Zeolite-Supported Ni Catalysts for CO2 Methanation: Effect of Zeolite Structure and Si/Al Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Characterization
2.3. Catalytic Activity
3. Results and Discussion
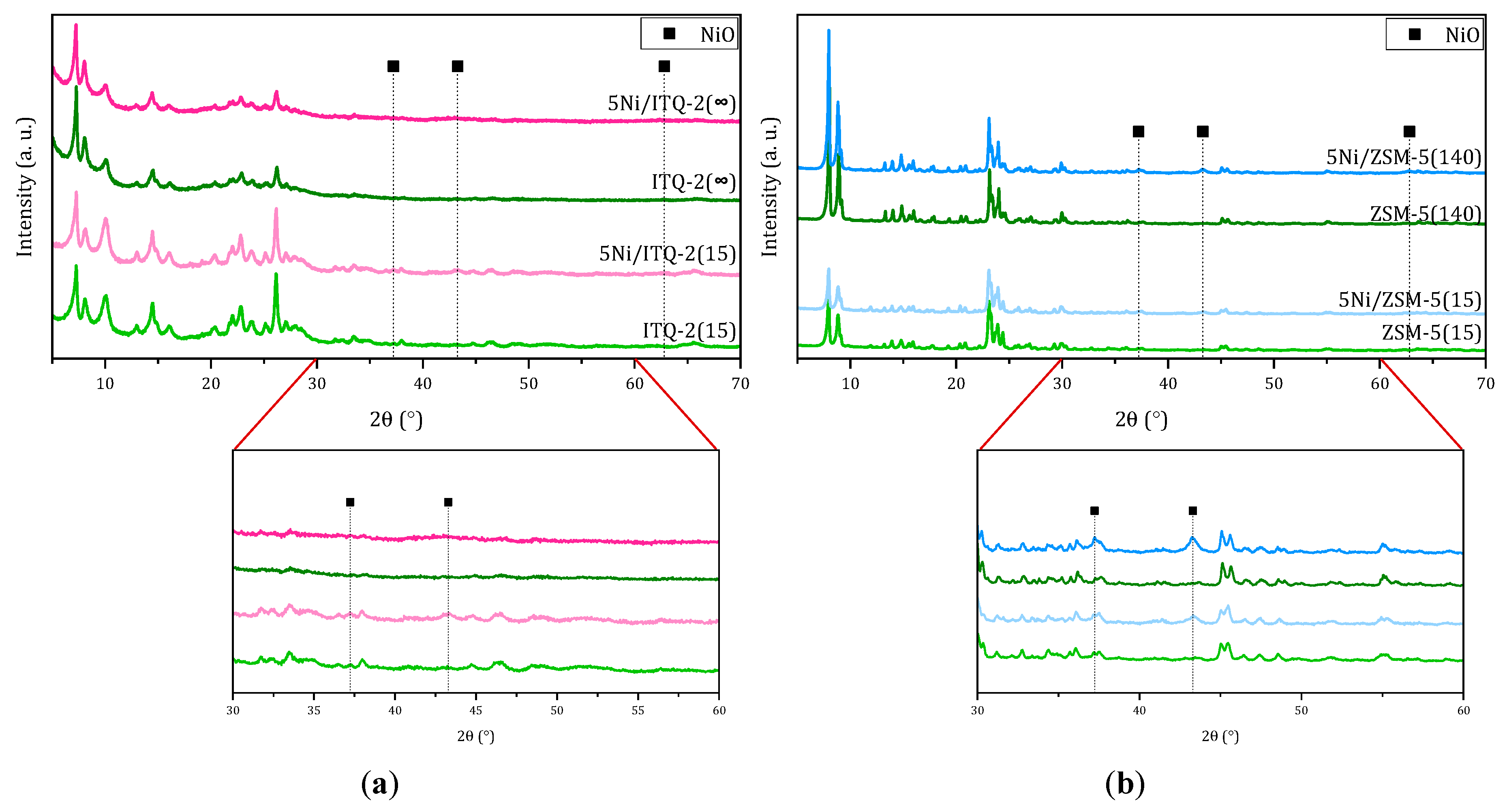
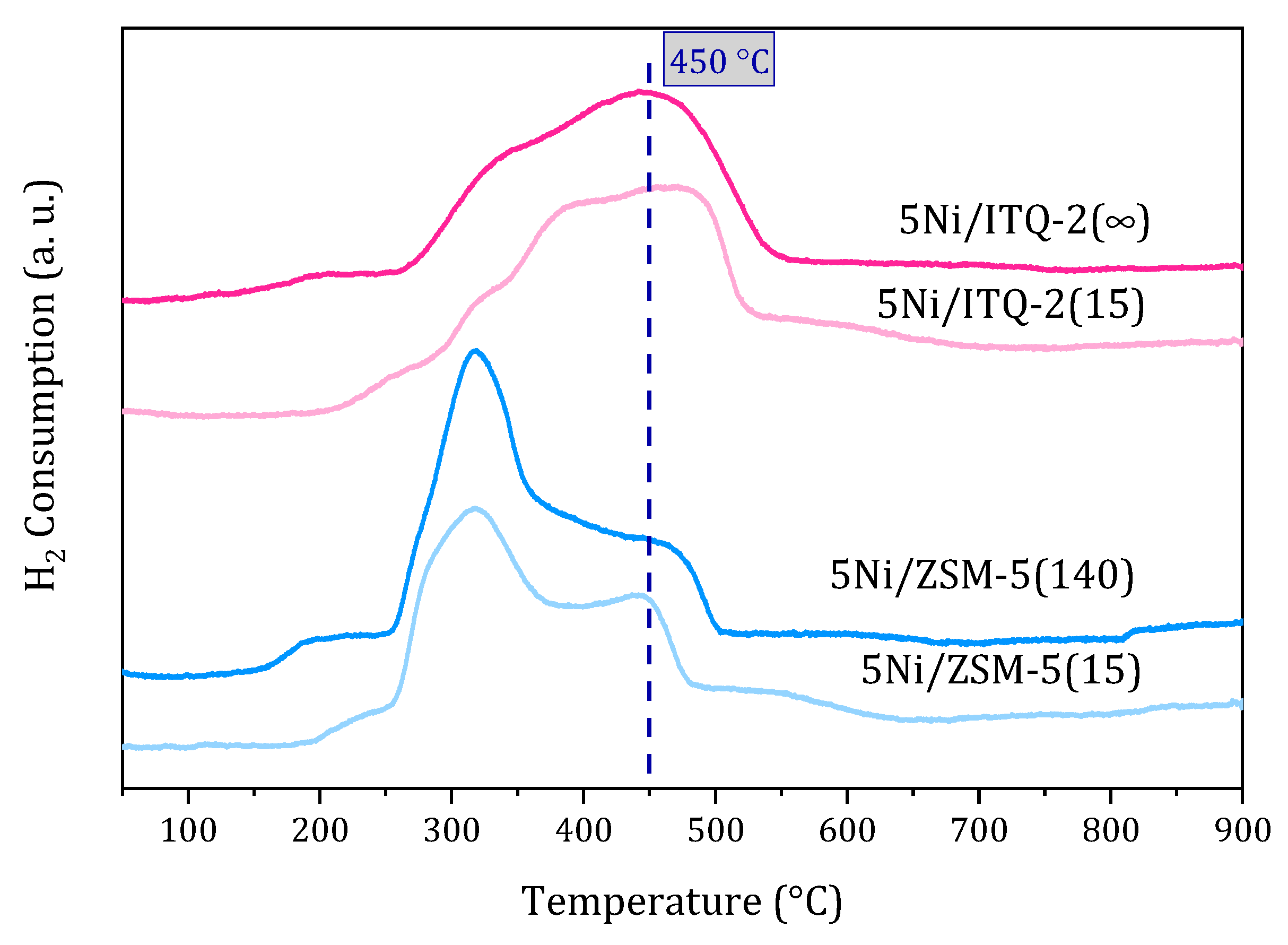
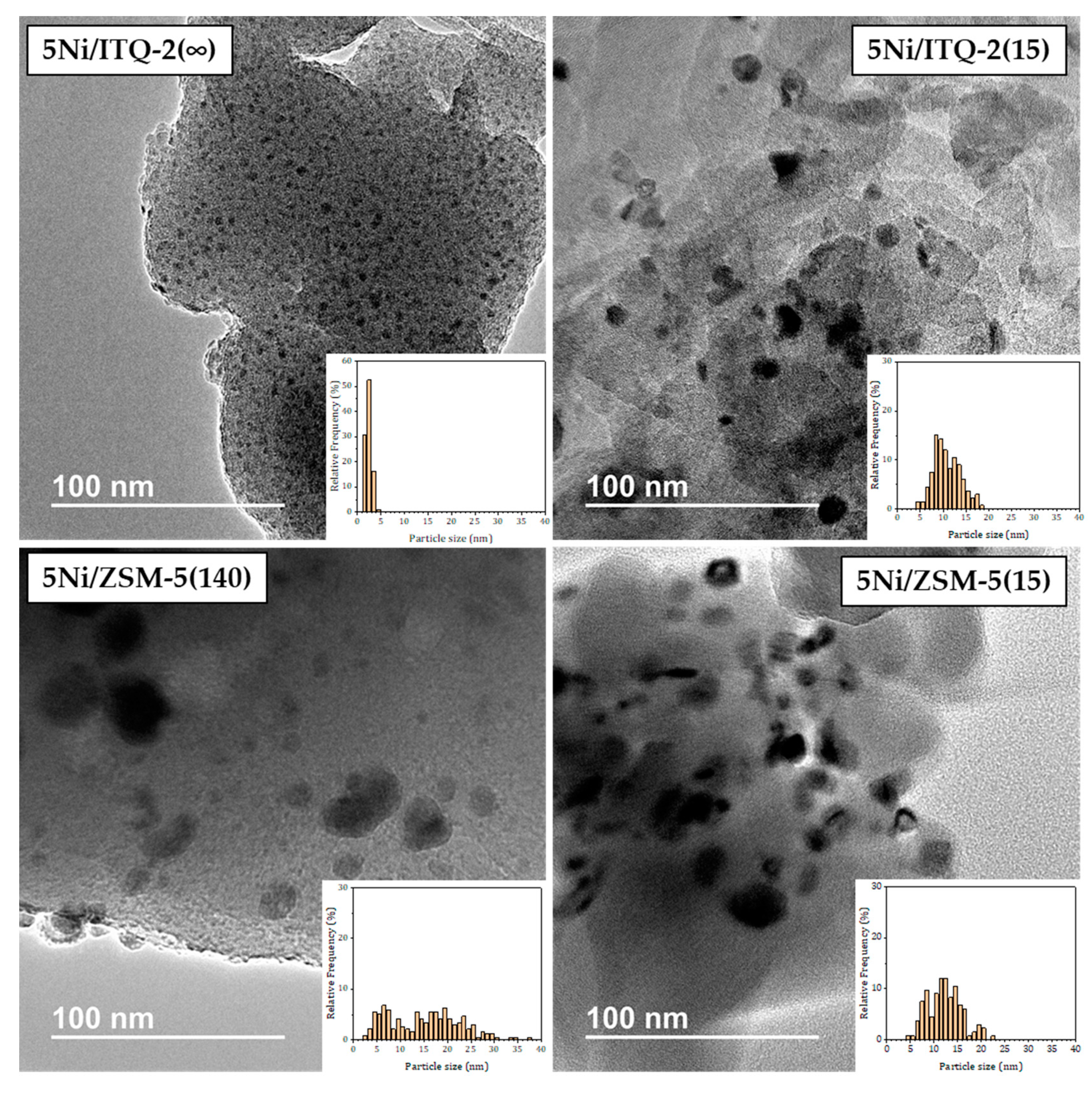
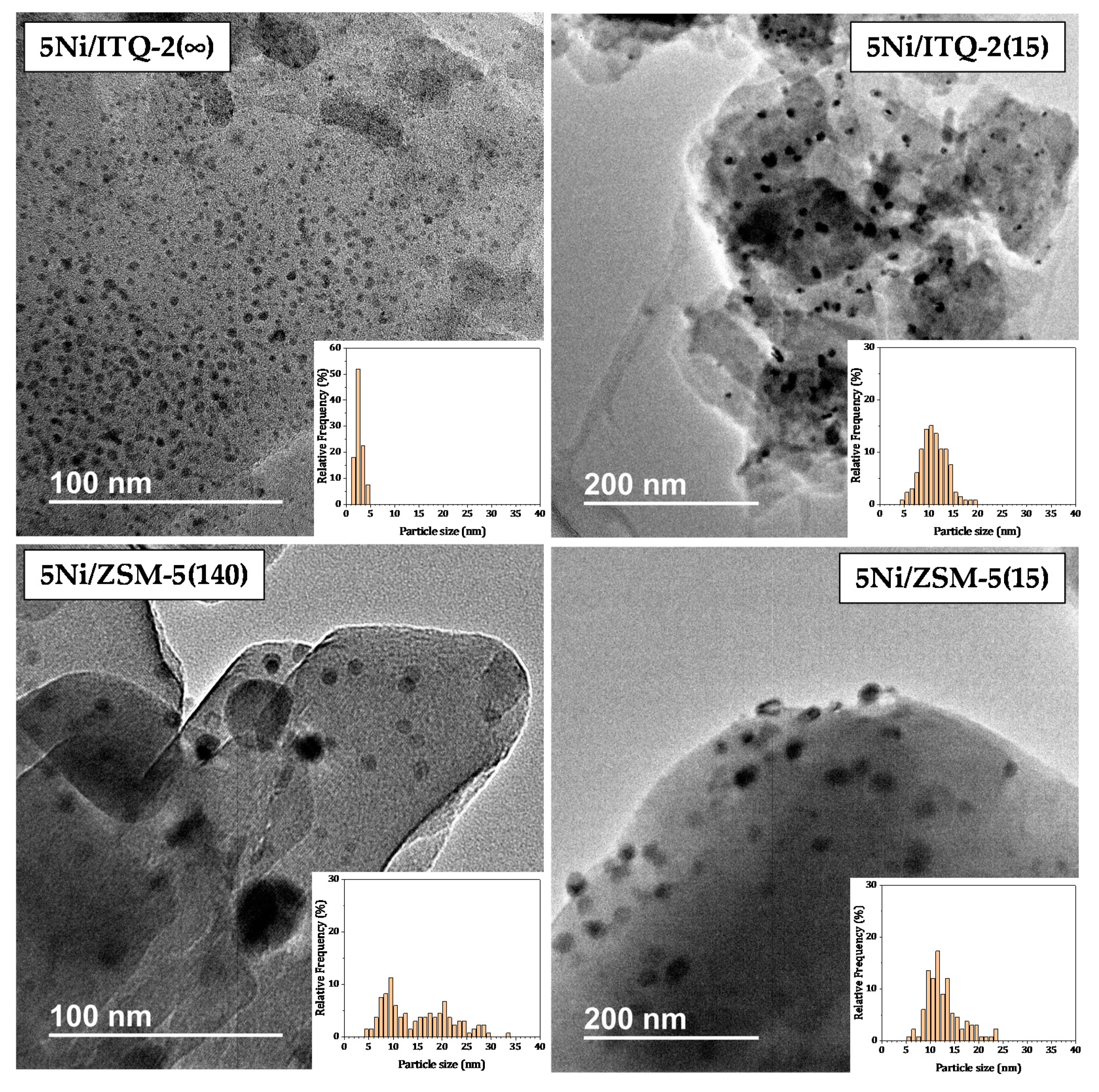
3.1. Characterization
3.2. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Available online: https://www.ipcc.ch/report/ar5/syr/ (accessed on 26 May 2020).
- Le Quéré, C.; Jackson, R.B.; Jones, M.W.; Smith, A.J.P.; Abernethy, S.; Andrew, R.M.; De-Gol, A.J.; Willis, D.R.; Shan, Y.; Canadell, J.G.; et al. Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nat. Clim. Chang. 2020, 19, 1–7. [Google Scholar]
- Muhammad, S.; Long, X.; Salman, M. COVID-19 pandemic and environmental pollution: A blessing in disguise? Sci. Total Environ. 2020, 728, 138820. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Monserrate, M.A.; Ruano, M.A.; Sanchez-Alcalde, L. Indirect effects of COVID-19 on the environment. Sci. Total Environ. 2020, 728, 138813. [Google Scholar] [CrossRef] [PubMed]
- United Nations Framework Convention on Climate Change: The Paris Agreement. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 26 May 2020).
- IPCC: Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. Available online: https://www.ipcc.ch/sr15/download/#full (accessed on 26 May 2020).
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Sahebdelfar, S.; Takht Ravanchi, M. Carbon dioxide utilization for methane production: A thermodynamic analysis. J. Petrol. Sci. Eng. 2015, 134, 14–22. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported catalysts for CO2 methanation: A review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Recent trend in thermal catalytic low temperature CO2 methanation: A critical review. Available online: https://doi.org/10.1016/j.cattod.2020.02.017 (accessed on 24 July 2020).
- Younas, M.; Loong Kong, L.; Bashir, M.J.K.; Nadeem, H.; Shehzad, A.; Sethupathi, S. Recent Advancements, Fundamental Challenges, and Opportunities in Catalytic Methanation of CO2. Energy & Fuels 2016, 30, 8815–8831. [Google Scholar]
- Lu, X.; Gu, F.; Liu, Q.; Gao, J.; Liu, Y.; Li, H.; Jia, L.; Xu, G.; Zhong, Z.; Su, F. VOx promoted Ni catalysts supported on the modified bentonite for CO and CO2 methanation. Fuel Process. Technol. 2015, 135, 34–46. [Google Scholar] [CrossRef]
- Guilera, J.; del Valle, J.; Alarcón, A.; Díaz, J.A.; Andreu, T. Metal-oxide promoted Ni/Al2O3 as CO2 methanation micro-size catalysts. J. CO2 Util. 2019, 30, 11–17. [Google Scholar] [CrossRef]
- Quindimil, A.; De-La-Torre, U.; Pereda-Ayo, B.; González-Marcos, J.A.; González-Velasco, J.R. Ni catalysts with La as promoter supported over Y- and BETA- zeolites for CO2 methanation. Appl. Catal. B-Environ. 2018, 238, 393–403. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Kim, T.W.; Park, E.D. CO and CO2 methanation over supported Ni catalysts. Catal. Today 2017, 293–294, 89–96. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Mukti, R.R.; Taufiq-Yap, Y.H.; Sazegar, M.R. Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl. Catal. B-Environ. 2014, 147, 359–368. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Lopes, J.M.; Henriques, C. Tuning zeolite properties towards CO2 methanation: An overview. ChemCatChem 2019, 11, 2388–2400. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Westermann, A.; Ribeiro, M.F.; Lopes, J.M.; Henriques, C. CO2 Hydrogenation Over Ni-Based Zeolites: Effect of Catalysts Preparation and Pre-reduction Conditions on Methanation Performance. Top. Catal. 2016, 59, 314–325. [Google Scholar] [CrossRef]
- Influence of nickel precursors on the properties and performance of Ni impregnated zeolite 5A and 13X catalysts in CO2 methanation. Available online: https://doi.org/10.1016/j.cattod.2020.05.025 (accessed on 24 July 2020).
- Bacariza, M.C.; Graça, I.; Bebiano, S.S.; Lopes, J.M.; Henriques, C. Magnesium as Promoter of CO2 Methanation on Ni-Based USY Zeolites. Energy & Fuels 2017, 31, 9776–9789. [Google Scholar]
- Bacariza, M.C.; Graça, I.; Lopes, J.M.; Henriques, C. Ni-Ce/Zeolites for CO2 Hydrogenation to CH4: Effect of the Metal Incorporation Order. ChemCatChem 2018, 10, 2773–2781. [Google Scholar] [CrossRef]
- Graça, I.; González, L.V.; Bacariza, M.C.; Fernandes, A.; Henriques, C.; Lopes, J.M.; Ribeiro, M.F. CO2 hydrogenation into CH4 on NiHNaUSY zeolites. Appl. Catal. B-Environ. 2014, 147, 101–110. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Maleval, M.; Graça, I.; Lopes, J.M.; Henriques, C. Power-to-methane over Ni/zeolites: Influence of the framework type. Microporous Mesoporous Mater. 2019, 274, 102–112. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Lopes, J.M.; Henriques, C. Enhanced activity of CO2 hydrogenation to CH4 over Ni based zeolites through the optimization of the Si/Al ratio. Microporous Mesoporous Mater. 2018, 267, 9–19. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Bértolo, R.; Graça, I.; Lopes, J.M.; Henriques, C. The effect of the compensating cation on the catalytic performances of Ni/USY zeolites towards CO2 methanation. J. CO2 Util. 2017, 21, 280–291. [Google Scholar] [CrossRef]
- Nicolopoulos, S.; González-Calbet, J.M.; Vallet-Regi, M.; Camblor, M.A.; Corell, C.; Corma, A.; Diaz-Cabañas, M.J. Use of Electron Microscopy and Microdiffraction for Zeolite Framework Comparison. J. Am. Chem. Soc. 1997, 119, 11000–11005. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Uvarov, V.; Popov, I. Metrological characterization of X-ray diffraction methods for determination of crystallite size in nano-scale materials. Mater. Charact. 2007, 58, 883–891. [Google Scholar] [CrossRef]
- Concepción, P.; López, C.; Martínez, A.; Puntes, V.F. Characterization and catalytic properties of cobalt supported on delaminated ITQ-6 and ITQ-2 zeolites for the Fischer—Tropsch synthesis reaction. J. Catal. 2004, 228, 321–332. [Google Scholar] [CrossRef]
- Da Costa-Serra, J.F.; Navarro, M.T.; Rey, F.; Chica, A. Bioethanol steam reforming on Ni-based modified mordenite. Effect of mesoporosity, acid sites and alkaline metals. Int. J. Hydrog. Energy 2012, 37, 7101–7108. [Google Scholar] [CrossRef]
- Mariscal, R.; Navarro, R.M.; Pawelec, B.; Fierro, J.L.G. Factors affecting Ni-sulfide formation in Y-type zeolites: A combined Fourier transform infrared and X-ray photoelectron spectroscopy study. Microporous Mesoporous Mater. 2000, 34, 181–194. [Google Scholar] [CrossRef]
- Romero, M.D.; Calles, J.A.; Rodríguez, A.; Cabanelas, J.C. The Influence of Calcination Treatment over Bifunctional Ni/HZSM-5 Catalysts. Ind. Eng. Chem. Res. 1998, 37, 3846–3852. [Google Scholar] [CrossRef]
- Frontera, P.; Testa, F.; Aiello, R.; Candamano, S.; Nagy, J.B. Transformation of MCM-22(P) into ITQ-2: The role of framework aluminium. Microporous Mesoporous Mater. 2007, 106, 107–114. [Google Scholar] [CrossRef]
- Chica, A.; Sayas, S. Effective and stable bioethanol steam reforming catalyst based on Ni and Co supported on all-silica delaminated ITQ-2 zeolite. Catal. Today 2009, 146, 37–43. [Google Scholar] [CrossRef]
- Maia, A.J.; Louis, B.; Lam, Y.L.; Pereira, M.M. Ni-ZSM-5 catalysts: Detailed characterization of metal sites for proper catalyst design. J. Catal. 2010, 269, 103–109. [Google Scholar] [CrossRef]
- Mohan, V.; Raghavendra, C.; Pramod, C.V.; Raju, B.D.; Rama Rao, K.S. Ni/H-ZSM-5 as a promising catalyst for vapour phase hydrogenation of levulinic acid at atmospheric pressure. RSC Adv. 2014, 4, 9660–9668. [Google Scholar] [CrossRef]
- Martínez, A.; Peris, E.; Sastre, G. Dehydroaromatization of methane under non-oxidative conditions over bifunctional Mo/ITQ-2 catalysts. Catal. Today 2005, 107–108, 676–684. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Ott, K.C.; Borup, R.L.; Greene, H.L. Role of acidity on the hydrolysis of dimethyl ether (DME) to methanol. Appl. Catal. B-Environ. 2005, 61, 281–287. [Google Scholar] [CrossRef]
- Yonli, A.H.; Batonneau-Gener, I.; Koulidiati, J. Adsorptive removal of α-endosulfan from water by hydrophobic zeolites. An isothermal study. J. Hazard. Mater. 2012, 203–204, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Yonli, A.H.; Gener, I.; Mignard, S. Comparative study of the hydrophobicity of BEA, HZSM-5 and HY zeolites determined by competitive adsorption. Microporous Mesoporous Mater. 2010, 132, 37–42. [Google Scholar] [CrossRef]
- Borgschulte, A.; Gallandat, N.; Probst, B.; Suter, R.; Callini, E.; Ferri, D.; Arroyo, Y.; Erni, R.; Geerlings, H.; Züttel, A. Sorption enhanced CO2 methanation. Phys. Chem. Chem. Phys. 2013, 15, 9620–9625. [Google Scholar] [CrossRef]
- Changkhamchom, S.; Sirivat, A. High proton conductivity ZSM-5/sulfonated poly(ether ketone ether sulfone) (S-PEKES) composite proton exchange membrane for using in direct methanol fuel cell. Solid State Ionics 2014, 263, 161–166. [Google Scholar] [CrossRef]
- Rossetti, I.; Compagnoni, M.; Finocchio, E.; Ramis, G.; Di Michele, A.; Zucchini, A.; Dzwigaj, S. Syngas production via steam reforming of bioethanol over Ni—BEA catalysts: A BTL strategy. Int. J. Hydrog. Energy 2016, 41, 16878–16889. [Google Scholar] [CrossRef]
- Guo, X.; Traitangwong, A.; Hu, M.; Zuo, C.; Meeyoo, V.; Peng, Z.; Li, C. Carbon Dioxide Methanation over Nickel-Based Catalysts Supported on Various Mesoporous Material. Energy & Fuels 2018, 32, 3681–3689. [Google Scholar]


| Sample | Si/Al Ratio 1 | Ni Content 1 (wt.%) | SBET (m2/g) | Sexternal (m2/g) | Smicropore (m2/g) |
|---|---|---|---|---|---|
| ITQ-2(∞) | 712 | 528 | 185 | ||
| 5Ni/ITQ-2(∞) | 5.2 | 579 | 458 (−13%) | 120 (−35%) | |
| ITQ-2(15) | 10 | 610 | 284 | 325 | |
| 5Ni/ITQ-2(15) | 10 | 5.7 | 520 | 213 (−25%) | 307 (−6%) |
| ZSM-5(140) | 117 | 394 | 43 | 351 | |
| 5Ni/ZSM-5(140) | 117 | 5.7 | 368 | 37 (−14%) | 331 (−6%) |
| ZSM-5(15) | 14 | 429 | 40 | 389 | |
| 5Ni/ZSM-5(15) | 15 | 5.3 | 373 | 40 (0%) | 333 (−14%) |
| Sample | Brønsted Acidity (mmol Pyridine/gcat) | ||
|---|---|---|---|
| 150 °C | 250 °C | 350 °C | |
| 5Ni/ITQ-2(∞) | 0.000 | 0.000 | 0.000 |
| 5Ni/ITQ-2(15) | 0.072 | 0.026 | 0.000 |
| 5Ni/ZSM-5(140) | 0.059 | 0.047 | 0.037 |
| 5Ni/ZSM-5(15) | 0.260 | 0.240 | 0.177 |
| Sample1 | NiO Size (nm) | Ni0 Size (nm) | GHSV | Temperature (°C) | CO2 Conversion (%) | CH4 Selectivity (%) | x2 | Ref. |
|---|---|---|---|---|---|---|---|---|
| 5Ni/ITQ-2(∞) | <3 | 9000 mL/(gcat·h) 3500 h−1 | 250 | 6 | 97 | 154 | This work | |
| 300 | 28 | 97 | 718 | |||||
| 350 | 68 | 99 | 1744 | |||||
| 400 | 82 | 99 | 2103 | |||||
| 5Ni13X-Cit | 13,333 mL/(gcat·h) | 240 | 17 | 71 | 378 | [20] | ||
| 10Ni/ZSM-5 | 14.3 | 2000 h−1 | 400 | 76 | ≈75 | [45] | ||
| 10Ni/Na-BETA | 20.1 | 10,000 h−1 | 350 | 33 | 88 | 660 | [15] | |
| 10Ni-10La2O3/Na-BETA | 8.7 | 10,000 h−1 | 350 | 65 | 99 | 1300 | [15] | |
| 14Ni7Ce/USY | 15,000 h−1 | 400 | 77 | 99 | 1023 | [23] | ||
| 14Ni7Ce/USY | 43,000 h−1 | 400 | 68 | 95 | 2600 | [23] | ||
| 15Ni/Na-USY | 18 | 25 | 43,000 h−1 | 350 | 55 | 95 | [24] | |
| 15Ni/Na-BEA | 16 | 18 | 43,000 h−1 | 350 | 43 | 92 | [24] | |
| 15Ni/Na-ZSM-5 | 21 | 24 | 43,000 h−1 | 350 | 40 | 85 | [24] | |
| 15Ni/Na-MOR | 26 | 23 | 43,000 h−1 | 350 | 40 | 87 | [24] | |
| 15Ni-20Ce/Cs-USY | 43,000 h−1 | 350 | ≈70 | ≈98 | [22] | |||
| 5Ni/Na-USY | 19.4 | 43,000 h−1 | 400 | ≈20 | ≈60 | [22] | ||
| 5Ni/3Ce/Na-USY | 13.3 | 43,000 h−1 | 400 | ≈34 | ≈70 | [22] | ||
| 3Ce/5Ni/Na-USY | 2.5 | 43,000 h−1 | 400 | ≈38 | ≈75 | [22] | ||
| 5Ni-3Ce/Na-USY | 2.4 | 43,000 h−1 | 400 | ≈40 | ≈80 | [22] | ||
| 9Mg 13Ni/USY | 43,000 h−1 | 400 | ≈60 | ≈95 | [21] | |||
| 5Ni/MSN | 9.9 | 50,000 mL/(gcat·h) | 300 | 64 | 6400 | [17] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa-Serra, J.F.; Cerdá-Moreno, C.; Chica, A. Zeolite-Supported Ni Catalysts for CO2 Methanation: Effect of Zeolite Structure and Si/Al Ratio. Appl. Sci. 2020, 10, 5131. https://doi.org/10.3390/app10155131
da Costa-Serra JF, Cerdá-Moreno C, Chica A. Zeolite-Supported Ni Catalysts for CO2 Methanation: Effect of Zeolite Structure and Si/Al Ratio. Applied Sciences. 2020; 10(15):5131. https://doi.org/10.3390/app10155131
Chicago/Turabian Styleda Costa-Serra, Javier Francisco, Cristina Cerdá-Moreno, and Antonio Chica. 2020. "Zeolite-Supported Ni Catalysts for CO2 Methanation: Effect of Zeolite Structure and Si/Al Ratio" Applied Sciences 10, no. 15: 5131. https://doi.org/10.3390/app10155131
APA Styleda Costa-Serra, J. F., Cerdá-Moreno, C., & Chica, A. (2020). Zeolite-Supported Ni Catalysts for CO2 Methanation: Effect of Zeolite Structure and Si/Al Ratio. Applied Sciences, 10(15), 5131. https://doi.org/10.3390/app10155131




