Developments in the Use of Lipase Transesterification for Biodiesel Production from Animal Fat Waste
Abstract
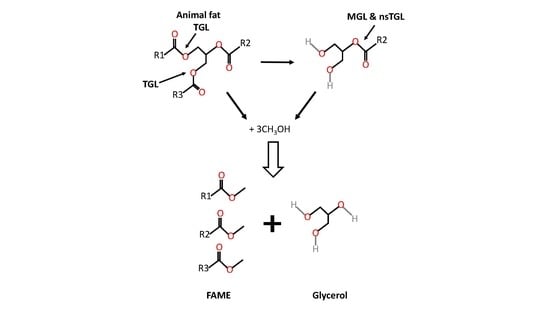
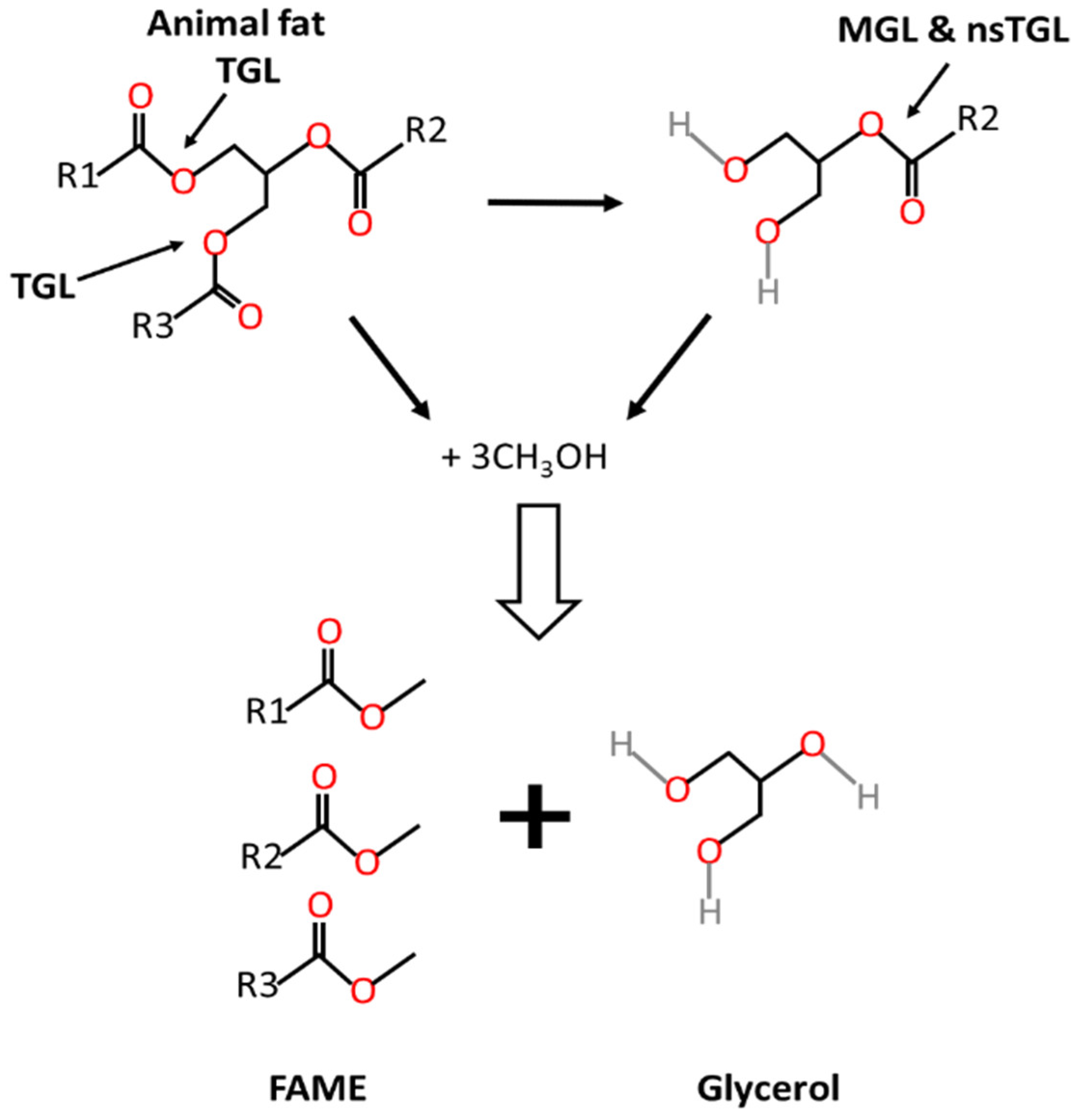
1. Introduction
2. Mechanisms of Action of Lipases
3. Sources of Lipases
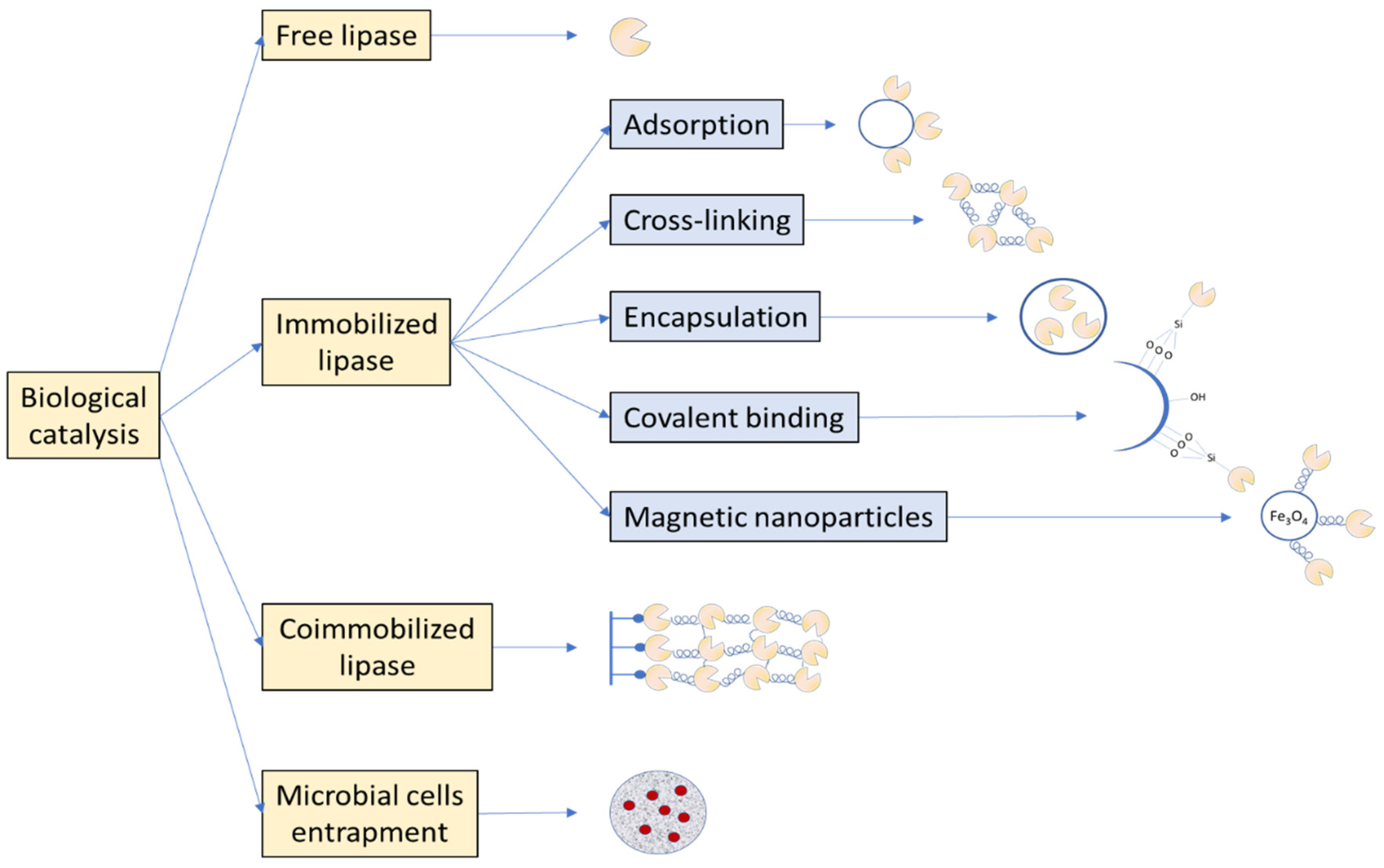
4. Free Lipase
5. Immobilized Lipase
5.1. Types of Supports and Immobilization Procedures
5.2. Magnetic Nanocarriers
5.3. Coimmobilization
6. Industrial Applications of Lipase-Catalyzed Biodiesel
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosson, E.; Sgarbossa, P.; Pedrielli, F.; Mozzon, M.; Bertani, R. Bioliquids from raw waste animal fats: An alternative renewable energy source. Biomass Convers. Biorefin. 2020, 1–16. [Google Scholar] [CrossRef]
- EFPRA. Rendering in Numbers. 2016. Available online: https://efpra.eu/wp-content/uploads/2016/11/Rendering-in-numbers-Infographic.pdf (accessed on 20 March 2020).
- Toldrá, F.; Mora, L.; Reig, M.; Mora, L. New insights into meat by-product utilization. Meat Sci. 2016, 120, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Akhil, U.S.; Alagumalai, A. A Short Review on Valorization of Slaughterhouse Wastes for Biodiesel Production. ChemistrySelect 2019, 4, 13356–13362. [Google Scholar] [CrossRef]
- Barik, D.; Vijayaraghavan, R. Effects of waste chicken fat derived biodiesel on the performance and emission characteristics of a compression ignition engine. Int. J. Ambient Energy 2018, 41, 88–97. [Google Scholar] [CrossRef]
- Prates, J.; Alfaia, C.; Alves, S.; Bessa, R. Fatty acids. In Handbook of Analysis of Edible Animal by-Products; Nollet, L.M.L., Toldrá, F., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 137–159. [Google Scholar]
- Mora, L.; Toldrá-Reig, F.; Prates, J.A.M.; Toldrá, F. Cattle by-products. In Byproducts from Agriculture and Fisheries: Adding Value for Food, Feed, Pharma and Fuels; Simpson, B.K., Aryee, A.N., Toldrá, F., Eds.; Wiley: Chichester, UK, 2020; pp. 43–55. [Google Scholar]
- Baladincz, P.; Hancsók, J. Fuel from waste animal fats. Chem. Eng. J. 2015, 282, 152–160. [Google Scholar] [CrossRef]
- Mora, L.; Toldrá-Reig, F.; Reig, M.; Toldrá, F. Possible uses of processed slaughter by-products. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press/Elsevier: London, UK, 2019; pp. 145–160. [Google Scholar]
- Banković-Ilić, I.B.; Stojković, I.J.; Stamenković, O.S.; Veljkovic, V.B.; Hung, Y.-T. Waste animal fats as feed stocks for biodiesel production. Renew. Sustain. Energy Rev. 2014, 32, 238–254. [Google Scholar] [CrossRef]
- Ramos, M.; Dias, A.P.S.; Puna, J.F.; Gomes, J.; Bordado, J.C. Biodiesel Production Processes and Sustainable Raw Materials. Energies 2019, 12, 4408. [Google Scholar] [CrossRef]
- Flach, B.; Lieberz, S.; Bolla, S. EU Biofuels Annual 2019, Gain Report NL9022, USDA Foreign Agricultural Service. 2019. Available online: http://gain.fas.usda.gov/Pages/Default.aspx (accessed on 6 May 2020).
- US Energy Information Administration. Monthly Biodiesel Production Report; US Department of Energy: Washington, DC, USA, 2020. Available online: https://www.eia.gov/biofuels/biodiesel/production/biodiesel.pdf (accessed on 5 May 2020).
- IPPR. Time for Change: A New Vision for the British Economy—The Interim Report of the IPPR Commission on Economic Justice. 2017. Available online: http://www.ippr.org/cej-time-for-change (accessed on 25 March 2020).
- IRENA. Global Energy Transformation: A Roadmap to 2050. 2018. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2018/Apr/IRENA_Report_GET_2018.pdf (accessed on 14 April 2020).
- Cernat, A.; Pana, C.; Negurescu, N.; Lazaroiu, G.; Nutu, C.; Fuiorescu, D.; Toma, M.; Nicolici, A. Combustion of preheated raw animal fats-diesel fuel blends at diesel engine. J. Therm. Anal. Calorim. 2019, 140, 2369–2375. [Google Scholar] [CrossRef]
- Toldrá-Reig, F.; Mora, L.; Toldrá, F. Trends in biodiesel production from animal fat waste. Appl. Sci. 2020, 10, 3644. [Google Scholar]
- Lawan, I.; Garba, Z.N.; Zhou, W.; Zhang, M.; Yuan, Z. Synergies between the microwave reactor and CaO/zeolite catalyst in waste lard biodiesel production. Renew. Energy 2020, 145, 2550–2560. [Google Scholar] [CrossRef]
- Bušić, A.; Kundas, S.; Morzak, G.; Belskaya, H.; Marđetko, N.; Santek, M.I.; Komes, D.; Novak, S.; Šantek, B. Recent Trends in Biodiesel and Biogas Production. Food Technol. Biotechnol. 2018, 56, 152–173. [Google Scholar] [CrossRef] [PubMed]
- Mahlia, T.; Syazmi, Z.; Mofijur, M.; Abas, A.P.; Bilad, M.; Ong, H.C.; Silitonga, A. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Bockey, D. The significance and perspective of biodiesel production—A European and global view. OCL 2019, 26, 40. [Google Scholar] [CrossRef]
- Biodiesel. Available online: https://www.biodiesel.org/what-is-biodiesel/biodiesel-basics (accessed on 21 March 2020).
- Kristi, M.; Milbrandt, A.; Lewis, J.; Schwab, A. Bioenergy Industry Status 2017 Report; National Renewable Energy Laboratory: Golden, CO, USA, 2018. Available online: https://www.nrel.gov/docs/fy20osti/75776.pdf (accessed on 5 May 2020).
- Canakci, M.; Sanli, H. Biodiesel production from various feedstocks and their effects on the fuel properties. J. Ind. Microbiol. Biotechnol. 2008, 35, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Wancura, J.H.C.; Tres, M.V.; Jahn, S.L.; de Oliveira, J.V. Lipases in liquid formulation for biodiesel production: Current status and challenges. Biotechnol. Appl. Biochem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-Y.; Lee, S.-H.; Ryu, J.-H.; Bae, S.-Y. Biodiesel production from waste lard using supercritical methanol. J. Supercrit. Fluids 2012, 61, 134–138. [Google Scholar] [CrossRef]
- Marulanda, V.F.; Anitescu, G.; Tavlarides, L.L. Investigations on supercritical transesterification of chicken fat for biodiesel production from low-cost lipid feedstocks. J. Supercrit. Fluids 2010, 54, 53–60. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.-R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef]
- Vakros, J. Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review. Catalysts 2018, 8, 562. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J.-M.; Oh, J.-I.; Ok, Y.S.; Lee, S.-R.; Kwon, E.E. Evaluating the effectiveness of various biochars as porous media for biodiesel synthesis via pseudo-catalytic transesterification. Bioresour. Technol. 2017, 231, 59–64. [Google Scholar] [CrossRef]
- Issariyakul, T.; Kulkarni, M.G.; Dalai, A.K.; Bakhshi, N.N. Production of biodiesel from waste fryer grease using mixed methanol/ethanol system. Fuel Process Technol. 2007, 88, 429–436. [Google Scholar] [CrossRef]
- Melani, N.B.; Tambourgi, E.B.; Silveira, E. Lipases: From Production to Applications. Sep. Purif. Rev. 2019, 49, 143–158. [Google Scholar] [CrossRef]
- Maldonado, R.R.; Lopes, D.B.; Aguiar-Oliveira, E.; Kamimura, E.S.; Macedo, G.A. A review on geotrichum lipases: Production, purification, immobilization and applications. Chem. Biochem. Eng. 2016, 30, 439–454. [Google Scholar] [CrossRef]
- Alzuhair, S.; Ling, F.W.; Limsong, J. Proposed kinetic mechanism of the production of biodiesel from palm oil using lipase. Process Biochem. 2007, 42, 951–960. [Google Scholar] [CrossRef]
- Sugihara, A.; Tani, T.; Tominaga, Y. Purification and characterization of a novel thermostable lipase from Bacillus sp. J. Biochem. 1991, 109, 211–216. [Google Scholar]
- Lanser, A.C.; Manthey, L.K.; Hou, C.T. Regioselectivity of new bacterial lipases determined by hydrolysis of triolein. Curr. Microbiol. 2002, 44, 336–340. [Google Scholar] [CrossRef]
- Tsurumura, T.; Tsuge, H. Substrate selectivity of bacterial monoacylglycerol lipase based on crystal structure. J. Struct. Funct. Genom. 2014, 15, 83–89. [Google Scholar] [CrossRef]
- Li, P.-Y.; Zhang, Y.-Q.; Zhang, Y.; Jiang, W.-X.; Wang, Y.-J.; Zhang, Y.-S.; Sun, Z.-Z.; Li, C.-Y.; Zhang, Y.-Z.; Shi, M.; et al. Study on a Novel Cold-Active and Halotolerant Monoacylglycerol Lipase Widespread in Marine Bacteria Reveals a New Group of Bacterial Monoacylglycerol Lipases Containing Unusual C(A/S)HSMG Catalytic Motifs. Front. Microbiol. 2020, 11, 9. [Google Scholar] [CrossRef]
- Davranov, K. Microbial lipases in biotechnology (review). Appl. Biochem. Microbiol. 1994, 30, 527–534. [Google Scholar]
- Jaeger, K.-E.; Ransac, S.; Dijkstra, B.W.; Colson, C.; Heuvel, M.; van Misset, O. Bacterial lipases. FEMS Microbiol. Rev. 1994, 15, 29–63. [Google Scholar] [CrossRef]
- Wei, L.; Li, R.W.; Qiang, L.; Wei, D.; Liu, D.H. Acyl migration and kinetics study of 1(3)-positional specific lipase of Rhizopus oryzae-catalyzed methanolysis of triglyceride for biodiesel production. Process Biochem. 2010, 45, 1888–1893. [Google Scholar]
- Sánchez, D.A.; Tonetto, G.M.; Ferreira, M.L. Burkholderia cepacia lipase: A versatile catalyst in synthesis reactions. Biotechnol. Bioeng. 2018, 115, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Encinar, J.M.; González, J.F.; Sánchez, N.; Nogales-Delgado, S. Sunflower oil transesterification with methanol using immobilized lipase enzymes. Bioproc. Biosyst. Eng. 2019, 42, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Riyadi, F.A.; Alam, M.Z.; Salleh, M.N.; Salleh, H.M. Optimization of thermostable organic solvent-tolerant lipase production by thermotolerant Rhizopus sp. Using solid-state fermentation of palm kernel cake. 3 Biotech 2017, 7, 300. [Google Scholar] [CrossRef]
- Oliveira, F.; Moreira, C.; Salgado, J.M.; Abrunhosa, L.; Venancio, A.; Belo, I. Olive pomace valorization by Aspergillus species: Lipase production using solid-state fermentation. J. Sci. Food Agric. 2016, 96, 3583–3589. [Google Scholar] [CrossRef]
- Pandey, N.; Dhakar, K.; Jain, R.; Pandey, A. Temperature dependent lipase production from cold and pH tolerant species of Penicillium. Mycosphere 2016, 7, 1533–1545. [Google Scholar] [CrossRef]
- Calabrò, V.; Ricca, E.; De Paola, M.G.; Curcio, S.; Iorio, G. Kinetics of enzymatic trans-esterification of glycerides for biodiesel production. Bioproc. Biosyst. Eng. 2010, 33, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Dizge, N.; Aydiner, C.; Imer, D.Y.; Bayramoglu, M.; Tanriseven, A.; Keskinler, B. Biodiesel production from sunflower, soybean, and waste cooking oils by transesterification using lipase immobilized onto a novel microporous polymer. Bioresour. Technol. 2009, 100, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Gog, A.; Roman, M.; Tos, M.; Paizs, C.; Dan Irimie, F. Biodiesel production using enzymatic transesterification e Current state and Perspectives. Renew. Energy 2012, 39, 10–16. [Google Scholar] [CrossRef]
- Shah, S.; Gupta, M.N. Lipase catalyzed preparation of biodiesel from Jatropha oil in a solvent free system. Process Biochem. 2007, 42, 409–414. [Google Scholar] [CrossRef]
- Cubides-Roman, D.C.; Perez, V.H.; de Castro, H.F.; Orrego, C.E.; Giraldo, O.H.; Silveira, E.G.; David, G.F. Ethyl esters (biodiesel) production by Pseudomonas fluorescens lipase immobilized on chitosan with magnetic properties in a bioreactor assisted by electromagnetic field. Fuel 2017, 196, 481–487. [Google Scholar] [CrossRef]
- Li, W.; Du, W.; Liu, D. Rhizopus oryzae IFO 4697 whole cell catalyzed methanolysis of crude and acidified rapeseed oils for biodiesel production in tert-butanol system. Process Biochem. 2007, 42, 1481–1485. [Google Scholar] [CrossRef]
- Kaieda, M.; Samukawa, T.; Kondo, A.; Fukuda, H. Effect of methanol and water contents on production of biodiesel fuel from plant oil catalyzed by various lipases in a solvent-free system. J. Biosci. Bioeng. 2001, 91, 12–15. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; He, Y.; Cui, G.; Abdulrazaq, M.A.; Yan, Y. Enhancing enzyme activity and enantioselectivity of Burkholderia cepacia lipase via immobilization on melamine glutaraldehyde dendrimer modified magnetic nanoparticles. Chem. Eng. J. 2018, 351, 258–268. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.; Park, S.; Kim, H.K. Biodiesel production using cross-linked Staphylococcus haemolyticus lipase immobilized on solid polymeric carriers. J. Mol. Catal. B Enzym. 2013, 85–86, 10–16. [Google Scholar] [CrossRef]
- Shah, S.; Sharma, S.; Gupta, M.N. Biodiesel preparation by lipase-catalyzed transesterification of Jatropha oil. Energy Fuels 2004, 18, 154–159. [Google Scholar] [CrossRef]
- Ji, Q.; Wang, B.; Tan, J.; Zhu, L.; Li, L. Immobilized multienzymatic systems for catalysis of cascade reactions. Process Biochem. 2016, 51, 1193–1203. [Google Scholar] [CrossRef]
- Handayani, R.; Wahyuningrum, D.; Zulfikar, M.A.; Nurbaiti, S.; Radiman, C.L.; Buchari, R. The synthesis of biodiesel catalyzed by Mucor miehei lipase immobilized onto aminated polyethersulfone membranes. Bioresour. Bioproc. 2016, 3, 22. [Google Scholar] [CrossRef]
- Costa Rodrigues, R.; Volpato, G.; Ayub, M.A.Z.; Wada, K. Lipase-catalysed ethanolysis of soybean oil in a solvent-free system using central composite design and response surface methodology. J. Chem. Technol. Biotechnol. 2008, 83, 849–854. [Google Scholar] [CrossRef]
- Ashjari, M.; Garmroodi, M.; Asl, F.A.; Emampour, M.; Yousefi, M.; Lish, M.P.; Habibi, Z.; Mohammadi, M. Application of multi-component reaction for covalent immobilization of two lipases on aldehyde-functionalized magnetic nanoparticles; production of biodiesel from waste cooking oil. Process Biochem. 2020, 90, 156–167. [Google Scholar] [CrossRef]
- Chen, G.; Ying, M.; Li, W. Enzymatic conversion of waste-cooking oils into alternative fuel biodiesel. Appl. Biochem. Biotechnol. 2006, 132, 911–921. [Google Scholar] [CrossRef]
- Arumugam, A.; Ponnusami, V. Production of biodiesel by enzymatic transesterification of waste sardine oil and evaluation of its engine performance. Heliyon 2017, 3, 00486. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Dai, L.; Du, W.; Liu, D. Effect of water on lipase NS81006-catalyzed alcoholysis for biodiesel production. Process Biochem. 2017, 58, 239–244. [Google Scholar] [CrossRef]
- Touqeer, T.; Mumtaz, M.W.; Mukhtar, H.; Irfan, A.; Akram, S.; Shabbir, A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Fe3O4-PDA-Lipase as Surface Functionalized NanoBiocatalyst for the Production of Biodiesel Using Waste Cooking Oil as Feedstock: Characterization and Process Optimization. Energies 2020, 13, 177. [Google Scholar] [CrossRef]
- Kaieda, M.; Samukawa, T.; Matsumoto, T.; Ban, K.; Kondo, A.; Shimada, Y.; Noda, H.; Nomoto, F.; Ohtsuka, K.; Izumoto, E.; et al. Biodiesel fuel production from plant oil catalyzed by Rhizopus oryzae lipase in a water-containing system without an organic solvent. J. Biosci. Bioeng. 1999, 88, 627–631. [Google Scholar] [CrossRef]
- Duarte, S.H.; Hernández, G.L.P.; Canet, A.; Benaiges, M.D.; Maugeria, F.; Valero, F. Enzymatic biodiesel synthesis from yeast oil using immobilized recombinant Rhizopus oryzae lipase. Bioresour. Technol. 2015, 183, 175–180. [Google Scholar] [CrossRef]
- Shahedi, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M.; As’habi, M.A. Co-immobilization of Rhizomucor miehei lipase and Candida antarctica lipase B and optimization of biocatalytic biodiesel production from palm oil using response surface methodology. Renew. Energy 2019, 141, 847–857. [Google Scholar] [CrossRef]
- Matsuda, T.; Marukado, R.; Mukouyama, M.; Harada, T.; Nakamura, K. Asymmetric reduction of ketones by Geotrichum candidum: Immobilization and application to reactions using supercritical carbon dioxide. Tetrahedron Asymmetry 2008, 19, 2272–2275. [Google Scholar] [CrossRef]
- Adewale, P.; Dumont, J.-M.; Ngadi, M. Enzyme-catalyzed synthesis and kinetics of ultrasonic assisted methanolysis of waste lard for biodiesel production. Chem. Eng. J. 2016, 284, 158–165. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Jiang, Y.; Liu, P.; Zhou, L.; Ma, L.; He, Y.; Li, H.; Gao, J. Silica Nanoflowers-Stabilized Pickering Emulsion as a Robust Biocatalysis Platform for Enzymatic Production of Biodiesel. Catalysts 2019, 9, 1026. [Google Scholar] [CrossRef]
- Antonio, D.C.; Amancio, L.P.; Rosset, I.G. Biocatalytic Ethanolysis of Waste Chicken Fat for Biodiesel Production. Catal. Lett. 2018, 148, 3214–3222. [Google Scholar] [CrossRef]
- Lara, P.V.; Park, E.Y. Potential application of waste activated bleaching earth on the production of fatty acid alkyl esters using Candida cylindracea lipase in organic solvent system. Enzym. Microb. Technol. 2004, 34, 270–277. [Google Scholar] [CrossRef]
- Matinja, A.I.; Zain, N.A.M.; Suhaimi, M.S.; Alhassan, A.J. Optimization of biodiesel production from palm oil mill effluent using lipase immobilized in PVA alginate- sulfate beads. Renew. Energy 2019, 135, 1178–1185. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.B.; Yoo, H.Y.; Lee, J.H.; Han, S.O.; Park, C.; Kim, S.W. Co-immobilization of Candida rugosa and Rhyzopus oryzae lipases and biodiesel production. Korean J. Chem. Eng. 2013, 30, 1335–1338. [Google Scholar] [CrossRef]
- Christopher, L.P.; Kumar, H.; Zambare, V.P. Enzymatic biodiesel: Challenges and opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Bandikari, R.; Qian, J.; Baskaran, R.; Liu, Z.; Wu, G. Bio-affinity mediated immobilization of lipase onto magnetic cellulose nanospheres for high yield biodiesel in one time addition of methanol. Bioresour. Technol. 2018, 249, 354–360. [Google Scholar] [CrossRef]
- López, E.N.; Medina, A.R.; Moreno, P.A.G.; Cerdán, L.E.; Valverde, L.M.; Grima, E.M. Biodiesel production from Nannochloropsis gaditana lipids through transesterification catalyzed by Rhizopus oryzae lipase. Bioresour. Technol. 2016, 203, 236–244. [Google Scholar] [CrossRef]
- Iso, M.; Chen, B.; Eguchi, M.; Kudo, T.; Shrestha, S. Production of biodiesel fuel from triglycerides and alcohol using immobilized lipase. J. Mol. Catal. B Enzym. 2001, 16, 53–58. [Google Scholar] [CrossRef]
- Goembira, F.; Saka, S. Optimization of biodiesel production by supercritical methyl acetate. Bioresour. Technol. 2013, 131, 47–52. [Google Scholar] [CrossRef]
- Razack, S.A.; Duraiarasan, S. Response surface methodology assisted biodiesel production from waste cooking oil using encapsulated mixed enzyme. Waste Manage. 2016, 47, 98–104. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.; Li, D.; Yang, B.; Wang, Y. One-step synthesis of high-yield biodiesel from waste cooking oils by a novel and highly methanol-tolerant immobilized lipase. Bioresour. Technol. 2017, 235, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.T.; Qi, F.; Yuan, C.; Zhao, X.; Ramkrishna, D.; Liu, D.; Varma, A. Lipase-Catalyzed Process for Biodiesel Production: Protein Engineering and Lipase Production. Biotechnol. Bioeng. 2014, 111, 639. [Google Scholar] [CrossRef] [PubMed]
- Pollardo, A.A.; Lee, H.; Lee, D.; Kim, S.; Kim, J. Solvent effect on the enzymatic production of biodiesel from waste animal fat. J. Clean. Prod. 2018, 185, 382–388. [Google Scholar] [CrossRef]
- Kumar, D.; Das, T.; Giri, B.S.; Verma, B. Preparation and characterization of novel hybrid bio-support material immobilized from Pseudomonas cepacia lipase and its application to enhance biodiesel production. Renew. Energy 2020, 147, 11–24. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Hasan, M.; Ramachandran, K. Kinetics of the enzymatic hydrolysis of palm oil by lipase. Process Biochem. 2003, 38, 1155–1163. [Google Scholar] [CrossRef]
- Chesterfield, D.M.; Rogers, P.L.; Al-Zaini, E.O.; Adesina, A.A. Production of biodiesel via ethanolysis of waste cooking oil using immobilised lipase. Chem. Eng. J. 2012, 207, 701–710. [Google Scholar] [CrossRef]
- Remonatto, D.; Santin, C.M.T.; Oliveira, D.; Di Luccio, M.; Oliveira, J.V. FAME Production from Waste Oils Through Commercial Soluble Lipase Eversa®Catalysis. Ind. Biotechnol. 2016, 12, 254–262. [Google Scholar] [CrossRef]
- Wancura, J.H.C.; Rosset, D.V.; Tres, M.V.; Oliveira, J.V.; Mazutti, M.A.; Jahn, S.L. Production of biodiesel catalyzed by lipase from Thermomyces lanuginosus in its soluble form. Can. J. Chem. Eng. 2018, 96, 2361–2368. [Google Scholar] [CrossRef]
- Lee, K.T.; Foglia, T.A.; Chang, K.S. Production of alkyl ester as biodiesel from fractionated lard and restaurant grease. J. Am. Oil Chem. Soc. 2002, 79, 191–195. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Yan, Y. Optimization of Lipase-Catalyzed Transesterification of lard for Biodiesel Production Using Response Surface Methodology. Appl. Microbiol. Biotechnol. 2010, 160, 504–515. [Google Scholar] [CrossRef]
- Lu, J.; Nie, K.; Xie, F.; Wang, F.; Tan, T. Enzymatic synthesis of fatty acid methyl esters from lard with immobilized Candida sp. 99–125. Process Biochem. 2007, 42, 1367–1370. [Google Scholar] [CrossRef]
- Da Silva, J.R.P.; da Costa, F.P.; Lerin, L.A.; Ninow, J.L.; Oliveira, V.; de Oliveira, D. Application of Different Methodologies to Produce Fatty Acid Esters Using the Waste Chicken Fat Catalyzed by Free NS 40116 Lipase. Ind. Biotechnol. 2019, 5, 293–302. [Google Scholar] [CrossRef]
- Poppe, J.K.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Záchia Ayub, M.A. Enzymatic reactors for biodiesel synthesis: Present status and future prospects. Biotechnol. Adv. 2015, 33, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, K.; He, Y.; Wang, Y.; Han, X.; Yan, Y. Enhanced performance of lipase immobilized onto Co2+-chelated magnetic nanoparticles and its application in biodiesel production. Fuel 2019, 255, 115794. [Google Scholar] [CrossRef]
- Cui, J.D.; Jia, S.R. Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: Current development and future challenges. Crit. Rev. Biotechnol. 2015, 35, 15–28. [Google Scholar] [CrossRef]
- Yan, J.; Yan, Y.; Liu, S.; Hu, J.; Wang, G. Preparation of cross-linked lipase-coated micro-crystals for biodiesel production from waste cooking oil. Bioresour. Technol. 2011, 102, 4755–4758. [Google Scholar] [CrossRef]
- Lopresto, C.G.; De Paola, M.G.; Albo, L.; Policicchio, M.F.; Chakraborty, S.; Calabro, V. Comparative analysis of immobilized biocatalyst: Study of process variables in trans-esterification reaction. 3 Biotech 2019, 9, 443. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Zhou, Z.; Hartmann, M. Progress in enzyme immobilization in ordered mesoporous materials and related applications. Chem. Soc. Rev. 2013, 42, 3894–3912. [Google Scholar] [CrossRef]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M. Process optimization for biodiesel production from waste cooking oil using multi-enzyme systems through response surface methodology. Renew. Energy 2017, 105, 465–472. [Google Scholar] [CrossRef]
- Da Rós, P.C.M.; Silva, G.A.M.; Mendes, A.A.; Santos, J.C.; de Castro, H.F. Evaluation of the catalytic properties of Burkholderia cepacia lipase immobilized on non-commercial matrices to be used in biodiesel synthesis from different feedstocks. Bioresour. Technol. 2010, 101, 5508–5516. [Google Scholar] [CrossRef] [PubMed]
- Angulo, B.; Fraile, J.M.; Gil, L.; Herrerías, C.I. Comparison of Chemical and Enzymatic Methods for the Transesterification of Waste Fish Oil Fatty Ethyl Esters with Different Alcohols. ACS Omega 2020, 5, 1479−1487. [Google Scholar] [CrossRef] [PubMed]
- Marin-Suarez, M.; Mendez-Mateos, D.; Guadix, A.; Guadix, E.M. Reuse of immobilized lipases in the transesterification of waste fish oil for the production of biodiesel. Renew. Energy 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [PubMed]
- Manoel, E.A.; dos Santos, J.C.; Freire, D.M.; Rueda, N. Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzym. Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Quilles, J.C., Jr.; Ferrarezi, A.L.; Borges, J.P.; Rossi, J.S.; Bocchini, D.A.; Gomes, E.; da Silva, R.; Boscolo, M. Ultrasound affects the selectivity and activity of immobilized lipases applied to fatty acid ethyl ester synthesis. Acta Sci-Technol. 2020, 42, 46582. [Google Scholar] [CrossRef]
- Zhou, W.J.; Fang, L.; Fan, Z.; Albela, B.; Bonneviot, L.; De Campo, F.; Pera Titus, M.; Clacens, J.M. Tunable catalysts for solvent-free biphasic systems: Pickering interfacial catalysts over amphiphilic silica nanoparticles. J. Am. Chem. Soc. 2014, 136, 4869–4872. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, X.; Chen, Y.; Zhou, L.; He, Y.; Ma, L.; Gao, J. Pickering emulsion stabilized by lipase-containing periodic mesoporous organosilica particles: A robust biocatalyst system for biodiesel production. Bioresour. Technol. 2014, 153, 278–283. [Google Scholar] [CrossRef]
- Zhang, W.; Fu, L.; Yang, H. Micrometer-Scale Mixing with Pickering Emulsions: Biphasic Reactions without Stirring. ChemSusChem 2014, 7, 391–396. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Jiang, Y.; Zhou, L.; Ma, L.; He, Y.; Gao, J. Biocatalytic Pickering Emulsions Stabilized by Lipase-Immobilized Carbon Nanotubes for Biodiesel Production. Catalysts 2018, 8, 587. [Google Scholar] [CrossRef]
- Shi, J.; Wang, X.; Zhang, S.; Tang, L.; Jiang, Z. Enzyme-conjugated ZIF-8 particles as efficient and stable Pickering interfacial biocatalysts for biphasic biocatalysis. J. Mater. Chem. B 2016, 4, 2654–2661. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, C.; Ju, E.; Ji, H.; Ren, J.; Binks, B.P.; Qu, X. Design of Surface-Active Artificial Enzyme Particles to Stabilize Pickering Emulsions for High-Performance Biphasic Biocatalysis. Adv. Mater. 2016, 28, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Leclercq, L.; Clacensb, J.M.; Rataj, V.N. Acidic/amphiphilic silica nanoparticles: New eco-friendly Pickering interfacial catalysis for biodiesel production. Green Chem. 2017, 19, 4552–4562. [Google Scholar] [CrossRef]
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; El Gabaly, F.; Yoon, H.P.; et al. Tunable electrical conductivity in metal-organic framework thin-film devices. Science 2014, 343, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, Y.; Chen, S.; Le, X.; Zhou, X.; Zhao, Z.; Ou, Y.; Yang, J. Reversible immobilization of laccase onto metal-ion-chelated magnetic microspheres for bisphenol A removal. Int. J. Biol. Macromol. 2016, 84, 189–199. [Google Scholar] [CrossRef]
- Netto, C.G.; Toma, H.E.; Andrade, L.H. Superparamagnetic nanoparticles as versatile carriers and supporting materials for enzymes. J. Mol. Catal. B Enzym. 2013, 85, 71–92. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W. Surfactant Imprinting Hyperactivated Immobilized Lipase as Efficient Biocatalyst for Biodiesel Production from Waste Cooking Oil. Catalysts 2019, 9, 914. [Google Scholar] [CrossRef]
- Ngo, T.P.N.; Li, A.; Tiew, K.-W.; Li, Z. Efficient transformation of grease to biodiesel using highly active and easily recyclable magnetic nanobiocatalyst aggregates. Bioresour. Technol. 2013, 145, 233–239. [Google Scholar] [CrossRef]
- Chiaradia, V.; Soares, N.S.; Valério, A.; de Oliveira, D.; Araújo, P.H.H.; Sayer, C. Immobilization of Candida antarctica Lipase B on Magnetic Poly(Urea-Urethane) Nanoparticles. Appl. Biochem. Biotechnol. 2016, 180, 558–575. [Google Scholar] [CrossRef]
- Miao, C.; Yang, L.; Wang, Z.; Luo, W.; Li, H.; Lv, P.; Yuan, Z. Lipase immobilization on amino-silane modified superparamagnetic Fe3O4 nanoparticles as biocatalyst for biodiesel production. Fuel 2018, 224, 774–782. [Google Scholar] [CrossRef]
- Mijone, P.D.; Vilas-Boas, R.N.; Bento, H.B.S.; Rodrigues, B.S.B.; de Castro, R.H.F. Coating and incorporation of iron oxides into a magnetic-polymer composite to be used as lipase support for ester syntheses. Renew. Energy 2020, 149, 1167–1173. [Google Scholar] [CrossRef]
- Tran, D.-T.; Chen, C.-L.; Chang, J.-S. Immobilization of Burkholderia sp. Lipase on a ferric silica nanocomposite for biodiesel production. J. Biotechnol. 2012, 158, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Wang, J. Immobilized lipase on magnetic chitosan microspheres for transesterification of soybean oil. Biomass. Bioenergy 2012, 36, 373–380. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Rios, N.S.; Mendez-Sanchez, C.; Lokha, Y.; Carballares, D.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Coimmobilization of different lipases: Simple layer by layer enzyme spatial ordering. Int. J. Biol. Macromol. 2020, 145, 856–864. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 5, 7461–7490. [Google Scholar] [CrossRef]
- Nielsen, P.M. Production of Fatty Acid Alkyl Esters. World Patent WO/2012/098114, 26 July 2012. [Google Scholar]
- Fraga, F.C.; Valério, A.; de Oliveira, V.A.; Di Luccio, M.; de Oliveira, D. Effect of magnetic field on the Eversa®Transform 2.0 enzyme: Enzymatic activity and structural conformation. Int. J. Biol. Macromol. 2019, 122, 653–658. [Google Scholar] [CrossRef]
- Viesel. Available online: https://gstarbio.com/es/ (accessed on 25 June 2020).
- Chen, X.; Li, L.; Deng, L.; Pedersen, J.N.; Li, L.; Guo, Z.; Cong, F.; Xu, X. Biodiesel Production Using Lipases. In Lipid Modification by Enzymes and Engineered Microbes; Bornscheuer, U.T., Ed.; Academic Press/AOCS Press: London, UK, 2018; pp. 343–373. [Google Scholar]
- Kotrba, R. Realizing the Vision to Reclaim, Recycle, Refuel. Biodiesel Magazine. 2015. Available online: http://www.biodieselmagazine.com/articles/265452/realizing-the-vision-to-reclaim-recycle-refuel (accessed on 22 June 2020).
- Wancura, J.H.C.; Rosset, D.V.; Brondani, M.; Mazutti, M.A.; de Oliveira, J.V.; Tres, M.V.; Jahn, S.L. Soluble lipase-catalyzed synthesis of methyl esters using a blend of edible and nonedible raw materials. Bioproc. Biosyst. Eng. 2018, 41, 1185–1193. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, A.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380. [Google Scholar] [CrossRef]
- Sobhi, B. Modified-Immobilized Enzymes of High Tolerance to Hydrophilic Substrates in Organic Media. US Patent US9,068,175 B2, 30 June 2015. [Google Scholar]
- Enzymocore. Available online: https://enzymocore.com/about-us/our-enzymatic-technology/ (accessed on 22 June 2020).
| Lipase Origin | Reference |
|---|---|
| Pseudomonas fluorescens | [52] |
| Burkholderia cepacia | [42,50,53,54] |
| Staphylococcus haemolyticus | [55] |
| Chromobacterium viscosum | [56] |
| Phichia pastoris | [57] |
| Mucor miehei | [58] |
| Thermomyces lanuginosus | [59,60] |
| Aspergillus oryzae | [61] |
| Aspergillus niger | [62,63] |
| Aspergillus terreus | [64] |
| Rhizopus oryzae | [41,65,66] |
| Rhizomucor miehei | [60,67] |
| Geotrichum candidum | [68] |
| Candida antarctica | [66,69,70,71] |
| Candida cylindracea | [72] |
| Candida rugosa | [50,73,74] |
| Lipase Source | Feedstock | Conditions (T, alcohol:oil, t) | Yield (%) | References |
|---|---|---|---|---|
| A. niger | Waste cooking oil | 45 °C, 4.2:1, 30 h | 90 a | [63] |
| T. lanoginosus | Beef tallow | 35 °C, 4.5:1, 6 h | 84.6 a | [88] |
| C. antarctica | Lard | 30 °C, 1:1, 72 h | 74 a | [89] |
| C. antarctica | Lard | 50 °C, 5:1, 20 h | 97.2 b | [90] |
| Candida sp | Lard | 40 °C, 3:1, 30 h | 87.4 b | [91] |
| T. lanoginosus | Chicken fat | - | 89.04 b | [92] |
| C. antarctica | Chicken fat | 32 °C, 3:1, 24 h | 96 a | [71] |
| Lipase Source | Feedstock | Immobilization | Yield a (%) | No. Cycles | References |
|---|---|---|---|---|---|
| A. niger | Sardine oil | Activated carbon | 94.5 a | 5 | [62] |
| T. lanoginosus | Lard | Silica gel | 97.6 a | 20 | [90] |
| C. antarctica | Waste cooking oil | Silica nanoflower pickering emulsion | 98.5 a | 15 | [70] |
| B. cepacia | Castor oil | Polyvinylalcohol alginate | 75 b | 6 | [85] |
| R. miehei and C. antarctica | Waste cooking oil | Epoxy functionalized silica | 91.5 a | 14 | [100] |
| Streptomyces sp | Waste cooking oil | XAD 1180 resin | 95.45 a | 4 | [79] |
| B. cepacia | Beef tallow | Polysiloxane–polyvinyl alcohol (SiO2–PVA) hybrid composite | 89 a | - | [101] |
| C. antarctica | Waste fish oil | Acrylic resin | 95 a | 4 | [102] |
| C. antarctica | Waste fish oil | Acrylic resin | 75 a | 10 | [103] |
| Lipase Source | Carrier | Immobilization | Yield a (%) | No. Cycles | References |
|---|---|---|---|---|---|
| B. cepacia | Silica coated hydroxyapatite and glutaraldehyde | Encapsulation | 98 | 4 | [118] |
| A. terreus | Iron oxide polydopamine | Covalent bonding | 92 | 7 | [64] |
| T. lanuginosis & C. antarctica | Core-shell structured iron oxide | Covalent bonding | 99 | 11 | [119] |
| P. fluorescens | Co2+ chelated, with 3-glycidoxypropyltrimethoxysylane | Adsorption | 95 | 10 | [70] |
| R. miehei and T. lanuginosa | Silica core shell iron oxide with tryethylamine | Covalent bonding | 93.1 | 5 | [60] |
| C. antarctica | Poly(urea-urethane) encapsulated magnetite | Encapsulation | 95 | 8 | [120] |
| C. antarctica | Magnetic iron oxide with 1-Butyl-3-methylimidazolium tetrafluoroborate & 3-aminopropyltriethoxysilane | Covalent bonding | 89.4 | 5 | [121] |
| B. cepacia | Polysiloxane–polyvinyl alcohol hybrid magnetic-polymer composite | Covalent bonding | 96.5 | - | [122] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toldrá-Reig, F.; Mora, L.; Toldrá, F. Developments in the Use of Lipase Transesterification for Biodiesel Production from Animal Fat Waste. Appl. Sci. 2020, 10, 5085. https://doi.org/10.3390/app10155085
Toldrá-Reig F, Mora L, Toldrá F. Developments in the Use of Lipase Transesterification for Biodiesel Production from Animal Fat Waste. Applied Sciences. 2020; 10(15):5085. https://doi.org/10.3390/app10155085
Chicago/Turabian StyleToldrá-Reig, Fidel, Leticia Mora, and Fidel Toldrá. 2020. "Developments in the Use of Lipase Transesterification for Biodiesel Production from Animal Fat Waste" Applied Sciences 10, no. 15: 5085. https://doi.org/10.3390/app10155085
APA StyleToldrá-Reig, F., Mora, L., & Toldrá, F. (2020). Developments in the Use of Lipase Transesterification for Biodiesel Production from Animal Fat Waste. Applied Sciences, 10(15), 5085. https://doi.org/10.3390/app10155085