Optimization of Optical Trapping and Laser Interferometry in Biological Cells
Abstract
1. Introduction
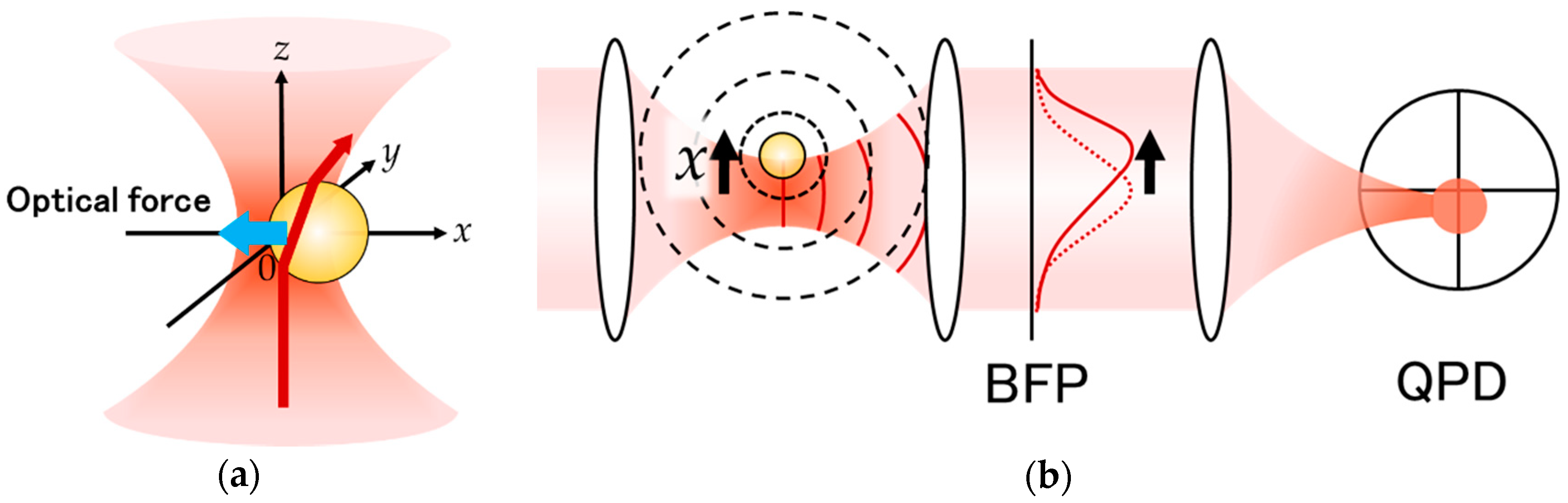
2. Laser Interferometry
3. Materials and Methods
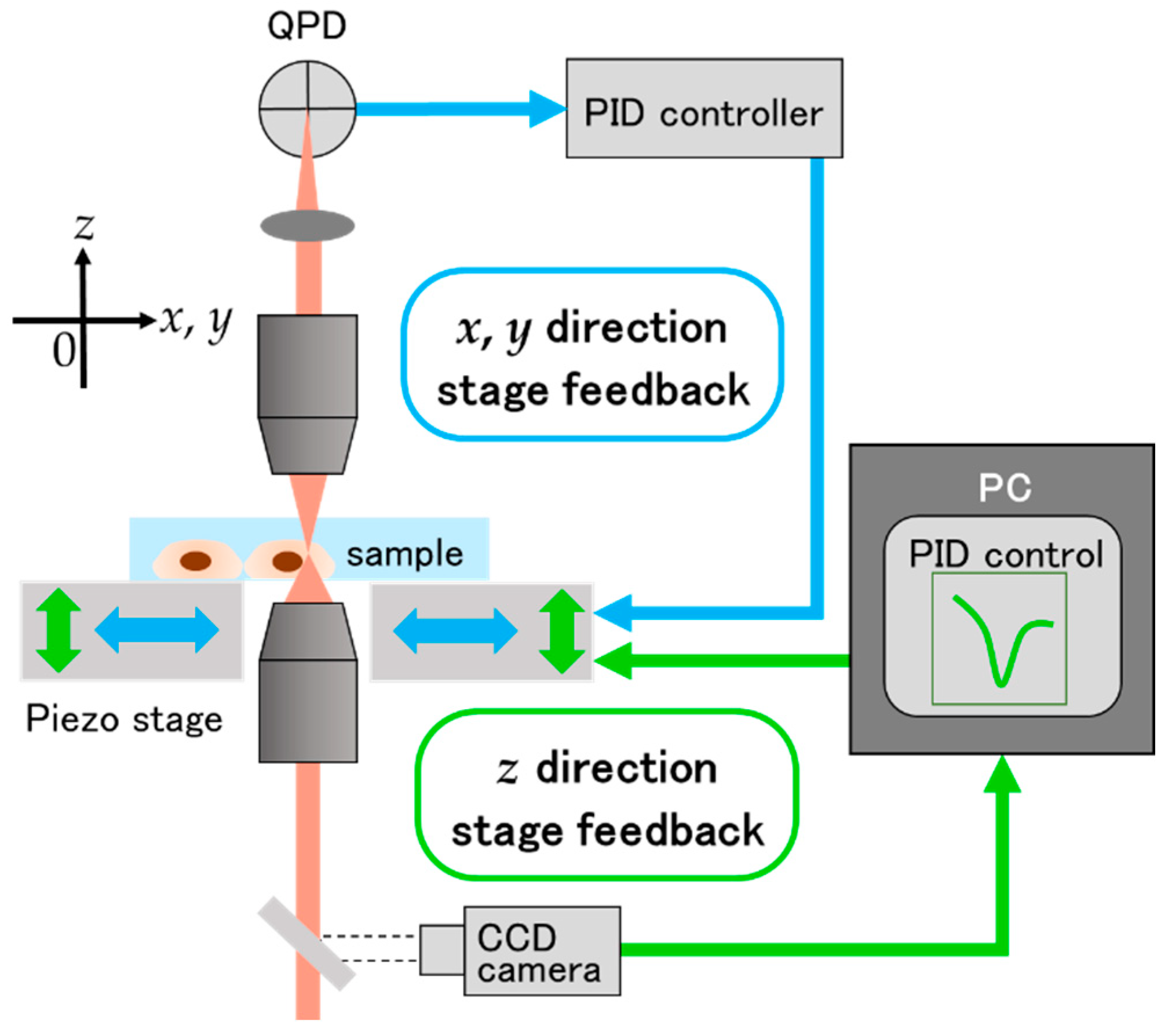
3.1. Experimental Setup
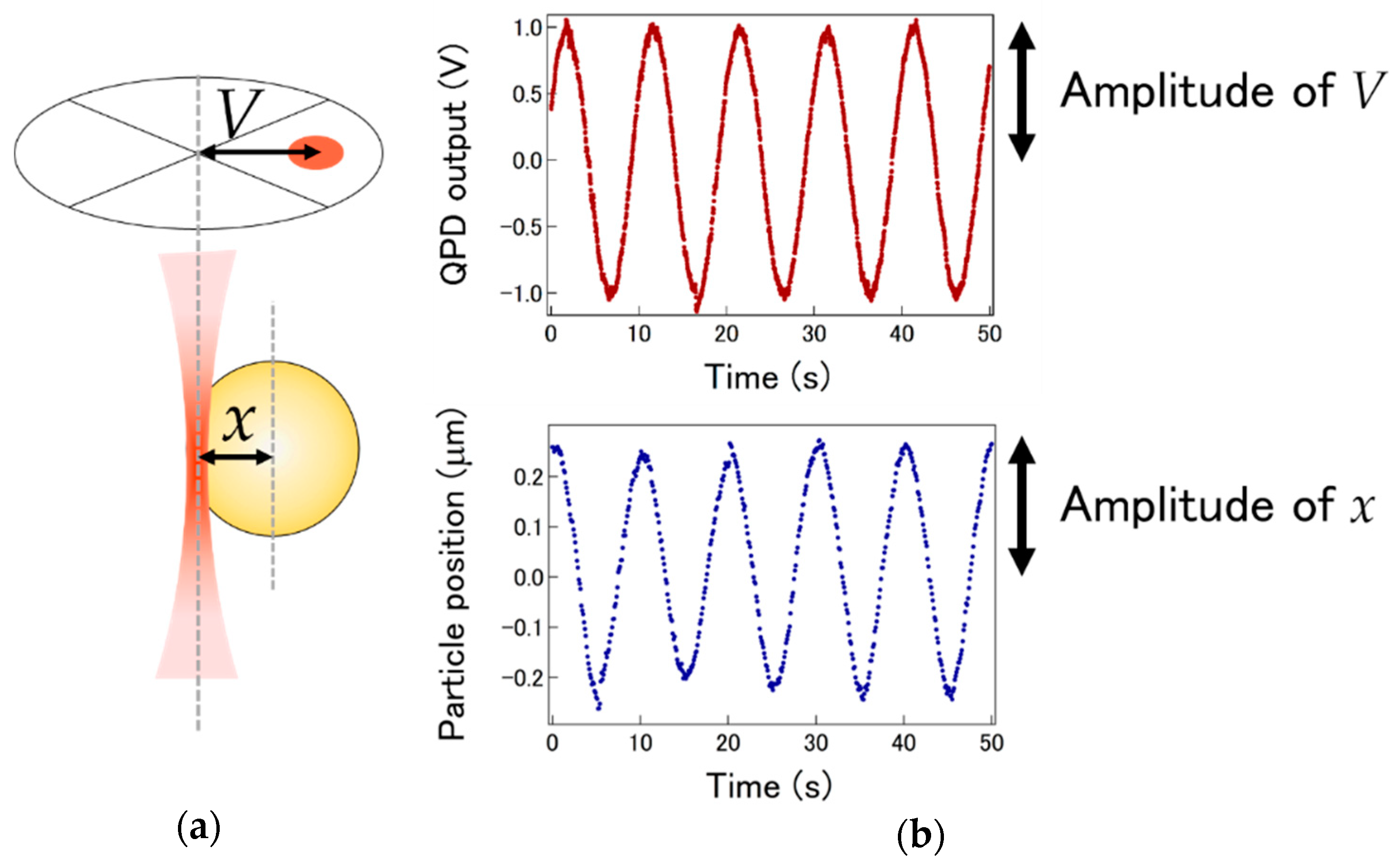
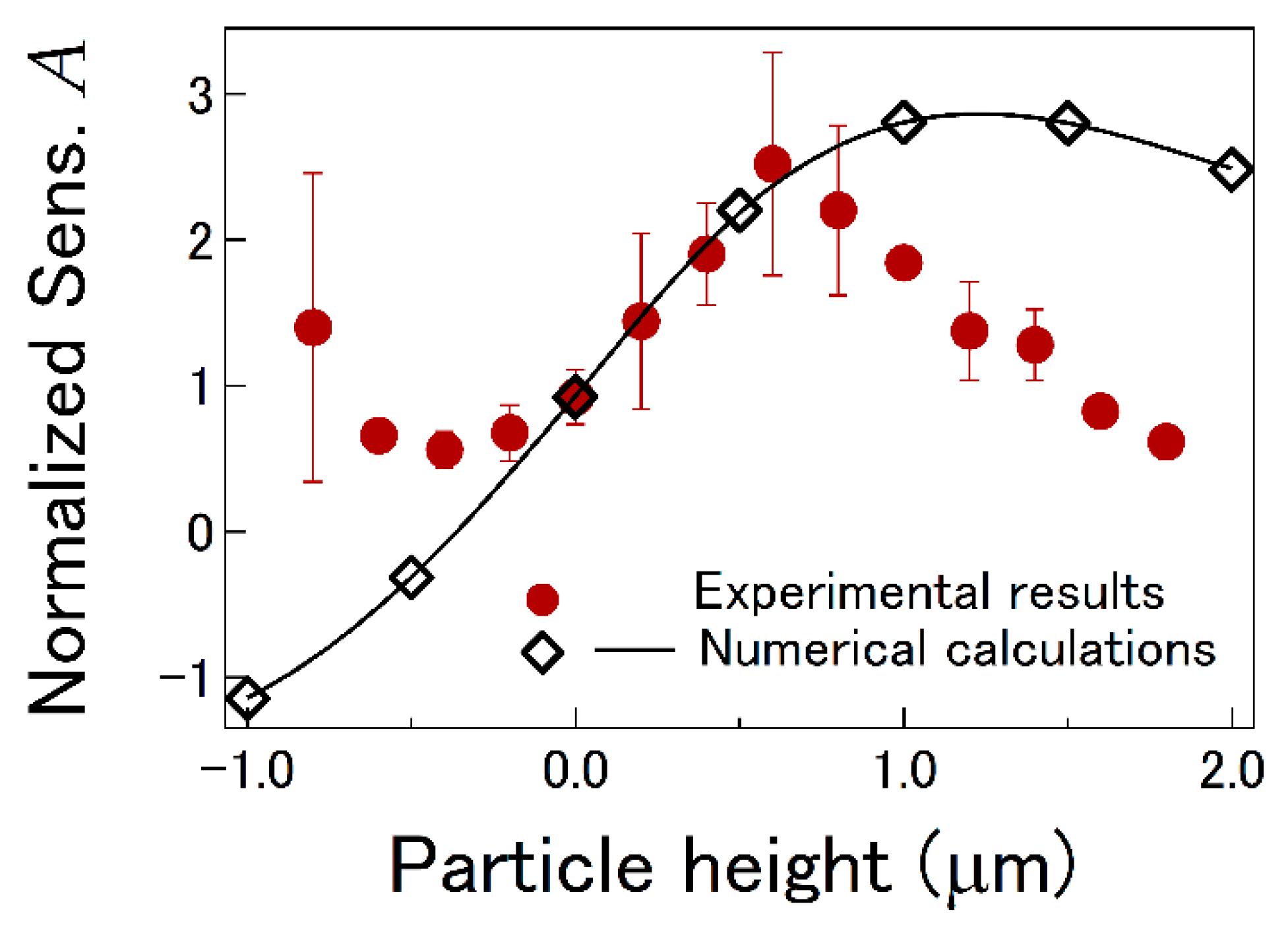
3.2. LI Sensitivity
3.3. Numerical Calculation of LI Sensitivity
3.4. Gel Preparation
3.5. Cell Preparation
3.6. Control of Intracellular Concentrations
4. Experimental Results and Discussions
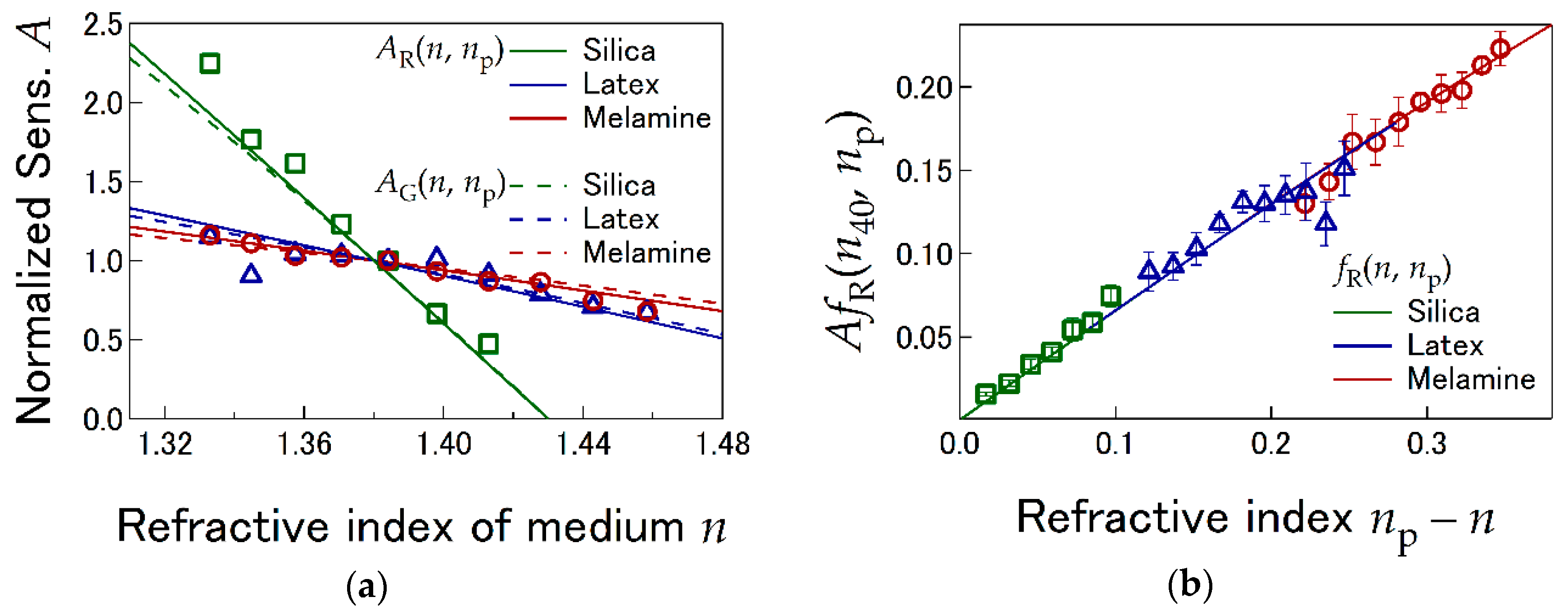
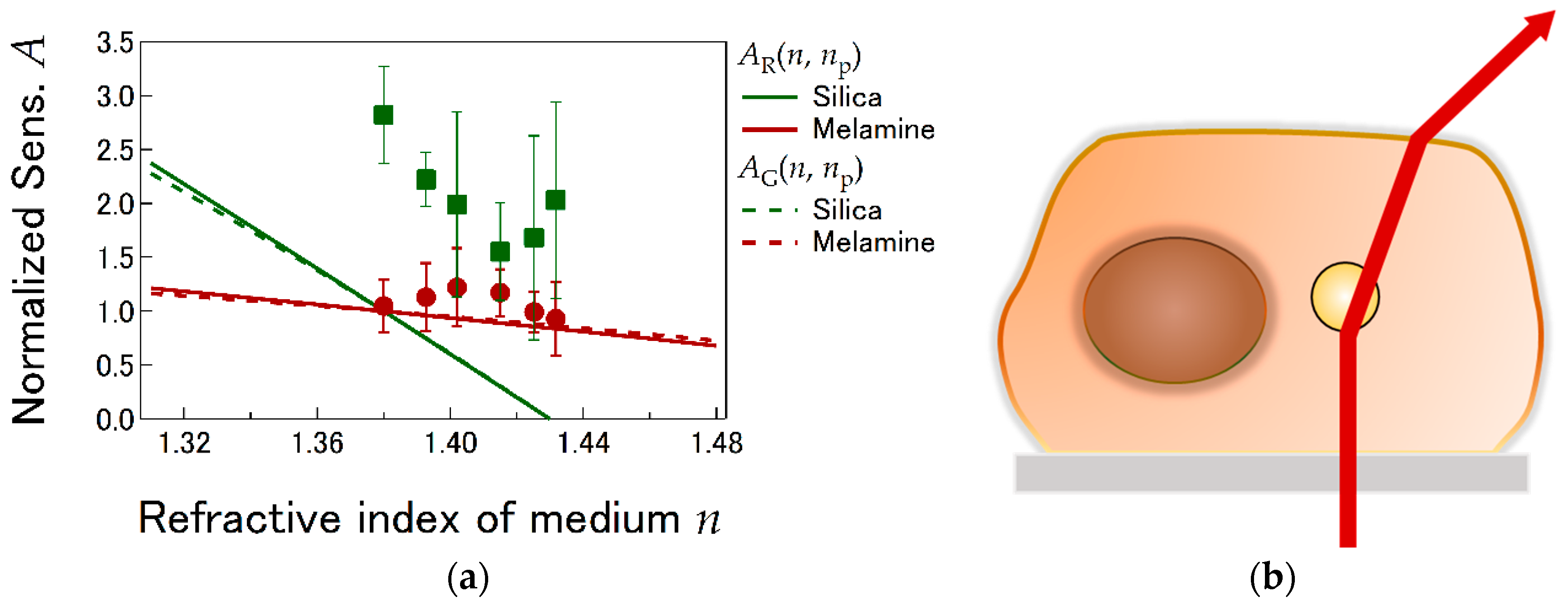
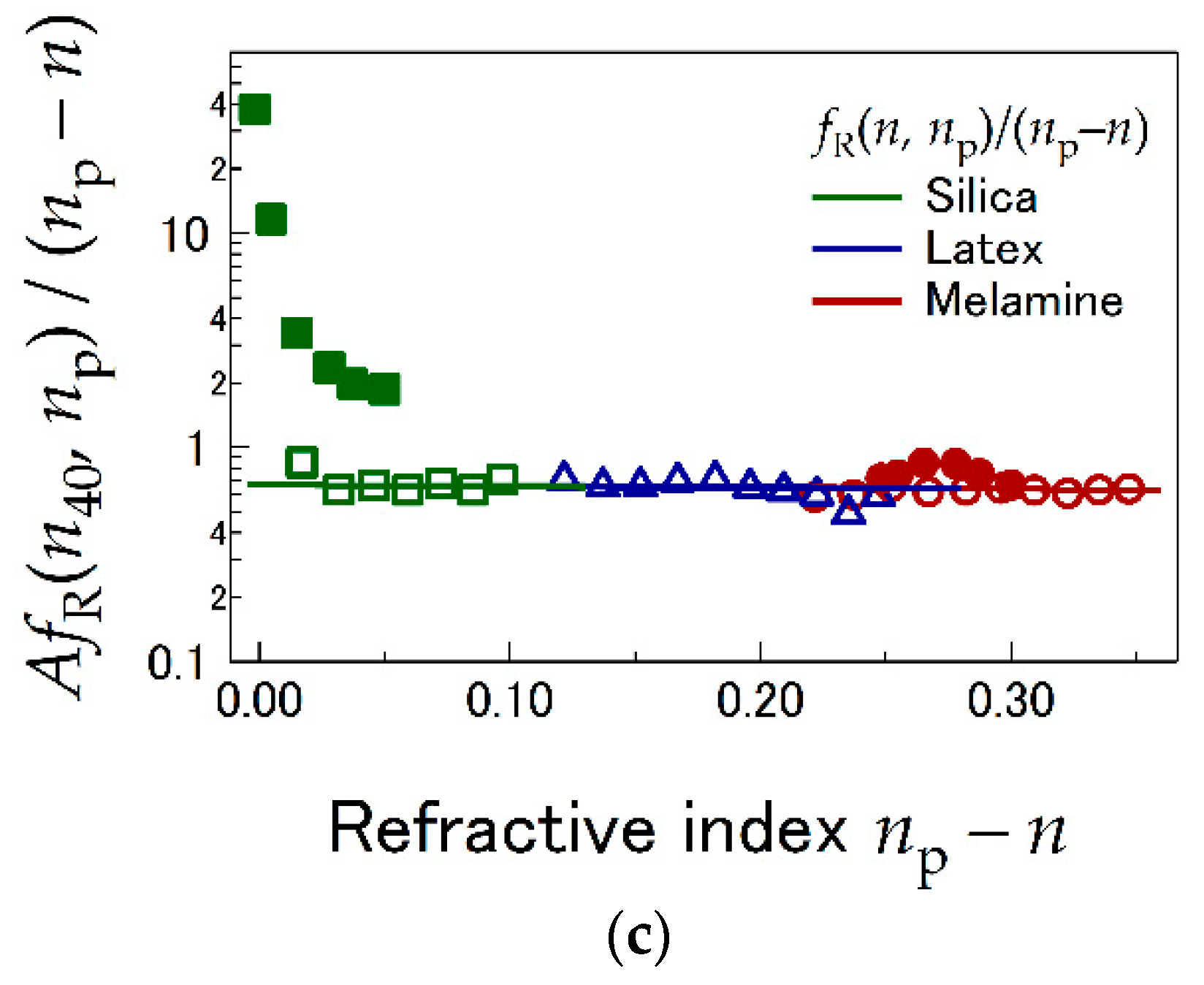
4.1. LI Sensitivity Depending on Optical Properties
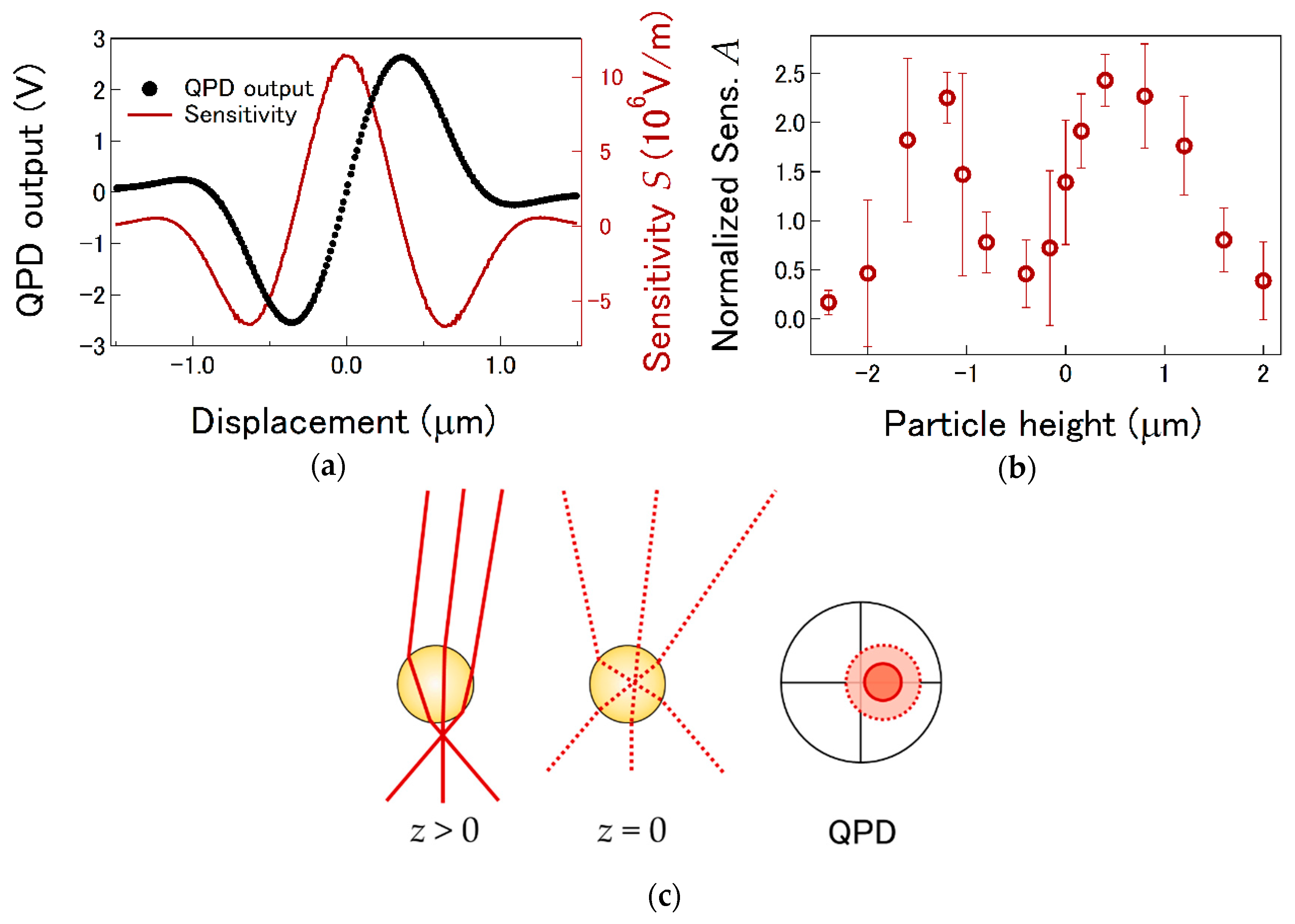
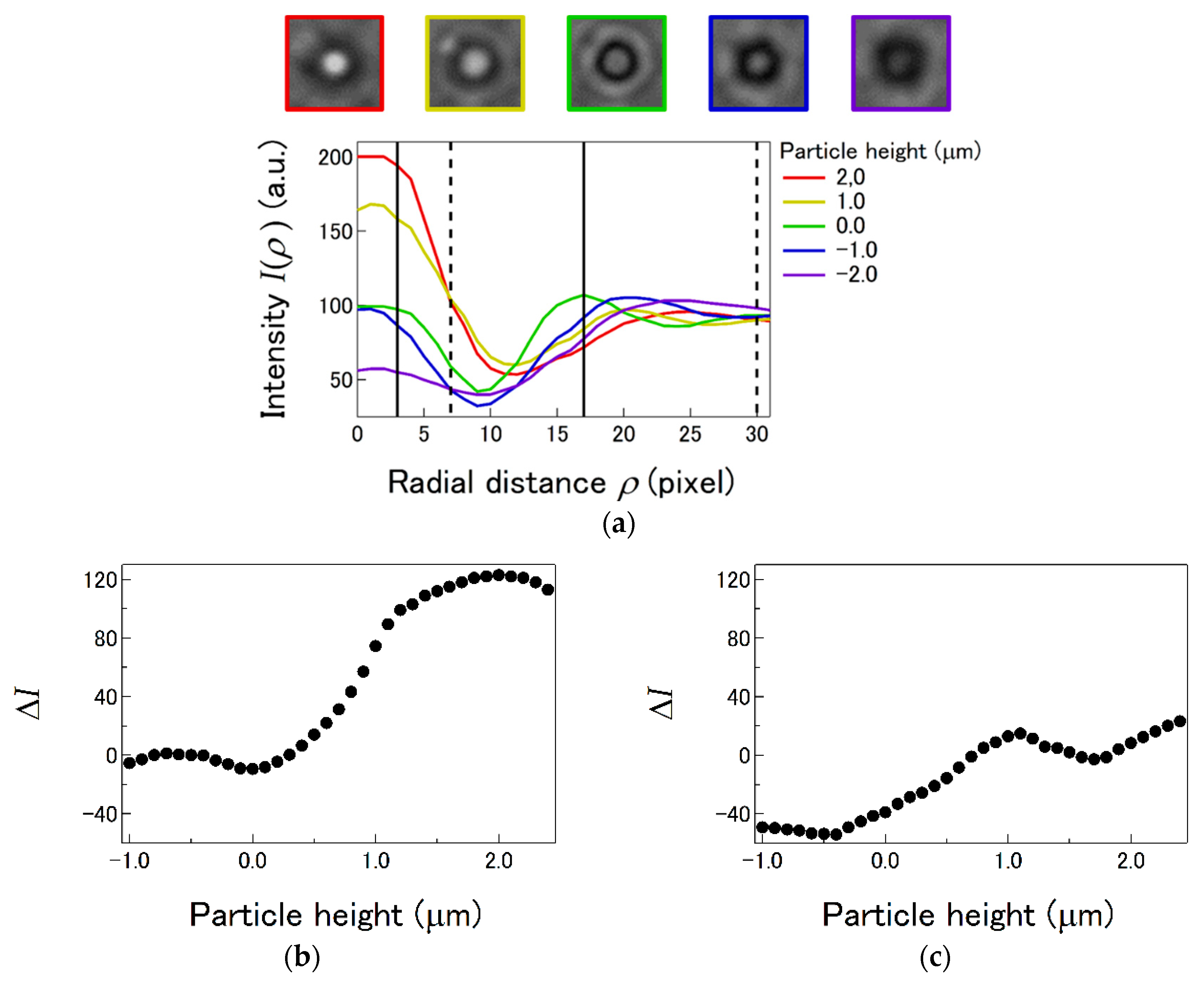
4.2. LI Sensitivity Depending on Radial and Axial Offsets
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ashkin, A.; Dziedzic, J.M.; Bjorkholm, J.E.; Chu, S. Observation of a Single-Beam Gradient Force Optical Trap for Dielectric Particles. Opt. Lett. 1986, 11, 288–290. [Google Scholar] [CrossRef]
- Ashkin, A. Optical trapping and manipulation of neutral particles using lasers. Proc. Natl. Acad. Sci. USA 1997, 94, 4853–4860. [Google Scholar] [CrossRef]
- Svoboda, K.; Block, S.M. Biological Applications of Optical Forces. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 247–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, S.; Kong, M.; Wang, Z.; Costa, K.D.; Li, R.A.; Sun, D. Enhanced cell sorting and manipulation with combined optical tweezer and microfluidic chip technologies. Lab Chip 2011, 11, 3656–3662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, K.K. Optical tweezers for single cells. J. R. Soc. Interface 2008, 5, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Gittes, F.; Schmidt, C.F. Interference model for back-focal-plane displacement detection in optical tweezers. Opt. Lett. 1998, 23, 7–9. [Google Scholar] [CrossRef]
- Ariga, T.; Tomishige, M.; Mizuno, D. Nonequilibrium Energetics of Molecular Motor Kinesin. Phys. Rev. Lett. 2018, 121, 218101. [Google Scholar] [CrossRef]
- Svoboda, K.; Schmidt, C.F.; Schnapp, B.J.; Block, S.M. Direct Observation of Kinesin Stepping by Optical Trapping Interferometry. Nature 1993, 365, 721–727. [Google Scholar] [CrossRef]
- Schurmann, M.; Scholze, J.; Muller, P.; Guck, J.; Chan, C.J. Cell nuclei have lower refractive index and mass density than cytoplasm. J. Biophotonics 2016, 9, 1068–1076. [Google Scholar] [CrossRef]
- Przibilla, S.; Dartmann, S.; Vollmer, A.; Ketelhut, S.; Greve, B.; von Bally, G.; Kemper, B. Sensing dynamic cytoplasm refractive index changes of adherent cells with quantitative phase microscopy using incorporated microspheres as optical probes. J. Biomed. Opt. 2012, 17, 097001. [Google Scholar] [CrossRef]
- Kam, Z.; Hanser, B.; Gustafsson, M.G.; Agard, D.A.; Sedat, J.W. Computational adaptive optics for live three-dimensional biological imaging. Proc. Natl. Acad. Sci. USA 2001, 98, 3790–3795. [Google Scholar] [CrossRef] [PubMed]
- Mullenbroich, M.C.; McAlinden, N.; Wright, A.J. Adaptive optics in an optical trapping system for enhanced lateral trap stiffness at depth. J. Opt. UK 2013, 15, 075305. [Google Scholar] [CrossRef]
- Seitz, P.C.; Stelzer, E.H.K.; Rohrbach, A. Interferometric tracking of optically trapped probes behind structured surfaces: A phase correction method. Appl. Opt. 2006, 45, 7309–7315. [Google Scholar] [CrossRef] [PubMed]
- Norregaard, K.; Metzler, R.; Ritter, C.M.; Berg-Sorensen, K.; Oddershede, L.B. Manipulation and Motion of Organelles and Single Molecules in Living Cells. Chem. Rev. 2017, 117, 4342–4375. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, K.; Bremerich, M.; Ayade, H.; Schmidt, C.F.; Ariga, T.; Mizuno, D. Feedback-tracking microrheology in living cells. Sci. Adv. 2017, 3, e1700318. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, A.; Zolotovskii, I.; Stoliarov, D.; Vorsina, S.; Liamina, D.; Pogodina, E.; Fotiadi, A.A.; Sokolovski, S.G.; Saenko, Y.; Rafailov, E.U. The Photobiomodulation of Vital Parameters of the Cancer Cell Culture by Low Dose of Near-IR Laser Irradiation. IEEE J. Sel. Top. Quant. Electron. 2019, 25, 1–10. [Google Scholar] [CrossRef]
- Konig, K.; Liang, H.; Berns, M.W.; Tromberg, B.J. Cell damage by near-IR microbeams. Nature 1995, 377, 20–21. [Google Scholar] [CrossRef]
- Neuman, K.C.; Chadd, E.H.; Liou, G.F.; Bergman, K.; Block, S.M. Characterization of photodamage to Escherichia coli in optical traps. Biophys. J. 1999, 77, 2856–2863. [Google Scholar] [CrossRef]
- Hulst, H.C.; van de Hulst, H.C. Light Scattering by Small Particles; Dover Publications: New York, NY, USA, 1981. [Google Scholar]
- Vermeulen, K.C.; Wuite, G.J.; Stienen, G.J.; Schmidt, C.F. Optical trap stiffness in the presence and absence of spherical aberrations. Appl. Opt. 2006, 45, 1812–1819. [Google Scholar] [CrossRef]
- Ashkin, A. Forces of a Single-Beam Gradient Laser Trap on a Dielectric Sphere in the Ray Optics Regime. Biophys. J. 1992, 61, 569–582. [Google Scholar] [CrossRef]
- Jones, P.H.; Maragò, O.M.; Volpe, G. Optical Tweezers: Principles and Applications; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar] [CrossRef]
- Mizuno, D.; Head, D.A.; MacKintosh, F.C.; Schmidt, C.F. Active and Passive Microrheology in Equilibrium and Nonequilibrium Systems. Macromolecules 2008, 41, 7194–7202. [Google Scholar] [CrossRef]
- Gittes, F.; Schmidt, C.F. Signals and noise in micromechanical measurements. Methods Cell Biol. 1998, 55, 129–156. [Google Scholar] [CrossRef]
- Phillips, K.G.; Jacques, S.L.; McCarty, O.J.T. Measurement of Single Cell Refractive Index, Dry Mass, Volume, and Density Using a Transillumination Microscope. Phys. Rev. Lett. 2012, 109, 118105. [Google Scholar] [CrossRef] [PubMed]
- Lue, N.; Popescu, G.; Ikeda, T.; Dasari, R.R.; Badizadegan, K.; Feld, M.S. Live cell refractometry using microfluidic devices. Opt. Lett. 2006, 31, 2759–2761. [Google Scholar] [CrossRef]
- Sernetz, M.; Thaer, A. Immersion Refractometry on Living Cells with Method of Refractive Index Gradient. Z. Anal. Chem. Fresenius 1970, 252, 90. [Google Scholar] [CrossRef]
- Barer, R.; Joseph, S. Refractometry of Living Cells 1. Basic Principles. Q. J. Microsc. Sci. 1954, 95, 399–423. [Google Scholar] [CrossRef]
- Rappaz, B.; Marquet, P.; Cuche, E.; Emery, Y.; Depeursinge, C.; Magistretti, P.J. Measurement of the integral refractive index and dynamic cell morphometry of living cells with digital holographic microscopy. Opt. Express 2005, 13, 9361–9373. [Google Scholar] [CrossRef]
- Curl, C.L.; Bellair, C.J.; Harris, T.; Allman, B.E.; Harris, P.J.; Stewart, A.G.; Roberts, A.; Nugent, K.A.; Delbridge, L.M.D. Refractive index measurement in viable cells using quantitative phase-amplitude microscopy and confocal microscopy. Cytom. Part A 2005, 65, 88–92. [Google Scholar] [CrossRef]
- Glycerine Producers Association. Physical Properties of Glycerine and Its Solutions; Glycerine Producers Association: New York, NY, USA, 1963. [Google Scholar]
- Laven, P. Simulation of rainbows, coronas, and glories by use of Mie theory. Appl. Opt. 2003, 42, 436–444. [Google Scholar] [CrossRef]
- Ting-Beall, H.P.; Needham, D.; Hochmuth, R.M. Volume and osmotic properties of human neutrophils. Blood 1993, 81, 2774–2780. [Google Scholar] [CrossRef]
- Guilak, F.; Erickson, G.R.; Ting-Beall, H.P. The effects of osmotic stress on the viscoelastic and physical properties of articular chondrocytes. Biophys. J. 2002, 82, 720–727. [Google Scholar] [CrossRef]
- Zhou, E.H.; Trepat, X.; Park, C.Y.; Lenormand, G.; Oliver, M.N.; Mijailovich, S.M.; Hardin, C.; Weitz, D.A.; Butler, J.P.; Fredberg, J.J. Universal behavior of the osmotically compressed cell and its analogy to the colloidal glass transition. Proc. Natl. Acad. Sci. USA 2009, 106, 10632–10637. [Google Scholar] [CrossRef] [PubMed]
- Tasic, A.Z.; Djordjevic, B.D.; Grozdanic, D.K.; Radojkovic, N. Use of Mixing Rules in Predicting Refractive-Indexes and Specific Refractivities for Some Binary-Liquid Mixtures. J. Chem. Eng. Data 1992, 37, 310–313. [Google Scholar] [CrossRef]
- Chang, A.T.; Chang, Y.R.; Chi, S.; Hsu, L. Optimization of probe-laser focal offsets for single-particle tracking. Appl. Opt. 2012, 51, 5643–5648. [Google Scholar] [CrossRef] [PubMed]
- Bodensiek, K.; Li, W.X.; Sanchez, P.; Nawaz, S.; Schaap, I.A.T. A high-speed vertical optical trap for the mechanical testing of living cells at piconewton forces. Rev. Sci. Instrum. 2013, 84, 113707. [Google Scholar] [CrossRef]
- Lee, S.H.; Grier, D.G. Holographic microscopy of holographically trapped three-dimensional structures. Opt. Express 2007, 15, 1505–1512. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugino, Y.; Ikenaga, M.; Mizuno, D. Optimization of Optical Trapping and Laser Interferometry in Biological Cells. Appl. Sci. 2020, 10, 4970. https://doi.org/10.3390/app10144970
Sugino Y, Ikenaga M, Mizuno D. Optimization of Optical Trapping and Laser Interferometry in Biological Cells. Applied Sciences. 2020; 10(14):4970. https://doi.org/10.3390/app10144970
Chicago/Turabian StyleSugino, Yujiro, Masahiro Ikenaga, and Daisuke Mizuno. 2020. "Optimization of Optical Trapping and Laser Interferometry in Biological Cells" Applied Sciences 10, no. 14: 4970. https://doi.org/10.3390/app10144970
APA StyleSugino, Y., Ikenaga, M., & Mizuno, D. (2020). Optimization of Optical Trapping and Laser Interferometry in Biological Cells. Applied Sciences, 10(14), 4970. https://doi.org/10.3390/app10144970




