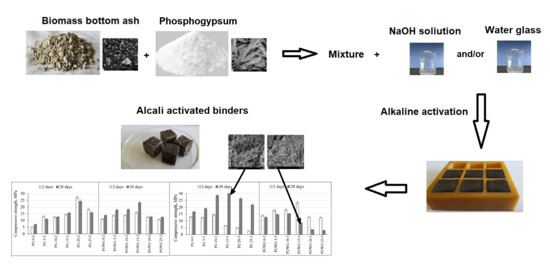
Alkali Activated Paste and Concrete Based on of Biomass Bottom Ash with Phosphogypsum
Abstract
1. Introduction
2. Methodology
2.1. The Characterization of Raw Materials and the Mixing Composition of Alkali Activated Biomass Bottom Ash Pastes and Concretes
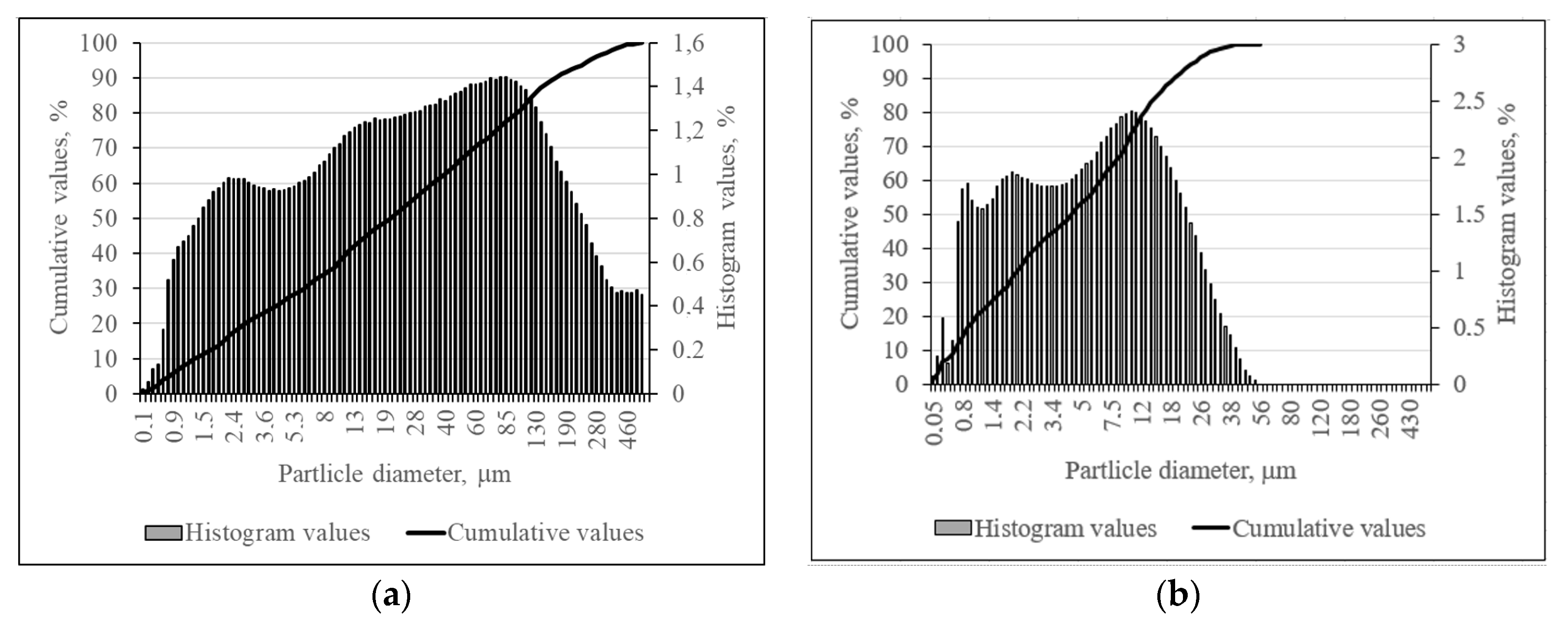
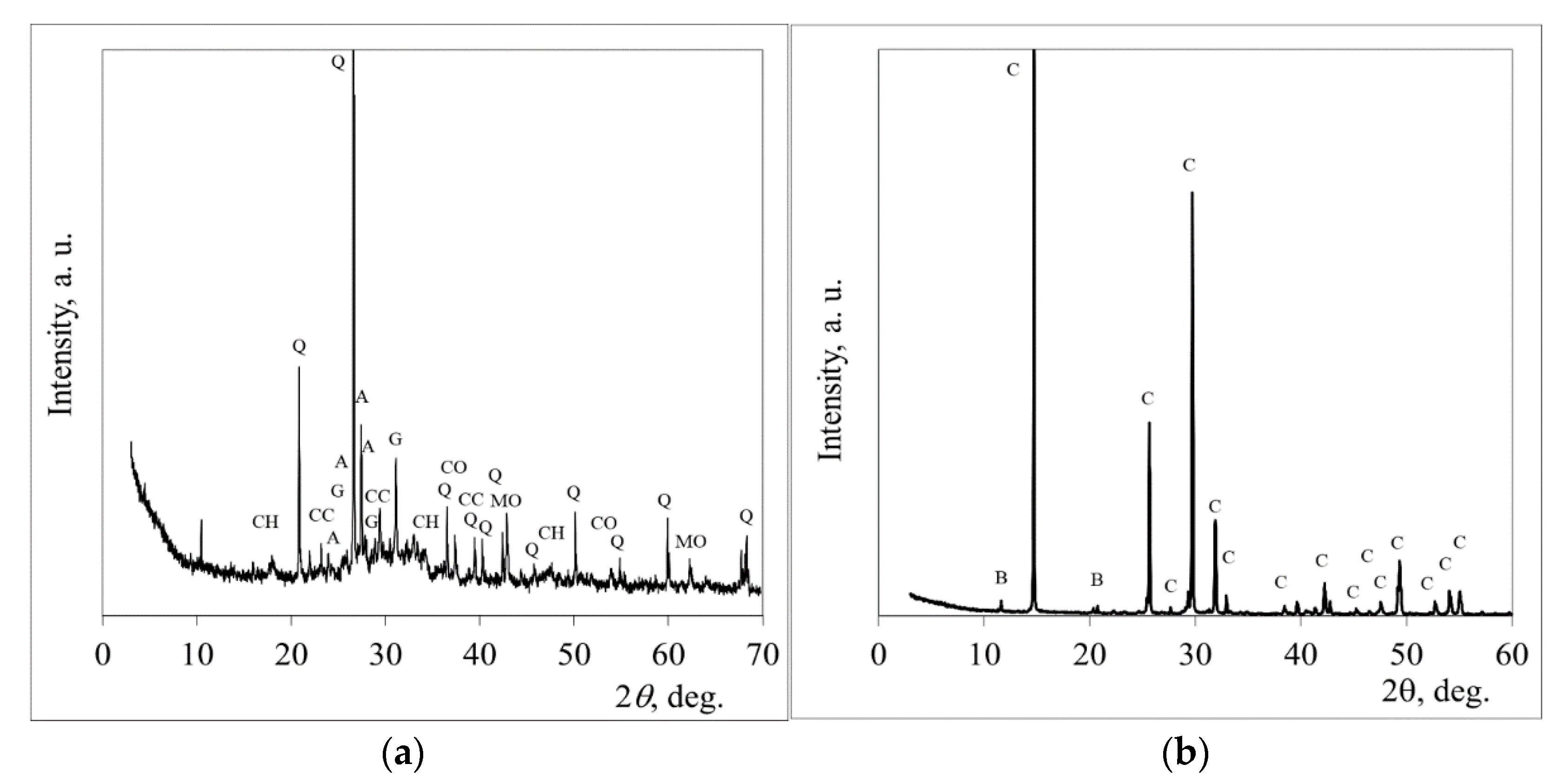
2.2. The Experimental Techniques
3. Results and Discussion
4. Conclusions
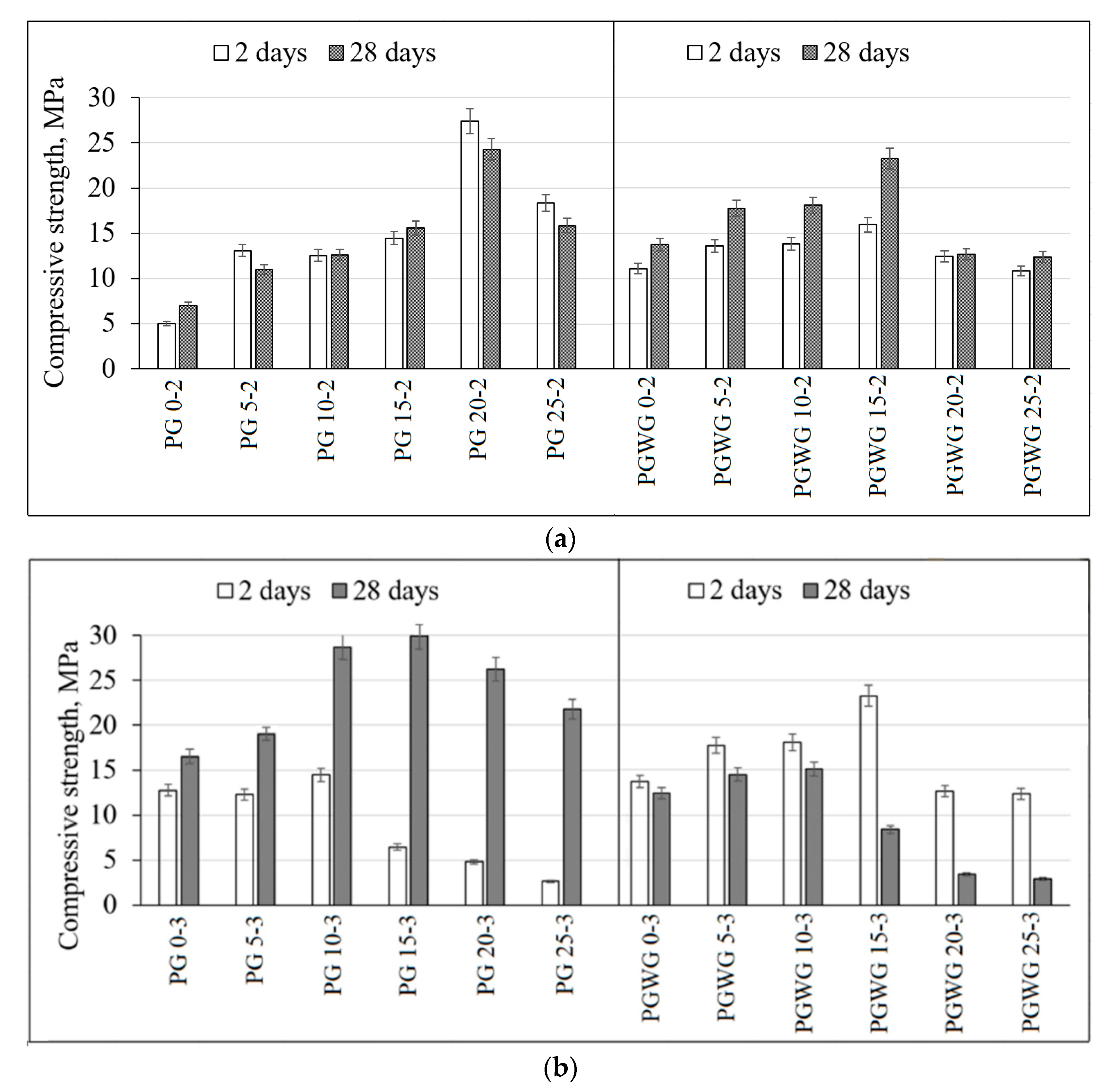
- The higher values of compressive strength were detected when SiO2/Na2O molar ratio was three compared with the samples where samples had SiO2/Na2O = 2. When SiO2/Na2O molar ratio was two, the samples containing 20% PG substitute had the highest compressive strength (24.3 MPa) after 28 days, while compressive strength of the samples with SiO2/Na2O molar ratio three peaked after 28 days at 30.0 MPa;
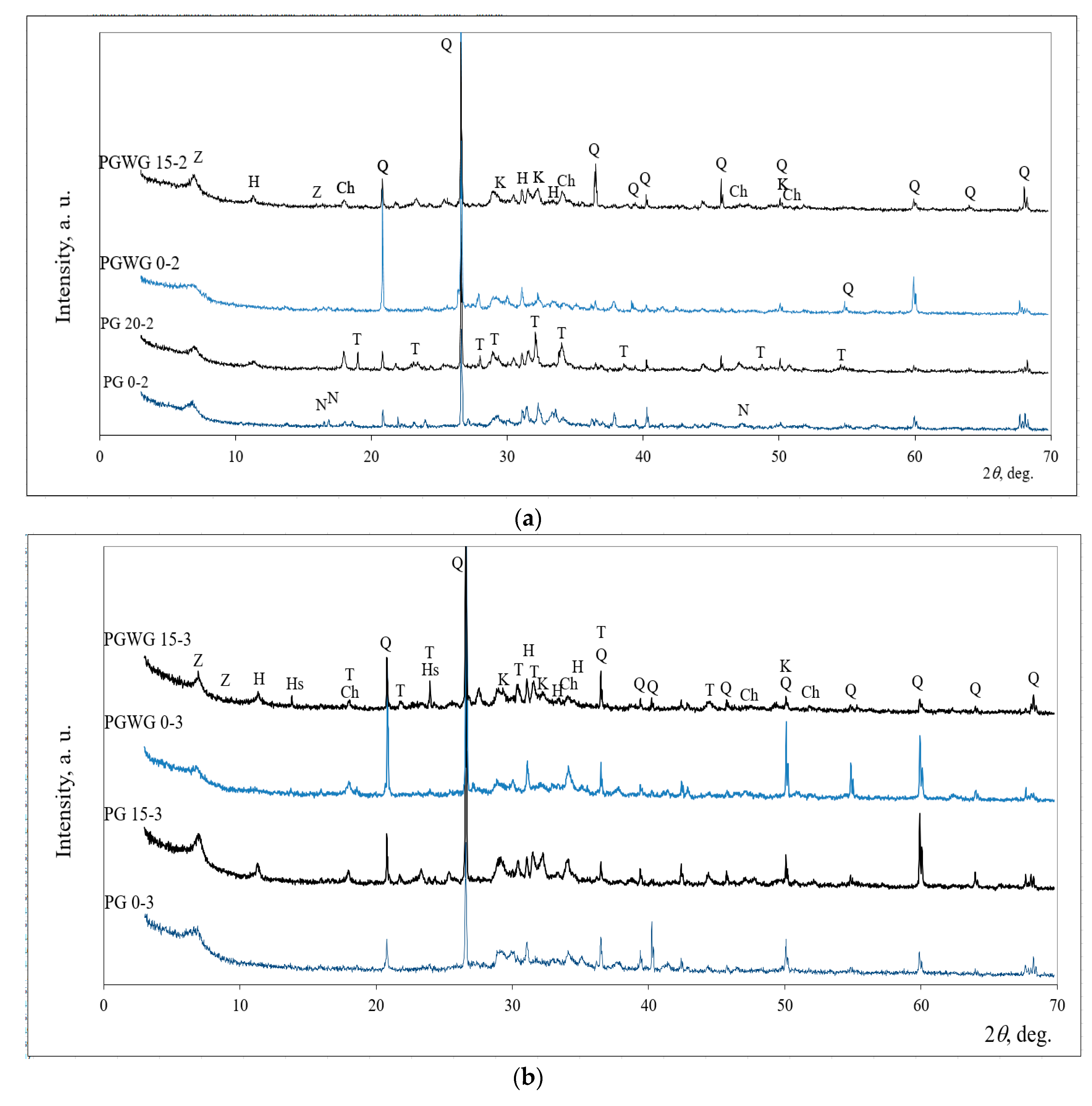
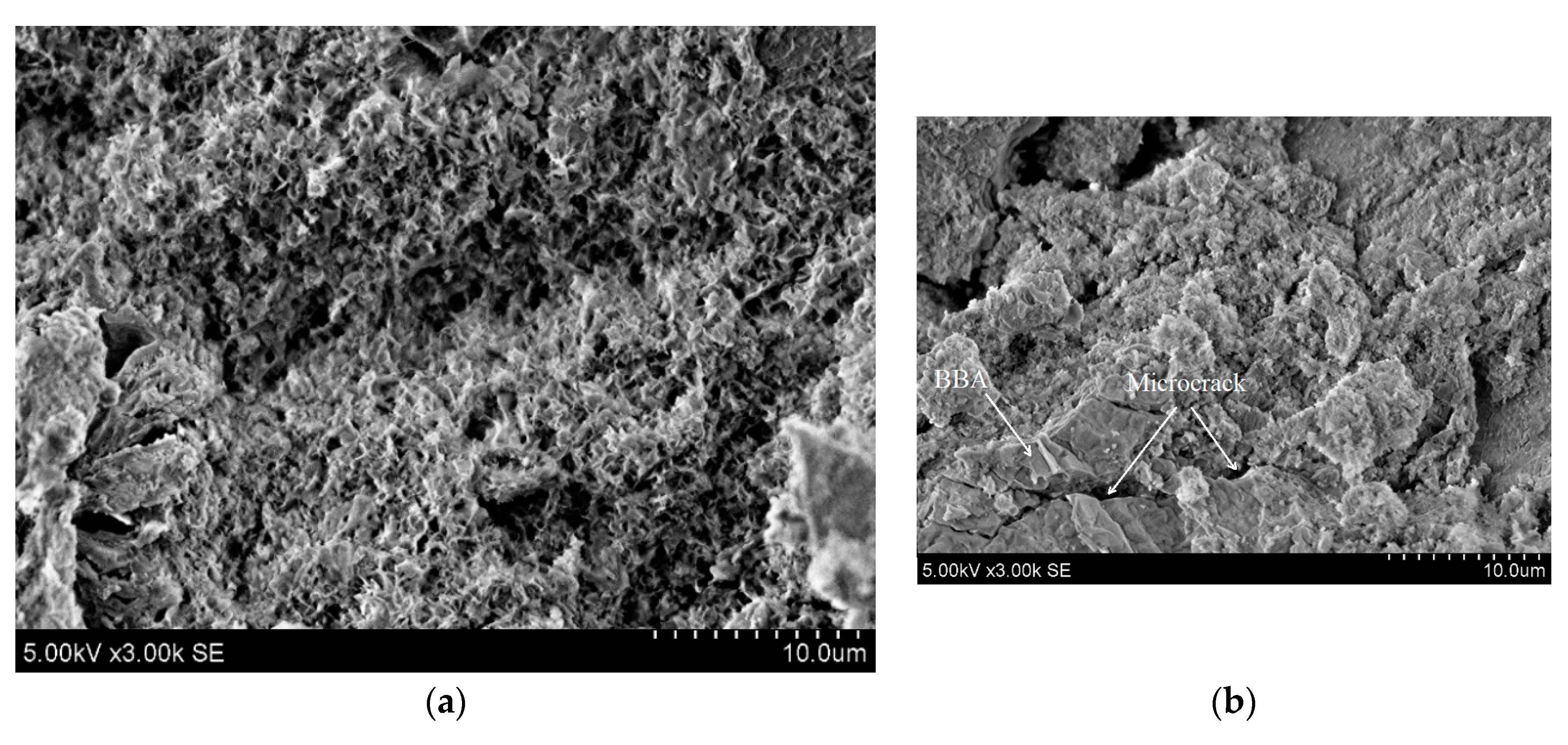
- Overall, paste samples activated with NaOH/Na2SiO3 solution were weaker compared activated with NaOH. The compressive strength peaked at 23.0 MPa after seven days with 15% PG substitute, but after 28 days was reduced to 8.0 MPa. The reduction could be attributed to the cracking caused by the drying shrinkage. In addition, SEM images showed a higher degree of microcracking and unreacted the particle of BBA were detected when NaOH/Na2SiO3 solution was activator;
- The amount of PG substitute was 15%–20% of BBA mass and it is recommended not to exceed 25%. The higher amount of PG significantly reduced the compressive strength;
- In contrary to paste samples, fine-grained concrete samples have shown different tendencies. When BBA with 15% PG was activated with NaOH/Na2SiO3 solution compressive strength reached 15.4 MPa after 28 days, but when the same precursors were activated with NaOH compressive strength was reduced by 16% (12.9 MPa). The higher compressive strength could be related with the higher amount of active silicon;
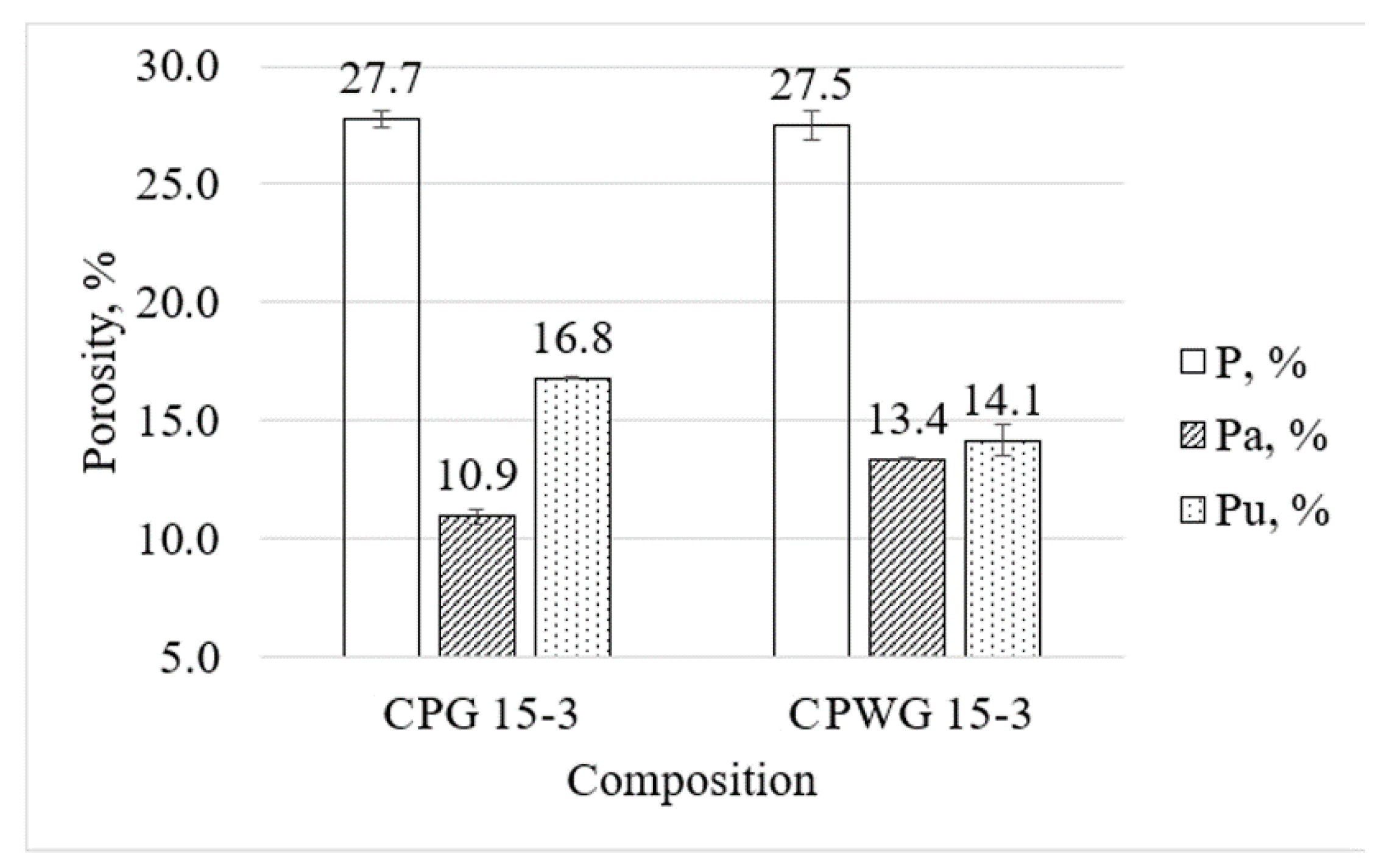
- Even though total porosity of fine-grained concrete activated by NaOH and NaOH/Na2SiO3 solution was nearly the same −27.5% and 27.7% respectfully, there is difference in open and closed porosity. When sodium silicate was present the open porosity was reduced from 16.8% to 14.1%. Hence, the fine-grained concrete activated with the NaOH and Na2SiO3 solution should have higher freeze–thaw resistance;
- After 28 days of hydration the highest compressive strength reached 30.0 MPa the samples activated NaOH solution and by using 15% of BBA substitution to PG. The possibility of potential use of BBA (silicon and aluminum sources) and PG (calcium source) as binder precursor for production of AAMs was confirmed. This can suggest a solution for both alkaline binders industry to provide a new precursor and fertilizers industry to solve the storage problems of PG.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carrasco-Hurtado, B.; Corpas-Iglesias, F.A.; Cruz-Pérez, N.; Terrados-Cepeda, J.; Pérez-Villarejo, L. Addition of bottom ash from biomass in calcium silicate masonry units for use as construction material with thermal insulating properties. Constr. Build. Mater. 2014, 52, 155–165. [Google Scholar] [CrossRef]
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124, 105826. [Google Scholar] [CrossRef]
- Nowoświat, A.; Gołaszewski, J. Influence of the variability of calcareous fly ash properties on rheological properties of fresh mortar with its addition. Materials 2019, 12, 1942. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ming, F.; Li, D.; Chen, L.; Liu, Y. Influence of water content on mechanical strength and microstructure of alkali-activated fly ash/GGBFS mortars cured at cold and polar regions. Materials 2020, 13, 138. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Bonet-Martínez, E.; Eliche-Quesada, D.; Sánchez-Soto, P.J. Biomass bottom ash and aluminium industry slags-based geopolymers. In Vitrification and Geopolimerization of Wastes for Immobilization or Recycling, Proceedings of the Vitrogeowastes, Elche, Spain, 14–15 September 2017; Rincón, J.M., Vidal, M.J., Rincón, J.M., Vidal, M.J., Eds.; Universidad Miguel Hernández: Elche, Spain, 2017; Available online: http://innovacionumh.es/editorial/Vitrogeowastes.pdf (accessed on 15 September 2017).
- Onori, R.; Will, J.; Hoppe, A.; Polettini, A.; Pomi, R.; Boccaccini, A.R. Bottom ash-based geopolymer materials: Mechanical and environmental properties. In Ceramic Engineering and Science Proceedings; Wiley: Hoboken, NJ, USA, 2011; Volume 32, pp. 71–82. [Google Scholar] [CrossRef]
- ul Haq, E.; Padmanabhan, S.K.; Licciulli, A. Synthesis and characteristics of fly ash and bottom ash based geopolymers—A comparative study. Ceram. Int. 2014, 40, 2965–2971. [Google Scholar] [CrossRef]
- Borg, R.P.; Briguglio, C.; Bocullo, V.; Vaičiukynienė, D. Preliminary investigation of geopolymer binder from waste materials. Rom. J. Mater. 2017, 47, 370–378. [Google Scholar]
- Bocullo, V.; Vaičiukynienė, D.; Gečys, R.; Daukšys, M. Effect of ordinary portland cement and water glass on the properties of alkali activated fly ash concrete. J. Miner. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- JSC Lifosa, Ekstrakcinė fosforo rūgštis. Available online: https://www.lifosa.com/lt/ekstrakcine-fosforo-rugstis (accessed on 13 March 2020).
- Mashifana, T.P. Chemical treatment of phosphogypsum and its potential application for building and construction. Proced. Manufact. 2019, 35, 641–648. [Google Scholar] [CrossRef]
- Pérez-López, R.; Macías, F.; Cánovas, C.R.; Sarmiento, A.M.; Pérez-Moreno, S.M. Pollutant flows from a phosphogypsum disposal area to an estuarine environment: An insight from geochemical signatures. Sci. Total Environ. 2016, 553, 42–51. [Google Scholar] [CrossRef]
- Gijbels, K.; Iacobescu, R.I.; Pontikes, Y.; Schreurs, S.; Schroeyers, W. Alkali-activated binders based on ground granulated blast furnace slag and phosphogypsum. Constr. Build. Mater. 2019, 215, 371–380. [Google Scholar] [CrossRef]
- Rashad, A.M. Potential use of phosphogypsum in alkali-activated fly ash under the effects of elevated temperatures and thermal shock cycles. J. Clean. Prod. 2015, 87, 717–725. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Nizevičienė, D.; Kielė, A.; Janavičius, E.; Pupeikis, D. Effect of phosphogypsum on the stability upon firing treatment of alkali-activated slag. Constr. Build. Mater. 2018, 184, 485–491. [Google Scholar] [CrossRef]
- Boonserm, K.; Sata, V.; Pimraksa, K.; Chindaprasirt, P. Improved geopolymerization of bottom ash by incorporating fly ash and using waste gypsum as additive. Cem. Concr. Comp. 2012, 34, 819–824. [Google Scholar] [CrossRef]
- Khater, H.M.; Zedane, S.R. Geopolymerization of industrial by-products and study of their stability upon firing treatment. Int. J. Engineer. Technol. 2012, 2, 308–316. [Google Scholar]
- Chang, J.J.; Yeih, W.; Hung, C.C. Effects of gypsum and phosphoric acid on the properties of sodium silicate-based alkali-activated slag pastes. Cem. Concr. Compos. 2005, 27, 85–91. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Nizevičienė, D.; Šeduikytė, L. Sustainable approach of the utilization of production waste: The use of phosphogypsum and AlF3 production waste in building materials. In Energy Efficient, Sustainable Building Materials and Products; Hager, I., Ed.; Wydawnictwo Politechniki Krakowskiej: Kraków, Poland, 2017; pp. 183–197. [Google Scholar]
- Darsanasiri, A.G.N.D.; Matalkah, F.; Ramli, S.; Al-Jalode, K.; Balachandra, A.; Soroushian, P. Ternary alkali aluminosilicate cement based on rice husk ash, slag and coal fly ash. J. Build. Eng. 2018, 19, 36–41. [Google Scholar] [CrossRef]
- Rajković, M.B.; Tošković, D.V. Phosphogypsum surface characterization using scanning electron microscopy. Acta Period. Technol. 2003, 34, 61–70. [Google Scholar] [CrossRef]
- Bruker, D8 Advance Diffractometer (Bruker AXS) Technical Details. Available online: https://www.bruker.com/products/x-ray-difractionand-elemental-analysis/x-ray-difraction/d8-advance.html (accessed on 13 March 2020).
- Bruker, X-ray S8 Tiger WD Series 2 Technical Details. Available online: https://www.bruker.com/products/x-ray-diffraction-andelemental-analysis/x-ray-fuorescence/s8-tiger.html (accessed on 13 March 2020).
- Zeiss. EVO MA and LS Series Scanning Electron Microscopes for Materials Analysis and Life Science. Operator User Guide, Version 1.0; Zeiss: Cambridge, MA, USA, 2008. [Google Scholar]
- CILAS, 1090 Particle Size Analyzer. Available online: https://www.pharmaceuticalonline.com/doc/cilas-1090-particle-size-analyzer-0002 (accessed on 13 March 2020).
- Girskas, G.; Skripkiūnas, G. The effect of synthetic zeolite on hardened cement paste microstructure and freeze-thaw durability of concrete. Constr. Build. Mater. 2017, 142, 117–127. [Google Scholar] [CrossRef]
- Topçu, İ.B.; Toprak, M.U.; Uygunoğlu, T. Durability and microstructure characteristics of alkali activated coal bottom ash geopolymer cement. J. Clean. Prod. 2014, 81, 211–217. [Google Scholar] [CrossRef]
- Samantasinghar, S.; Singh, S. Effects of curing environment on strength and microstructure of alkali-activated fly ash-slag binder. Constr. Build. Mater. 2020, 235, 117481. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Phoo-ngernkham, T.; Damrongwiriyanupap, N. Comparative study using Portland cement and calcium carbide residue as a promoter in bottom ash geopolymer mortar. Constr. Build. Mater. 2017, 133, 128–134. [Google Scholar] [CrossRef]
- Kovalchuk, G.; Fernández-Jiménez, A.; Palomo, A. Alkali-activated fly ash. Relationship between mechanical strength gains and initial ash chemistry. Mater. De Construcción 2008, 58, 35–52. [Google Scholar] [CrossRef]
- Douglas, E.; Brandstetr, J. A preliminary study on the alkali activation of ground granulated blast-furnace slag. Cem. Concr. Res. 1990, 20, 746–756. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; Monzo, M.; Vincent, M.; Barba, A.; Palomo, A. Alkaline activation of metakaolin—Fly ash mixtures: Obtain of Zeoceramics and Zeocements. Micropor. Mesopor. Mater. 2008, 108, 41–49. [Google Scholar] [CrossRef]
- Karim, M.R.; Hossain, M.M.; Elahi, M.M.A.; Zain, M.F.M. Effects of source materials, fineness and curing methods on the strength development of alkali-activated binder. J. Build. Eng. 2020, 29, 101147. [Google Scholar] [CrossRef]
- Santa, R.A.A.B.; Bernardin, A.M.; Riella, H.G.; Kuhnen, N.C. Geopolymer synthetized from bottom coal ash and calcined paper sludge. J. Clean. Prod. 2013, 57, 302–307. [Google Scholar] [CrossRef]
- Gholampour, A.; Ho, V.D.; Ozbakkaloglu, T. Ambient-cured geopolymer mortars prepared with waste-based sands: Mechanical and durability-related properties and microstructure. Compos. Part. B Eng. 2019, 160, 519–534. [Google Scholar] [CrossRef]
- Ding, Y.C.; Cheng, T.W.; Dai, Y.S. Application of geopolymer paste for concrete repair. Struct. Concr. 2017, 18, 561–570. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Zhang, L.; Liu, X.; Liu, B. Effect of activated silica on polymerization mechanism and strength development of MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2019, 201, 90–99. [Google Scholar] [CrossRef]
- Nagrockienė, D.; Skripkiūnas, G.; Girskas, G. Predicting frost resistance of concrete with different coarse aggregate concentration by porosity parameters. Mater. Sci. 2011, 17, 203–207. [Google Scholar] [CrossRef]

| CaO | SiO2 | Na2O | Al2O3 | MnO | MgO | K2O | Fe2O3 | P2O5 | TiO2 | SO3 | F | Other | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBA | 49.0 | 22.4 | 0.28 | 2.51 | 0.30 | 8.29 | 8.69 | 2.18 | 5.05 | 0.33 | 0.58 | – | 0.39 |
| PG | 38.60 | 0.37 | – | 0.13 | – | 0.04 | – | 0.03 | 0.81 | – | 53.48 | 0.14 | 6.4 |
| Samples | BBA, g | PG, g | WG, g | H2O, g | NaOH, g | SiO2/Al2O3 | SiO2/Na2O |
|---|---|---|---|---|---|---|---|
| PG 0-2 | 100 | 0 | – | 23 | 20.1 | 12.0 | 2 |
| PG 5-2 | 95 | 5 | – | 28 | 19.9 | 12.0 | 2 |
| PG 10-2 | 90 | 10 | – | 29 | 18.8 | 12.0 | 2 |
| PG 15-2 | 85 | 15 | – | 30 | 17.8 | 12.0 | 2 |
| PG 20-2 | 80 | 20 | – | 30 | 16.7 | 12.0 | 2 |
| PG 25-2 | 75 | 25 | – | 30 | 15.7 | 12.0 | 2 |
| PGWG 0-2 | 100 | 0 | 20.0 | 23 | 24.5 | 14.8 | 2 |
| PGWG 5-2 | 95 | 5 | 15.8 | 28 | 22.6 | 14.3 | 2 |
| PGWG 10-2 | 90 | 10 | 14.0 | 29 | 21.2 | 14.2 | 2 |
| PGWG 15-2 | 85 | 15 | 15.7 | 30 | 20.5 | 14.6 | 2 |
| PGWG 20-2 | 80 | 20 | 16.5 | 30 | 19.7 | 14.9 | 2 |
| PGWG 25-2 | 75 | 25 | 19.5 | 30 | 19.4 | 15.6 | 2 |
| PG 0-3 | 100 | 0 | – | 23 | 13.0 | 12.0 | 3 |
| PG 5-3 | 95 | 5 | – | 29 | 12.4 | 12.0 | 3 |
| PG 10-3 | 90 | 10 | – | 30 | 11.7 | 12.0 | 3 |
| PG 15-3 | 85 | 15 | – | 30 | 11.1 | 12.0 | 3 |
| PG 20-3 | 80 | 20 | – | 30 | 10.4 | 12.0 | 3 |
| PG 25-3 | 75 | 25 | – | 30 | 9.8 | 12.0 | 3 |
| PGWG 0-3 | 100 | 0 | 6.4 | 23 | 13.0 | 13.3 | 3 |
| PGWG 5-3 | 95 | 5 | 7.1 | 27 | 12.4 | 13.5 | 3 |
| PGWG 10-3 | 90 | 10 | 6.0 | 28 | 11.7 | 13.3 | 3 |
| PGWG 15-3 | 85 | 15 | 7.3 | 30 | 11.1 | 13.7 | 3 |
| PGWG 20-3 | 80 | 20 | 7.6 | 30 | 10.4 | 13.9 | 3 |
| PGWG 25-3 | 75 | 25 | 9.7 | 30 | 9.8 | 18.4 | 3 |
| Ingredients | The Activation with NaOH | The Activation with NaOH + Na2OnSiO2-mH2O | ||||
|---|---|---|---|---|---|---|
| CPG 0-3 | CPG 15-3 | CPG 20-3 | CPWG 0-3 | CPWG 15-3 | CPWG 20-3 | |
| BBA | 450 | 383 | 360 | 450 | 383 | 360 |
| PG | 0 | 67.5 | 90 | 0 | 67.5 | 90 |
| Sand 0/4 | 1350 | 1350 | 1350 | 1350 | 1350 | 1350 |
| NaOH | 58.5 | 49.7 | 46.8 | 58.5 | 49.7 | 46.8 |
| Water glass | 0 | 0 | 0 | 28.8 | 32.9 | 34.2 |
| Water | 210 | 210 | 210 | 200 | 200 | 200 |
| Samples | Compressive Strength, MPa | Flexural Strength, MPa | Compressive Strength, MPa | Flexural Strength, MPa | Density (After 28 Days), kg/m3 |
|---|---|---|---|---|---|
| After 7 Days of Hardening | After 28 Days of Hardening | ||||
| CPG 0-3 | 4.51 ± 0.26 | 1.52 ± 0.05 | 10.94 ± 0.48 | 2.30 ± 0.12 | 1965 ± 14 |
| CPG 15-3 | 4.79 ± 0.23 | 2.22 ± 0.06 | 12.90 ± 0.45 | 2.31 ± 0.15 | 1971 ± 14 |
| CPG 20-3 | 3.94 ± 0.22 | 1.39 ± 0.06 | 9.33 ± 0.41 | 2.00 ± 0.17 | 1853 ± 15 |
| CPWG 0-3 | 6.42 ± 0.28 | 1.32 ± 0.05 | 10.73 ± 0.42 | 1.84 ± 0.16 | 1998 ± 15 |
| CPWG 15-3 | 8.40 ± 0.24 | 2.18 ± 0.08 | 15.42 ± 0.43 | 2.41 ± 0.11 | 2019 ± 14 |
| CPWG 20-3 | 6.20 ± 0.27 | 1.20 ± 0.07 | 11.94 ± 0.31 | 1.54 ± 0.13 | 1885 ± 13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaičiukynienė, D.; Nizevičienė, D.; Kantautas, A.; Bocullo, V.; Kielė, A. Alkali Activated Paste and Concrete Based on of Biomass Bottom Ash with Phosphogypsum. Appl. Sci. 2020, 10, 5190. https://doi.org/10.3390/app10155190
Vaičiukynienė D, Nizevičienė D, Kantautas A, Bocullo V, Kielė A. Alkali Activated Paste and Concrete Based on of Biomass Bottom Ash with Phosphogypsum. Applied Sciences. 2020; 10(15):5190. https://doi.org/10.3390/app10155190
Chicago/Turabian StyleVaičiukynienė, Danutė, Dalia Nizevičienė, Aras Kantautas, Vytautas Bocullo, and Andrius Kielė. 2020. "Alkali Activated Paste and Concrete Based on of Biomass Bottom Ash with Phosphogypsum" Applied Sciences 10, no. 15: 5190. https://doi.org/10.3390/app10155190
APA StyleVaičiukynienė, D., Nizevičienė, D., Kantautas, A., Bocullo, V., & Kielė, A. (2020). Alkali Activated Paste and Concrete Based on of Biomass Bottom Ash with Phosphogypsum. Applied Sciences, 10(15), 5190. https://doi.org/10.3390/app10155190