Materials and Methods
2.1. Drugs and Chemicals
Ginkgo was obtained as a commercial preparation Ginkgo Biloba® (Future Pharmaceuticals for industries for EMA Pharma, Cairo, Egypt). Ginkgo contains 24% Ginkgoflavones glycosides and 6% terpenoids. Ginkgo was given orally using stomach gavage. Cis was received from Sigma Chemicals (Sigma-Aldrich Louis, MO, USA).
2.2. Animals
Forty male Wistar Albino rats weighing (180–250 g) were received from the animal facility at El Nahda University (Beni-Suef, Egypt). Rats were adapted to laboratory conditions for two weeks before the start of the experimental work, then, rats were put in metal cages (each one contained 3 rats), kept under standard laboratory conditions with an optimum temperature (25 ± 2 °C), humidity (70%), 12 h of dark/light cycle and free access to rat chow and drinking water. The present work was approved by the Experimental Animal Ethics Committee at the faculty of Veterinary Medicine, Beni-Suef University, Egypt and all procedures used during dealing with animals were in harmony with the National Institutes of Health (NIH) guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978) and the body weights were recorded weekly.
2.3. Experimental Design
As shown in Figure 1 and after the accommodation period, rats were randomly divided into four groups (n = 10) as follows:
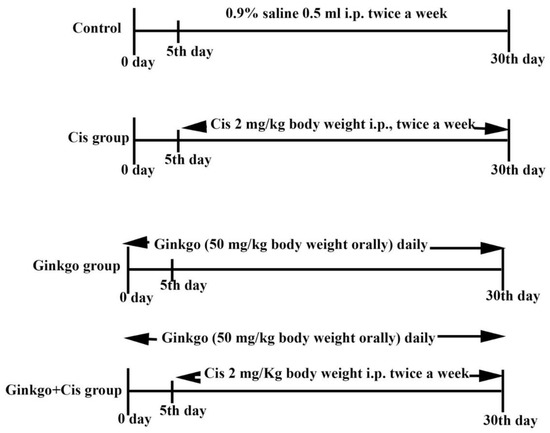
Figure 1.
Experimental design including all treated groups. Experimental design outlining the cisplatin (Cis) induced cerebral damage model and Ginkgo treatment protocol where n = 10 rats for each group. Control group: Rats were injected with 0.9% saline 0.5 mL i.p. twice a week on the 5th day, for 30 days. Cis group: Rats were given Cis 2 mg/kg body weight i.p. twice a week starting on the 5th day for 30 days. Ginkgo group: Rats received Ginkgo (50 mg/kg body weight orally) daily for 30 consecutive days. Ginkgo+Cis group: Rats received Ginkgo (50 mg/kg body weight orally) daily for 30 days, and on the 5th day, rats were injected with Cis 2 mg/Kg body weight i.p. twice a week for 30 days. All measured parameters were blind examined to confirm the accuracy of the results.
Control group: Rats were injected with 0.9% saline 0.5 mL i.p., twice a week on the 5th day, for 30 days.
Cis group: Rats were given Cis 2 mg/kg body weight i.p., twice a week starting on the 5th day for 30 days [36].
Ginkgo group: Rats received Ginkgo (50 mg/kg body weight PO) daily for 30 consecutive days [37].
Ginkgo+Cis group: Rats received Ginkgo (50 mg/kg body weight PO) daily 30 days [38]. On the 5th day, rats were injected with Cis 2 mg/Kg body weight IP twice a week for 30 days [36].
Twenty-four hours after the last treatment, the rats were fasted overnight and then sacrificed by decapitation. The brain tissues were collected, weighed then washed with phosphate-buffered saline (PBS) and divided into two parts. The first part was used to obtain a uniform suspension, 0.5 g of brain tissue was suspended in 5 mL PBS (pH: 7) and homogenized by using tissue homogenizer (Ortoalresa, Spain). The supernatant was kept at −80 °C for further biochemical evaluation. The second part was kept for molecular investigations (Western blot analysis and real-time polymerase chain reaction (RT-PCR) analyses) according to the instruction kits.
2.4. Methods
2.4.1. Biochemical Investigations
All used kits were purchased from Sigma-Aldrich Chemicals, St Louis, MO, USA. Acetyl Cholinesterase activity (CHE) was measured by using CHE assay kit (Cat. No. 119BJ11A25) according to [39]. Gamma glutamyl transferase activity (GGT) was measured by using GGT assay kit (Cat. No. 6A03K07840) according to [40]. Reduced glutathione concentration (GSH) was measured by using GSH assay kit (Cat. No. 099M4064V) according to [41]. Super oxide dismutase activity (SOD) was measured by using SOD assay kit (Cat. No. BCCC1068) according to the method of [42]. Total antioxidant capacity (TAC) was measured by using TAC assay kit (Cat. No. 059M4154V) according to the method of [43]. Malondialdehyde content (MDA) was measured by using MDA assay kit (Cat. No. 6A20K07390) according to the method of [44].
2.4.2. Western Blot Technique for Measurement of (APP, Aβ, X1AP and Caspase-3)
The concentration of cerebral APP, Aβ, X1AP and caspase-3 were quantified through immune blotting with the corresponding antibody and then the proteins were separated according to their molecular weight by gel electrophoresis. We used TGX Stain-Free™ Fast-Cast™ Acrylamide Kit (sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)) which was provided by Bio-Rad Laboratories, TNC, USA, Catalog No. 161-0181. The SDS-PAGE TGX Stain-Free Fast Cast was prepared according to the manufacturer’s instructions. Bands were visualized using Clarity™ Western ECL substrate (Bio-Rad, USA cat#170-5060), and the intensity of the bands was assessed against that of β-actin with the image analysis software Chemi Doc MP imager (Markham Ontario L3R 8T4 Canada) [45,46].
2.4.3. Determination of BDNF, P2Y12R and P2X7R Gene Expression by Real-Time-Polymerase Chain Reaction (RT-PCR)
By using RNeasy Purification Reagent (Qiagen, Valencia, CA, USA), RNA of the brain tissue was isolated. Primers specific for BDNF, P2Y12R and P2X7R were used and are listed in Table 1. Real time quantitative PCR was used to determine gene expression according to the instructions for Applied Biosystems version 3.1 software and SYBR Green I (Step One™, USA). All data are expressed relative to the β-actin gene [47].

Table 1.
Primers used for real-time quantitative PCR.
2.4.4. DNA fragmentation%
The percentage of DNA fragmentation in the brain tissue was measured according to the method described by [48], and the percentage was calculated by using the following equation:
2.5. Statistical Analysis
All obtained data are described as arithmetic means ± standard error (SE). The results were assessed by SPSS 20 (SPSS, Chicago, IL, USA). One-way ANOVA followed by Tukey’s post hoc test were done to evaluate and compare the significance between testing groups. Values of p < 0.05 were referred to as significant.
3. Results
Administration of Ginkgo alone in the Ginkgo group caused no significance at (p < 0.05) as compared to control group in all measured parameters.
3.1. Effect of Ginkgo on CHE and GGT Activities in Brain Toxicity
The activities of cholinesterase (Figure 2A) and GGT (Figure 2B) were significantly (p < 0.05) increased in the Cis group in comparison with the control group. Administration of Ginkgo with Cis in Ginkgo+Cis group decreased the activities of CHE and GGT compared to Cis only in Cis group at (p < 0.05).
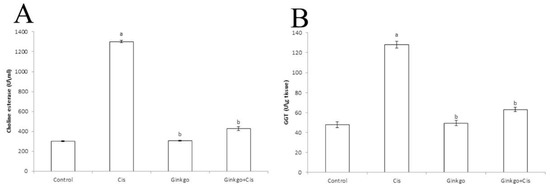
Figure 2.
Changes of Cholinestrase (CHE) and Gamma glutamyl transferase (GGT) activities of different groups. The effect of Cis, Ginkgo, and their combination on choline esterase (A) and GGT (B). Data are presented as mean ± SE (n = 10). a indicates a significant difference compared to control group, b indicates a significant change compared to Cis group. a and b indicate statistical significance at p < 0.05 using ANOVA followed by Tukey–Kramer as a post ANOVA test.
3.2. Effect of Ginkgo on Oxidant/Antioxidant Parameters in Brain Toxicity
Cis administration caused a significant decrease of GSH concentration (Figure 3A), SOD activity (Figure 3B), TAC concentration (Figure 3C) and a significant increase of MDA concentration (Figure 3D) in comparison with the control group (p < 0.05). Administration of Ginkgo with Cis reversed the adverse effect of Cis as Ginkgo+Cis group indicated a significant boost of GSH concentration, SOD activity, TAC concentration and a significant reduction of MDA in comparison with the Cis group (p < 0.05).
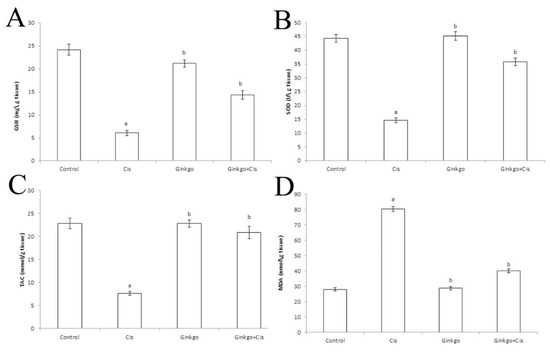
Figure 3.
The changes of oxidant/antioxidant parameters of different groups. The Effect of Cis, Ginkgo, and their combination on reduced glutathione (GSH) (A), super oxide dismutase (SOD) (B), Total antioxidant capacity (TAC) (C) and Malondialdehyde (MDA) (D). Data were presented as mean ± SE (n = 10). a indicates a significant difference compared to control group, b indicates a significant change compared to Cis group. a and b indicate statistical significance at p < 0.05 using ANOVA followed by Tukey–Kramer as a post ANOVA test.
3.3. Effect of Ginkgo on APP, Aβ, XIAP and Caspase-3
Cis caused a significant increase of APP (Figure 4A) and Aβ (Figure 4B), decrease of XIAP (Figure 4C) and increase of caspase-3 (Figure 4D) in comparison with the control group (p < 0.05). Ginkgo reversed the effect of Cis as Ginkgo administration in the Ginkgo+Cis group decreased APP, Aβ and caspase-3 and increased XIAP in comparison with the Cis group (p < 0.05).
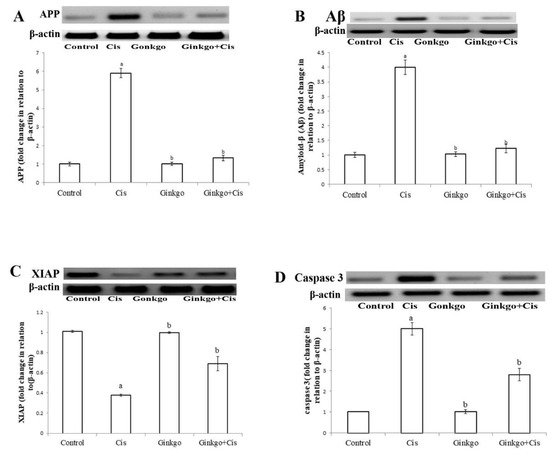
Figure 4.
The changes of amyloid precursor protein (APP), Aβ, XIAP and caspase-3 in different groups. The effect of Cis, Ginkgo, and their combination on the expression levels of APP (A), Amyloid β (Aβ) (B), XIAP (C), caspase-3 (D) in rat brain tissues. Data present as mean ± SE (n = 10). a indicates a significant difference compared to control group, b indicates a significant change compared to Cis group. a and b indicate statistical significance at p < 0.05 using ANOVA followed by Tukey–Kramer as a post ANOVA test.
3.4. Effect of Ginkgo on BDNF, P2Y12R and P2X7R mRNA Expressions in Brain Toxicity
Administration of Cis caused a significant reduction of BDNF mRNA expression (p < 0.05) in comparison with the control group (Figure 5A). Moreover, Cis caused a significant increase of P2Y12R (Figure 5B) and P2X7R (Figure 5C) mRNA expressions (p < 0.05) in comparison with the control group. Ginkgo caused a significant augmentation of BDNF mRNA expression and decreased P2Y12R and P2X7R mRNA expressions in the Ginkgo+Cis group in comparison with the Cis group (p < 0.05).
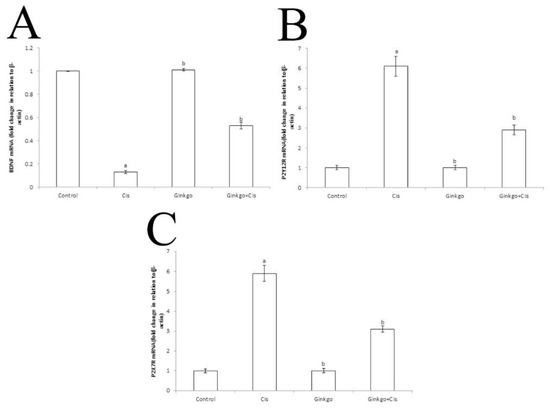
Figure 5.
The changes of BDNF, P2Y12R and P2X7R mRNA expressions in different group. The Effect of Cis, Ginkgo, and their combination on the expression levels of BDNF (A), PY12R (B) and P2X7R (C) in rat brain tissues. Data were presented as mean ± SE (n = 10). a indicates a significant difference compared to control group, b indicates a significant change compared to Cis group. a and b indicate statistical significance at p < 0.05 using ANOVA followed by Tukey–Kramer as a post ANOVA test.
3.5. DNA Fragmentation%
Cis caused a significant increase of DNA fragmentation% (Figure 6) in comparison with the control group (p < 0.05). Ginkgo ameliorates the effect of Cis as Ginkgo decreased DNA fragmentation% in comparison with Cis group (p < 0.05).
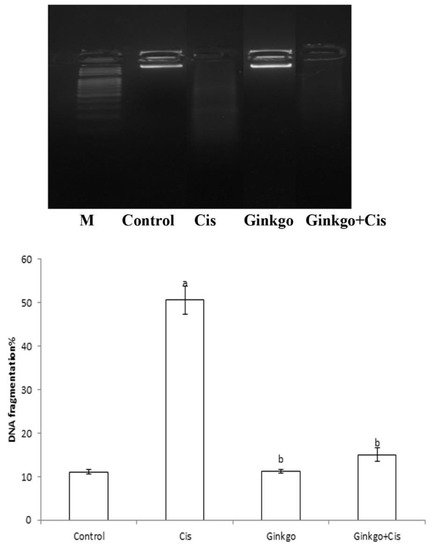
Figure 6.
The changes of DNA fragmentation% in different groups. The effect of Cis, Ginkgo, and their combination on the expression level of DNA fragmentation% in rat brain tissues. Data present as mean ± SE (n = 10). a indicates a significant difference compared to control group, b indicates a significant change compared to Cis group. a and b indicate statistical significance at p < 0.05 using ANOVA followed by Tukey–Kramer as a post ANOVA test.
4. Discussion
Although Cis is a potent chemotherapeutic drug, it has many harmful complications. In the existing study we focused on the toxic effect of Cis on the brain; the main target of Cis. It crosses the BBB and inhibits the proliferation of neuronal stem cells [3]. During Cis therapy, a marked decrease in the hippocampus neurons number has been identified [49].
Our results showed that Cis causes severe cerebral damage which is manifested by a significant increase of CHE and GGT activities in the Cis group in comparison with the control group. These findings are in agreement with [50,51,52]. References [53,54] reported that GGT is posited as an early and a delicate marker for brain damage. CHE is an important enzyme involved in acetyl choline catabolism at cholinergic synapses into acetic acid and choline [55]. Also, CHE is considered a target enzyme for Cis induced cerebral damage as the inhibition of CHE activity leads to the disturbance of the neurotransmitter acetylcholine [56] and an obvious disturbance in the nerve impulse transmission will take place [57].
Treatment with Ginkgo significantly reduced CHE and GGT activities compared to the Cis group. This effect might be explained by the efficacy of Ginkgo to scavenge the free radicals and suppress the leakage of enzymes through plasma membranes [58].
Furthermore, our results showed that Cis causes severe cerebral oxidative stress which is revealed by the severe depletion of GSH concentration [59], SOD activity, TAC with a concurrent augmentation of MDA. These findings are in accordance with [60]. Stimulation of lipid membrane peroxidation via augmentation of free oxygen radicals generation together with down regulation of antioxidant defense could explain the oxidative harmful effects of Cis [61]. Based on the existing work, the potent antioxidant outcome of Ginkgo on Cis-stimulated cerebral oxidative stress was so clear and could be inferred by the significant reduction of MDA [62] and increase in cerebral antioxidant defense GSH and SOD [63]. The antioxidant properties of Ginkgo are related to its efficacy to work as a free radical scavenger by preventing the lipid peroxidation [58]. The bio-flavonoid contents of Ginkgo have the upper hand in its antioxidant properties [64] through the direct scavenging effect on ROS, removing pro-oxidant transitional metal ions and boosting antioxidant proteins [65].
Apoptosis, which is clarified as a programmed cell death, controls cell repair and the removal of injured cells [66]. Cis induces neuronal loss by promoting neuronal apoptosis and inhibiting neurogenesis [67].
A novel finding is the ability of Cis to increase the production of APP and accumulation of Aβ, which is generated by proteolytic processing of the APP via β-secretase (β-amyloid cleavage enzyme) and γ-secretase complex [68]. Over accumulation of Aβ is one of the most acceptable hypotheses explaining the induction of neuronal oxidative damage and cell expiration in Alzheimer’s disease (AD) [15,69,70]. The neuronal apoptosis of AD is distinguished by the presence of many apoptotic markers which are found in the brains of AD sufferers after death, such as inducing caspase activities and DNA fragmentations [71,72].
Based on our findings, Cis increases the expression of some purinergic receptors such as P2Y12R and P2X7R. It is recorded that purinergic receptors significantly increased the neurotoxicity [73]. They are implicated in several CNS disorders, such as neuronal damage [74] and Alzheimer’s disease [75]. These receptors are activated by binding certain nucleotides that are released from injured neurons [76], and then activated in microglia [77]. Various pro-inflammatory mediators are generated by the activated microglia such as cyclooxygenase-2 (COX-2), tumor necrosis factor (TNF-a), and interleukin 6 (IL-6) [78], leading to neuronal damage, disability of tissue renovate and neuronal lesions [79]. A previous study reported that over concentration of Aβ in the brain tissue leads to the over expression of P2Y12R and P2X7R in the brain tissue [16].
Another apoptotic pathway of Cis is the inhibition of XIAP. XIAP belongs to the IAP family [80]. Reference [81] stated that XIAP has a powerful neuroprotective effect by reducing cerebral apoptosis. Our results showed that Cis administration diminished cerebral XIAP concentration with a concurrent up-regulation of caspase-3 which activates apoptotic cell death through a pathway of signal transduction Figure 7. The down-regulation of XIAP inhibits the phosphorylation of the tropomyosin linked kinase-B (TrkB) receptor; one of the most important membrane receptors of BDNF [82] and this could explain the significant reduction of BDNF expression in Cis treated rats. Added to that, Peng stated that too much accumulation of Aβ reduces the BDNF expression [83]. The reduction of BDNF inhibits Bcl-2 which is an important factor inhibiting apoptosis. Thus, caspase-3 is augmented and the intrinsic pathway of apoptosis is initiated in the brain tissue [84].
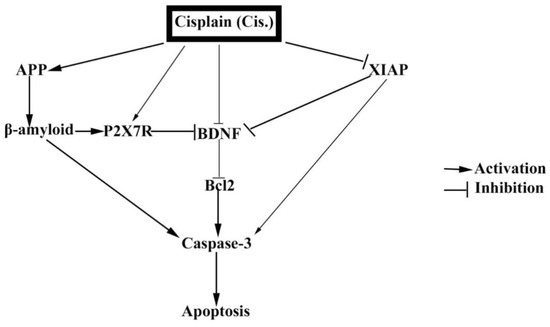
Figure 7.
The mechanistic cascades of Cis induced brain apoptosis. The integration of possible mechanistic cascades of Cis induced brain apoptosis. Cis directly activates APP, β-amyloid and P2X7R. On the contrary, Cis can inhibit BDNF directly and indirectly via inhibiting XIAP. The decrease of DDNF is subsequently associated with the decrease of Bcl2, and so caspase-3 is activated and finally cerebral apoptosis is induced.
From what has been previously mentioned, using anti-apoptotic agent such as Ginkgo is a logic approach to protect against cis induced cerebral apoptosis. Ginkgo has a powerful protective effect against the induced neuronal apoptosis which may be developed due to many sets of conditions, such as oxidative stress [85], and aggregation of Aβ [86]. Our data reported that co-administration of Ginkgo and Cis reduced APP causes a consequent reduction of Aβ accumulation in the brain tissue [87,88].
Our obtained results showed a significant reduction of P2Y12R, P2X7R and up-regulation of XIAP in the Ginkgo+Cis group. These changes were followed by a significant increase of BDNF. The overexpression of BDNF is in accordance with [89] who reported that Ginkgo protects against acrylamide induced brain toxicity.
The increased BDNF expression promotes the neuronal regeneration [89], inhibits many inflammatory mediators, up-regulates anti-apoptotic proteins such as Bcl-2 [90], thus blocking apoptotic markers such as caspase-3 [91].
5. Conclusions
The results of the existing study verify that concurrent treatment with Ginkgo attenuated Cis-induced cerebral apoptosis in rats. Thus, Ginkgo is considered an effective adjuvant for Cis as it has the ability to inhibit cerebral oxidative stress and apoptosis via regulating XIAP and BDNF levels and inhibiting APP, P2Y12R, P2X7R and caspase-3 in the brain tissue.
Author Contributions
Conceptualization, K.S.H., D.H.G. and A.M.M.H.E.; methodology, K.S.H. and D.H.G.; software, W.G.H. and H.A.-s. and A.M.M.H.E.; original draft preparation; K.S.H., D.H.G. and A.M.M.H.E.; writing—review and editing, K.S.H., D.H.G. and A.M.M.H.E. All authors have read and agreed to the published version of the manuscript.
Funding
The authors did not receive any sort of funding or financial help.
Acknowledgments
The authors extend their appreciation to the staff members of the Biochemistry Department, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef, Egypt.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Mehmood, R.K. Review of Cisplatin and oxaliplatin in current immunogenic and monoclonal antibody treatments. Oncol. Rev. 2014, 8. [Google Scholar] [CrossRef]
- Kasznicki, J.; Sliwinska, A.; Drzewoski, J. Metformin in cancer prevention and therapy. Ann. Transl. Med. 2014, 2. [Google Scholar]
- Takahara, P.M.; Rosenzweig, A.C.; Frederick, C.A.; Lippard, S.J. Crystal structure of double-stranded DNA containing the major adduct of the anticancer drug cisplatin. Nature 1995, 377, 649. [Google Scholar] [CrossRef]
- Ahmad, S. Platinum–DNA interactions and subsequent cellular processes controlling sensitivity to anticancer platinum complexes. Chem. Biodivers. 2010, 7, 543–566. [Google Scholar] [CrossRef] [PubMed]
- Santabarbara, G.; Maione, P.; Rossi, A.; Gridelli, C. Pharmacotherapeutic options for treating adverse effects of Cisplatin chemotherapy. Expert Opin. Pharmacother. 2016, 17, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Bobylev, I.; Joshi, A.R.; Barham, M.; Neiss, W.F.; Lehmann, H.C. Depletion of mitofusin-2 causes mitochondrial damage in cisplatin-induced neuropathy. Mol. Neurobiol. 2018, 55, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Seigers, R.; Schagen, S.; Van Tellingen, O.; Dietrich, J. Chemotherapy-related cognitive dysfunction: Current animal studies and future directions. Brain Imaging Behav. 2013, 7, 453–459. [Google Scholar] [CrossRef]
- Aydin, B.; Unsal, M.; Sekeroglu, Z.A.; Gülbahar, Y. The antioxidant and antigenotoxic effects of Pycnogenol® on rats treated with cisplatin. Biol. Trace Elem. Res. 2011, 142, 638–650. [Google Scholar] [CrossRef]
- Saad, S.Y.; Najjar, T.A.; Alashari, M. Role of non-selective adenosine receptor blockade and phosphodiesterase inhibition in cisplatin-induced nephrogonadal toxicity in rats. Clin. Exp. Pharmacol. Physiol. 2004, 31, 862–867. [Google Scholar] [CrossRef]
- Kart, A.; Cigremis, Y.; Karaman, M.; Ozen, H. Caffeic acid phenethyl ester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit. Exp. Toxicol. Pathol. 2010, 62, 45–52. [Google Scholar] [CrossRef]
- Gorgun, M.F.; Zhuo, M.; Englander, E.W. Cisplatin toxicity in dorsal root ganglion neurons is relieved by meclizine via diminution of mitochondrial compromise and improved clearance of DNA damage. Mol. Neurobiol. 2017, 54, 7883–7895. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid β-peptide. Trends Mol. Med. 2001, 7, 548–554. [Google Scholar] [CrossRef]
- Westmark, C. What’s hAPPening at synapses? The role of amyloid β-protein precursor and β-amyloid in neurological disorders. Mol. Psychiatry 2013, 18, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Lecanu, L.; Yao, W.; Teper, G.L.; Yao, Z.-X.; Greeson, J.; Papadopoulos, V. Identification of naturally occurring spirostenols preventing β-amyloid-induced neurotoxicity. Steroids 2004, 69, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Orellana, F.; Fuentes-Fuentes, M.C.; Godoy, P.A.; Silva-Grecchi, T.; Panes, J.D.; Guzmán, L.; Yévenes, G.E.; Gavilán, J.; Egan, T.M.; Aguayo, L.G. P2X receptor overexpression induced by soluble oligomers of amyloid beta peptide potentiates synaptic failure and neuronal dyshomeostasis in cellular models of Alzheimer’s disease. Neuropharmacology 2018, 128, 366–378. [Google Scholar] [CrossRef]
- Skaper, S.D.; Debetto, P.; Giusti, P. The P2X7 purinergic receptor: From physiology to neurological disorders. Faseb J. 2010, 24, 337–345. [Google Scholar] [CrossRef]
- Monif, M.; Burnstock, G.; Williams, D.A. Microglia: Proliferation and activation driven by the P2X7 receptor. Int. J. Biochem. Cell Biol. 2010, 42, 1753–1756. [Google Scholar] [CrossRef]
- Bernardino, L.; Balosso, S.; Ravizza, T.; Marchi, N.; Ku, G.; Randle, J.C.; Malva, J.O.; Vezzani, A. Inflammatory events in hippocampal slice cultures prime neuronal susceptibility to excitotoxic injury: A crucial role of P2X7 receptor-mediated IL-1β release. J. Neurochem. 2008, 106, 271–280. [Google Scholar] [CrossRef]
- Srinivasula, S.M.; Ashwell, J.D. IAPs: What’s in a name? Mol. Cell 2008, 30, 123–135. [Google Scholar] [CrossRef]
- Hartmann, D.; Drummond, J.; Handberg, E.; Ewell, S.; Pozzo-Miller, L. Multiple approaches to investigate the transport and activity-dependent release of BDNF and their application in neurogenetic disorders. Neural Plast. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Degos, V.; Chhor, V.; Brissaud, O.; Lebon, S.; Schwendimann, L.; Bednareck, N.; Passemard, S.; Mantz, J.; Gressens, P. Neuroprotective effects of dexmedetomidine against glutamate agonist-induced neuronal cell death are related to increased astrocyte brain-derived neurotrophic factor expression. Anesthesiol. J. Am. Soc. Anesthesiol. 2013, 118, 1123–1132. [Google Scholar] [CrossRef]
- Man, S.M.; Kanneganti, T.-D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-F.; Hu, Y.-C.; Kang, B.-H.; Tseng, Y.-K.; Wu, P.-C.; Liang, C.-C.; Hou, Y.-Y.; Fu, T.-Y.; Liou, H.-H.; Hsieh, I.-C. Expression levels of cleaved caspase-3 and caspase-3 in tumorigenesis and prognosis of oral tongue squamous cell carcinoma. PLoS ONE 2017, 12, e0180620. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.A.; Nada, S.E.; Doré, S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience 2011, 180, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.; Heiser, J.; Leuner, K. Effects of the standardized Ginkgo biloba extract EGb 761® on neuroplasticity. Int. Psychogeriatr. 2012, 24, S21–S24. [Google Scholar] [CrossRef]
- Kim, M.-S.; Bang, J.H.; Lee, J.; Han, J.-S.; Baik, T.G.; Jeon, W.K. Ginkgo biloba L. extract protects against chronic cerebral hypoperfusion by modulating neuroinflammation and the cholinergic system. Phytomedicine 2016, 23, 1356–1364. [Google Scholar] [CrossRef]
- Maclennan, K.M.; Darlington, C.L.; Smith, P.F. The CNS effects of Ginkgo biloba extracts and ginkgolide B. Prog. Neurobiol. 2002, 67, 235–257. [Google Scholar] [CrossRef]
- Aydin, D.; Peker, E.G.; Karakurt, M.D.; Gurel, A.; Ayyildiz, M.; Cevher, Ş.C.; Agar, E.; Dane, S. Effects of Ginkgo biloba extract on brain oxidative condition after cisplatin exposure. Clin. Investig. Med. 2016, S100–S105. [Google Scholar] [CrossRef]
- Oyama, Y.; Ueha, T.; Hayashi, A.; Chikahisa, L.; Noda, K. Flow cytometric estimation of the effect of Ginkgo biloba extract on the content of hydrogen peroxide in dissociated mammalian brain neurons. Jpn. J. Pharmacol. 1992, 60, 385–388. [Google Scholar] [CrossRef][Green Version]
- Oyama, Y.; Chikahisa, L.; Ueha, T.; Kanemaru, K.; Noda, K. Ginkgo biloba extract protects brain neurons against oxidative stress induced by hydrogen peroxide. Brain Res. 1996, 712, 349–352. [Google Scholar] [CrossRef]
- Abdel-Kader, R.; Hauptmann, S.; Keil, U.; Scherping, I.; Leuner, K.; Eckert, A.; Müller, W.E. Stabilization of mitochondrial function by Ginkgo biloba extract (EGb 761). Pharmacol. Res. 2007, 56, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Chen, R.; Wang, F.; Ren, C.; Zhang, P.; Li, Q.; Li, H.-H.; Guo, K.-T.; Geng, D.-Q.; Liu, C.-F. EGb-761 attenuates the anti-proliferative activity of fluoride via DDK1 in PC-12 cells. Neurochem. Res. 2017, 42, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhao, X.; Zhang, X.; Liu, S.; Zhao, H.; Chen, Y. Effect of Ginkgo biloba extract on apoptosis of brain tissues in rats with acute cerebral infarction and related gene expression. Genet. Mol. Res 2015, 14, 6387–6394. [Google Scholar] [CrossRef]
- Song, W.; Zhao, J.; Yan, X.-S.; Fang, X.; Huo, D.-S.; Wang, H.; Jia, J.-X.; Yang, Z.-J. Mechanisms Associated with Protective Effects of Ginkgo Biloba Leaf Extracton in Rat Cerebral Ischemia Reperfusion Injury. J. Toxicol. Environ. Health Part A 2019, 82, 1045–1051. [Google Scholar] [CrossRef]
- Kandeil, M.A.; Mahmoud, M.O.; Abdel-Razik, A.-R.H.; Gomaa, S.B. Thymoquinone and geraniol alleviate cisplatin-induced neurotoxicity in rats through downregulating the p38 MAPK/STAT-1 pathway and oxidative stress. Life Sci. 2019, 228, 145–151. [Google Scholar] [CrossRef]
- Zaki, H.F.; Shafey, G.M.; Amin, N.; Attia, A.S.; El-Ghazaly, M.A. Neuroprotective effects of ginkgo biloba extract on brain damage induced by γ-radiation and lead acetate. Int. J. Sci. Res. Publ. 2015, 5, 2250–3153. [Google Scholar]
- Dias, M.C.; Furtado, K.S.; Rodrigues, M.A.M.; Barbisan, L.F. Effects of Ginkgo biloba on chemically-induced mammary tumors in rats receiving tamoxifen. Bmc Complement. Altern. Med. 2013, 13, 93. [Google Scholar] [CrossRef]
- Kovarik, Z.; Radić, Z.; Berman, H.A.; Simeon-Rudolf, V.; Reiner, E.; Taylor, P. Acetylcholinesterase active centre and gorge conformations analysed by combinatorial mutations and enantiomeric phosphonates. Biochem. J. 2003, 373, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Persijn, J.; Van der Slik, W. A new method for the determination of γ-glutamyltransferase in serum. Clin. Chem. Lab. Med. 1976, 14, 421–428. [Google Scholar] [CrossRef]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. [30] Microsomal lipid peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 52, pp. 302–310. [Google Scholar]
- Marks, H.M. The Progress of Experiment: Science and Therapeutic Reform in the United States, 1900–1990; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Wang, J.; Edeen, K.; Manzer, R.; Chang, Y.; Wang, S.; Chen, X.; Funk, C.J.; Cosgrove, G.P.; Fang, X.; Mason, R.J. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am. J. Respir. Cell Mol. Biol. 2007, 36, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Burton, K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. J. 1956, 62, 315. [Google Scholar] [CrossRef]
- Dietrich, J.; Han, R.; Yang, Y.; Mayer-Pröschel, M.; Noble, M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J. Biol. 2006, 5, 22. [Google Scholar] [CrossRef]
- Chen, Y.; Jungsuwadee, P.; Vore, M.; Butterfield, D.A.; St Clair, D.K. Collateral damage in cancer chemotherapy: Oxidative stress in nontargeted tissues. Mol. Interv. 2007, 7, 147. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; El-Gizawy, M.M.; Sorour, S.M.; Sawie, H.G.; Hosny, E.N. Effect of curcumin nanoparticles on the cisplatin-induced neurotoxicity in rat. Drug Chem. Toxicol. 2019, 42, 194–202. [Google Scholar] [CrossRef]
- Owoeye, O.; Adedara, I.A.; Farombi, E.O. Pretreatment with taurine prevented brain injury and exploratory behaviour associated with administration of anticancer drug cisplatin in rats. Biomed. Pharmacother. 2018, 102, 375–384. [Google Scholar] [CrossRef]
- Lee, D.-H.; Blomhoff, R.; Jacobs, D.R. Review is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic. Res. 2004, 38, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Paolicchi, A.; Emdin, M.; Passino, C.; Lorenzini, E.; Titta, F.; Marchi, S.; Malvaldi, G.; Pompella, A. β-Lipoprotein-and LDL-associated serum γ-glutamyltransferase in patients with coronary atherosclerosis. Atherosclerosis 2006, 186, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Worek, F.; Reiter, G.; Eyer, P.; Szinicz, L. Reactivation kinetics of acetylcholinesterase from different species inhibited by highly toxic organophosphates. Arch. Toxicol. 2002, 76, 523–529. [Google Scholar] [PubMed]
- Caloni, F.; Cortinovis, C.; Rivolta, M.; Davanzo, F. Suspected poisoning of domestic animals by pesticides. Sci. Total Environ. 2016, 539, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Petroianu, G.; Lorke, D.E. α7-nicotinic acetylcholine receptors: New therapeutic avenues in Alzheimer’s disease. In Nicotinic Acetylcholine Receptor Technologies; Springer: Cham, Switzerland, 2016; pp. 149–169. [Google Scholar]
- Huang, X.; Whitworth, C.A.; Rybak, L.P. Ginkgo biloba extract (EGb 761) protects against cisplatin-induced ototoxicity in rats. Otol. Neurotol. 2007, 28, 828–833. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta (Bba)-Gen. Subj. 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Turan, M.; Cayir, A.; Cetin, N.; Suleyman, H.; Turan, I.S.; Tan, H. An investigation of the effect of thiamine pyrophosphate on cisplatin-induced oxidative stress and DNA damage in rat brain tissue compared with thiamine: Thiamine and thiamine pyrophosphate effects on cisplatin neurotoxicity. Hum. Exp. Toxicol. 2014, 33, 14–21. [Google Scholar] [CrossRef]
- Pabla, N.; Dong, G.; Jiang, M.; Huang, S.; Kumar, M.V.; Messing, R.O.; Dong, Z. Inhibition of PKCδ reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J. Clin. Investig. 2011, 121, 2709–2722. [Google Scholar] [CrossRef]
- Eckert, A. Mitochondrial effects of Ginkgo biloba extract. Int. Psychogeriatr. 2012, 24, S18–S20. [Google Scholar] [CrossRef]
- Li, W.Z.; Wu, W.Y.; Huang, H.; Wu, Y.Y.; Yin, Y.Y. Protective effect of bilobalide on learning and memory impairment in rats with vascular dementia. Mol. Med. Rep. 2013, 8, 935–941. [Google Scholar] [CrossRef]
- Laukeviciene, A.; Cecen, S.; Masteikova, R.; Civinskiene, G.; Zelbiene, E.; Burkauskienė, A.; Velziene, S.; Bernatoniene, J. Reduction of small arteries contractility with improving the relaxation properties by Ginkgo biloba extract. J. Med. Plants Res. 2012, 6, 4785–4789. [Google Scholar] [CrossRef]
- Smith, J.; Luo, Y. Studies on molecular mechanisms of Ginkgo biloba extract. Appl. Microbiol. Biotechnol. 2004, 64, 465–472. [Google Scholar]
- Xia, T.; Kovochich, M.; Liong, M.; Madler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. Acs Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Winocur, G.; Wojtowicz, J.M.; Tannock, I.F. Memory loss in chemotherapy-treated rats is exacerbated in high-interference conditions and related to suppression of hippocampal neurogenesis. Behav. Brain Res. 2015, 281, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Manczak, M.; Mao, P.; Calkins, M.J.; Reddy, A.P.; Shirendeb, U. Amyloid-β and mitochondria in aging and Alzheimer’s disease: Implications for synaptic damage and cognitive decline. J. Alzheimer’s Dis. 2010, 20, S499–S512. [Google Scholar] [CrossRef]
- Karran, E.; Mercken, M.; De Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205. [Google Scholar] [CrossRef]
- Ankarcrona, M.; Winblad, B. Biomarkers for apoptosis in Alzheimer’s disease. Int. J. Geriatr. Psychiatry A J. Psychiatry Late Life Allied Sci. 2005, 20, 101–105. [Google Scholar] [CrossRef]
- Uribe, V.; Wong, B.K.; Graham, R.K.; Cusack, C.L.; Skotte, N.H.; Pouladi, M.A.; Xie, Y.; Feinberg, K.; Ou, Y.; Ouyang, Y. Rescue from excitotoxicity and axonal degeneration accompanied by age-dependent behavioral and neuroanatomical alterations in caspase-6-deficient mice. Hum. Mol. Genet. 2012, 21, 1954–1967. [Google Scholar] [CrossRef]
- Makoto, T.; Hidetoshi, T.S.; Kazuhide, I. P2X4R and P2X7R in neuropathic pain. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 513–521. [Google Scholar] [CrossRef]
- Carmo, M.R.; Menezes, A.P.F.; Nunes, A.C.L.; Pliássova, A.; Rolo, A.P.; Palmeira, C.M.; Cunha, R.A.; Canas, P.M.; Andrade, G.M. The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology 2014, 81, 142–152. [Google Scholar] [CrossRef]
- Lee, H.G.; Won, S.M.; Gwag, B.J.; Lee, Y.B. Microglial P2X 7 receptor expression is accompanied by neuronal damage in the cerebral cortex of the APP swe/PS1dE9 mouse model of Alzheimer’s disease. Exp. Mol. Med. 2011, 43, 7. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D. Ion channels on microglia: Therapeutic targets for neuroprotection. Cns Neurol. Disord. Drug Targets (Former. Curr. Drug Targets-Cns Neurol. Disord.) 2011, 10, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K. Purinergic systems in microglia. Cell. Mol. Life Sci. 2008, 65, 3074–3080. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.K.; Koppula, S.; Suk, K. Inhibitors of microglial neurotoxicity: Focus on natural products. Molecules 2011, 16, 1021–1043. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Su, B.-C.; Mo, F.-E. CCN1 enables Fas ligand-induced apoptosis in cardiomyoblast H9c2 cells by disrupting caspase inhibitor XIAP. Cell. Signal. 2014, 26, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, H.; Roughton, K.; Wang, X.; Kroemer, G.; Blomgren, K.; Zhu, C. Lithium reduces apoptosis and autophagy after neonatal hypoxia–ischemia. Cell Death Dis. 2010, 1, e56. [Google Scholar] [CrossRef]
- Kairisalo, M.; Korhonen, L.; Sepp, M.; Pruunsild, P.; Kukkonen, J.P.; Kivinen, J.; Timmusk, T.; Blomgren, K.; Lindholm, D. NF-κB-dependent regulation of brain-derived neurotrophic factor in hippocampal neurons by X-linked inhibitor of apoptosis protein. Eur. J. Neurosci. 2009, 30, 958–966. [Google Scholar] [CrossRef]
- Peng, S.; Garzon, D.J.; Marchese, M.; Klein, W.; Ginsberg, S.D.; Francis, B.M.; Mount, H.T.; Mufson, E.J.; Salehi, A.; Fahnestock, M. Decreased brain-derived neurotrophic factor depends on amyloid aggregation state in transgenic mouse models of Alzheimer’s disease. J. Neurosci. 2009, 29, 9321–9329. [Google Scholar] [CrossRef]
- Green, D.R.; Llambi, F. Cell death signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.; Navarro, A.; Ordónez, C.; del Valle, E.; Tolivia, J. Oxidative stress induces apolipoprotein D overexpression in hippocampus during aging and Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 36, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Bate, C.; Tayebi, M.; Williams, A. Ginkgolides protect against amyloid-β 1–42-mediated synapse damage in vitro. Mol. Neurodegener. 2008, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Smith, J.V.; Paramasivam, V.; Burdick, A.; Curry, K.J.; Buford, J.P.; Khan, I.; Netzer, W.J.; Xu, H.; Butko, P. Inhibition of amyloid-β aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc. Natl. Acad. Sci. USA 2002, 99, 12197–12202. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, S.; Park, Y. Multifaceted therapeutic benefits of Ginkgo biloba L.: Chemistry, efficacy, safety, and uses. J. Food Sci. 2008, 73, R14–R19. [Google Scholar] [CrossRef]
- Huang, W.-L.; Ma, Y.-X.; Fan, Y.-B.; Lai, S.-M.; Liu, H.-Q.; Liu, J.; Luo, L.; Li, G.-Y.; Tian, S.-M. Extract of Ginkgo biloba promotes neuronal regeneration in the hippocampus after exposure to acrylamide. Neural Regen. Res. 2017, 12, 1287. [Google Scholar]
- Wu, C.L.; Hwang, C.S.; Chen, S.D.; Yin, J.H.; Yang, D.I. Neuroprotective mechanisms of brain-derived neurotrophic factor against 3-nitropropionic acid toxicity: Therapeutic implications for Huntington’s disease. Ann. N. Y. Acad. Sci. 2010, 1201, 8–12. [Google Scholar] [CrossRef]
- Han, B.H.; D’Costa, A.; Back, S.A.; Parsadanian, M.; Patel, S.; Shah, A.R.; Gidday, J.M.; Srinivasan, A.; Deshmukh, M.; Holtzman, D.M. BDNF blocks caspase-3 activation in neonatal hypoxia–ischemia. Neurobiol. Dis. 2000, 7, 38–53. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).