Zinc Oxide Nanorod Surface-Enhanced Raman Scattering Substrates without and with Gold Nanoparticles Fabricated through Pulsed-Laser-Induced Photolysis
Abstract
1. Introduction
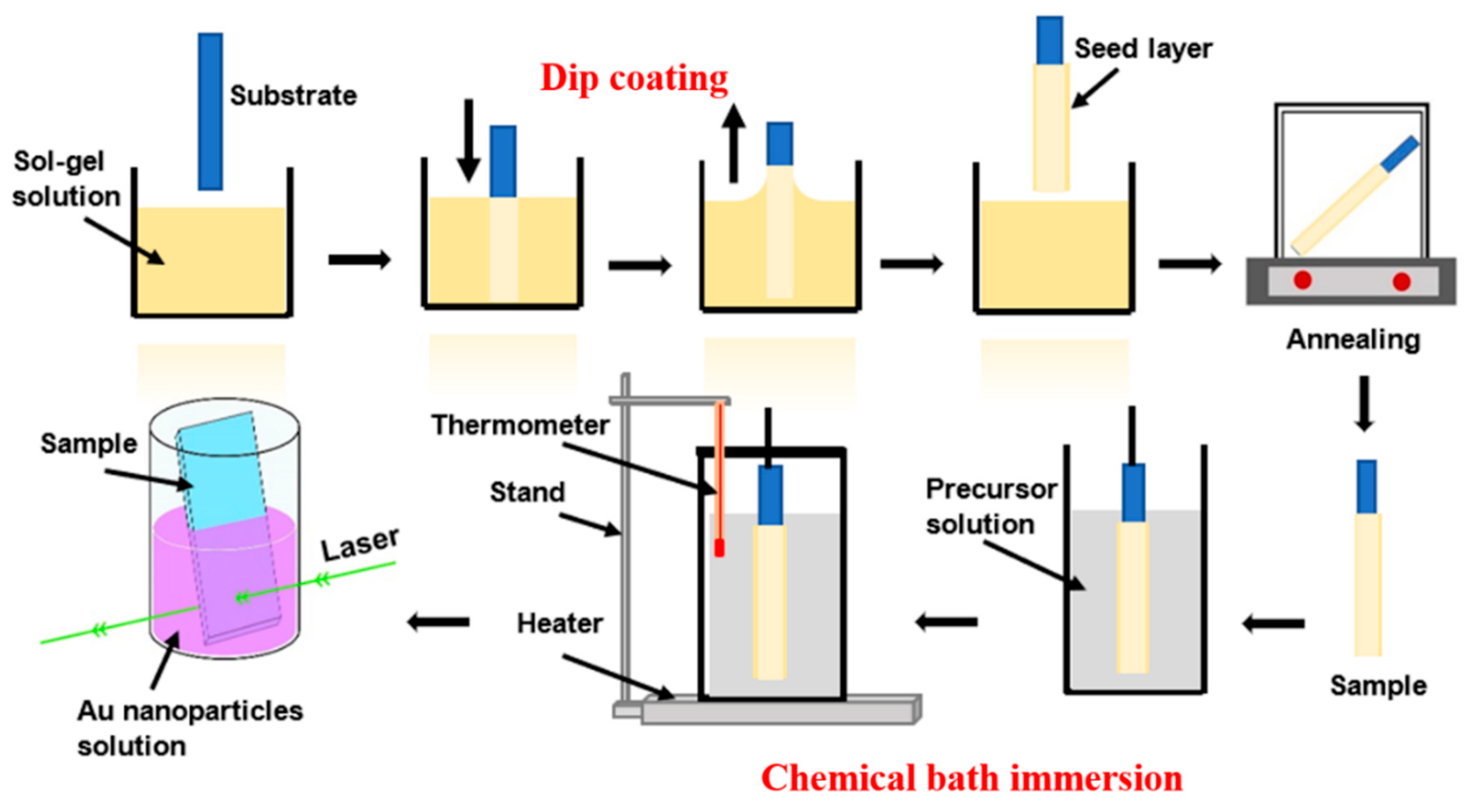
2. Materials and Methods
3. Results and Discussion
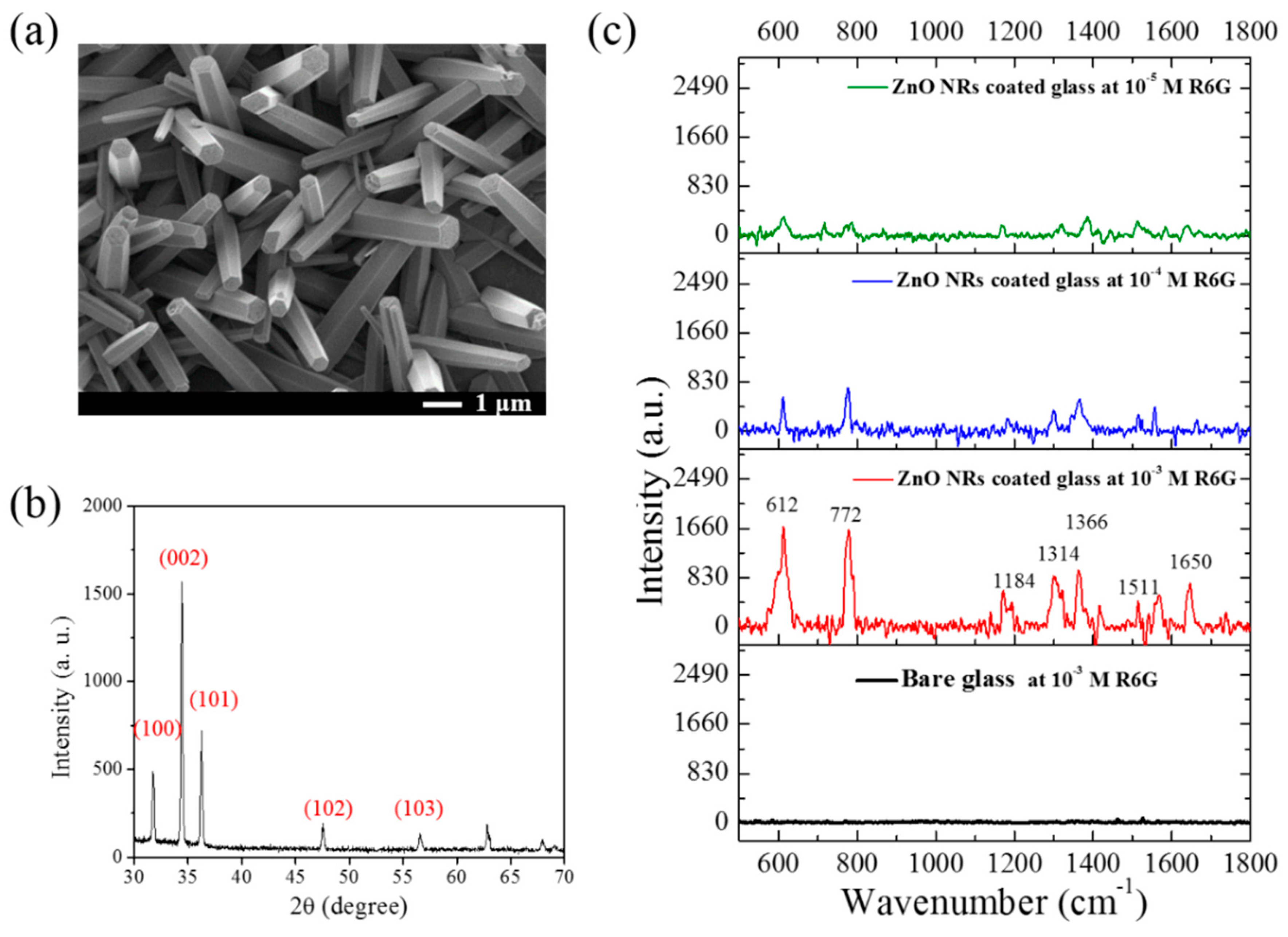
3.1. Surface Morphology, XRD and SERS Characteristic of ZnO NRs
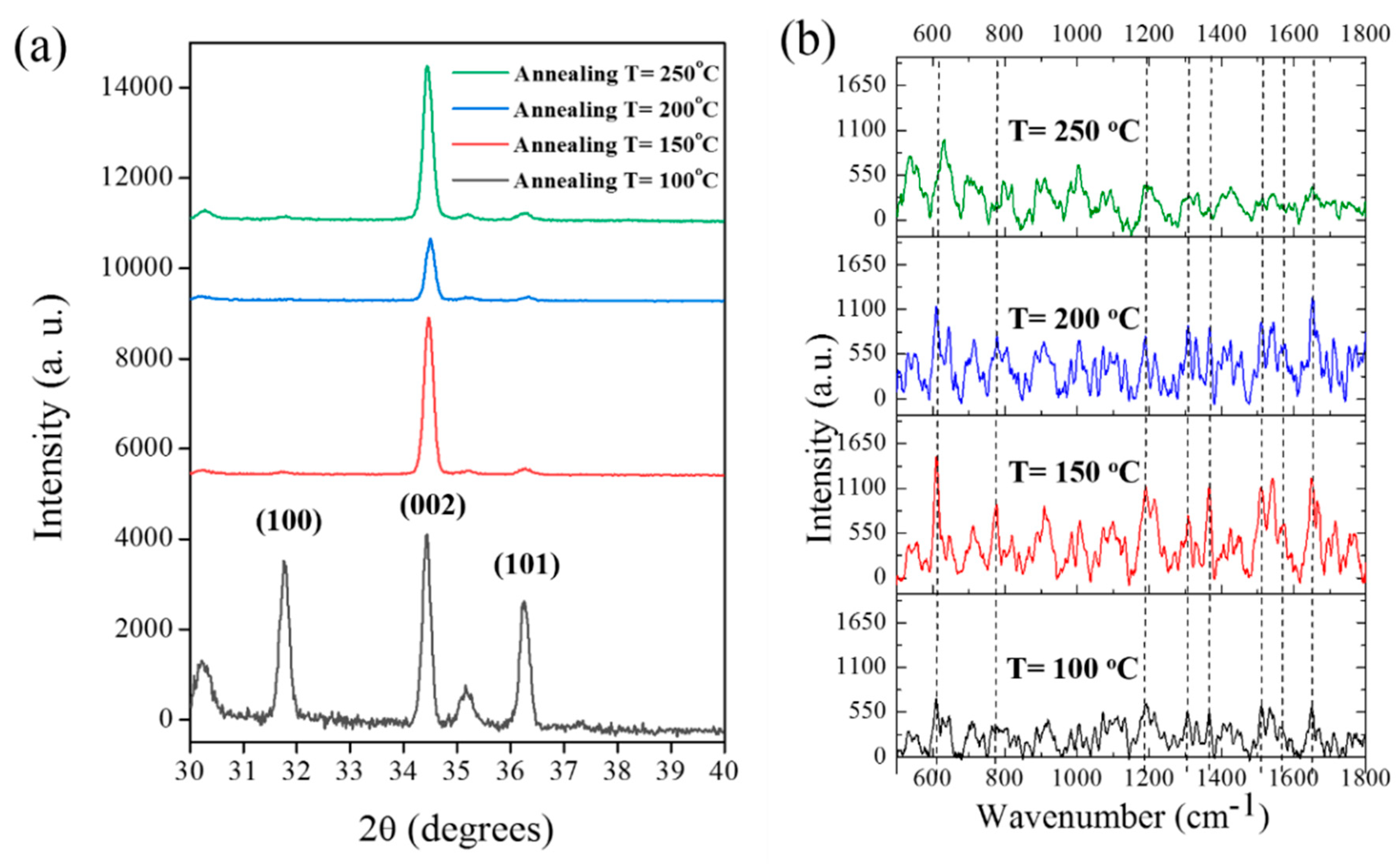
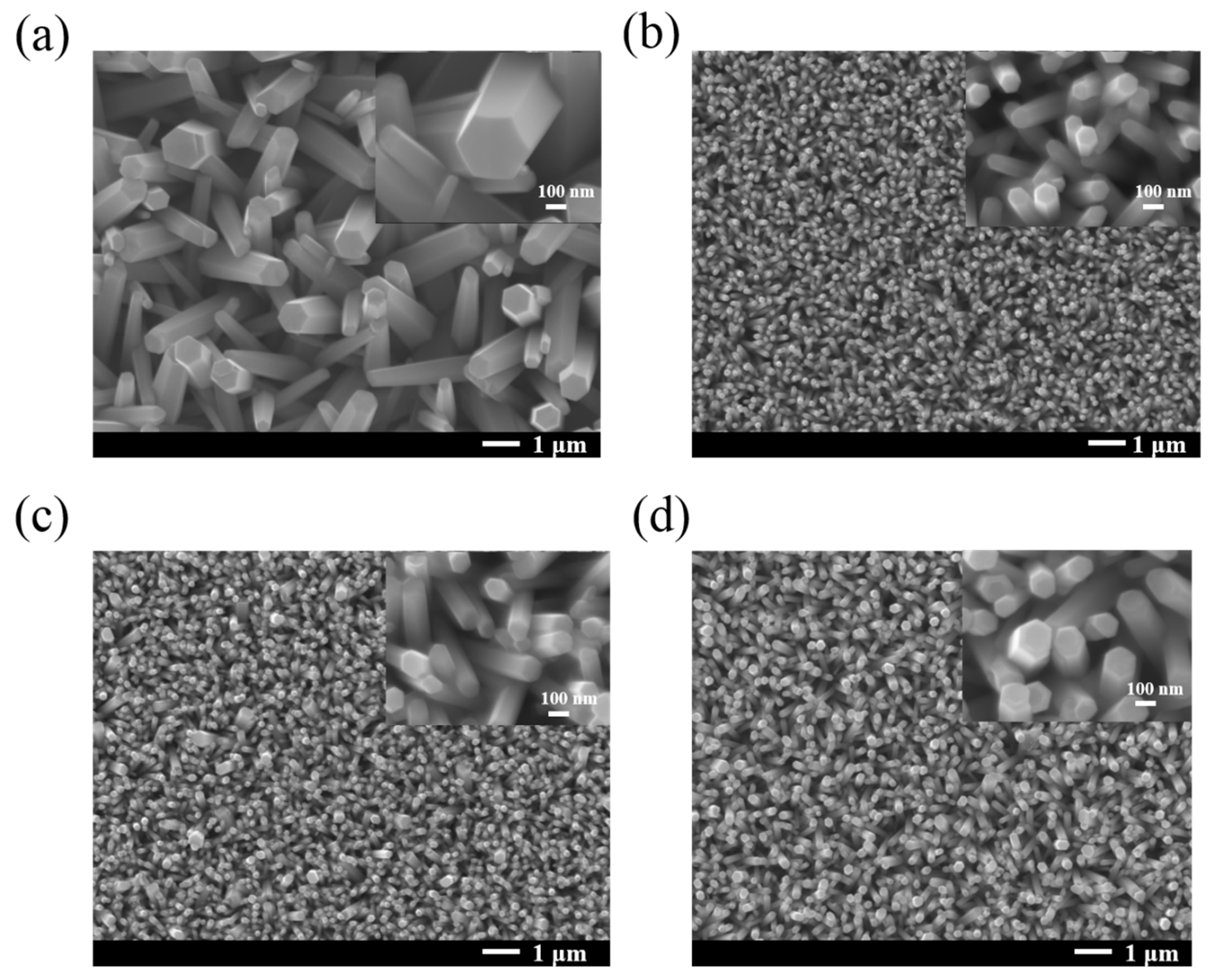
3.2. XRD, SERS and Surface Morphology of Annealed ZnO NRs
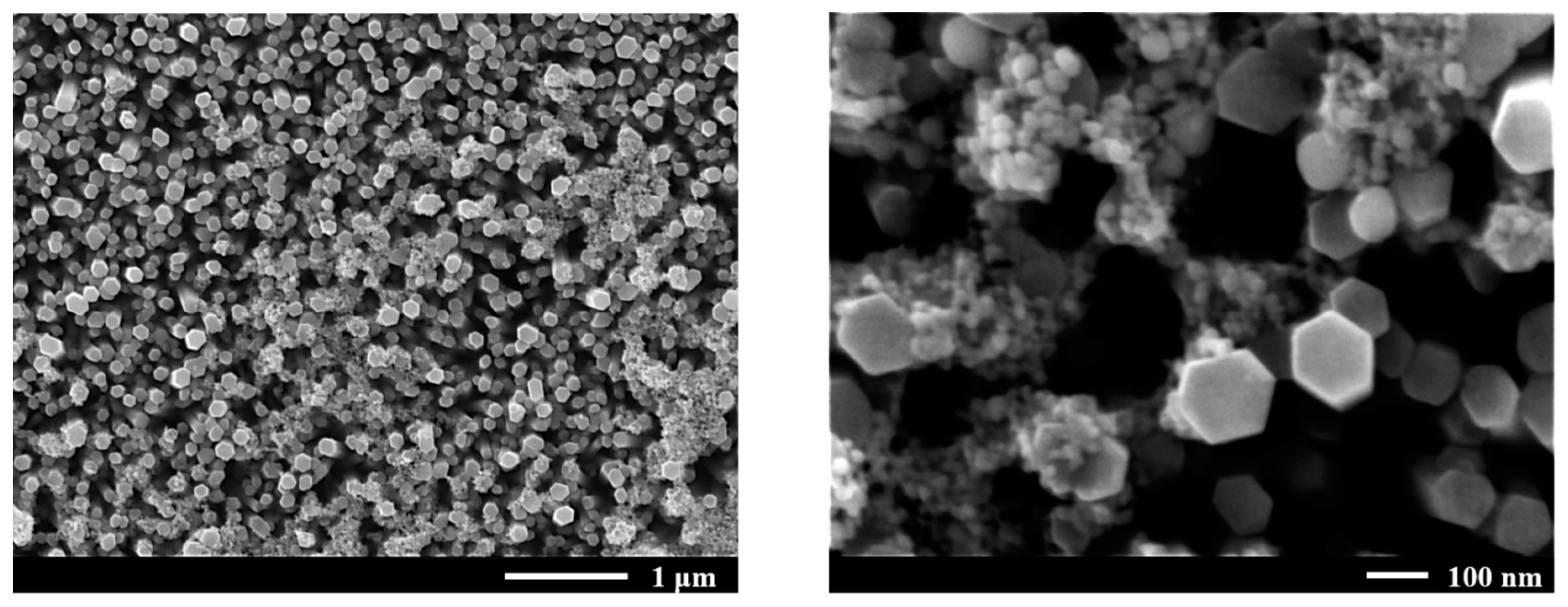
3.3. SERS and Surface Morphology of AuNP-Decorated ZnO NRs
3.4. SERS and Surface Morphology of AuNP-Decorated ZnO NRs by Changing the PLIP Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.; McQuillan, A. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Alessandri, I.; Lombardi, J.R. Enhanced Raman Scattering with Dielectrics. Chem. Rev. 2016, 116, 14921–14981. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Wang, Y.; Ruan, W.; Zhang, J.; Yang, B.; Xu, W.; Zhao, B.; Lombardi, J.R. Direct observation of surface-Enhanced Raman scattering in ZnO nanocrystals. J. Raman Spectrosc. 2009, 40, 1072–1077. [Google Scholar] [CrossRef]
- Wang, X.; She, G.; Xu, H.; Mu, L.; Shi, W. The surface-Enhanced Raman scattering from ZnO nanorod arrays and its application for chemosensors. Sens. Actuators B Chem. 2014, 193, 745–751. [Google Scholar] [CrossRef]
- Wang, X.; Shi, W.; She, G.; Mu, L. Surface-Enhanced Raman Scattering (SERS) on transition metal and semiconductor nanostructures. Phys. Chem. 2012, 14, 5891–5901. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, C.; Yang, J.; Zhao, B.; Lombardi, J.R. Nanoparticle Metal−Semiconductor Charge Transfer in ZnO/PATP/Ag Assemblies by Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2008, 112, 6093–6098. [Google Scholar] [CrossRef]
- Cheng, C.; Yan, B.; Wong, S.M.; Li, X.; Zhou, W.; Yu, T.; Shen, Z.; Yu, H.; Fan, H.J. Fabrication and SERS Performance of Silver-Nanoparticle-Decorated Si/ZnO Nanotrees in Ordered Arrays. ACS Appl. Mater. Interfaces 2010, 2, 1824–1828. [Google Scholar] [CrossRef]
- Tang, H.; Meng, G.; Huang, Q.; Zhang, Z.; Huang, Z.; Zhu, C. Arrays of Cone-Shaped ZnO Nanorods Decorated with Ag Nanoparticles as 3D Surface-Enhanced Raman Scattering Substrates for Rapid Detection of Trace Polychlorinated Biphenyls. Adv. Funct. Mater. 2011, 22, 218–224. [Google Scholar] [CrossRef]
- Liu, K.; Li, D.; Li, R.; Wang, Q.; Pan, S.; Peng, W.; Chen, M. Silver-Decorated ZnO hexagonal nanoplate arrays as SERS-Active substrates: An experimental and simulation study. J. Mater. Res. 2013, 28, 3374–3383. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, S.; Mao, Z.; Li, P.; Zhao, C.; Cohick, Z.; Huang, P.-H.; Huang, T.J. In Situ Fabrication of 3D Ag@ZnO Nanostructures for Microfluidic Surface-Enhanced Raman Scattering Systems. ACS Nano 2014, 8, 12175–12184. [Google Scholar] [CrossRef] [PubMed]
- Kandjani, A.E.; Mohammadtaheri, M.; Thakkar, A.; Bhargava, S.K.; Bansal, V. Zinc oxide/silver nanoarrays as reusable SERS substrates with controllable ‘Hot-Spots’ for highly reproducible molecular sensing. J. Colloid Interface Sci. 2014, 436, 251–257. [Google Scholar] [CrossRef]
- Tao, Q.; Li, S.; Ma, C.; Liu, K.; Zhang, Q.-Y. A highly sensitive and recyclable SERS substrate based on Ag-nanoparticle-Decorated ZnO nanoflowers in ordered arrays. Dalton Trans. 2015, 44, 3447–3453. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xu, C.; Lu, J.; Li, Z.; Xu, C. 3D Ag/ZnO hybrids for sensitive surface-Enhanced Raman scattering detection. Appl. Surf. Sci. 2016, 365, 291–295. [Google Scholar] [CrossRef]
- Tang, F.; Zhang, M.; Li, Z.; Du, Z.; Chen, B.; He, X.; Zhao, S. Hexagonally arranged arrays of urchin-Like Ag-Nanoparticle decorated ZnO-Nanorods grafted on PAN-Nanopillars as surface-Enhanced Raman scattering substrates. CrystEngComm. 2018, 20, 3550–3558. [Google Scholar] [CrossRef]
- Lin, R.; Hu, L.; Wang, J.; Zhang, W.; Ruan, S.; Zeng, Y.-J. Raman scattering enhancement of a single ZnO nanorod decorated with Ag nanoparticles: Synergies of defects and plasmons. Opt. Lett. 2018, 43, 2244–2247. [Google Scholar] [CrossRef]
- Lei, S.; Tao, C.; Li, J.; Zhao, X.; Wang, W. Visible light-induced charge transfer to improve sensitive surface-Enhanced Raman scattering of ZnO/Ag nanorod arrays. Appl. Surf. Sci. 2018, 452, 148–154. [Google Scholar] [CrossRef]
- Sedira, S.; Mounir, S. Surface enhanced Raman scattering (SERS) investigation and sensitive detection of zinc oxide nanorods (ZnO Nrds) deposited on silver nanoparticles (Ag NPs) substrate. Mater. Lett. 2019, 254, 112–115. [Google Scholar]
- Mishra, Y.K.; Mohapatra, S.; Singhal, R.; Avasthi, D.K.; Agarwal, D.C.; Ogale, S.B. Au–ZnO: A tunable localized surface plasmonic nanocomposite. Appl. Phys. Lett. 2008, 92, 043107. [Google Scholar] [CrossRef]
- Sakano, T.; Tanaka, Y.; Nishimura, R.; Nedyalkov, N.N.; Atanasov, P.A.; Saiki, T.; Obara, M. Surface enhanced Raman scattering properties using Au-Coated ZnO nanorods grown by two-Step, off-Axis pulsed laser deposition. J. Phys. D Appl. Phys. 2008, 41, 235304. [Google Scholar] [CrossRef]
- Chen, L.; Luo, L.; Chen, Z.; Zhang, M.; Zapien, J.A.; Lee, C.; Lee, S.T. ZnO/Au Composite Nanoarrays As Substrates for Surface-Enhanced Raman Scattering Detection. J. Phys. Chem. C 2009, 114, 93–100. [Google Scholar] [CrossRef]
- Sinha, G.; Depero, L.E.; Alessandri, I. Recyclable SERS Substrates Based on Au-Coated ZnO Nanorods. ACS Appl. Mater. Interfaces 2011, 3, 2557–2563. [Google Scholar] [CrossRef] [PubMed]
- Graniel, O.; Iatsunskyi, I.; Coy, E.; Humbert, C.; Barbillon, G.; Michel, T.; Maurin, D.; Balme, S.; Miele, P.; Bechelany, M. Au-Covered hollow urchin-Like ZnO nanostructures for surface-Enhanced Raman scattering sensing. J. Mater. Chem. C 2019, 7, 15066–15073. [Google Scholar] [CrossRef]
- Jue, M.; Lee, S.; Paulson, B.; Namgoong, J.-M.; Yu, H.Y.; Kim, G.; Jeon, S.; Shin, D.-M.; Choo, M.-S.; Joo, J.; et al. Optimization of ZnO Nanorod-Based Surface Enhanced Raman Scattering Substrates for Bio-Applications. Nanomaterials 2019, 9, 447. [Google Scholar] [CrossRef]
- Ohyama, M.; Kouzuka, H.; Yoko, T. Sol-Gel preparation of ZnO films with extremely preferred orientation along (002) plane from zinc acetate solution. Thin Solid Film. 1997, 306, 78–85. [Google Scholar] [CrossRef]
- Vayssieres, L. Growth of Arrayed Nanorods and Nanowires of ZnO from Aqueous Solutions. Adv. Mater. 2003, 15, 464–466. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Han, M.; Hao, X.-T.; Zhu, F. A General Low-Temperature Route for Large-Scale Fabrication of Highly Oriented ZnO Nanorod/Nanotube Arrays. J. Am. Chem. Soc. 2005, 127, 2378–2379. [Google Scholar] [CrossRef]
- Yamabi, S.; Imai, H. Growth conditions for wurtzite zinc oxide films in aqueous solutions. J. Mater. Chem. 2002, 12, 3773–3778. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, C.-M.; Thanh Thi, L.T.; Quynh Nhu, N.T.; Liao, S.-Y.; Fu, Y.-Z.; Hung, L.V.T.; Hsiao, V.K.S. Zinc Oxide Nanorod Surface-Enhanced Raman Scattering Substrates without and with Gold Nanoparticles Fabricated through Pulsed-Laser-Induced Photolysis. Appl. Sci. 2020, 10, 5015. https://doi.org/10.3390/app10145015
Chou C-M, Thanh Thi LT, Quynh Nhu NT, Liao S-Y, Fu Y-Z, Hung LVT, Hsiao VKS. Zinc Oxide Nanorod Surface-Enhanced Raman Scattering Substrates without and with Gold Nanoparticles Fabricated through Pulsed-Laser-Induced Photolysis. Applied Sciences. 2020; 10(14):5015. https://doi.org/10.3390/app10145015
Chicago/Turabian StyleChou, Chia-Man, Le Tran Thanh Thi, Nguyen Thi Quynh Nhu, Su-Yu Liao, Yu-Zhi Fu, Le Vu Tuan Hung, and Vincent K. S. Hsiao. 2020. "Zinc Oxide Nanorod Surface-Enhanced Raman Scattering Substrates without and with Gold Nanoparticles Fabricated through Pulsed-Laser-Induced Photolysis" Applied Sciences 10, no. 14: 5015. https://doi.org/10.3390/app10145015
APA StyleChou, C.-M., Thanh Thi, L. T., Quynh Nhu, N. T., Liao, S.-Y., Fu, Y.-Z., Hung, L. V. T., & Hsiao, V. K. S. (2020). Zinc Oxide Nanorod Surface-Enhanced Raman Scattering Substrates without and with Gold Nanoparticles Fabricated through Pulsed-Laser-Induced Photolysis. Applied Sciences, 10(14), 5015. https://doi.org/10.3390/app10145015





