Advances in Materials Design for All-Solid-state Batteries: From Bulk to Thin Films
Abstract
Featured Application
Abstract
1. Introduction
2. Electrodes and Electrolytes for Lithium-Based Batteries
2.1. Electrodes
2.1.1. Anodes
2.1.2. Cathodes
2.2. Solid Electrolytes
3. Interfacial Phenomena between Solid-State Electrolytes and Electrodes
3.1. Deposition Techniques
3.1.1. Physical Deposition Methods
3.1.2. Chemical Deposition Methods
3.2. Buffer Layers
3.2.1. Buffer Layers at the Electrolyte/Cathode Interface
3.2.2. Buffer Layers at Solid-State Electrolyte/Anode
4. Electrodes and Electrolytes for SSTFBs
4.1. Thin-film Electrodes
4.1.1. Anodes
4.1.2. Cathodes
4.2. Thin-film Electrolytes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.; Zhang, L.; Sun, F.; Wang, Z. An overview on thermal safety issues of lithium-ion batteries for electric vehicle application. IEEE Access 2018, 6, 23848–23863. [Google Scholar] [CrossRef]
- Dubarry, M.; Devie, A. Battery durability and reliability under electric utility grid operations: Representative usage aging and calendar aging. J. Energy Storage 2018, 18, 185–195. [Google Scholar] [CrossRef]
- Lopez, J.; Gonzalez, M.; Viera, J.C.; Blanco, C. Fast-charge in lithium-ion batteries for portable applications. In Proceedings of the INTELEC 2004. 26th Annual International Telecommunications Energy Conference, Chicago, IL, USA, 19–23 September 2004. [Google Scholar]
- Karden, E.; Ploumen, S.; Fricke, B.; Miller, T.; Snyder, K. Energy storage devices for future hybrid electric vehicles. J. Power Sources 2007, 168, 2–11. [Google Scholar] [CrossRef]
- Nishi, Y. Lithium ion secondary batteries: Past 10 years and the future. J. Power Sources 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Ge, S.; Leng, Y.; Liu, T.; Longchamps, R.S.; Yang, X.G.; Gao, Y.; Wang, D.; Wang, D.; Wang, C.Y. A new approach to both high safety and high performance of lithium-ion batteries. Sci. Adv. 2020, 6, eaay7633. [Google Scholar] [CrossRef]
- Schmidt, M.; Neuschütz, M. Lithium-ion batteries key component electrolyte. ATZ Worldw. 2011, 6, 10–15. [Google Scholar] [CrossRef]
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Plug-in Electric Light Vehicle Sales Worldwide 2015–2019. Available online: https://www.statista.com/statistics/665774/global-sales-of-plug-in-light-vehicles/#:~:text=In%202019%2C%20around%202.2%20million,(PEV)%20were%20sold%20worldwide (accessed on 11 June 2020).
- Peljo, P.; Girault, H.H. Electrochemical potential window of battery electrolytes: The HOMO-LUMO misconception. Energy Environ. Sci. 2018, 11, 2306–2309. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Grugeon, S.; Laruelle, S.; Boyanov, S.; Lecocq, A.; Bertrand, J.P.; Marlair, G. In-depth safety-focused analysis of solvents used in electrolytes for large scale lithium ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 9145–9155. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lu, S.; Shi, L.; Cheng, X.; Zhang, H. Combustion characteristics of electrolyte pool fires for lithium ion batteries. J. Electrochem. Soc. 2016, 163, A2022–A2028. [Google Scholar] [CrossRef]
- Fan, H.; Qi, L.; Yoshio, M.; Wang, H. Hexafluorophosphate intercalation into graphite electrode from ethylene carbonate/ethylmethyl carbonate. Solid State Ion. 2017, 304, 107–112. [Google Scholar] [CrossRef]
- Ding, W.; Lei, X.; Ouyang, C. Coordination of lithium ion with ethylene carbonate electrolyte solvent: A computational study. Int. J. Quantum Chem. 2016, 116, 97–102. [Google Scholar] [CrossRef]
- Younesi, R.; Veith, G.M.; Johansson, P.; Edström, K.; Vegge, T. Lithium salts for advanced lithium batteries: Li-metal, Li-O2, and Li-S. Energy Environ. Sci. 2015, 8, 1905–1922. [Google Scholar] [CrossRef]
- Liu, L.; Gu, S.; Wang, S.; Zhang, X.; Chen, S. A LiPO2F2/LiPF6 dual-salt electrolyte enabled stable cycling performance of nickel-rich lithium ion batteries. RSC Adv. 2020, 10, 1704–1710. [Google Scholar] [CrossRef]
- Miller, T.F.; Wang, Z.G.; Coates, G.W.; Balsara, N.P. Designing polymer electrolytes for safe and high capacity rechargeable lithium batteries. Acc. Chem. Res. 2017, 50, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Arbizzani, C.; Gabrielli, G.; Mastragostino, M. Thermal stability and flammability of electrolytes for lithium-ion batteries. J. Power Sources 2011, 196, 4801–4805. [Google Scholar] [CrossRef]
- Takehara, Z.I. Future prospects of the lithium metal anode. J. Power Sources 1997, 68, 82–86. [Google Scholar] [CrossRef]
- Takeda, Y.; Yamamoto, O.; Imanishi, N. Lithium dendrite formation on a lithium metal anode from liquid, polymer and solid electrolytes. Electrochemistry 2016, 84, 210–218. [Google Scholar] [CrossRef]
- Xu, R.C.; Wang, X.L.; Zhang, S.Z.; Xia, Y.; Xia, X.H.; Wu, J.B.; Tu, J.P. Rational coating of Li7P3S11 solid electrolyte on MoS2 electrode for all-solid-state lithium ion batteries. J. Power Sources 2018, 374, 107–112. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Zhang, G.; Ma, A.; Chen, W.; Shao, L.; Shen, C.; Xie, K. High-performance solid composite polymer electrolyte for all solid-state lithium battery through facile microstructure regulation. Front. Chem. 2019, 7, 338. [Google Scholar] [CrossRef]
- Xu, H.; Chien, P.H.; Shi, J.; Li, Y.; Wu, N.; Liu, Y.; Hu, Y.Y.; Goodenough, J.B. High-performance all-solid-state batteries enabled by salt bonding to perovskite in poly (ethylene oxide). Proc. Natl. Acad. Sci. USA 2019, 116, 18815–18821. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Zhu, Y.; Jiang, P. Cellulose nanofiber supported 3D interconnected BN nanosheets for epoxy nanocomposites with ultrahigh thermal management capability. Adv. Funct. Mater. 2017, 27, 1604754. [Google Scholar] [CrossRef]
- Hou, H.; Xu, Q.; Pang, Y.; Li, L.; Wang, J.; Zhang, C.; Sun, C. Efficient storing energy harvested by triboelectric nanogenerators using a safe and durable all-solid-state sodium-ion battery. Adv. Sci. 2017, 4, 1700072. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, E.; Carewska, M.; Maresca, G.; De Francesco, M.; Appetecchi, G.B. Highly conductive, ionic liquid-based polymer electrolytes. J. Electrochem. Soc. 2016, 164, A6213–A6219. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Wu, X.; Yao, S. Flexible electrode materials based on WO3 nanotube bundles for high performance energy storage devices. Nano Energy 2017, 42, 143–150. [Google Scholar] [CrossRef]
- Wu, F.; Yushin, G. Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ. Sci. 2017, 10, 435–459. [Google Scholar] [CrossRef]
- Taberna, P.L.; Mitra, S.; Poizot, P.; Simon, P.; Tarascon, J.M. High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nat. Mater. 2006, 5, 567–573. [Google Scholar] [CrossRef]
- Liu, W.; Lin, D.; Pei, A.; Cui, Y. Stabilizing lithium metal anodes by uniform Li-ion flux distribution in nanochannel confinement. J. Am. Chem. Soc. 2016, 138, 15443–15450. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Bruce, P.G. Synthesis of layered LiMnO2 as an electrode for rechargeable lithium batteries. Nature 1996, 381, 499–500. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, W.; Huq, A.; Misture, S.T.; Zhang, B.; Guo, S.; Wu, L.; Zhu, Y.; Chen, Z.; Amine, K.; et al. In Situ probing and synthetic control of cationic ordering in Ni-rich layered oxide cathodes. Adv. Energy Mater. 2017, 7, 1601266. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, T.; Hu, Z.; Wei, Y.; Song, X.; Ren, Y.; Wang, W.; Rao, M.; Lin, Y.; Chen, Z.; et al. Tuning of thermal stability in layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 2016, 138, 13326–13334. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, G.; Yan, K.; Xie, J.; Li, Y.; Liao, L.; Jin, Y.; Liu, K.; Hsu, P.C.; Wang, J.; et al. Air-stable and freestanding lithium alloy/graphene foil as an alternative to lithium metal anodes. Nat. Nanotechnol. 2017, 12, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Tang, H.; Li, L.; Hu, Q.; Zhang, L.; Xue, H.; Pang, H. Hierarchically nanostructured transition metal oxides for lithium-ion batteries. Adv. Sci. 2018, 5, 1700592. [Google Scholar] [CrossRef]
- Liu, H.; Bugnet, M.; Tessaro, M.Z.; Harris, K.J.; Dunham, M.J.R.; Jiang, M.; Goward, G.R.; Botton, G.A. Spatially resolved surface valence gradient and structural transformation of lithium transition metal oxides in lithium-ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 29064–29075. [Google Scholar] [CrossRef]
- Shukla, A.K.; Ramasse, Q.M.; Ophus, C.; Kepaptsoglou, D.M.; Hage, F.S.; Gammer, C.; Bowling, C.; Gallegos, P.A.H.; Venkatachalam, S. Effect of composition on the structure of lithium- and manganese-rich transition metal oxides. Energy Environ. Sci. 2018, 11, 830–840. [Google Scholar] [CrossRef]
- Chan, C.K.; Zhang, X.F.; Cui, Y. High capacity Li ion battery anodes using Ge nanowires. Nano Lett. 2008, 8, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Meduri, P.; Pendyala, C.; Kumar, V.; Sumanasekera, G.U.; Sunkara, M.K. Hybrid tin oxide nanowires as stable and high capacity anodes for Li-ion batteries. Nano Lett. 2009, 9, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Guyomard, D.; Sigala, C.; de Gal La Salle, A.; Piffard, Y. New amorphous oxides as high capacity negative electrodes for lithium batteries: The LixMVO4 (M = Ni, Co, Cd, Zn; 1 < x ≤ 8) series. J. Power Sources 1997, 68, 692–697. [Google Scholar]
- Piffard, Y.; Leroux, F.; Guyomard, D.; Mansot, J.L.; Tournoux, M. The amorphous oxides MnV2O6+δ (0 < δ < 1) as high capacity negative electrode materials for lithium batteries. J. Power Sources 1997, 68, 698–703. [Google Scholar]
- Shodai, T.; Okada, S.; Tobishima, S.; Yamaki, J. Anode performance of a new layered nitride Li3−xCoxN (x = 0.2–0.6). J. Power Sources 1997, 68, 515–518. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Xu, R.; Han, F.; Ji, X.; Fan, X.; Tu, J.; Wang, C. Interface engineering of sulfide electrolytes for all-solid-state lithium batteries. Nano Energy 2018, 53, 958–966. [Google Scholar] [CrossRef]
- Wang, G.X.; Bradhurst, D.H.; Dou, S.X.; Liu, H.K. LiTi2(PO4)3 with NASICON-type structure as lithium-storage materials. J. Power Sources 2003, 124, 231–236. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Chien, P.H.; Wu, N.; Xin, S.; Xue, L.; Park, K.; Hu, Y.Y.; Goodenough, J.B. A perovskite electrolyte that is stable in moist air for lithium-ion batteries. Angew. Chem. Int. Ed. 2018, 57, 8587–8591. [Google Scholar] [CrossRef]
- Al-Qawasmeh, A.; Holzwarth, N.A.W. Li14P2O3N6 and Li7PN4: Computational study of two nitrogen rich crystalline LiPON electrolyte materials. J. Power Sources 2017, 364, 410–419. [Google Scholar] [CrossRef]
- Asano, T.; Sakai, A.; Ouchi, S.; Sakaida, M.; Miyazaki, A.; Hasegawa, S. Solid halide electrolytes with high lithium-ion conductivity for application in 4 V class bulk-type all-solid-state batteries. Adv. Mater. 2018, 30, 1803075. [Google Scholar] [CrossRef]
- Maekawa, H.; Matsuo, M.; Takamura, H.; Ando, M.; Noda, Y.; Karahashi, T.; Orimo, S.I. Halide-stabilized LiBH4, a room-temperature lithium fast-ion conductor. J. Am. Chem. Soc. 2009, 131, 894–895. [Google Scholar] [CrossRef]
- Seino, Y.; Ota, T.; Takada, K.; Hayashi, A.; Tatsumisago, M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 2014, 7, 627–631. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.K.; Gong, Y.; Xu, S.; Zhu, Y.; Li, Y.; Dai, J.; Wang, C.; Liu, B.; Pastel, G.; Xie, H.; et al. Stabilizing the garnet solid-electrolyte/polysulfide interface in Li-S batteries. Chem. Mater. 2017, 29, 8037–8041. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.X.; Zheng, G.; Zhong, G.; Wang, D.; Fu, R.; Yang, Y. Toward understanding of ion dynamics in highly conductive lithium ion conductors: Some perspectives by solid state NMR techniques. Solid State Ion. 2018, 318, 19–26. [Google Scholar] [CrossRef]
- Loho, C.; Djenadic, R.; Bruns, M.; Clemens, O.; Hahn, H. Garnet-type Li7La3Zr2O12 solid electrolyte thin films grown by CO2-laser assisted CVD for all-solid-state batteries. J. Electrochem. Soc. 2016, 164, A6131–A6139. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, J.Z.; Xin, H.L.; Han, L.; Grillon, N.; Guy-Bouyssou, D.; Bouyssou, E.; Proust, M.; Meng, Y.S. Effects of cathode electrolyte interfacial (CEI) layer on long term cycling of all-solid-state thin-film batteries. J. Power Sources 2016, 324, 342–348. [Google Scholar] [CrossRef]
- Lobe, S.; Dellen, C.; Finsterbusch, M.; Gehrke, H.G.; Sebold, D.; Tsai, C.L.; Uhlenbruck, S.; Guillon, O. Radio frequency magnetron sputtering of Li7La3Zr2O12 thin films for solid-state batteries. J. Power Sources 2016, 307, 684–689. [Google Scholar] [CrossRef]
- Larfaillou, S.; Guy-Bouyssou, D.; le Cras, F.; Franger, S. Comprehensive characterization of all-solid-state thin films commercial microbatteries by electrochemical impedance spectroscopy. J. Power Sources 2016, 319, 139–146. [Google Scholar] [CrossRef]
- Yoon, M.; Lee, S.; Lee, D.; Kim, J.; Moon, J. All-solid-state thin film battery based on well-aligned slanted LiCoO2 nanowires fabricated by glancing angle deposition. Appl. Surf. Sci. 2017, 412, 537–544. [Google Scholar] [CrossRef]
- Wang, Y.; Roller, J.; Maric, R. Direct dry synthesis of thin nanostructured LiNi0.8Co0.2O2 film for lithium ion micro-battery cathodes. Electrochim. Acta 2017, 241, 510–516. [Google Scholar] [CrossRef]
- Chan, C.K.; Ruffo, R.; Hong, S.S.; Huggins, R.A.; Cui, Y. Structural and electrochemical study of the reaction of lithium with silicon nanowires. J. Power Sources 2009, 189, 34–39. [Google Scholar] [CrossRef]
- Chen, J.S.; Lou, X.W. Anatase TiO2 nanosheet: An ideal host structure for fast and efficient lithium insertion/extraction. Electrochem. Commun. 2009, 11, 2332–2335. [Google Scholar] [CrossRef]
- Chou, S.L.; Wang, J.Z.; Sun, J.Z.; Wexler, D.; Forsyth, M.; Liu, H.K.; MacFarlane, D.R.; Dou, S.X. High capacity, safety, and enhanced cyclability of lithium metal battery using a V2O5 nanomaterial cathode and room temperature ionic liquid electrolyte. Chem. Mater. 2008, 20, 7044–7051. [Google Scholar] [CrossRef]
- Dokko, K.; Nakata, N.; Kanamura, K. High rate discharge capability of single particle electrode of LiCoO2. J. Power Sources 2009, 189, 783–785. [Google Scholar] [CrossRef]
- Jang, Y.I.; Huang, B.; Wang, H.; Sadoway, D.R.; Chiang, Y.M. Electrochemical cycling-induced spinel formation in high-charge-capacity orthorhombic LiMnO2. J. Electrochem. Soc. 1999, 146, 3217. [Google Scholar] [CrossRef]
- Landi, B.J.; Ganter, M.J.; Cress, C.D.; DiLeo, R.A.; Raffaelle, R.P. Carbon nanotubes for lithium ion batteries. Energy Environ. Sci. 2009, 2, 638–654. [Google Scholar] [CrossRef]
- Mauger, A.; Xie, H.; Julien, C.M. Composite anodes for lithium-ion batteries: Status and trends. AIMS Mater. Sci. 2016, 3, 1054. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Nagayama, M. Electrochemistry and structural chemistry of LiNiO2 (R3m) for 4 volt secondary lithium cells. J. Electrochem. Soc. 1993, 140, 1862. [Google Scholar] [CrossRef]
- Reddy, T.B. Linden’s Handbook of Batteries, 4th ed.; Mcgraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Song, M.; Ahn, D. Improvement in the cycling performance of LiMn2O4 by the substitution of Fe for Mn. Solid State Ion. 1998, 112, 245–248. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Y.; Meng, Y.; Wang, Y.; Ou, J.; Guo, Y.; Xiao, D. Phytic acid derived LiFePO4 beyond theoretical capacity as high-energy density cathode for lithium ion battery. Nano Energy 2017, 34, 408–420. [Google Scholar] [CrossRef]
- Zou, Z.; Yuan, Q.; Wang, J.; Gao, Y.; Wu, Y.; Long, F.; Han, S.; Wan, Z. Hydrothermal synthesis of high specific capacity Al-doped V6O13 cathode material for lithium-ion battery. Int. J. Electrochem. Sci. 2017, 12, 1670–1679. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Zhao, C.Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Dietrich, C.; Weber, D.A.; Sedlmaier, S.J.; Indris, S.; Culver, S.P.; Walter, D.; Janek, J.; Zeier, W.G. Lithium ion conductivity in Li2S-P2S5 glasses–building units and local structure evolution during the crystallization of superionic conductors Li3PS4, Li7P3S11 and Li4P2S7. J. Mater. Chem. A 2017, 5, 18111–18119. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Zhang, X.; Liu, T.; Lin, Y.H.; Shen, Y.; Li, L.; Nan, C.W. High-conductivity argyrodite Li6PS5Cl solid electrolytes prepared via optimized sintering processes for all-solid-state lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2018, 10, 42279–42285. [Google Scholar] [CrossRef] [PubMed]
- Moitzheim, S.; Put, B.; Vereecken, P.M. Advances in 3D thin-film Li-ion batteries. Adv. Mater. Interfaces 2019, 6, 1900805. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, H. Advanced thin film cathodes for lithium ion batteries. Research 2020, 2020, 24. [Google Scholar] [CrossRef]
- Salah, M.; Murphy, P.; Hall, C.; Francis, C.; Kerr, R.; Fabretto, M. Pure silicon thin-film anodes for lithium-ion batteries: A review. J. Power Sources 2019, 414, 48–67. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Haering, R.R.; Stiles, J.A.R.; Brandt, K. Lithium Molybdenum Disulphide Battery Cathode. U.S. Patent 4,224,390, 23 September 1980. [Google Scholar]
- Li, W.; Yao, H.; Yan, K.; Zheng, G.; Liang, Z.; Chiang, Y.M.; Cui, Y. The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth. Nat. Commun. 2015, 6, 7436. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.K.; Gong, Y.; Liu, B.; Zhu, Y.; Xu, S.; Yao, Y.; Luo, W.; Wang, C.; Lacey, S.D.; Dai, J.; et al. Toward garnet electrolyte-based Li metal batteries: An ultrathin, highly effective, artificial solid-state electrolyte/metallic Li interface. Sci. Adv. 2017, 3, e1601659. [Google Scholar] [CrossRef]
- Wang, C.; Gong, Y.; Liu, B.; Fu, K.; Yao, Y.; Hitz, E.; Li, Y.; Dai, J.; Xu, S.; Luo, W.; et al. Conformal, nanoscale ZnO surface modification of garnet-based solid-state electrolyte for lithium metal anodes. Nano Lett. 2017, 17, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Gong, Y.; Hitz, G.T.; McOwen, D.W.; Li, Y.; Xu, S.; Wen, Y.; Zhang, L.; Wang, C.; Pastel, G.; et al. Three-dimensional bilayer garnet solid electrolyte based high energy density lithium metal-sulfur batteries. Energy Environ. Sci. 2017, 10, 1568–1575. [Google Scholar] [CrossRef]
- Lushta, V.; Dietzel, D.; Roling, B.; Schirmeisen, A. Nanoscale characterization of ion mobility by temperature-controlled Li-nanoparticle growth. ACS Appl. Mater. Interfaces 2019, 11, 5476–5483. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; Mcilwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liao, L.; Lee, H.R.; Shi, F.; Huang, W.; Zhao, J.; Pei, A.; Tang, J.; Zheng, X.; Chen, W.; et al. Surface-engineered mesoporous silicon microparticles as high-coulombic-efficiency anodes for lithium-ion batteries. Nano Energy 2019, 61, 404–410. [Google Scholar] [CrossRef]
- Graetz, J.A.; Fultz, B.T.; Ahn, C.; Yazami, R. High-Capacity Nanostructured Silicon and Lithium Alloys Thereof. U.S. Patent 20,040,126,659, 1 July 2004. [Google Scholar]
- Hou, G.; Cheng, B.; Yang, Y.; Du, Y.; Zhang, Y.; Li, B.; He, J.; Zhou, Y.; Yi, D.; Zhao, N.; et al. Multiscale buffering engineering in silicon-carbon anode for ultrastable Li-ion storage. ACS Nano 2019, 13, 10179–10190. [Google Scholar] [CrossRef]
- Schneier, D.; Harpak, N.; Menkin, S.; Davidi, G.; Goor, M.; Mados, E.; Ardel, G.; Patolsky, F.; Golodnitsky, D.; Peled, E. Analysis of scale-up parameters in 3D silicon-nanowire lithium-battery anodes. J. Electrochem. Soc. 2020, 167, 050511. [Google Scholar] [CrossRef]
- Ai, Q.; Li, D.; Guo, J.; Hou, G.; Sun, Q.; Sun, Q.; Xu, X.; Zhai, W.; Zhang, L.; Feng, J.; et al. Artificial solid electrolyte interphase coating to reduce lithium trapping in silicon anode for high performance lithium-ion batteries. Adv. Mater. Interfaces 2019, 6, 1901187. [Google Scholar] [CrossRef]
- Shang, H.; Zuo, Z.; Yu, L.; Wang, F.; He, F.; Li, Y. Low-temperature growth of all-carbon graphdiyne on a silicon anode for high-performance lithium-ion batteries. Adv. Mater. 2018, 30, e1801459. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Y.; Hou, G.; Yi, D.; Zhou, B.; Chen, S.; Lam, T.D.; Yuan, F.; Golberg, D.; Wang, X. Stress-relieving defects enable ultra-stable silicon anode for Li-ion storage. Nano Energy 2020, 70, 104568. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Chen, R.; Bai, Y.; Li, T.; Jin, H.; Wang, J.; Xia, H. A green-synthetic spiderweb-like Si@graphene-oxide anode material with multifunctional citric acid binder for high energy-density Li-ion batteries. Carbon 2020, 157, 330–339. [Google Scholar] [CrossRef]
- Hwang, C.; Lee, K.; Um, H.D.; Lee, Y.; Seo, K.; Song, H.K. Conductive and porous silicon nanowire anodes for lithium ion batteries. J. Electrochem. Soc. 2017, 164, A1564–A1568. [Google Scholar] [CrossRef]
- Li, J.Y.; Li, G.; Zhang, J.; Yin, Y.X.; Yue, F.S.; Xu, Q.; Guo, Y.G. Rational design of robust Si/C microspheres for high-tap-density anode materials. ACS Appl. Mater. Interfaces 2019, 11, 4057–4064. [Google Scholar] [CrossRef]
- Kuhne, M.; Borrnert, F.; Fecher, S.; Ghorbani-Asl, M.; Biskupek, J.; Samuelis, D.; Krasheninnikov, A.V.; Kaiser, U.; Smet, J.H. Reversible superdense ordering of lithium between two graphene sheets. Nature 2018, 564, 234–239. [Google Scholar] [CrossRef]
- Nandi, S.; Das, S.K. Realizing a low-cost and sustainable rechargeable aqueous aluminum-metal battery with exfoliated graphite cathode. ACS Sustain. Chem. Eng. 2019, 7, 19839–19847. [Google Scholar] [CrossRef]
- Yang, C.; Chen, J.; Ji, X.; Pollard, T.P.; Lu, X.; Sun, C.J.; Hou, S.; Liu, Q.; Liu, C.; Qing, T.; et al. Aqueous Li-ion battery enabled by halogen conversion-intercalation chemistry in graphite. Nature 2019, 569, 245–250. [Google Scholar] [CrossRef]
- Mortazavi, B.; Shahrokhi, M.; Madjet, M.E.; Makaremi, M.; Ahzi, S.; Rabczuk, T. N-, P-, As-triphenylene-graphdiyne: Strong and stable 2D semiconductors with outstanding capacities as anodes for Li-ion batteries. Carbon 2019, 141, 291–303. [Google Scholar] [CrossRef]
- Chen, Z.; Belharouak, I.; Sun, Y.K.; Amine, K. Titanium-based anode materials for safe lithium-ion batteries. Adv. Funct. Mater. 2013, 23, 959–969. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Dong, Y.; Zhang, Z.; Tang, Z. Recent progress in Ti-based nanocomposite anodes for lithium ion batteries. J. Adv. Ceram. 2019, 8, 1–18. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhang, X.; Yao, C.; Wang, R.; Xu, C.; Lei, J. Porous spheres of TiO2 (B)/anatase entwined by graphene nanoribbons for high Li+ rate performance. Electrochim. Acta 2019, 298, 14–21. [Google Scholar] [CrossRef]
- Bai, X.; Li, T.; Wei, C.; Sun, Y.K.; Qi, Y.X.; Zhu, H.L.; Lun, N.; Bai, Y.J. Enhancing the long-term cyclability and rate capability of Li4Ti5O12 by simple copper-modification. Electrochim. Acta 2015, 155, 132–139. [Google Scholar] [CrossRef]
- Aravindan, V.; Lee, Y.S.; Yazami, R.; Madhavi, S. TiO2 polymorphs in ‘rocking-chair’ Li-ion batteries. Mater. Today 2015, 18, 345–351. [Google Scholar] [CrossRef]
- Liu, G.; Wu, H.H.; Meng, Q.; Zhang, T.; Sun, D.; Jin, X.; Guo, D.; Wu, N.; Liu, X.; Kim, J.K. Role of the anatase/TiO2(B) heterointerface for ultrastable high-rate lithium and sodium energy storage performance. Nanoscale Horiz. 2020, 5, 150–162. [Google Scholar] [CrossRef]
- Wang, S.; Quan, W.; Zhu, Z.; Yang, Y.; Liu, Q.; Ren, Y.; Zhang, X.; Xu, R.; Hong, Y.; Zhang, Z.; et al. Lithium titanate hydrates with superfast and stable cycling in lithium ion batteries. Nat. Commun. 2017, 8, 627. [Google Scholar] [CrossRef]
- Xu, G.; Yang, L.; Wei, X.; Ding, J.; Zhong, J.; Chu, P.K. MoS2-quantum-dot-interspersed Li4TiO12 nanosheets with enhanced performance for Li- and Na-ion batteries. Adv. Funct. Mater. 2016, 26, 3349–3358. [Google Scholar] [CrossRef]
- Christensen, C.K.; Mamakhel, M.A.H.; Balakrishna, A.R.; Iversen, B.B.; Chiang, Y.M.; Ravnsbaek, D.B. Order-disorder transition in nano-rutile TiO2 anodes: A high capacity low-volume change Li-ion battery material. Nanoscale 2019, 11, 12347–12357. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Q.; Yang, X.; He, S.; Khan, J.; Meng, Y.; Zhu, X.; Tong, S.; Wu, M. Chestnut-like TiO2@alpha-Fe2O3 core-shell nanostructures with abundant interfaces for efficient and ultralong life lithium-ion storage. ACS Appl. Mater. Interfaces 2017, 9, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Han, J.T.; Huang, Y.H.; Goodenough, J.B. New anode framework for rechargeable lithium batteries. Chem. Mater. 2011, 23, 2027–2029. [Google Scholar] [CrossRef]
- Lu, X.; Jian, Z.; Fang, Z.; Gu, L.; Hu, Y.S.; Chen, W.; Wang, Z.; Chen, L. Atomic-scale investigation on lithium storage mechanism in TiNb2O7. Energy Environ. Sci. 2011, 4, 2638–2644. [Google Scholar] [CrossRef]
- Song, H.; Kim, Y.T. A Mo-doped TiNb2O7 anode for lithium-ion batteries with high rate capability due to charge redistribution. Chem. Commun. 2015, 51, 9849–9852. [Google Scholar] [CrossRef]
- Takami, N.; Ise, K.; Harada, Y.; Iwasaki, T.; Kishi, T.; Hoshina, K. High-energy, fast-charging, long-life lithium-ion batteries using TiNb2O7 anodes for automotive applications. J. Power Sources 2018, 396, 429–436. [Google Scholar] [CrossRef]
- Tang, K.; Mu, X.; van Aken, P.A.; Yu, Y.; Maier, J. “Nano-pearl-string” TiNb2O7 as anodes for rechargeable lithium batteries. Adv. Energy Mater. 2013, 3, 49–53. [Google Scholar] [CrossRef]
- Park, H.; Shin, D.H.; Song, T.; Park, W.I.; Paik, U. Synthesis of hierarchical porous TiNb2O7 nanotubes with controllable porosity and their application in high power Li-ion batteries. J. Mater. Chem. A 2017, 5, 6958–6965. [Google Scholar] [CrossRef]
- Guo, B.; Yu, X.; Sun, X.G.; Chi, M.; Qiao, Z.A.; Liu, J.; Hu, Y.S.; Yang, X.Q.; Goodenough, J.B.; Dai, S. A long-life lithium-ion battery with a highly porous TiNb2O7 anode for large-scale electrical energy storage. Energy Environ. Sci. 2014, 7, 2220–2226. [Google Scholar] [CrossRef]
- Jo, C.; Kim, Y.; Hwang, J.; Shim, J.; Chun, J.; Lee, J. Block copolymer directed ordered mesostructured TiNb2O7 multimetallic oxide constructed of nanocrystals as high power Li-ion battery anodes. Chem. Mater. 2014, 26, 3508–3514. [Google Scholar] [CrossRef]
- Amatucci, G.G. CoO2, The end member of the LixCoO2 solid solution. J. Electrochem. Soc. 1996, 143, 1114–1123. [Google Scholar] [CrossRef]
- Van der Ven, A.; Aydinol, M.K.; Ceder, G.; Kresse, G.; Hafner, J. First-principles investigation of phase stability in LixCoO2. Phys. Rev. B 1998, 58, 2975–2987. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x ≤ 1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar]
- Hu, B.; Lou, X.; Li, C.; Geng, F.; Zhao, C.; Wang, J.; Shen, M.; Hu, B. Reversible phase transition enabled by binary Ba and Ti-based surface modification for high voltage LiCoO2 cathode. J. Power Sources 2019, 438, 226954. [Google Scholar] [CrossRef]
- Liu, Q.; Su, X.; Lei, D.; Qin, Y.; Wen, J.; Guo, F.; Wu, Y.A.; Rong, Y.; Kou, R.; Xiao, X.; et al. Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping. Nat. Energy 2018, 3, 936–943. [Google Scholar] [CrossRef]
- Uyama, T.; Mukai, K.; Yamada, I. High-pressure synthesis and electrochemical properties of tetragonal LiMnO2. RSC Adv. 2018, 8, 26325–26334. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Y.; Wang, Z.; Chen, L. Understanding structural stability of monoclinic LiMnO2 and NaMnO2 upon de-intercalation. Phys. Chem. Chem. Phys. 2016, 18, 17345–17350. [Google Scholar] [CrossRef] [PubMed]
- Ammundsen, B.; Paulsen, J. Novel lithium-ion cathode materials based on layered manganese oxides. Adv. Mater. 2001, 13, 943–956. [Google Scholar] [CrossRef]
- Bhandari, A.; Bhattacharya, J. Review-Manganese dissolution from spinel cathode: Few unanswered questions. J. Electrochem. Soc. 2016, 164, A106–A127. [Google Scholar] [CrossRef]
- Zhan, C.; Wu, T.; Lu, J.; Amine, K. Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodes–a critical review. Energy Environ. Sci. 2018, 11, 243–257. [Google Scholar] [CrossRef]
- Hirayama, M.; Ido, H.; Kim, K.; Cho, W.; Tamura, K.; Mizuki, J.; Kanno, R. Dynamic structural changes at LiMn2O4/electrolyte interface during lithium battery reaction. J. Am. Chem. Soc. 2010, 132, 15268–15276. [Google Scholar] [CrossRef]
- Tang, D.; Sun, Y.; Yang, Z.; Ben, L.; Gu, L.; Huang, X. Surface structure evolution of LiMn2O4 cathode material upon charge/discharge. Chem. Mater. 2014, 26, 3535–3543. [Google Scholar] [CrossRef]
- Cai, Z.; Ma, Y.; Huang, X.; Yan, X.; Yu, Z.; Zhang, S.; Song, G.; Xu, Y.; Wen, C.; Yang, W. High electrochemical stability Al-doped spinel LiMn2O4 cathode material for Li-ion batteries. J. Energy Storage 2020, 27, 101036. [Google Scholar] [CrossRef]
- Xu, J.; Le, T.; Yu, Z.; Yang, Y. Yttrium-doped LiMn2O4 spheres with long cycle life as lithium-ion battery cathode. J. Mater. Sci.: Mater. Electron. 2019, 30, 19450–19456. [Google Scholar] [CrossRef]
- Michalska, M.; Ziółkowska, D.A.; Jasiński, J.B.; Lee, P.H.; Ławniczak, P.; Andrzejewski, B.; Ostrowski, A.; Bednarski, W.; Wu, S.H.; Lin, J.Y. Improved electrochemical performance of LiMn2O4 cathode material by Ce doping. Electrochim. Acta 2018, 276, 37–46. [Google Scholar] [CrossRef]
- Li, W.; Siqin, G.W.; Zhu, Z.; Qi, L.; Tian, W.H. Electrochemical properties of niobium and phosphate doped spherical Li-rich spinel LiMn2O4 synthesized by ion implantation method. Chin. Chem. Lett. 2017, 28, 1438–1446. [Google Scholar] [CrossRef]
- Fey, G.T.K. LiNiVO4: A 4.8 volt electrode material for lithium cells. J. Electrochem. Soc. 1994, 141, 2279. [Google Scholar] [CrossRef]
- Prabaharan, S.R.S.; Michael, M.S.; Radhakrishna, S.; Julien, C. Novel low-temperature synthesis and characterization of LiNiVO4 for high-voltage Li ion batteries. J. Mater. Chem. 1997, 7, 1791–1796. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Zaghib, K.; Liu, D. High voltage cathode materials. In Rechargeable Batteries: Materials, Technologies and New Trends; Zhang, Z., Zhang, S.S., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 477–509. [Google Scholar]
- Kazakopoulos, A.; Sarafidis, C.; Chrissafis, K.; Kalogirou, O. Synthesis and characterization of inverse spinel LiNiVO4 and LiCoVO4 with impedance spectroscopy. Solid State Ion. 2008, 179, 1980–1985. [Google Scholar] [CrossRef]
- Fey, G.T.K.; Huang, D.L. Synthesis, characterization and cell performance of inverse spinel electrode materials for lithium secondary batteries. Electrochim. Acta 1999, 45, 295–314. [Google Scholar] [CrossRef]
- Thongtem, T.; Kaowphong, S.; Thongtem, S. Malic acid complex method for preparation of LiNiVO4 nano-crystallites. J. Mater. Sci. 2007, 42, 3923–3927. [Google Scholar] [CrossRef]
- Thongtem, T.; Kaowphong, S.; Thongtem, S. Preparation of LiNiVO4 nano-powder using tartaric acid as a complexing agent. Ceram. Int. 2007, 33, 1449–1453. [Google Scholar] [CrossRef]
- Prakash, D.; Masuda, Y.; Sanjeeviraja, C. Synthesis and structure refinement studies of LiNiVO4 electrode material for lithium rechargeable batteries. Ionics 2013, 19, 17–23. [Google Scholar] [CrossRef]
- Liu, R.S.; Cheng, Y.C.; Gundakaram, R.; Jang, L.Y. Crystal and electronic structures of inverse spinel-type LiNiVO4. Mater. Res. Bull. 2001, 36, 1479–1486. [Google Scholar] [CrossRef]
- Qin, M.L.; Liu, W.M.; Liang, S.Q.; Pan, A.Q. Facile synthesis of porous LiNiVO4 powder as high-voltage cathode material for lithium-ion batteries. Trans. Nonferrous Met. Soc. 2016, 26, 3232–3237. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Zhang, Z. First principles investigation of electronic structure change and energy transfer by redox in inverse spinel cathodes LiNiVO4 and LiCoVO4. J. Mater. Chem. 2012, 22, 18968–18974. [Google Scholar] [CrossRef]
- Ohzuku, T.; Makimura, Y. Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries. Chem. Lett. 2001, 30, 642–643. [Google Scholar] [CrossRef]
- Hwang, B.J.; Tsai, Y.W.; Carlier, D.; Ceder, G. A combined computational/experimental study on LiNi1/3Co1/3Mn1/3O2. Chem. Mater. 2003, 15, 3676–3682. [Google Scholar] [CrossRef]
- Koyama, Y.; Tanaka, I.; Adachi, H.; Makimura, Y.; Ohzuku, T. Crystal and electronic structures of superstructural Li1−x[Co1/3Ni1/3Mn1/3]O2 (0 ≤ x ≤ 1). J. Power Sources 2003, 119-121, 644–648. [Google Scholar] [CrossRef]
- Xu, J.; Lin, F.; Doeff, M.M.; Tong, W. A review of Ni-based layered oxides for rechargeable Li-ion batteries. J. Mater. Chem. A 2017, 5, 874–901. [Google Scholar] [CrossRef]
- Jung, S.K.; Gwon, H.; Hong, J.; Park, K.Y.; Seo, D.H.; Kim, H.; Hyun, J.; Yang, W.; Kang, K. Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries. Adv. Energy Mater. 2014, 4, 1300787. [Google Scholar] [CrossRef]
- Weigel, T.; Schipper, F.; Erickson, E.M.; Susai, F.A.; Markovsky, B.; Aurbach, D. Structural and electrochemical aspects of LiNi0.8Co0.1Mn0.1O2 cathode materials doped by various cations. ACS Energy Lett. 2019, 4, 508–516. [Google Scholar] [CrossRef]
- Fan, X.; Hu, G.; Zhang, B.; Ou, X.; Zhang, J.; Zhao, W.; Jia, H.; Zou, L.; Li, P.; Yang, Y. Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano Energy 2020, 70, 104450. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, M.; Yin, J.; Ma, C.; Dai, Y.; Wang, D.; Mi, S.; Qiang, W.; Huang, B.; Chen, Y. Regulating surface and grain-boundary structures of Ni-rich layered cathodes for ultrahigh cycle stability. Small 2020, 16, e1906433. [Google Scholar] [CrossRef]
- Fan, X.; Chen, L.; Borodin, O.; Ji, X.; Chen, J.; Hou, S.; Deng, T.; Zheng, J.; Yang, C.; Liou, S.C.; et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 2018, 13, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Li, J.; Shang, H.; Liu, W.; Wan, Q.; Chen, M.; Qu, M.; Peng, G. Improving cyclic stability of LiNi0.6Co0.2Mn0.2O2-SiOx/graphite full cell using tris (trimethylsilyl) phosphite and fluoroethylene carbonate as combinative electrolyte additive. Ionics 2020, 26, 2247–2257. [Google Scholar] [CrossRef]
- Dahn, J.R.; Xia, J.; Wang, Y.; Petibon, R.; Ma, L.; Nelson, K.; Downie, L.E. Electrolyte Additives for Lithium Ion Batteries. U.S. Patent 20,170,025,706, 26 January 2017. [Google Scholar]
- Dahn, J.R.; Hynes, T.; Hall, D.S. Dioxazolones and Nitrile Sulfites as Electrolyte Additives for Lithium-Ion Batteries. U.S. Patent 20,190,393,546, 26 December 2019. [Google Scholar]
- Lv, Y.; Cheng, X.; Qiang, W.; Huang, B. Improved electrochemical performances of Ni-rich LiNi0.83Co0.12Mn0.05O2 by Mg-doping. J. Power Sources 2020, 450, 227718. [Google Scholar] [CrossRef]
- Wu, L.; Tang, X.; Chen, X.; Rong, Z.; Dang, W.; Wang, Y.; Li, X.; Huang, L.; Zhang, Y. Improvement of electrochemical reversibility of the Ni-rich cathode material by gallium doping. J. Power Sources 2020, 445, 227337. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Wu, L.; Feng, L.; Jin, S.; Zhang, R.; Jin, M. Effect of Ti ion doping on electrochemical performance of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode material. Electrochim. Acta 2019, 328, 135086. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, B.; Wang, M.; Yang, X.; Gu, Y. Facile synthesis of fluorine doped single crystal Ni-rich cathode material for lithium-ion batteries. Solid State Ion. 2019, 342, 115065. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, T.; Zhang, Q.; Zheng, M.; Xu, K.; Yan, W. Enhanced electrochemical performance of La and F co-modified Ni-rich cathode. Ionics 2019, 26, 1165–1171. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Y.; Zhao, L.; Wu, J.; Dai, C.; Li, Y.; Wang, X.; Ding, F. Improving the electrochemical performance of lithium-rich cathode materials Li1.2Mn0.54Ni0.13Co0.13O2 by a method of tungsten doping. Ionics 2019, 25, 5239–5247. [Google Scholar] [CrossRef]
- Hashigami, S.; Kato, Y.; Yoshimi, K.; Fukumoto, A.; Cao, Z.; Yoshida, H.; Inagaki, T.; Hashinokuchi, M.; Haruta, M.; Doi, T.; et al. Effect of lithium silicate addition on the microstructure and crack formation of LiNi0.8Co0.1Mn0.1O2 cathode particles. ACS Appl. Mater. Interfaces 2019, 11, 39910–39920. [Google Scholar] [CrossRef]
- Feng, Z.; Rajagopalan, R.; Sun, D.; Tang, Y.; Wang, H. In-situ formation of hybrid Li3PO4-AlPO4-Al(PO3)3 coating layer on LiNi0.8Co0.1Mn0.1O2 cathode with enhanced electrochemical properties for lithium-ion battery. Chem. Eng. J. 2020, 382, 122959. [Google Scholar] [CrossRef]
- Liao, Y.; Li, J.; Deng, B.; Wang, H.; Chen, T.; Li, X.; Qu, M.; Li, X.; Peng, G. Surface modification of Li1.144Ni0.136Co0.136Mn0.544O2 by hybrid protection layer with enhanced rate capability. Energy Technol. 2020, 8, 1901133. [Google Scholar] [CrossRef]
- Xiao, Z.; Chi, Z.; Song, L.; Cao, Z.; Li, A. LiTa2PO8 coated nickel-rich cathode material for improved electrochemical performance at high voltage. Ceram. Int. 2020, 46, 8328–8333. [Google Scholar] [CrossRef]
- Arbi, K.; Bucheli, W.; Jiménez, R.; Sanz, J. High lithium ion conducting solid electrolytes based on NASICON Li1+xAlxM2−x(PO4)3 materials (M = Ti, Ge and 0 ≤ x ≤ 0.5). J. Eur. Ceram. Soc. 2015, 35, 1477–1484. [Google Scholar] [CrossRef]
- Thokchom, J.S.; Kumar, B. The effects of crystallization parameters on the ionic conductivity of a lithium aluminum germanium phosphate glass–ceramic. J. Power Sources 2010, 195, 2870–2876. [Google Scholar] [CrossRef]
- Kuwano, J.; West, A.R. New Li+ ion conductors in the system, Li4GeO4-Li3VO4. Mater. Res. Bull. 1980, 15, 1661–1667. [Google Scholar] [CrossRef]
- Song, S.; Lu, J.; Zheng, F.; Duong, H.M.; Lu, L. A facile strategy to achieve high conduction and excellent chemical stability of lithium solid electrolytes. RSC Adv. 2015, 5, 6588–6594. [Google Scholar] [CrossRef]
- Kanno, R.; Murayama, M. Lithium ionic conductor thio-LISICON: The Li2S-GeS2-P2S5 system. J. Electrochem. Soc. 2001, 148, A742–A746. [Google Scholar] [CrossRef]
- Bates, J.; Dudney, N.; Gruzalski, G.; Zuhr, R.; Choudhury, A.; Luck, C.; Robertson, J. Electrical properties of amorphous lithium electrolyte thin films. Solid State Ion. 1992, 53, 647–654. [Google Scholar] [CrossRef]
- Fleutot, B.; Pecquenard, B.; Martinez, H.; Letellier, M.; Levasseur, A. Investigation of the local structure of LiPON thin films to better understand the role of nitrogen on their performance. Solid State Ion. 2011, 186, 29–36. [Google Scholar] [CrossRef]
- Su, Y.; Falgenhauer, J.; Polity, A.; Leichtweiß, T.; Kronenberger, A.; Obel, J.; Zhou, S.; Schlettwein, D.; Janek, J.; Meyer, B.K. LiPON thin films with high nitrogen content for application in lithium batteries and electrochromic devices prepared by RF magnetron sputtering. Solid State Ion. 2015, 282, 63–69. [Google Scholar] [CrossRef]
- Inaguma, Y.; Liquan, C.; Itoh, M.; Nakamura, T.; Uchida, T.; Ikuta, H.; Wakihara, M. High ionic conductivity in lithium lanthanum titanate. Solid State Commun. 1993, 86, 689–693. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Ding, Y. Structure, stability, and ionic conductivity of perovskite Li2x-ySr1-x-yLayTiO3 solid electrolytes. Ceram. Int. 2020, 46, 7741–7747. [Google Scholar] [CrossRef]
- Hu, Z.; Sheng, J.; Chen, J.; Sheng, G.; Li, Y.; Fu, X.Z.; Wang, L.; Sun, R.; Wong, C.P. Enhanced Li ion conductivity in Ge-doped Li0.33La0.56TiO3 perovskite solid electrolytes for all-solid-state Li-ion batteries. New J. Chem. 2018, 42, 9074–9079. [Google Scholar] [CrossRef]
- Chen, C. Stable lithium-ion conducting perovskite lithium-strontium-tantalum-zirconium-oxide system. Solid State Ion. 2004, 167, 263–272. [Google Scholar] [CrossRef]
- Inada, R.; Kimura, K.; Kusakabe, K.; Tojo, T.; Sakurai, Y. Synthesis and lithium-ion conductivity for perovskite-type Li3/8Sr7/16Ta3/4Zr1/4O3 solid electrolyte by powder-bed sintering. Solid State Ion. 2014, 261, 95–99. [Google Scholar] [CrossRef]
- Huang, B.; Xu, B.; Li, Y.; Zhou, W.; You, Y.; Zhong, S.; Wang, C.A.; Goodenough, J.B. Li-ion conduction and stability of perovskite Li3/8Sr7/16Hf1/4Ta3/4O3. ACS Appl. Mater. Interfaces 2016, 8, 14552–14557. [Google Scholar] [CrossRef]
- Huang, B.; Zhong, S.; Luo, J.; Huang, Z.; Wang, C.A. Highly dense perovskite electrolyte with a high Li+ conductivity for Li-ion batteries. J. Power Sources 2019, 429, 75–79. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, C.H.; Jarry, A.; Chen, W.; Ye, Y.; Zhu, J.; Kostecki, R.; Persson, K.; Guo, J.; Salmeron, M.; et al. Interrelationships among grain size, surface composition, air stability, and interfacial resistance of Al-substituted Li7La3Zr2O12 solid electrolytes. ACS Appl. Mater. Interfaces 2015, 7, 17649–17655. [Google Scholar] [CrossRef] [PubMed]
- Thangadurai, V.; Kaack, H.; Weppner, W.J.F. Novel fast lithium ion conduction in garnet-type Li5La3M2O12 (M = Nb, Ta). J. Am. Ceram. Soc. 2003, 86, 437–440. [Google Scholar] [CrossRef]
- Allen, J.L.; Wolfenstine, J.; Rangasamy, E.; Sakamoto, J. Effect of substitution (Ta, Al, Ga) on the conductivity of Li7La3Zr2O12. J. Power Sources 2012, 206, 315–319. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Manalastas, W.; Lopez del Amo, J.M.; Aguadero, A.; Aguesse, F.; Kilner, J.A. Atmosphere controlled processing of Ga-substituted garnets for high Li-ion conductivity ceramics. Chem. Mat. 2014, 26, 3610–3617. [Google Scholar] [CrossRef]
- Zhao, Y.; Daemen, L.L. Superionic conductivity in lithium-rich anti-perovskites. J. Am. Chem. Soc. 2012, 134, 15042–15047. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, W.; Xin, S.; Li, S.; Zhu, J.; Lu, X.; Cui, Z.; Jia, Q.; Zhou, J.; Zhao, Y.; et al. Fluorine-doped antiperovskite electrolyte for all-solid-state lithium-ion batteries. Angew. Chem. Int. Ed. Engl. 2016, 55, 9965–9968. [Google Scholar] [CrossRef]
- Rao, R.P.; Adams, S. Studies of lithium argyrodite solid electrolytes for all-solid-state batteries. Phys. Status Solidi (a) 2011, 208, 1804–1807. [Google Scholar] [CrossRef]
- Schneider, H.; Du, H.; Kelley, T.; Leitner, K.; ter Maat, J.; Scordilis-Kelley, C.; Sanchez-Carrera, R.; Kovalev, I.; Mudalige, A.; Kulisch, J.; et al. A novel class of halogen-free, super-conductive lithium argyrodites: Synthesis and characterization. J. Power Sources 2017, 366, 151–160. [Google Scholar] [CrossRef]
- Wang, P.; Liu, H.; Patel, S.; Feng, X.; Chien, P.H.; Wang, Y.; Hu, Y.Y. Fast ion conduction and its origin in Li6–xPS5–xBr1+x. Chem. Mater. 2020, 32, 3833–3840. [Google Scholar] [CrossRef]
- Jung, W.D.; Kim, J.S.; Choi, S.; Kim, S.; Jeon, M.; Jung, H.G.; Chung, K.Y.; Lee, J.H.; Kim, B.K.; Lee, J.H.; et al. Superionic halogen-rich Li-argyrodites using in situ nanocrystal nucleation and rapid crystal growth. Nano Lett. 2020, 20, 2303–2309. [Google Scholar] [CrossRef]
- Hagman, L.O.; Kierkegaard, P.; Karvonen, P. The crystal structure of NaMe2IV(PO4)3; MeIV = Ge, Ti, Zr. Acta Chem. Scand. 1968, 22, 1822–1832. [Google Scholar] [CrossRef]
- Anantharamulu, N.; Koteswara Rao, K.; Rambabu, G.; Vijaya Kumar, B.; Radha, V.; Vithal, M. A wide-ranging review on NASICON type materials. J. Mater. Sci. 2011, 46, 2821–2837. [Google Scholar] [CrossRef]
- Safanama, D.; Adams, S. High efficiency aqueous and hybrid lithium-air batteries enabled by Li1.5Al0.5Ge1.5(PO4)3 ceramic anode-protecting membranes. J. Power Sources 2017, 340, 294–301. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Li, B.; Song, H.; Cheng, Z.; Chen, M.; He, P.; Zhou, H. Germanium thin film protected lithium aluminum germanium phosphate for solid-state Li batteries. Adv. Energy Mater. 2018, 8, 1702374. [Google Scholar] [CrossRef]
- Hong, H.P. Crystal structure and ionic conductivity of Li14Zn(GeO4)4 and other new Li+ superionic conductors. Mater. Res. Bull. 1978, 13, 117–124. [Google Scholar] [CrossRef]
- Knauth, P. Inorganic solid Li ion conductors: An overview. Solid State Ion. 2009, 180, 911–916. [Google Scholar] [CrossRef]
- Tintignac, S.; Baddour-Hadjean, R.; Pereira-Ramos, J.P.; Salot, R. High rate bias sputtered LiCoO2 thin films as positive electrode for all-solid-state lithium microbatteries. Electrochim. Acta 2014, 146, 472–476. [Google Scholar] [CrossRef]
- Eftekhari, A. Fabrication of 5 V lithium rechargeable micro-battery. J. Power Sources 2004, 132, 240–243. [Google Scholar] [CrossRef]
- Lethien, C.; Zegaoui, M.; Roussel, P.; Tilmant, P.; Rolland, N.; Rolland, P.A. Micro-patterning of LiPON and lithium iron phosphate material deposited onto silicon nanopillars array for lithium ion solid state 3D micro-battery. Microelectron. Eng. 2011, 88, 3172–3177. [Google Scholar] [CrossRef]
- Alonso, J.A.; Sanz, J.; Santamaría, J.; León, C.; Várez, A.; Fernández-Díaz, M.T. On the location of Li+ cations in the fast Li-cation conductor La0.5Li0.5TiO3 perovskite. Angew. Chem. Int. Ed. 2000, 39, 619–621. [Google Scholar] [CrossRef]
- Jay, E.E.; Rushton, M.J.; Chroneos, A.; Grimes, R.W.; Kilner, J.A. Genetics of superionic conductivity in lithium lanthanum titanates. Phys. Chem. Chem. Phys. 2015, 17, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Bohnke, O. Mechanism of ionic conduction and electrochemical intercalation of lithium into the perovskite lanthanum lithium titanate. Solid State Ion. 1996, 91, 21–31. [Google Scholar] [CrossRef]
- Birke, P. Electrolytic stability limit and rapid lithium insertion in the fast-ion-conducting Li0.29La0.57TiO3 perovskite-type compound. J. Electrochem. Soc. 1997, 144, L167–L169. [Google Scholar] [CrossRef]
- Chen, C. Ionic conductivity, lithium insertion and extraction of lanthanum lithium titanate. Solid State Ion. 2001, 144, 51–57. [Google Scholar] [CrossRef]
- Yu, R.; Du, Q.X.; Zou, B.K.; Wen, Z.Y.; Chen, C.H. Synthesis and characterization of perovskite-type (Li,Sr)(Zr,Nb)O3 quaternary solid electrolyte for all-solid-state batteries. J. Power Sources 2016, 306, 623–629. [Google Scholar] [CrossRef]
- Jalem, R.; Nakayama, M.; Manalastas, W.; Kilner, J.A.; Grimes, R.W.; Kasuga, T.; Kanamura, K. Insights into the lithium-ion conduction mechanism of garnet-type cubic Li5La3Ta2O12 by ab-initio calculations. J. Phys. Chem. C 2015, 119, 20783–20791. [Google Scholar] [CrossRef]
- Awaka, J.; Kijima, N.; Hayakawa, H.; Akimoto, J. Synthesis and structure analysis of tetragonal Li7La3Zr2O12 with the garnet-related type structure. J. Solid State Chem. 2009, 182, 2046–2052. [Google Scholar] [CrossRef]
- Awaka, J.; Takashima, A.; Kataoka, K.; Kijima, N.; Idemoto, Y.; Akimoto, J. Crystal structure of fast lithium-ion-conducting cubic Li7La3Zr2O12. Chem. Lett. 2011, 40, 60–62. [Google Scholar] [CrossRef]
- Li, Y.; Han, J.T.; Wang, C.A.; Xie, H.; Goodenough, J.B. Optimizing Li+ conductivity in a garnet framework. J. Mater. Chem. 2012, 22, 15357–15361. [Google Scholar] [CrossRef]
- Kang, S.G.; Sholl, D.S. First-principles study of chemical stability of the lithium oxide garnets Li7La3M2O12 (M = Zr, Sn, or Hf). J. Phys. Chem. C 2014, 118, 17402–17406. [Google Scholar] [CrossRef]
- Xia, W.; Xu, B.; Duan, H.; Tang, X.; Guo, Y.; Kang, H.; Li, H.; Liu, H. Reaction mechanisms of lithium garnet pellets in ambient air: The effect of humidity and CO2. J. Am. Ceram. Soc. 2017, 100, 2832–2839. [Google Scholar] [CrossRef]
- Orera, A.; Larraz, G.; Rodriguez-Velamazan, J.A.; Campo, J.; Sanjuan, M.L. Influence of Li+ and H+ distribution on the crystal structure of Li7-xHxLa3Zr2O12 (0 ≤ x ≤ 5) garnets. Inorg. Chem. 2016, 55, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Larraz, G.; Orera, A.; Sanjuán, M.L. Cubic phases of garnet-type Li7La3Zr2O12: The role of hydration. J. Mater. Chem. A 2013, 1, 11419. [Google Scholar] [CrossRef]
- Nemori, H.; Matsuda, Y.; Mitsuoka, S.; Matsui, M.; Yamamoto, O.; Takeda, Y.; Imanishi, N. Stability of garnet-type solid electrolyte LixLa3A2-yByO12 (A = Nb or Ta, B = Sc or Zr). Solid State Ion. 2015, 282, 7–12. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Investigations on electrical conductivity and chemical compatibility between fast lithium ion conducting garnet-like Li6BaLa2Ta2O12 and lithium battery cathodes. J. Power Sources 2005, 142, 339–344. [Google Scholar] [CrossRef]
- Miara, L.J.; Richards, W.D.; Wang, Y.E.; Ceder, G. First-principles studies on cation dopants and electrolyte|cathode interphases for lithium garnets. Chem. Mater. 2015, 27, 4040–4047. [Google Scholar] [CrossRef]
- Deiseroth, H.J.; Kong, S.T.; Eckert, H.; Vannahme, J.; Reiner, C.; Zaiß, T.; Schlosser, M. Li6PS5X: A class of crystalline Li-rich solids with an unusually high Li+ mobility. Angew. Chem. Int. Ed. 2008, 47, 755–758. [Google Scholar] [CrossRef]
- Lee, Y.G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D.S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S.; et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver-carbon composite anodes. Nat. Energy 2020, 5, 299–308. [Google Scholar] [CrossRef]
- Luntz, A.C.; Voss, J.; Reuter, K. Interfacial challenges in solid-state Li ion batteries. J. Phys. Chem. Lett. 2015, 6, 4599–4604. [Google Scholar] [CrossRef]
- Takada, K.; Ohta, N.; Zhang, L.; Fukuda, K.; Sakaguchi, I.; Ma, R.; Osada, M.; Sasaki, T. Interfacial modification for high-power solid-state lithium batteries. Solid State Ion. 2008, 179, 1333–1337. [Google Scholar] [CrossRef]
- Hartmann, P.; Leichtweiss, T.; Busche, M.R.; Schneider, M.; Reich, M.; Sann, J.; Adelhelm, P.; Janek, J. Degradation of NASICON-type materials in contact with lithium metal: Formation of mixed conducting interphases (MCI) on solid electrolytes. J. Phys. Chem. C. 2013, 117, 21064–21074. [Google Scholar] [CrossRef]
- Pan, Q.; Barbash, D.; Smith, D.M.; Qi, H.; Gleeson, S.E.; Li, C.Y. Correlating electrode-electrolyte interface and battery performance in hybrid solid polymer electrolyte-based lithium metal batteries. Adv. Energy Mater. 2017, 7, 1701231. [Google Scholar] [CrossRef]
- Wu, B.; Wang, S.; Evans Iv, W.J.; Deng, D.Z.; Yang, J.; Xiao, J. Interfacial behaviours between lithium ion conductors and electrode materials in various battery systems. J. Mater. Chem. A 2016, 4, 15266–15280. [Google Scholar] [CrossRef]
- Kitaura, H.; Hayashi, A.; Ohtomo, T.; Hama, S.; Tatsumisago, M. Fabrication of electrode-electrolyte interfaces in all-solid-state rechargeable lithium batteries by using a supercooled liquid state of the glassy electrolytes. J. Mater. Chem. 2011, 21, 118–124. [Google Scholar] [CrossRef]
- Zhang, W.; Schröder, D.; Arlt, T.; Manke, I.; Koerver, R.; Pinedo, R.; Weber, D.A.; Sann, J.; Zeier, W.G.; Janek, J. (Electro) chemical expansion during cycling: Monitoring the pressure changes in operating solid-state lithium batteries. J. Mater. Chem. A 2017, 5, 9929–9936. [Google Scholar] [CrossRef]
- Koerver, R.; Zhang, W.; de Biasi, L.; Schweidler, S.; Kondrakov, A.O.; Kolling, S.; Brezesinski, T.; Hartmann, P.; Zeier, W.G.; Janek, J. Chemo-mechanical expansion of lithium electrode materials–on the route to mechanically optimized all-solid-state batteries. Energy Environ. Sci. 2018, 11, 2142–2158. [Google Scholar] [CrossRef]
- Koerver, R.; Aygün, I.; Leichtweiß, T.; Dietrich, C.; Zhang, W.; Binder, J.O.; Hartmann, P.; Zeier, W.G.; Janek, J. Capacity fade in solid-state batteries: Interphase formation and chemomechanical processes in nickel-rich layered oxide cathodes and ithium thiophosphate solid electrolytes. Chem. Mater. 2017, 29, 5574–5582. [Google Scholar] [CrossRef]
- Bucci, G.; Talamini, B.; Renuka Balakrishna, A.; Chiang, Y.M.; Carter, W.C. Mechanical instability of electrode-electrolyte interfaces in solid-state batteries. Phys. Rev. Mater. 2018, 2, 105407. [Google Scholar] [CrossRef]
- Sakuda, A.; Kitaura, H.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. Improvement of high-rate performance of all-solid-state lithium secondary batteries using LiCoO2 coated with Li2O-SiO2 glasses. Electrochem. Solid-State Lett. 2008, 11, A1. [Google Scholar] [CrossRef]
- Ohta, S.; Komagata, S.; Seki, J.; Saeki, T.; Morishita, S.; Asaoka, T. All-solid-state lithium ion battery using garnet-type oxide and Li3BO3 solid electrolytes fabricated by screen-printing. J. Power Sources 2013, 238, 53–56. [Google Scholar] [CrossRef]
- Ohta, S.; Seki, J.; Yagi, Y.; Kihira, Y.; Tani, T.; Asaoka, T. Co-sinterable lithium garnet-type oxide electrolyte with cathode for all-solid-state lithium ion battery. J. Power Sources 2014, 265, 40–44. [Google Scholar] [CrossRef]
- Zhang, W.; Richter, F.H.; Culver, S.P.; Leichtweiss, T.; Lozano, J.G.; Dietrich, C.; Bruce, P.G.; Zeier, W.G.; Janek, J. Degradation mechanisms at the Li10GeP2S12/LiCoO2 cathode interface in an all-solid-state lithium-ion battery. ACS Appl. Mater. Interfaces 2018, 10, 22226–22236. [Google Scholar] [CrossRef]
- Morimoto, H.; Awano, H.; Terashima, J.; Shindo, Y.; Nakanishi, S.; Ito, N.; Ishikawa, K.; Tobishima, S.I. Preparation of lithium ion conducting solid electrolyte of NASICON-type Li1+xAlxTi2−x(PO4)3 (x = 0.3) obtained by using the mechanochemical method and its application as surface modification materials of LiCoO2 cathode for lithium cell. J. Power Sources 2013, 240, 636–643. [Google Scholar] [CrossRef]
- Richards, W.D.; Miara, L.J.; Wang, Y.; Kim, J.C.; Ceder, G. Interface stability in solid-state batteries. Chem. Mater. 2016, 28, 266–273. [Google Scholar] [CrossRef]
- Takada, K.; Ohta, N.; Tateyama, Y. Recent Progress in interfacial nanoarchitectonics in solid-state batteries. J. Inorg. Organomet. Polym. Mater. 2015, 25, 205–213. [Google Scholar] [CrossRef]
- Miara, L.; Windmüller, A.; Tsai, C.L.; Richards, W.D.; Ma, Q.; Uhlenbruck, S.; Guillon, O.; Ceder, G. About the compatibility between high voltage spinel cathode materials and solid oxide electrolytes as a function of temperature. ACS Appl. Mater. Interfaces 2016, 8, 26842–26850. [Google Scholar] [CrossRef]
- Yamamoto, K.; Iriyama, Y.; Asaka, T.; Hirayama, T.; Fujita, H.; Fisher, C.A.J.; Nonaka, K.; Sugita, Y.; Ogumi, Z. Dynamic visualization of the electric potential in an all-solid-state rechargeable lithium battery. Angew. Chem. 2010, 49, 4414–4417. [Google Scholar] [CrossRef]
- Masuda, H.; Ishida, N.; Ogata, Y.; Ito, D.; Fujita, D. Internal potential mapping of charged solid-state-lithium ion batteries using in situ Kelvin probe force microscopy. Nanoscale 2017, 9, 893–898. [Google Scholar] [CrossRef]
- Haruta, M.; Shiraki, S.; Suzuki, T.; Kumatani, A.; Ohsawa, T.; Takagi, Y.; Shimizu, R.; Hitosugi, T. Negligible “negative space-charge layer effects” at oxide-electrolyte/electrode interfaces of thin-film batteries. Nano Lett. 2015, 15, 1498–1502. [Google Scholar] [CrossRef]
- De Klerk, N.J.J.; Wagemaker, M. Space-charge layers in all-solid-state batteries; important or negligible? ACS Appl. Energy Mater. 2018, 1, 5609–5618. [Google Scholar] [CrossRef]
- Okumura, T.; Nakatsutsumi, T.; Ina, T.; Orikasa, Y.; Arai, H.; Fukutsuka, T.; Iriyama, Y.; Uruga, T.; Tanida, H.; Uchimoto, Y.; et al. Depth-resolved X-ray absorption spectroscopic study on nanoscale observation of the electrode-solid electrolyte interface for all solid state lithium ion batteries. J. Mater. Chem. 2011, 21, 10051–10060. [Google Scholar] [CrossRef]
- Wenzel, S.; Leichtweiss, T.; Krüger, D.; Sann, J.; Janek, J. Interphase formation on lithium solid electrolytes–an in situ approach to study interfacial reactions by photoelectron spectroscopy. Solid State Ion. 2015, 278, 98–105. [Google Scholar] [CrossRef]
- Bron, P.; Roling, B.; Dehnen, S. Impedance characterization reveals mixed conducting interphases between sulfidic superionic conductors and lithium metal electrodes. J. Power Sources 2017, 352, 127–134. [Google Scholar] [CrossRef]
- Mizuno, F.; Yada, C.; Iba, H. Solid-state lithium-ion batteries for electric vehicles. In Lithium-Ion Batteries, 1st ed.; Pistoia, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 273–291. [Google Scholar]
- Park, K.; Yu, B.C.; Jung, J.W.; Li, Y.; Zhou, W.; Gao, H.; Son, S.; Goodenough, J.B. Electrochemical nature of the cathode interface for a solid-state lithium-ion battery: Interface between LiCoO2 and garnet-Li7La3Zr2O12. Chem. Mater. 2016, 28, 8051–8059. [Google Scholar] [CrossRef]
- Wenzel, S.; Randau, S.; Leichtweiß, T.; Weber, D.A.; Sann, J.; Zeier, W.G.; Janek, J. Direct observation of the interfacial instability of the fast ionic conductor Li10GeP2S12 at the lithium metal anode. Chem. Mater. 2016, 28, 2400–2407. [Google Scholar] [CrossRef]
- Han, X.; Gong, Y.; Fu, K.; He, X.; Hitz, G.T.; Dai, J.; Pearse, A.; Liu, B.; Wang, H.; Rubloff, G.; et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 2017, 16, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Ohta, N.; Takada, K.; Zhang, L.; Ma, R.; Osada, M.; Sasaki, T. Enhancement of the high-rate capability of solid-state lithium batteries by nanoscale interfacial modification. Adv. Mater. 2006, 18, 2226–2229. [Google Scholar] [CrossRef]
- Malligavathy, M.; Ananth Kumar, R.T.; Das, C.; Asokan, S.; Pathinettam Padiyan, D. Growth and characteristics of amorphous Sb2Se3 thin films of various thicknesses for memory switching applications. J. Non-Cryst. Solids 2015, 429, 93–97. [Google Scholar] [CrossRef]
- Xing, Y.J.; Xi, Z.H.; Zhang, X.D.; Song, J.H.; Wang, R.M.; Xu, J.; Xue, Z.Q.; Yu, D.P. Thermal evaporation synthesis of zinc oxide nanowires. Appl. Phys. A 2005, 80, 1527–1530. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wu, J.; Wen, W.; Qi, Y. Fabrication of efficient metal halide perovskite solar cells by vacuum thermal evaporation: A progress review. Curr. Opin. Electrochem. 2018, 11, 130–140. [Google Scholar] [CrossRef]
- Quartarone, E.; Dall′Asta, V.; Resmini, A.; Tealdi, C.; Tredici, I.G.; Tamburini, U.A.; Mustarelli, P. Graphite-coated ZnO nanosheets as high-capacity, highly stable, and binder-free anodes for lithium-ion batteries. J. Power Sources 2016, 320, 314–321. [Google Scholar] [CrossRef]
- Ying, Z.; Wan, Q.; Cao, H.; Song, Z.T.; Feng, S.L. Characterization of SnO2 nanowires as an anode material for Li-ion batteries. Appl. Phys. Lett. 2005, 87, 113108. [Google Scholar] [CrossRef]
- Mattox, D.M. Chapter 6–vacuum evaporation and vacuum deposition. In Handbook of Physical Vapor Deposition (PVD) Processing, 2nd ed.; Mattox, D.M., Ed.; Elsevier: Boston, MA, USA, 2010; pp. 195–235. [Google Scholar]
- Christen, H.M.; Eres, G. Recent advances in pulsed-laser deposition of complex oxides. J. Phys. Condens. Matter. 2008, 20, 264005. [Google Scholar] [CrossRef] [PubMed]
- Safi, I. Recent aspects concerning DC reactive magnetron sputtering of thin films: A review. Surf. Coat. Technol. 2000, 127, 203–218. [Google Scholar] [CrossRef]
- Bobzin, K.; Bagcivan, N.; Immich, P.; Bolz, S.; Alami, J.; Cremer, R. Advantages of nanocomposite coatings deposited by high power pulse magnetron sputtering technology. J. Mater. Process. Technol. 2009, 209, 165–170. [Google Scholar] [CrossRef]
- Kumar, D.S.; Kumar, B.J.; Mahesh, H. Quantum nanostructures (QDs): An overview. In Synthesis of Inorganic Nanomaterials, 1st ed.; Bhagyaraj, S.M., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Elsevier: London, UK, 2018; pp. 59–88. [Google Scholar]
- Rao, B.G.; Mukherjee, D.; Reddy, B.M. Novel approaches for preparation of nanoparticles. In Nanostructures for Novel Therapy, 1st ed.; Ficai, D., Grumezescu, A., Eds.; Elsevier: London, UK, 2017; pp. 1–36. [Google Scholar]
- Best, S.; Marti, P. Mineral coatings for orthopaedic applications. In Coatings for Biomedical Applications, 1st ed.; Driver, M., Ed.; Elsevier: London, UK, 2012; pp. 43–74. [Google Scholar]
- Li, J.F.; Viehland, D.; Tani, T.; Lakeman, C.; Payne, D. Piezoelectric properties of sol-gel-derived ferroelectric and antiferroelectric thin layers. J. Appl. Phys. 1994, 75, 442–448. [Google Scholar] [CrossRef]
- Kakihana, M. Invited review “sol-gel” preparation of high temperature superconducting oxides. J. Sol-Gel Scie. Technol. 1996, 6, 7–55. [Google Scholar] [CrossRef]
- Niederberger, M. Nonaqueous sol-gel routes to metal oxide nanoparticles. Acc. Chem. Res. 2007, 40, 793–800. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Rebenne, H.E.; Bhat, D.G. Review of CVD TiN coatings for wear-resistant applications: Deposition processes, properties and performance. Surf. Coat. Technol. 1994, 63, 1–13. [Google Scholar] [CrossRef]
- Carlsson, J.O.; Martin, P.M. Chemical vapor deposition. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; Elsevier: London, UK, 2010; pp. 314–363. [Google Scholar]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Takada, K. Progress and prospective of solid-state lithium batteries. Acta Mater. 2013, 61, 759–770. [Google Scholar] [CrossRef]
- Jeong, E.; Hong, C.; Tak, Y.; Nam, S.C.; Cho, S. Investigation of interfacial resistance between LiCoO2 cathode and LiPON electrolyte in the thin film battery. J. Power Sources 2006, 159, 223–226. [Google Scholar] [CrossRef]
- Ito, S.; Fujiki, S.; Yamada, T.; Aihara, Y.; Park, Y.; Kim, T.Y.; Baek, S.W.; Lee, J.M.; Doo, S.; Machida, N. A rocking chair type all-solid-state lithium ion battery adopting Li2O-ZrO2 coated LiNi0.8Co0.15Al0.05O2 and a sulfide based electrolyte. J. Power Sources 2014, 248, 943–950. [Google Scholar] [CrossRef]
- Machida, N.; Kashiwagi, J.; Naito, M.; Shigematsu, T. Electrochemical properties of all-solid-state batteries with ZrO2-coated LiNi1/3Mn1/3Co1/3O2 as cathode materials. Solid State Ion. 2012, 225, 354–358. [Google Scholar] [CrossRef]
- Kitaura, H.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. Improvement of electrochemical performance of all-solid-state lithium secondary batteries by surface modification of LiMn2O4 positive electrode. Solid State Ion. 2011, 192, 304–307. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Noh, S.; Lee, G.; Shin, D. Enhanced electrochemical performance of surface modified LiCoO2 for all-solid-state lithium batteries. Ceram. Int. 2016, 42, 2140–2146. [Google Scholar] [CrossRef]
- Yang, G.J.; Kim, Y. Component-selective passivation of Li residues of Ni-based cathode materials by chemical mimicry of solid electrolyte interphase formation. ACS Appl. Energy Mater. 2019, 2, 217–221. [Google Scholar] [CrossRef]
- Yubuchi, S.; Ito, Y.; Matsuyama, T.; Hayashi, A.; Tatsumisago, M. 5V class LiNi0.5Mn1.5O4 positive electrode coated with Li3PO4 thin film for all-solid-state batteries using sulfide solid electrolyte. Solid State Ion. 2016, 285, 79–82. [Google Scholar] [CrossRef]
- Ito, Y.; Sakurai, Y.; Yubuchi, S.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. Application of LiCoO2 particles coated with lithium ortho-oxosalt thin films to sulfide-type all-solid-state lithium batteries. J. Electrochem. Soc. 2015, 162, A1610–A1616. [Google Scholar] [CrossRef]
- Sakurai, Y.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. Preparation of amorphous Li4SiO4-Li3PO4 thin films by pulsed laser deposition for all-solid-state lithium secondary batteries. Solid State Ion. 2011, 182, 59–63. [Google Scholar] [CrossRef]
- Okada, K.; Machida, N.; Naito, M.; Shigematsu, T.; Ito, S.; Fujiki, S.; Nakano, M.; Aihara, Y. Preparation and electrochemical properties of LiAlO2-coated Li(Ni1/3Mn1/3Co1/3)O2 for all-solid-state batteries. Solid State Ion. 2014, 255, 120–127. [Google Scholar] [CrossRef]
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Interfacial observation between LiCoO2 electrode and Li2S−P2S5 solid electrolytes of all-solid-state lithium secondary batteries using transmission electron microscopy. Chem. Mater. 2010, 22, 949–956. [Google Scholar] [CrossRef]
- Seino, Y.; Ota, T.; Takada, K. High rate capabilities of all-solid-state lithium secondary batteries using Li4Ti5O12-coated LiNi0.8Co0.15Al0.05O2 and a sulfide-based solid electrolyte. J. Power Sources 2011, 196, 6488–6492. [Google Scholar] [CrossRef]
- Kwak, H.W.; Park, Y.J. Li2MoO4 coated Ni-rich cathode for all-solid-state batteries. Thin Solid Films 2018, 660, 625–630. [Google Scholar] [CrossRef]
- Ding, Z.; Li, J.; Li, J.; An, C. Interfaces: Key issue to be solved for all solid-state lithium battery technologies. J. Electrochem. Soc. 2020, 167, 070541. [Google Scholar] [CrossRef]
- Xu, R.C.; Xia, X.H.; Zhang, S.Z.; Xie, D.; Wang, X.L.; Tu, J.P. Interfacial challenges and progress for inorganic all-solid-state lithium batteries. Electrochim. Acta 2018, 284, 177–187. [Google Scholar] [CrossRef]
- Ma, J.; Chen, B.; Wang, L.; Cui, G. Progress and prospect on failure mechanisms of solid-state lithium batteries. J. Power Sources 2018, 392, 94–115. [Google Scholar] [CrossRef]
- Zhu, Y.; He, X.; Mo, Y. Origin of outstanding stability in the lithium solid electrolyte materials: Insights from thermodynamic analyses based on first-principles calculations. ACS Appl. Mater. Interfaces 2015, 7, 23685–23693. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; He, X.; Mo, Y. First principles study on electrochemical and chemical stability of solid electrolyte-electrode interfaces in all-solid-state Li-ion batteries. J. Mater. Chem. A 2016, 4, 3253–3266. [Google Scholar] [CrossRef]
- Tadanaga, K.; Takano, R.; Ichinose, T.; Mori, S.; Hayashi, A.; Tatsumisago, M. Low temperature synthesis of highly ion conductive Li7La3Zr2O12-Li3BO3 composites. Electrochem. Commun. 2013, 33, 51–54. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Y.; Zhang, X.; Wang, L.; Zhao, S.X.; Lin, Y.H.; Shen, Y.; Luo, J.; Li, L.; Nan, C.W. Enhanced electrochemical performance of bulk type oxide ceramic lithium batteries enabled by interface modification. J. Mater. Chem. A 2018, 6, 4649–4657. [Google Scholar] [CrossRef]
- Lee, Y.N.; Yoon, Y.S. Cycle stability increase by insertion of Li-La-Ta-O thin-film electrolyte between cathode and solid electrolyte for all-solid-state battery. Thin Solid Films 2015, 579, 75–80. [Google Scholar] [CrossRef]
- Bai, L.; Xue, W.; Qin, H.; Li, Y.; Li, Y.; Sun, J. A novel dense LiCoO2 microcrystalline buffer layer on a cathode-electrolyte interface for all-solid-state lithium batteries prepared by the magnetron sputtering method. Electrochim. Acta 2019, 295, 677–683. [Google Scholar] [CrossRef]
- Ohta, N.; Takada, K.; Sakaguchi, I.; Zhang, L.; Ma, R.; Fukuda, K.; Osada, M.; Sasaki, T. LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries. Electrochem. Commun. 2007, 9, 1486–1490. [Google Scholar] [CrossRef]
- Haruyama, J.; Sodeyama, K.; Han, L.; Takada, K.; Tateyama, Y. Space-charge layer effect at interface between oxide cathode and sulfide electrolyte in all-solid-state lithium-ion battery. Chem. Mater. 2014, 26, 4248–4255. [Google Scholar] [CrossRef]
- Takada, K.; Ohta, N.; Zhang, L.; Xu, X.; Hang, B.T.; Ohnishi, T.; Osada, M.; Sasaki, T. Interfacial phenomena in solid-state lithium battery with sulfide solid electrolyte. Solid State Ion. 2012, 225, 594–597. [Google Scholar] [CrossRef]
- Vinado, C.; Wang, S.; He, Y.; Xiao, X.; Li, Y.; Wang, C.; Yang, J. Electrochemical and interfacial behavior of all solid state batteries using Li10SnP2S12 solid electrolyte. J. Power Sources 2018, 396, 824–830. [Google Scholar] [CrossRef]
- Takahashi, K.; Maekawa, H.; Takamura, H. Effects of intermediate layer on interfacial resistance for all-solid-state lithium batteries using lithium borohydride. Solid State Ion. 2014, 262, 179–182. [Google Scholar] [CrossRef]
- Woo, J.H.; Trevey, J.E.; Cavanagh, A.S.; Choi, Y.S.; Kim, S.C.; George, S.M.; Oh, K.H.; Lee, S.H. Nanoscale interface modification of LiCoO2 by Al2O3 atomic layer deposition for solid-state Li batteries. J. Electrochem. Soc. 2012, 159, A1120–A1124. [Google Scholar] [CrossRef]
- Chen, K.; Yamamoto, K.; Orikasa, Y.; Uchiyama, T.; Ito, Y.; Yubuchi, S.; Hayashi, A.; Tatsumisago, M.; Nitta, K.; Uruga, T.; et al. Effect of introducing interlayers into electrode/electrolyte interface in all-solid-state battery using sulfide electrolyte. Solid State Ion. 2018, 327, 150–156. [Google Scholar] [CrossRef]
- Kim, S.; Harada, K.; Toyama, N.; Oguchi, H.; Kisu, K.; Orimo, S.I. Room temperature operation of all-solid-state battery using a closo-type complex hydride solid electrolyte and a LiCoO2 cathode by interfacial modification. J. Energy Chem. 2020, 43, 47–51. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Wang, Z.; Song, D.; Zhang, H.; Shi, X.; Li, C.; Zhang, L.; Zhu, L. Outstanding electrochemical performances of the all-solid-state lithium battery using Ni-rich layered oxide cathode and sulfide electrolyte. J. Power Sources 2020, 456, 227997. [Google Scholar] [CrossRef]
- Li, X.; Jin, L.; Song, D.; Zhang, H.; Shi, X.; Wang, Z.; Zhang, L.; Zhu, L. LiNbO3-coated LiNi0.8Co0.1Mn0.1O2 cathode with high discharge capacity and rate performance for all-solid-state lithium battery. J. Energy Chem. 2020, 40, 39–45. [Google Scholar] [CrossRef]
- Han, F.; Westover, A.S.; Yue, J.; Fan, X.; Wang, F.; Chi, M.; Leonard, D.N.; Dudney, N.J.; Wang, H.; Wang, C. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 2019, 4, 187–196. [Google Scholar] [CrossRef]
- Wu, B.; Wang, S.; Lochala, J.; Desrochers, D.; Liu, B.; Zhang, W.; Yang, J.; Xiao, J. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries. Energy Environ. Sci. 2018, 11, 1803–1810. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Zhao, Y.; Wang, B.; Kaghazchi, P.; Adair, K.R.; Li, R.; Zhang, C.; Liu, J.; Kuo, L.Y.; et al. Stabilizing the interface of NASICON solid electrolyte against Li metal with atomic layer deposition. ACS Appl. Mater. Interfaces 2018, 10, 31240–31248. [Google Scholar] [CrossRef]
- Alexander, G.V.; Indu, M.S.; Kamakshy, S.; Murugan, R. Development of stable and conductive interface between garnet structured solid electrolyte and lithium metal anode for high performance solid-state battery. Electrochim. Acta 2020, 332, 135511. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, L.; Xu, S.; McOwen, D.W.; Gong, Y.; Yang, C.; Pastel, G.R.; Xie, H.; Fu, K.; Dai, J.; et al. 3D lithium metal anodes hosted in asymmetric garnet frameworks toward high energy density batteries. Energy Storage Mater. 2018, 14, 376–382. [Google Scholar] [CrossRef]
- Lou, J.; Wang, G.; Xia, Y.; Liang, C.; Huang, H.; Gan, Y.; Tao, X.; Zhang, J.; Zhang, W. Achieving efficient and stable interface between metallic lithium and garnet-type solid electrolyte through a thin indium tin oxide interlayer. J. Power Sources 2020, 448, 227440. [Google Scholar] [CrossRef]
- Dudney, N.; Neudecker, B. Solid state thin-film lithium battery systems. Curr. Opin. Solid State Mater. Sci. 1999, 4, 479–482. [Google Scholar]
- Wang, Y.; Liu, B.; Li, Q.; Cartmell, S.; Ferrara, S.; Deng, Z.D.; Xiao, J. Lithium and lithium ion batteries for applications in microelectronic devices: A review. J. Power Sources 2015, 286, 330–345. [Google Scholar] [CrossRef]
- Oudenhoven, J.F.; Baggetto, L.; Notten, P.H. All-solid-state lithium-ion microbatteries: A review of various three-dimensional concepts. Adv. Energy Mater. 2011, 1, 10–33. [Google Scholar] [CrossRef]
- Kanehori, K.; Matsumoto, K.; Miyauchi, K.; Kudo, T. Thin film solid electrolyte and its application to secondary lithium cell. Solid State Ion. 1983, 9, 1445–1448. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Yamaki, J.I. Electrical characteristics of Li2O-V2O5-SiO2 thin films. Solid State Ion. 1989, 35, 201–206. [Google Scholar] [CrossRef]
- Jourdaine, L.; Souquet, J.; Delord, V.; Ribes, M. Lithium solid state glass-based microgenerators. Solid State Ion. 1988, 28, 1490–1494. [Google Scholar] [CrossRef]
- Balkanski, M.; Julien, C.; Emery, J. Integrable lithium solid-state microbatteries. J. Power Sources 1989, 26, 615–622. [Google Scholar] [CrossRef]
- Bates, J.; Gruzalski, G.; Dudney, N.; Luck, C.; Yu, X. Rechargeable thin-film lithium batteries. Solid State Ion. 1994, 70, 619–628. [Google Scholar] [CrossRef]
- Long, J.W.; Dunn, B.; Rolison, D.R.; White, H.S. Three-dimensional battery architectures. Chem. Rev. 2004, 104, 4463–4492. [Google Scholar] [CrossRef]
- Garbayo, I.; Struzik, M.; Bowman, W.J.; Pfenninger, R.; Stilp, E.; Rupp, J.L. Glass-Type polyamorphism in Li-garnet thin film solid state battery conductors. Adv. Energy Mater. 2018, 8, 1702265. [Google Scholar] [CrossRef]
- Ferrari, S.; Loveridge, M.; Beattie, S.D.; Jahn, M.; Dashwood, R.J.; Bhagat, R. Latest advances in the manufacturing of 3D rechargeable lithium microbatteries. J. Power Sources 2015, 286, 25–46. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Pulsed laser deposited films for microbatteries. Coatings 2019, 9, 386. [Google Scholar] [CrossRef]
- Dudney, N.J. Solid-state thin-film rechargeable batteries. Mater. Sci. Eng.: B 2005, 116, 245–249. [Google Scholar] [CrossRef]
- Bates, J.; Gruzalski, G.; Dudney, N.; Luck, C.; Yu, X.; Jones, S. Rechargeable thin-film lithium microbatteries. Solid State Technol. 1993, 36, 59–64. [Google Scholar]
- Jones, S.D.; Akridge, J.R. A thin film solid state microbattery. Solid State Ion. 1992, 53, 628–634. [Google Scholar] [CrossRef]
- Lee, S.J.; Baik, H.K.; Lee, S.M. An all-solid-state thin film battery using LISIPON electrolyte and Si-V negative electrode films. Electrochem. Commun. 2003, 5, 32–35. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Xue, M.Z.; Fu, Z.W. Nanostructured thin film electrodes for lithium storage and all-solid-state thin-film lithium batteries. J. Power Sources 2013, 234, 310–332. [Google Scholar] [CrossRef]
- Patil, A.; Patil, V.; Shin, D.W.; Choi, J.W.; Paik, D.S.; Yoon, S.J. Issue and challenges facing rechargeable thin film lithium batteries. Mater. Res. Bull. 2008, 43, 1913–1942. [Google Scholar] [CrossRef]
- Randau, S.; Weber, D.A.; Kötz, O.; Koerver, R.; Braun, P.; Weber, A.; Ivers-Tiffée, E.; Adermann, T.; Kulisch, J.; Zeier, W.G.; et al. Benchmarking the performance of all-solid-state lithium batteries. Nat. Energy 2020, 5, 259–270. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. In Materials for Sustainable Energy; Dusastre, V., Ed.; World Scientific: Singapore, 2010; pp. 171–179. [Google Scholar]
- Zhang, W.; Liu, Y.; Guo, Z. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci. Adv. 2019, 5, eaav7412. [Google Scholar] [CrossRef]
- Bates, J.; Gruzalski, G.; Dudney, N.; Luck, C.; Yu, X. Thin-film rechargeable lithium batteries. In Proceedings of the Symposium on the Science of Advanced Batteries, Cleveland, OH, USA, 8–9 November 1993. [Google Scholar]
- Xia, H.; Tang, S.; Lu, L. Properties of amorphous Si thin film anodes prepared by pulsed laser deposition. Mater. Res. Bull. 2007, 42, 1301–1309. [Google Scholar] [CrossRef]
- Park, M.; Wang, G.; Liu, H.K.; Dou, S. Electrochemical properties of Si thin film prepared by pulsed laser deposition for lithium ion micro-batteries. Electrochim. Acta 2006, 51, 5246–5249. [Google Scholar] [CrossRef]
- Ohara, S.; Suzuki, J.; Sekine, K.; Takamura, T. A thin film silicon anode for Li-ion batteries having a very large specific capacity and long cycle life. J. Power Sources 2004, 136, 303–306. [Google Scholar] [CrossRef]
- Chen, L.; Xie, J.; Yu, H.; Wang, T. An amorphous Si thin film anode with high capacity and long cycling life for lithium ion batteries. J. Appl. Electrochem. 2009, 39, 1157–1162. [Google Scholar] [CrossRef]
- Saulnier, M.; Trudeau, C.; Cloutier, S.G.; Schougaard, S.B. Investigation of CVD multilayered graphene as negative electrode for lithium-ion batteries. Electrochim. Acta 2017, 244, 54–60. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis of nitrogen-doped graphene films for lithium battery application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef]
- Fan, Z.J.; Yan, J.; Wei, T.; Ning, G.Q.; Zhi, L.J.; Liu, J.C.; Cao, D.X.; Wang, G.L.; Wei, F. Nanographene-constructed carbon nanofibers grown on graphene sheets by chemical vapor deposition: High-performance anode materials for lithium ion batteries. ACS Nano 2011, 5, 2787–2794. [Google Scholar] [CrossRef]
- Shen, B.; Ding, J.; Yan, X.; Feng, W.; Li, J.; Xue, Q. Influence of different buffer gases on synthesis of few-layered graphene by arc discharge method. Appl. Surf. Sci. 2012, 258, 4523–4531. [Google Scholar] [CrossRef]
- Bleu, Y.; Bourquard, F.; Tite, T.; Loir, A.S.; Maddi, C.; Donnet, C.; Garrelie, F. Review of graphene growth from a solid carbon source by pulsed laser deposition (PLD). Front. Chem. 2018, 6, 572. [Google Scholar] [CrossRef]
- Hirayama, M.; Kim, K.; Toujigamori, T.; Cho, W.; Kanno, R. Epitaxial growth and electrochemical properties of Li4Ti5O12 thin-film lithium battery anodes. Dalton Trans. 2011, 40, 2882–2887. [Google Scholar] [CrossRef]
- Deng, J.; Lu, Z.; Chung, C.; Han, X.; Wang, Z.; Zhou, H. Electrochemical performance and kinetic behavior of lithium ion in Li4Ti5O12 thin film electrodes. Appl. Surf. Sci. 2014, 314, 936–941. [Google Scholar] [CrossRef]
- Deng, J.; Lu, Z.; Belharouak, I.; Amine, K.; Chung, C.Y. Preparation and electrochemical properties of Li4Ti5O12 thin film electrodes by pulsed laser deposition. J. Power Sources 2009, 193, 816–821. [Google Scholar] [CrossRef]
- Cunha, D.M.; Hendriks, T.A.; Vasileiadis, A.; Vos, C.M.; Verhallen, T.; Singh, D.P.; Wagemaker, M.; Huijben, M. Doubling reversible capacities in epitaxial Li4Ti5O12 thin film anodes for microbatteries. ACS Appl. Energy Mater. 2019, 2, 3410–3418. [Google Scholar] [CrossRef]
- Wunde, F.; Berkemeier, F.; Schmitz, G. Lithium diffusion in sputter-deposited Li4Ti5O12 thin films. J. Power Sources 2012, 215, 109–115. [Google Scholar] [CrossRef]
- Kumatani, A.; Shiraki, S.; Takagi, Y.; Suzuki, T.; Ohsawa, T.; Gao, X.; Ikuhara, Y.; Hitosugi, T. Epitaxial growth of Li4Ti5O12 thin films using RF magnetron sputtering. Jpn. J. Appl. Phys. 2014, 53, 058001. [Google Scholar] [CrossRef]
- Shen, C.M.; Zhang, X.G.; Zhou, Y.K.; Li, H.L. Preparation and characterization of nanocrystalline Li4Ti5O12 by sol-gel method. Mater. Chem. Phys. 2003, 78, 437–441. [Google Scholar] [CrossRef]
- Rho, Y.H.; Kanamura, K.; Fujisaki, M.; Hamagami, J.I.; Suda, S.I.; Umegaki, T. Preparation of Li4Ti5O12 and LiCoO2 thin film electrodes from precursors obtained by sol-gel method. Solid State Ion. 2002, 151, 151–157. [Google Scholar] [CrossRef]
- Tadanaga, K.; Yamaguchi, A.; Hayashi, A.; Tatsumisago, M.; Mosa, J.; Aparicio, M. Preparation of Li4Ti5O12 electrode thin films by a mist CVD process with aqueous precursor solution. J. Asian Ceram. Soc. 2015, 3, 88–91. [Google Scholar] [CrossRef]
- Tang, S.; Xia, H.; Lai, M.; Lu, L. Characterization of amorphous LiNiVO4 thin-film anode grown by pulsed laser deposition. J. Electrochem. Soc. 2006, 153, A875–A879. [Google Scholar] [CrossRef]
- Tang, S.; Xia, H.; Lai, M.; Lu, L. Amorphous LiNiVO4 thin-film anode for microbatteries grown by pulsed laser deposition. J. Power Sources 2006, 159, 685–689. [Google Scholar] [CrossRef]
- Tang, S.; Lai, M.; Lu, L. Growth and characterization of LiNiVO4 thin film cathode by pulsed laser deposition. Thin Solid Films 2008, 516, 1693–1698. [Google Scholar] [CrossRef]
- Reddy, M.; Wannek, C.; Pecquenard, B.; Vinatier, P.; Levasseur, A. LiNiVO4-promising thin films for use as anode material in microbatteries. J. Power Sources 2003, 119, 101–105. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, H.Y.; Ha, T.S.; Baik, H.K.; Lee, S.M. Amorphous lithium nickel vanadate thin-film anodes for rechargeable lithium microbatteries. Electrochem. Solid State Lett. 2002, 5, A138. [Google Scholar] [CrossRef]
- Reddy, M.; Pecquenard, B.; Vinatier, P.; Levasseur, A. Structural and electrochemical studies of annealed LiNiVO4 thin films. Surf. Interface Anal. 2007, 39, 653–659. [Google Scholar] [CrossRef]
- Daramalla, V.; Penki, T.R.; Munichandraiah, N.; Krupanidhi, S. Fabrication of TiNb2O7 thin film electrodes for Li-ion micro-batteries by pulsed laser deposition. Mater. Sci. Eng.: B 2016, 213, 90–97. [Google Scholar] [CrossRef]
- Daramalla, V.; Krupanidhi, S. Growth and characterization of titanium niobium oxide (TiNb2O7) thin films. Mater. Res. Soc. Symp. Proc. 2015, 1805. [Google Scholar] [CrossRef]
- Daramalla, V.; Dutta, S.; Krupanidhi, S. Temperature dependent dielectric properties and AC conductivity studies on titanium niobium oxide (TiNb2O7) thin films. J. Eur. Ceram. Soc. 2020, 40, 1293–1300. [Google Scholar] [CrossRef]
- Chang, M.C.; Huang, C.S.; Ho, Y.D.; Huang, C.L. Sol-gel derived TiNb2O7 dielectric thin films for transparent electronic applications. J. Am. Ceram. Soc. 2018, 101, 674–682. [Google Scholar] [CrossRef]
- Tan, J.; Tiwari, A. Fabrication and characterization of Li7La3Zr2O12 thin films for lithium ion battery. ECS Solid State Lett. 2012, 1, Q57. [Google Scholar] [CrossRef]
- Jeon, S.W.; Lim, J.K.; Lim, S.H.; Lee, S.M. As-deposited LiCoO2 thin film cathodes prepared by RF magnetron sputtering. Electrochim. Acta 2005, 51, 268–273. [Google Scholar] [CrossRef]
- Striebel, K.; Deng, C.; Wen, S.; Cairns, E. Electrochemical behavior of LiMn2O4 and LiCoO2 thin films produced with pulsed laser deposition. J. Electrochem. Soc. 1996, 143, 1821. [Google Scholar] [CrossRef]
- McGraw, J.M.; Bahn, C.S.; Parilla, P.A.; Perkins, J.D.; Readey, D.W.; Ginley, D.S. Li ion diffusion measurements in V2O5 and Li(Co1−xAlx)O2 thin-film battery cathodes. Electrochim. Acta 1999, 45, 187–196. [Google Scholar] [CrossRef]
- Julien, C.; Camacho-Lopez, M.; Escobar-Alarcon, L.; Haro-Poniatowski, E. Fabrication of LiCoO2 thin-film cathodes for rechargeable lithium microbatteries. Mater. Chem. Phys. 2001, 68, 210–216. [Google Scholar] [CrossRef]
- Escobar-Alarcon, L.; Haro-Poniatowski, E.; Jimenez-Jarquin, J.; Massot, M.; Julien, C. Physical properties of lithium-cobalt oxides grown by laser ablation. Mat. Res. Soc. Symp. Proc. 1998, 548, 223–228. [Google Scholar] [CrossRef]
- Julien, C.; Haro-Poniatowski, E.; Hussain, O.; Ramana, C. Structure and electrochemistry of thin-film oxides grown by laser-pulsed deposition. Ionics 2001, 7, 165–171. [Google Scholar] [CrossRef]
- Okada, K.; Ohnishi, T.; Mitsuishi, K.; Takada, K. Epitaxial growth of LiCoO2 thin films with (001) orientation. AIP Adv. 2017, 7, 115011. [Google Scholar] [CrossRef]
- Nishio, K.; Ohnishi, T.; Mitsuishi, K.; Ohta, N.; Watanabe, K.; Takada, K. Orientation alignment of epitaxial LiCoO2 thin films on vicinal SrTiO3 (100) substrates. J. Power Sources 2016, 325, 306–310. [Google Scholar] [CrossRef]
- Xie, J.; Imanishi, N.; Matsumura, T.; Hirano, A.; Takeda, Y.; Yamamoto, O. Orientation dependence of Li-ion diffusion kinetics in LiCoO2 thin films prepared by RF magnetron sputtering. Solid State Ion. 2008, 179, 362–370. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, Z.; Du, G.; Zhang, P.; Liu, H. LiCoO2 cathode thin film fabricated by RF sputtering for lithium ion microbatteries. Surf. Coat. Technol. 2010, 204, 1710–1714. [Google Scholar] [CrossRef]
- Liao, C.L.; Lee, Y.H.; Fung, K.Z. The film growth and electrochemical properties of RF-sputtered LiCoO2 thin films. J. Alloys Compd. 2007, 436, 303–308. [Google Scholar] [CrossRef]
- Donders, M.; Arnoldbik, W.; Knoops, H.; Kessels, W.; Notten, P. Atomic layer deposition of LiCoO2 thin-film electrodes for all-solid-state Li-ion micro-batteries. J. Electrochem. Soc. 2013, 160, A3066. [Google Scholar] [CrossRef]
- Nilsen, O.; Miikkulainen, V.; Gandrud, K.B.; Østreng, E.; Ruud, A.; Fjellvåg, H. Atomic layer deposition of functional films for Li-ion microbatteries. Phys. Status Solidi A 2014, 211, 357–367. [Google Scholar] [CrossRef]
- Cho, S.I.; Yoon, S.G. Characterization of LiCoO2 thin film cathodes deposited by liquid-delivery metallorganic chemical vapor deposition for rechargeable lithium batteries. J. Electrochem. Soc. 2002, 149, A1584–A1588. [Google Scholar] [CrossRef]
- Fragnaud, P.; Nagarajan, R.; Schleich, D.; Vujic, D. Thin-film cathodes for secondary lithium batteries. J. Power Sources 1995, 54, 362–366. [Google Scholar] [CrossRef]
- Nishio, K.; Ohnishi, T.; Akatsuka, K.; Takada, K. Crystal orientation of epitaxial LiCoO2 films grown on SrTiO3 substrates. J. Power Sources 2014, 247, 687–691. [Google Scholar] [CrossRef]
- Tang, S.; Xia, H.; Lai, M.; Lu, L. Characterization of LiMn2O4 thin films grown on Si substrates by pulsed laser deposition. J. Alloys Compd. 2008, 449, 322–325. [Google Scholar] [CrossRef]
- Sonoyama, N.; Iwase, K.; Takatsuka, H.; Matsumura, T.; Imanishi, N.; Takeda, Y.; Kanno, R. Electrochemistry of LiMn2O4 epitaxial films deposited on various single crystal substrates. J. Power Sources 2009, 189, 561–565. [Google Scholar] [CrossRef]
- Hendriks, R.; Cunha, D.M.; Singh, D.P.; Huijben, M. Enhanced lithium transport by control of crystal orientation in spinel LiMn2O4 thin film cathodes. ACS Appl. Energy Mater. 2018, 1, 7046–7051. [Google Scholar] [CrossRef]
- Camacho-Lopez, M.; Escobar-Alarcon, L.; Haro-Poniatowski, E.; Julien, C. LiMn2O4 films grown by pulsed-laser deposition. Ionics 1999, 5, 244–250. [Google Scholar] [CrossRef]
- Xie, J.; Tanaka, T.; Imanishi, N.; Matsumura, T.; Hirano, A.; Takeda, Y.; Yamamoto, O. Li-ion transport kinetics in LiMn2O4 thin films prepared by radio frequency magnetron sputtering. J. Power Sources 2008, 180, 576–581. [Google Scholar] [CrossRef]
- Prasad, K.H.; Vinoth, S.; Ratnakar, A.; Venkateswarlu, M.; Satyanarayana, N. Structural and electrical conductivity studies of spinel LiMn2O4 cathode films grown by RF Sputtering. Mater. Today: Proc. 2016, 3, 4046–4051. [Google Scholar] [CrossRef]
- Hwang, B.J.; Wang, C.Y.; Cheng, M.Y.; Santhanam, R. Structure, morphology, and electrochemical investigation of LiMn2O4 thin film cathodes deposited by Radio frequency sputtering for lithium microbatteries. J. Phys. Chem. C 2009, 113, 11373–11380. [Google Scholar] [CrossRef]
- Park, Y.; Kim, J.; Kim, M.; Chung, H.; Um, W.; Kim, M.; Kim, H. Fabrication of LiMn2O4 thin films by sol-gel method for cathode materials of microbattery. J. Power Sources 1998, 76, 41–47. [Google Scholar] [CrossRef]
- Park, Y.; Kim, J.; Kim, M.; Chung, H.; Kim, H.G. Preparation of LiMn2O4 thin films by a sol-gel method. Solid State Ion. 2000, 130, 203–214. [Google Scholar] [CrossRef]
- Tadanaga, K.; Yamaguchi, A.; Sakuda, A.; Hayashi, A.; Tatsumisago, M.; Duran, A.; Aparacio, M. Preparation of LiMn2O4 cathode thin films for thin film lithium secondary batteries by a mist CVD process. Mater. Res. Bull. 2014, 53, 196–198. [Google Scholar] [CrossRef]
- Tan, G.; Wu, F.; Lu, J.; Chen, R.; Li, L.; Amine, K. Controllable crystalline preferred orientation in Li-Co-Ni-Mn oxide cathode thin films for all-solid-state lithium batteries. Nanoscale 2014, 6, 10611–10622. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Lynch, T.; Chen, A.; Jian, J.; Wang, H. Highly textured Li(Ni0.5Mn0.3Co0.2)O2 thin films on stainless steel as cathode for lithium-ion battery. J. Power Sources 2013, 241, 410–414. [Google Scholar] [CrossRef]
- Prathibha, G.; Rosaiah, P.; Hussain, O. Growth and electrochemical properties of RF sputter deposited Li[Ni0.5Co0.25Mn0.25]O2 film cathodes. Mater. Today: Proc. 2019, 19, 388–391. [Google Scholar] [CrossRef]
- Jacob, C.; Jian, J.; Zhu, Y.; Su, Q.; Wang, H. A new approach to investigate Li2MnO3 and Li(Ni0.5Mn0.3Co0.2)O2 mixed phase cathode materials. J. Mater. Chem. A 2014, 2, 2283–2289. [Google Scholar] [CrossRef]
- Wu, C.Y.; Bao, Q.; Lai, Y.C.; Liu, X.; Lu, Y.C.; Tao, H.; Duh, J.G. In-situ thermal annealing Pt/Ti interphase layers for epitaxial growth of improved Li(Ni0.5Mn0.3Co0.2)O2 solid thin-film cathodes. Nano Energy 2019, 60, 784–793. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, L.; Yang, B.; Li, Q.; Wu, B.; Zhao, J.; Ma, L.; Liu, Y.; An, H. Preparation and ion conduction of Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte films using radio frequency sputtering. Solid State Ion. 2020, 346, 115224. [Google Scholar] [CrossRef]
- Ling, Q.; Yu, Z.; Xu, H.; Zhu, G.; Zhang, X.; Zhao, Y.; Yu, A. Preparation and electrical properties of amorphous Li-Al-Ti-PO thin film electrolyte. Mater. Lett. 2016, 169, 42–45. [Google Scholar] [CrossRef]
- Chen, H.; Tao, H.; Zhao, X.; Wu, Q. Fabrication and ionic conductivity of amorphous Li-Al-Ti-P-O thin film. J. Non-Cryst. Solids 2011, 357, 3267–3271. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, L.; Lu, Y.; Shi, G.; Li, J.; Ma, L.; Zhao, J.; An, H. Preparation and ionic conduction of Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte using inorganic germanium as precursor. J. Eur. Ceram. Soc. 2019, 39, 402–408. [Google Scholar] [CrossRef]
- Inada, R.; Ishida, K.I.; Tojo, M.; Okada, T.; Tojo, T.; Sakurai, Y. Properties of aerosol deposited NASICON-type Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte thin films. Ceram. Int. 2015, 41, 11136–11142. [Google Scholar] [CrossRef]
- Nakagawa, A.; Kuwata, N.; Matsuda, Y.; Kawamura, J. Characterization of stable solid electrolyte lithium silicate for thin film lithium battery. J. Phys. Soc. Jpn. 2010, 79, 98–101. [Google Scholar] [CrossRef]
- Kuwata, N.; Kumar, R.; Toribami, K.; Suzuki, T.; Hattori, T.; Kawamura, J. Thin film lithium ion batteries prepared only by pulsed laser deposition. Solid State Ion. 2006, 177, 2827–2832. [Google Scholar] [CrossRef]
- Kuwata, N.; Kawamura, J.; Toribami, K.; Hattori, T.; Sata, N. Thin-film lithium ion batteries with Li-V-Si-O amorphous solid electrolyte fabricated by pulsed laser deposition. In Solid State Ionics: The Science and Technology of Ions in Motion; Yoo, H.I., Chowdari, B.V.R., Choi, G.M., Lee, J.H., Eds.; World Scientific: Singapore, 2004; pp. 637–644. [Google Scholar]
- Kuwata, N.; Kawamura, J.; Toribami, K.; Hattori, T.; Sata, N. Thin-film lithium-ion battery with amorphous solid electrolyte fabricated by pulsed laser deposition. Electrochem. Commun. 2004, 6, 417–421. [Google Scholar] [CrossRef]
- Kawamura, J.; Kuwata, N.; Toribami, K.; Sata, N.; Kamishima, O.; Hattori, T. Preparation of amorphous lithium ion conductor thin films by pulsed laser deposition. Solid State Ion. 2004, 175, 273–276. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Yamaki, J.I. Preparation and electrical conductivity of Li2O-V2O5-SiO2 thin films. Jpn. J. Appl. Phys. 1989, 28, 2264. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Okada, S.; Yamaki, J.I. Solid state battery with Li2O-V2O5-SiO2 solid electrolyte thin film. Solid State Ion. 1990, 40, 964–966. [Google Scholar] [CrossRef]
- Hamon, Y.; Douard, A.; Sabary, F.; Marcel, C.; Vinatier, P.; Pecquenard, B.; Levasseur, A. Influence of sputtering conditions on ionic conductivity of LiPON thin films. Solid State Ion. 2006, 177, 257–261. [Google Scholar] [CrossRef]
- West, W.; Whitacre, J.; Lim, J. Chemical stability enhancement of lithium conducting solid electrolyte plates using sputtered LiPON thin films. J. Power Sources 2004, 126, 134–138. [Google Scholar] [CrossRef]
- Zhao, S.; Fu, Z.; Qin, Q. A solid-state electrolyte lithium phosphorus oxynitride film prepared by pulsed laser deposition. Thin Solid Films 2002, 415, 108–113. [Google Scholar] [CrossRef]
- West, W.C.; Hood, Z.D.; Adhikari, S.P.; Liang, C.; Lachgar, A.; Motoyama, M.; Iriyama, Y. Reduction of charge-transfer resistance at the solid electrolyte-electrode interface by pulsed laser deposition of films from a crystalline Li2PO2N source. J. Power Sources 2016, 312, 116–122. [Google Scholar] [CrossRef]
- Kozen, A.C.; Pearse, A.J.; Lin, C.F.; Noked, M.; Rubloff, G.W. Atomic layer deposition of the solid electrolyte LiPON. Chem. Mater. 2015, 27, 5324–5331. [Google Scholar] [CrossRef]
- Wei, J.; Ogawa, D.; Fukumura, T.; Hirose, Y.; Hasegawa, T. Epitaxial strain-controlled ionic conductivity in Li-ion solid electrolyte Li0.33La0.56TiO3 thin films. Cryst. Growth Des. 2015, 15, 2187–2191. [Google Scholar] [CrossRef]
- Kim, S.; Hirayama, M.; Suzuki, K.; Kanno, R. Hetero-epitaxial growth of Li0.17La0.61TiO3 solid electrolyte on LiMn2O4 electrode for all solid-state batteries. Solid State Ion. 2014, 262, 578–581. [Google Scholar] [CrossRef]
- Ohnishi, T.; Takada, K. Synthesis and orientation control of Li-ion conducting epitaxial Li0.33La0.56TiO3 solid electrolyte thin films by pulsed laser deposition. Solid State Ion. 2012, 228, 80–82. [Google Scholar] [CrossRef]
- Ohta, H.; Mizoguchi, T.; Aoki, N.; Yamamoto, T.; Sabarudin, A.; Umemura, T. Lithium-ion conducting La2/3−xLi3xTiO3 solid electrolyte thin films with stepped and terraced surfaces. Appl. Phys. Lett. 2012, 100, 173107. [Google Scholar] [CrossRef]
- Aaltonen, T.; Alnes, M.; Nilsen, O.; Costelle, L.; Fjellvåg, H. Lanthanum titanate and lithium lanthanum titanate thin films grown by atomic layer deposition. J. Mater. Chem. 2010, 20, 2877–2881. [Google Scholar] [CrossRef]
- Ohnishi, T.; Mitsuishi, K.; Nishio, K.; Takada, K. Epitaxy of Li3xLa2/3–xTiO3 films and the influence of La ordering on Li-ion conduction. Chem. Mater. 2015, 27, 1233–1241. [Google Scholar] [CrossRef]
- Aguesse, F.; Roddatis, V.; Roqueta, J.; García, P.; Pergolesi, D.; Santiso, J.; Kilner, J.A. Microstructure and ionic conductivity of LLTO thin films: Influence of different substrates and excess lithium in the target. Solid State Ion. 2015, 272, 1–8. [Google Scholar] [CrossRef]
- Teranishi, T.; Ishii, Y.; Hayashi, H.; Kishimoto, A. Lithium ion conductivity of oriented Li0.33La0.56TiO3 solid electrolyte films prepared by a sol-gel process. Solid State Ion. 2016, 284, 1–6. [Google Scholar] [CrossRef]
- Xiong, Y.; Tao, H.; Zhao, J.; Cheng, H.; Zhao, X. Effects of annealing temperature on structure and opt-electric properties of ion-conducting LLTO thin films prepared by RF magnetron sputtering. J. Alloys Compd. 2011, 509, 1910–1914. [Google Scholar] [CrossRef]
- Lee, J.Z.; Wang, Z.; Xin, H.L.; Wynn, T.A.; Meng, Y.S. Amorphous lithium lanthanum titanate for solid-state microbatteries. J. Electrochem. Soc. 2017, 164, A6268–A6273. [Google Scholar] [CrossRef]
- Kim, D.H.; Imashuku, S.; Wang, L.; Shao-Horn, Y.; Ross, C.A. Li loss during the growth of (Li, La)TiO3 thin films by pulsed laser deposition. J. Cryst. Growth 2013, 372, 9–14. [Google Scholar] [CrossRef]
- Furusawa, S.I.; Tabuchi, H.; Sugiyama, T.; Tao, S.; Irvine, J.T. Ionic conductivity of amorphous lithium lanthanum titanate thin film. Solid State Ion. 2005, 176, 553–558. [Google Scholar] [CrossRef]
- Stramare, S.; Thangadurai, V.; Weppner, W. Lithium lanthanum titanates: A review. Chem. Mater 2003, 15, 3974–3990. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef]
- Van Den Broek, J.; Afyon, S.; Rupp, J.L. Interface-engineered all-solid-state Li-ion batteries based on garnet-type fast Li+ conductors. Adv. Energy Mater. 2016, 6, 1600736. [Google Scholar] [CrossRef]
- Kim, S.; Hirayama, M.; Taminato, S.; Kanno, R. Epitaxial growth and lithium ion conductivity of lithium-oxide garnet for an all solid-state battery electrolyte. Dalton Trans. 2013, 42, 13112–13117. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Cheng, L.; Zorba, V.; Mehta, A.; Cabana, J.; Chen, G.; Doeff, M.M.; Richardson, T.J.; Park, J.H.; Son, J.W. Effects of crystallinity and impurities on the electrical conductivity of Li-La-Zr-O thin films. Thin Solid Films 2015, 576, 55–60. [Google Scholar] [CrossRef]
- Rawlence, M.; Garbayo, I.; Buecheler, S.; Rupp, J. On the chemical stability of post-lithiated garnet Al-stabilized Li7La3Zr2O12 solid state electrolyte thin films. Nanoscale 2016, 8, 14746–14753. [Google Scholar] [CrossRef]
- Kalita, D.; Lee, S.; Lee, K.; Ko, D.; Yoon, Y. Ionic conductivity properties of amorphous Li-La-Zr-O solid electrolyte for thin film batteries. Solid State Ion. 2012, 229, 14–19. [Google Scholar] [CrossRef]
- Tadanaga, K.; Egawa, H.; Hayashi, A.; Tatsumisago, M.; Mosa, J.; Aparicio, M.; Duran, A. Preparation of lithium ion conductive Al-doped Li7La3Zr2O12 thin films by a sol-gel process. J. Power Sources 2015, 273, 844–847. [Google Scholar] [CrossRef]
- Bitzer, M.; Van Gestel, T.; Uhlenbruck, S. Sol-gel synthesis of thin solid Li7La3Zr2O12 electrolyte films for Li-ion batteries. Thin Solid Films 2016, 615, 128–134. [Google Scholar] [CrossRef]
- Chen, R.J.; Huang, M.; Huang, W.Z.; Shen, Y.; Lin, Y.H.; Nan, C.W. Sol-gel derived Li-La-Zr-O thin films as solid electrolytes for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 13277–13282. [Google Scholar] [CrossRef]
- Katsui, H.; Goto, T. Preparation of cubic and tetragonal Li7La3Zr2O12 film by metal organic chemical vapor deposition. Thin Solid Films 2015, 584, 130–134. [Google Scholar] [CrossRef]
- Saccoccio, M.; Yu, J.; Lu, Z.; Kwok, S.C.; Wang, J.; Yeung, K.K.; Yuen, M.M.; Ciucci, F. Low temperature pulsed laser deposition of garnet Li6.4La3Zr1.4Ta0.6O12 films as all solid-state lithium battery electrolytes. J. Power Sources 2017, 365, 43–52. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Iwasa, Y.; Kawasaki, M.; Keimer, B.; Nagaosa, N.; Tokura, Y. Emergent phenomena at oxide interfaces. Nat. Mater. 2012, 11, 103–113. [Google Scholar]
- Zubko, P.; Gariglio, S.; Gabay, M.; Ghosez, P.; Triscone, J.M. Interface physics in complex oxide heterostructures. Annu. Rev. Condens. Matter Phys. 2011, 2, 141–165. [Google Scholar] [CrossRef]
- Brinkman, A.; Huijben, M.; van Zalk, M.; Huijben, J.; Zeitler, U.; Maan, J.C.; van der Wiel, W.G.; Rijnders, G.; Blank, D.H.A.; Hilgenkamp, H. Magnetic effects at the interface between non-magnetic oxides. Nat. Mater. 2007, 6, 493–496. [Google Scholar] [CrossRef]
- Ohtomo, A.; Hwang, H.Y. A high-mobility electron gas at the LaAlO3/SrTiO3 heterointerface. Nature 2004, 427, 423–426. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Gogotsi, Y. Two-dimensional heterostructures for energy storage. Nat. Energy 2017, 2, 17089. [Google Scholar] [CrossRef]
- Suzuki, K.; Kim, K.; Taminato, S.; Hirayama, M.; Kanno, R. Fabrication and electrochemical properties of LiMn2O4/SrRuO3 multi-layer epitaxial thin film electrodes. J. Power Sources 2013, 226, 340–345. [Google Scholar] [CrossRef]
- Aierken, Y.; Sevik, C.; Gülseren, O.; Peeters, F.M.; Çakır, D. MXenes/graphene heterostructures for Li battery applications: A first principles study. J. Mater. Chem. A 2018, 6, 2337–2345. [Google Scholar] [CrossRef]
- Chueh, W.C.; Hao, Y.; Jung, W.; Haile, S.M. High electrochemical activity of the oxide phase in model ceria-Pt and ceria-Ni composite anodes. Nat. Mater. 2012, 11, 155–161. [Google Scholar] [CrossRef]
- Lee, D.; Gao, X.; Sun, L.; Jee, Y.; Poplawsky, J.; Farmer, T.O.; Fan, L.; Guo, E.J.; Lu, Q.; Heller, W.T.; et al. Colossal oxygen vacancy formation at a fluorite-bixbyite interface. Nat. Commun. 2020, 11, 1371. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Z.; Wang, G.; Ruan, G.; Fan, X.; Li, L.; Fei, H.; Hauge, R.H.; Tour, J.M. Three-dimensional thin film for lithium-ion batteries and supercapacitors. ACS Nano 2014, 8, 7279–7287. [Google Scholar] [CrossRef]
- Choi, B.G.; Chang, S.J.; Lee, Y.B.; Bae, J.S.; Kim, H.J.; Huh, Y.S. 3D heterostructured architectures of Co3O4 nanoparticles deposited on porous graphene surfaces for high performance of lithium ion batteries. Nanoscale 2012, 4, 5924–5930. [Google Scholar] [CrossRef] [PubMed]
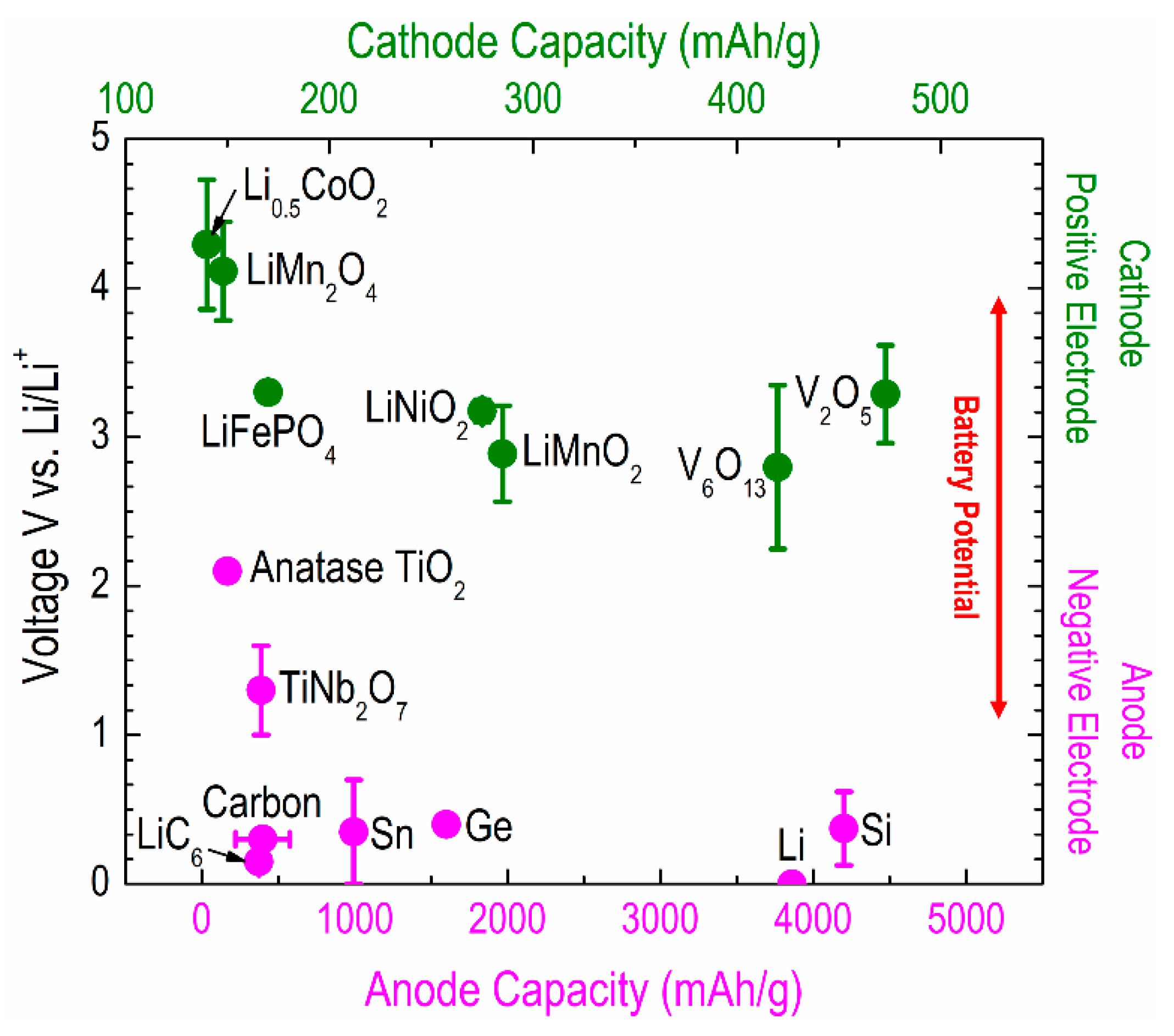
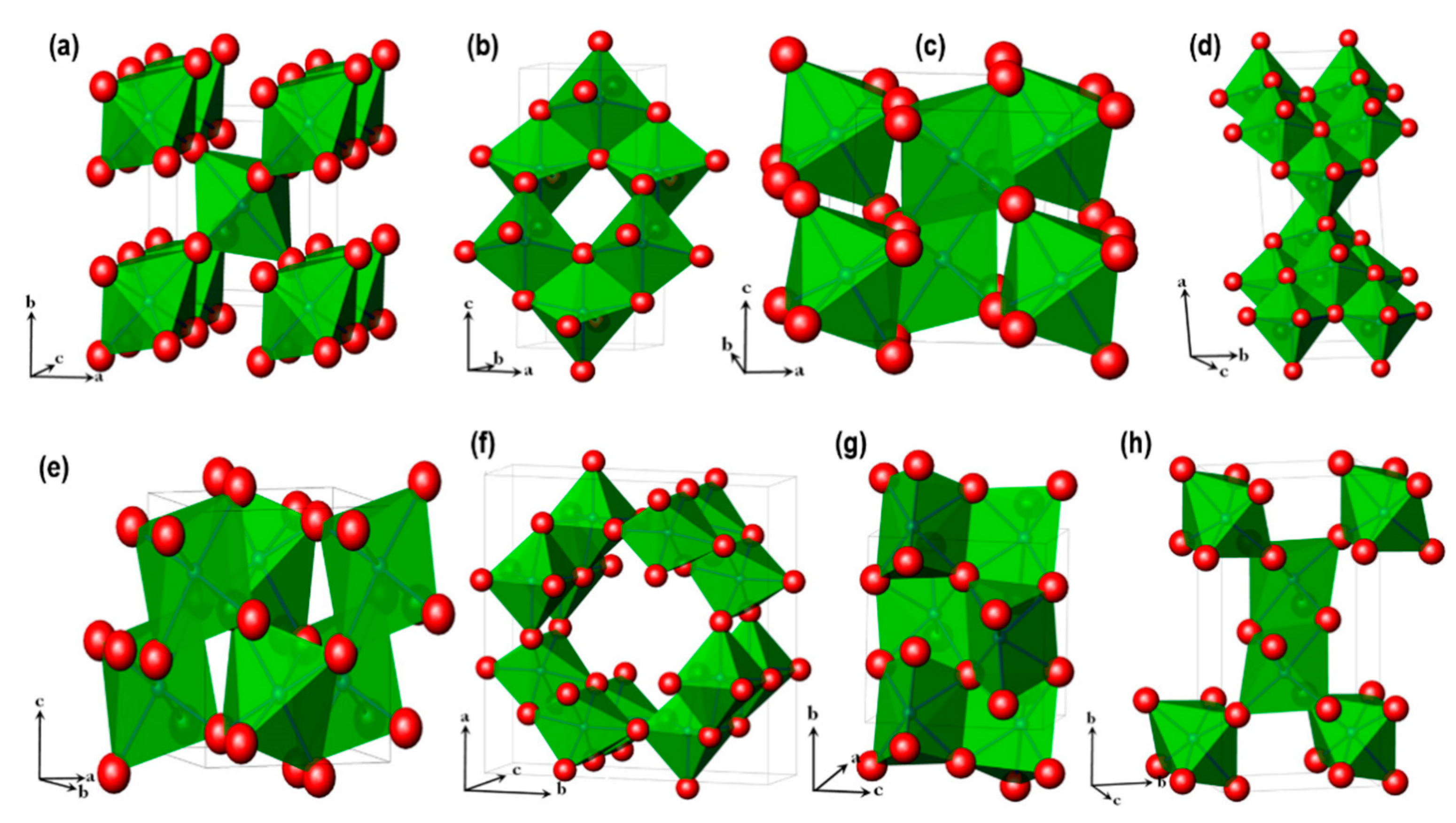
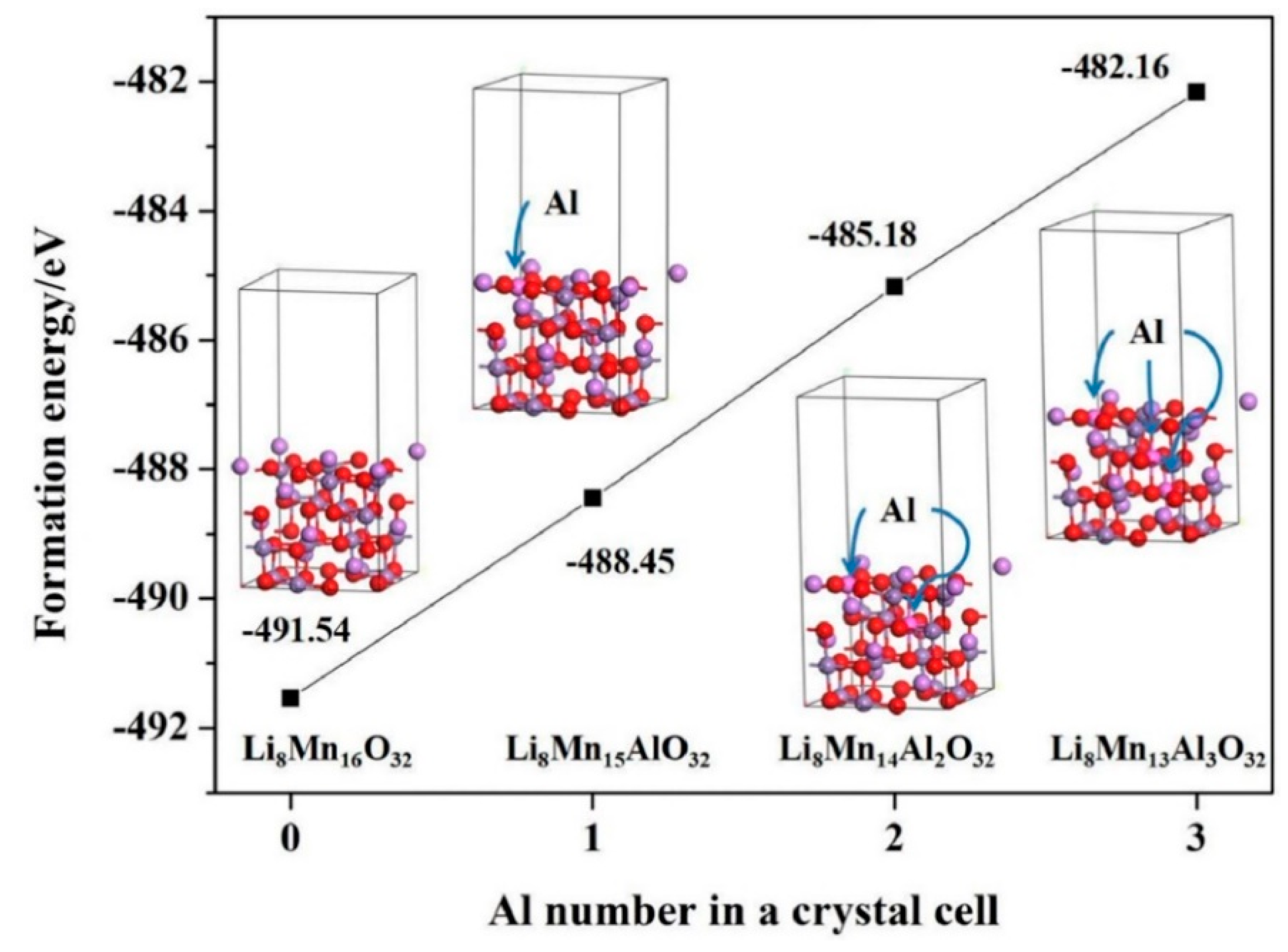
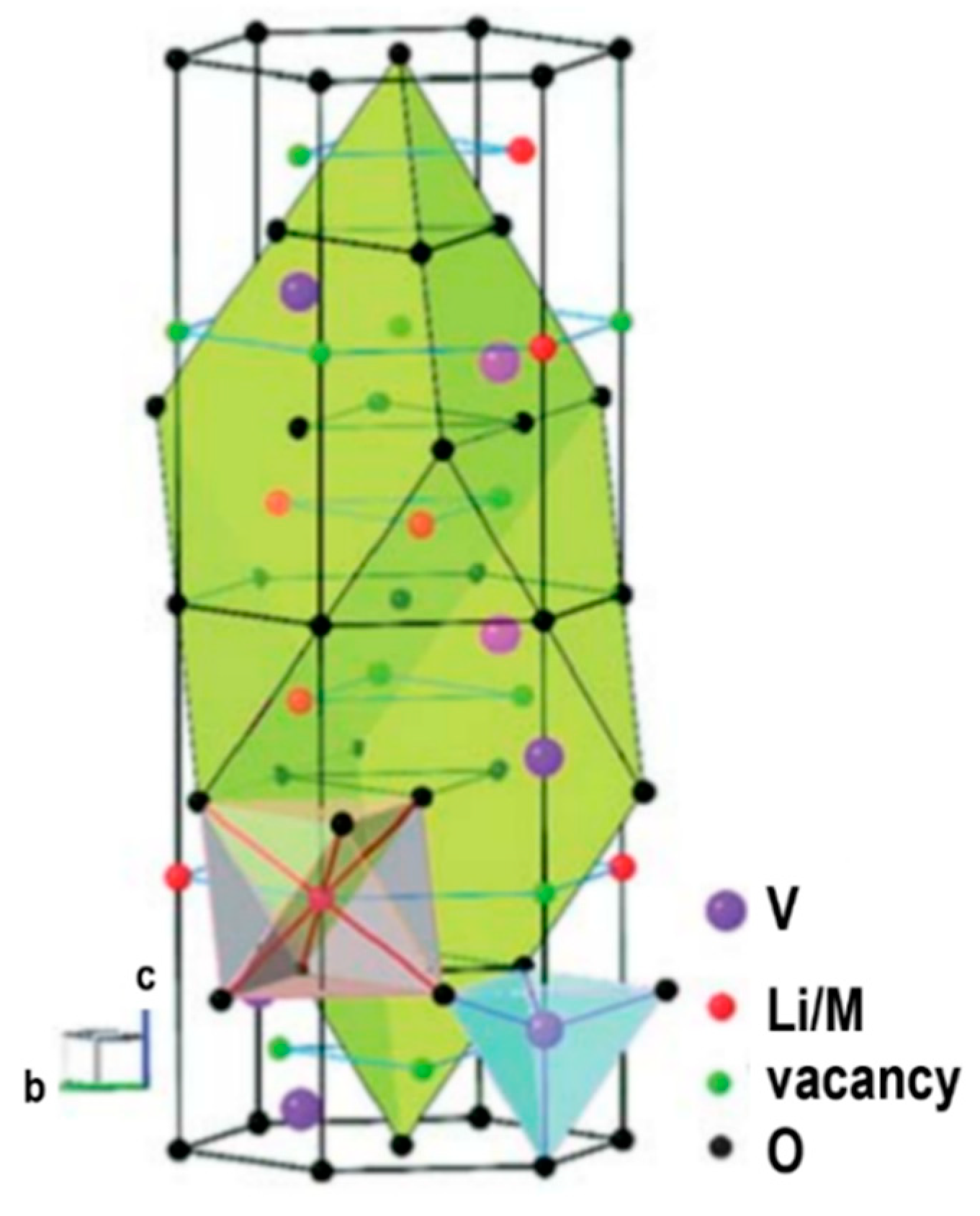
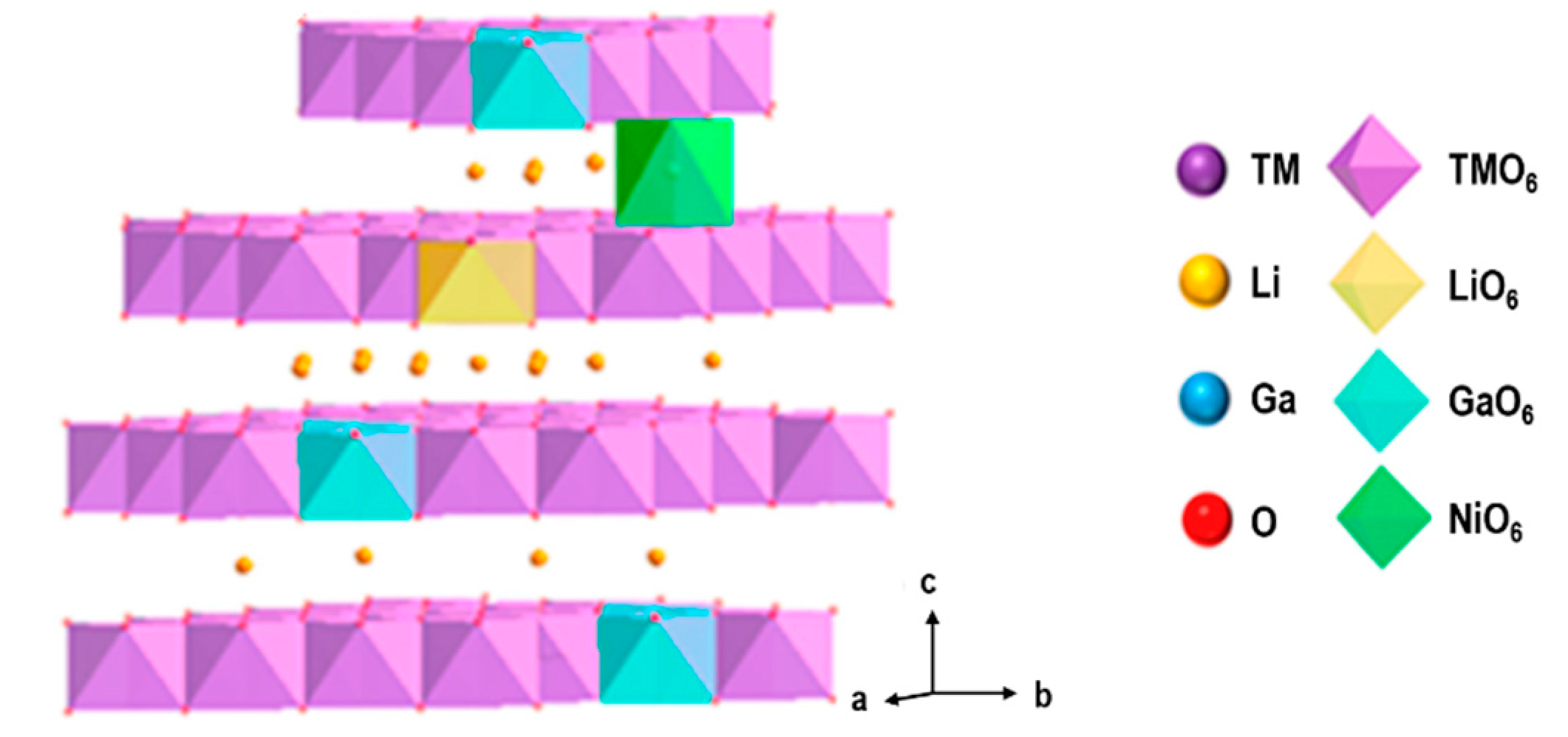
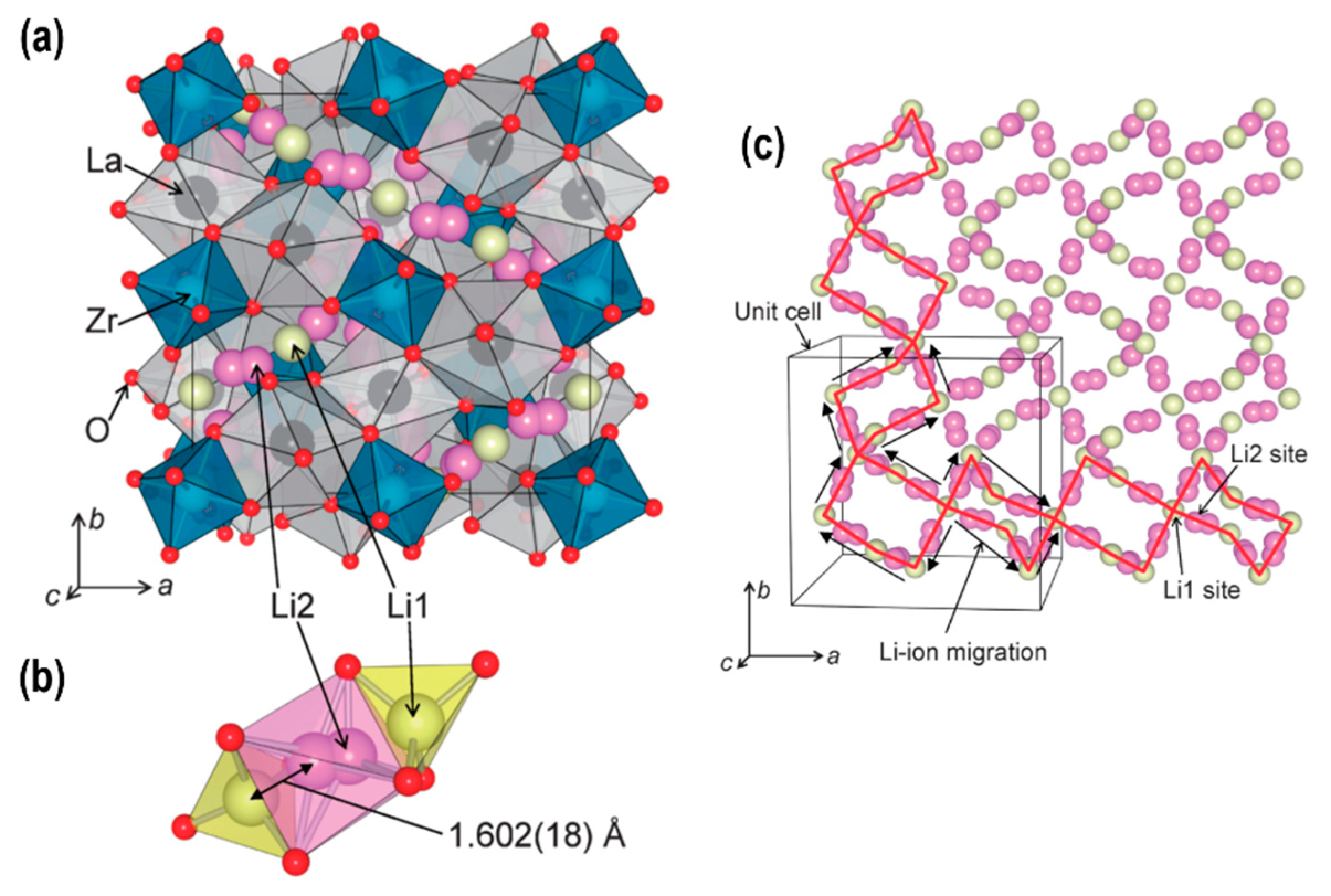
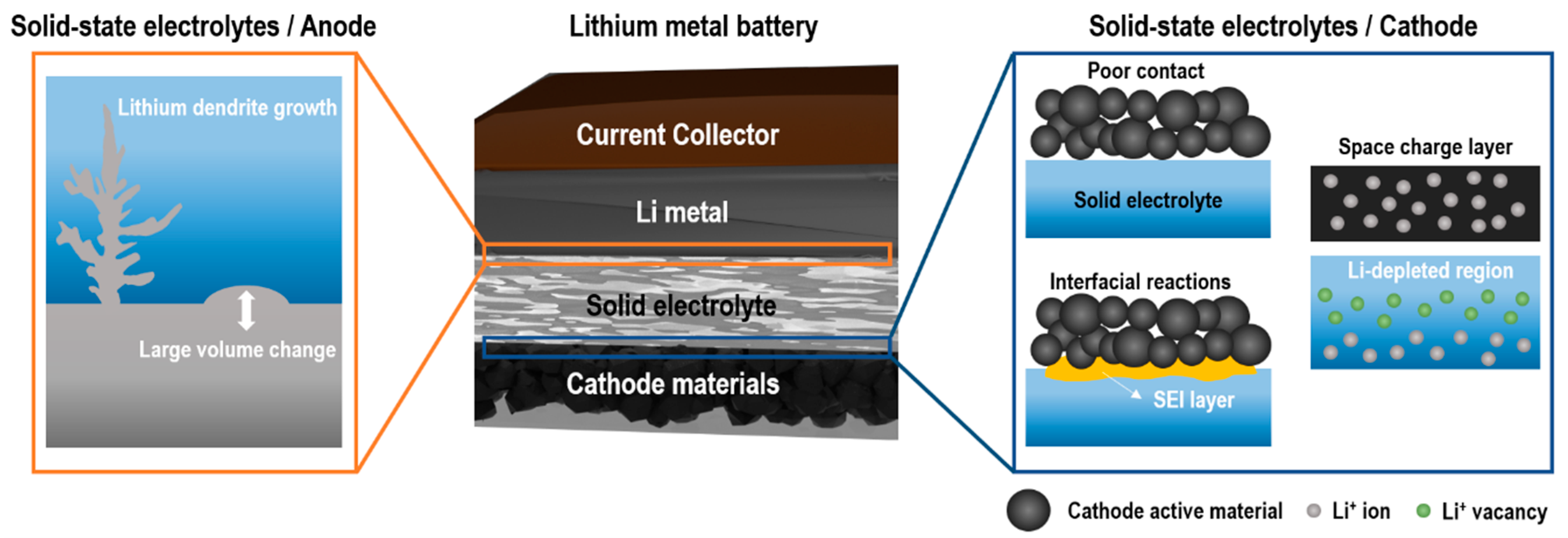
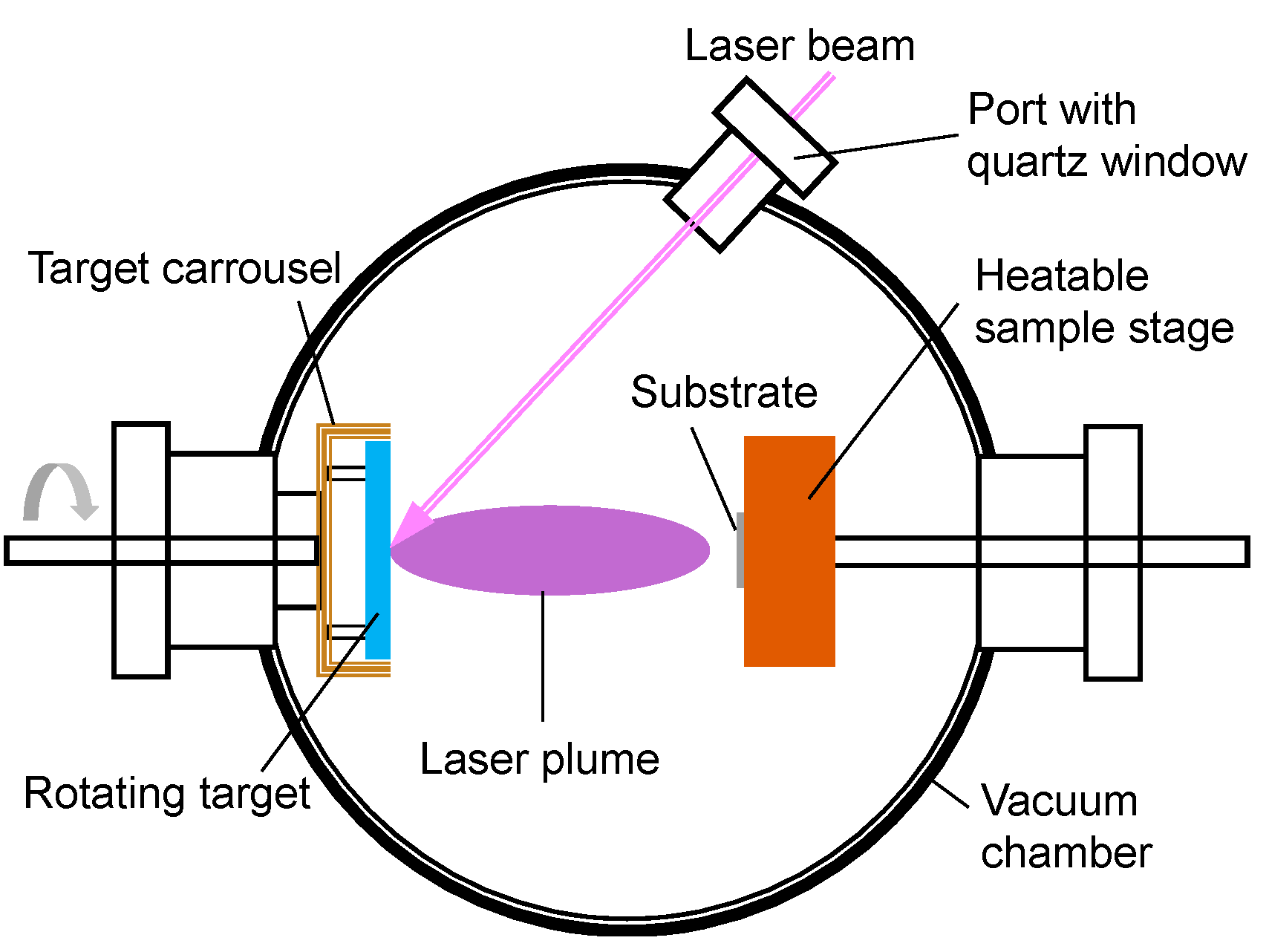
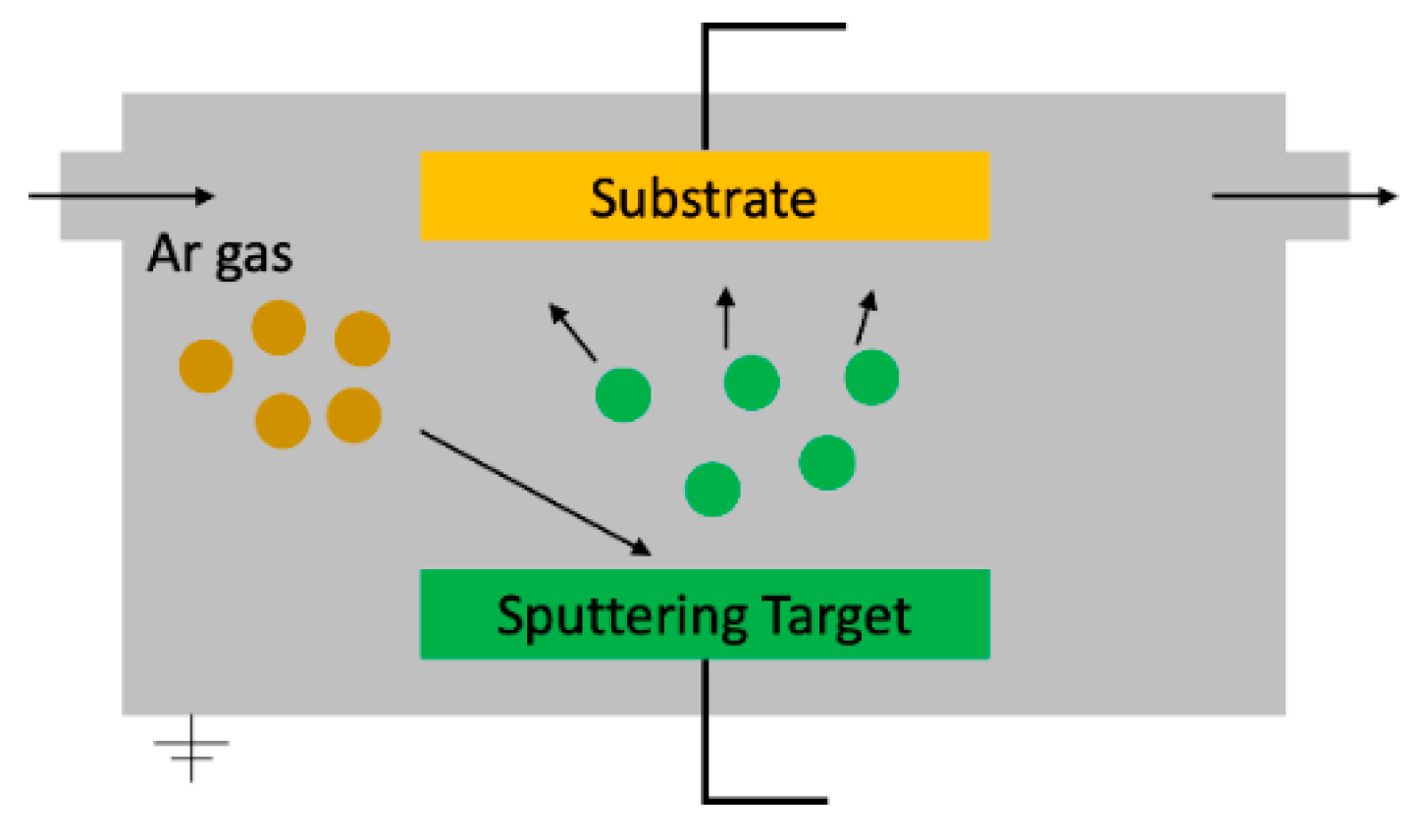
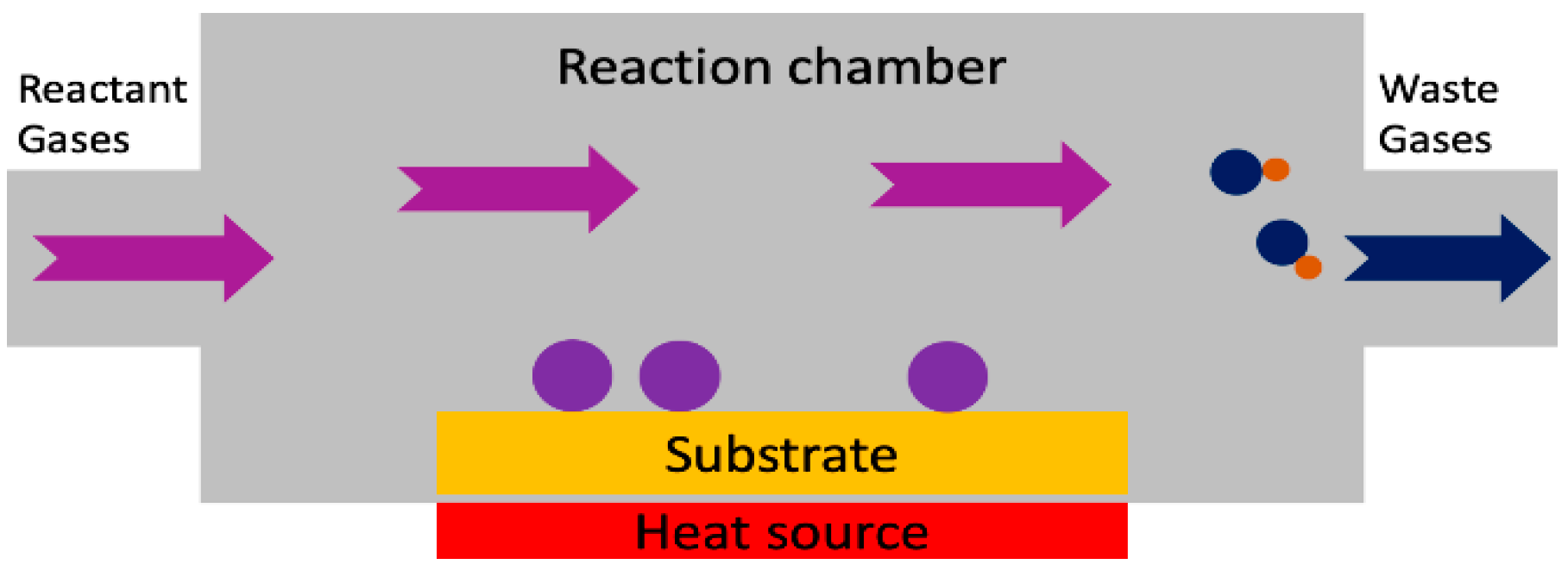
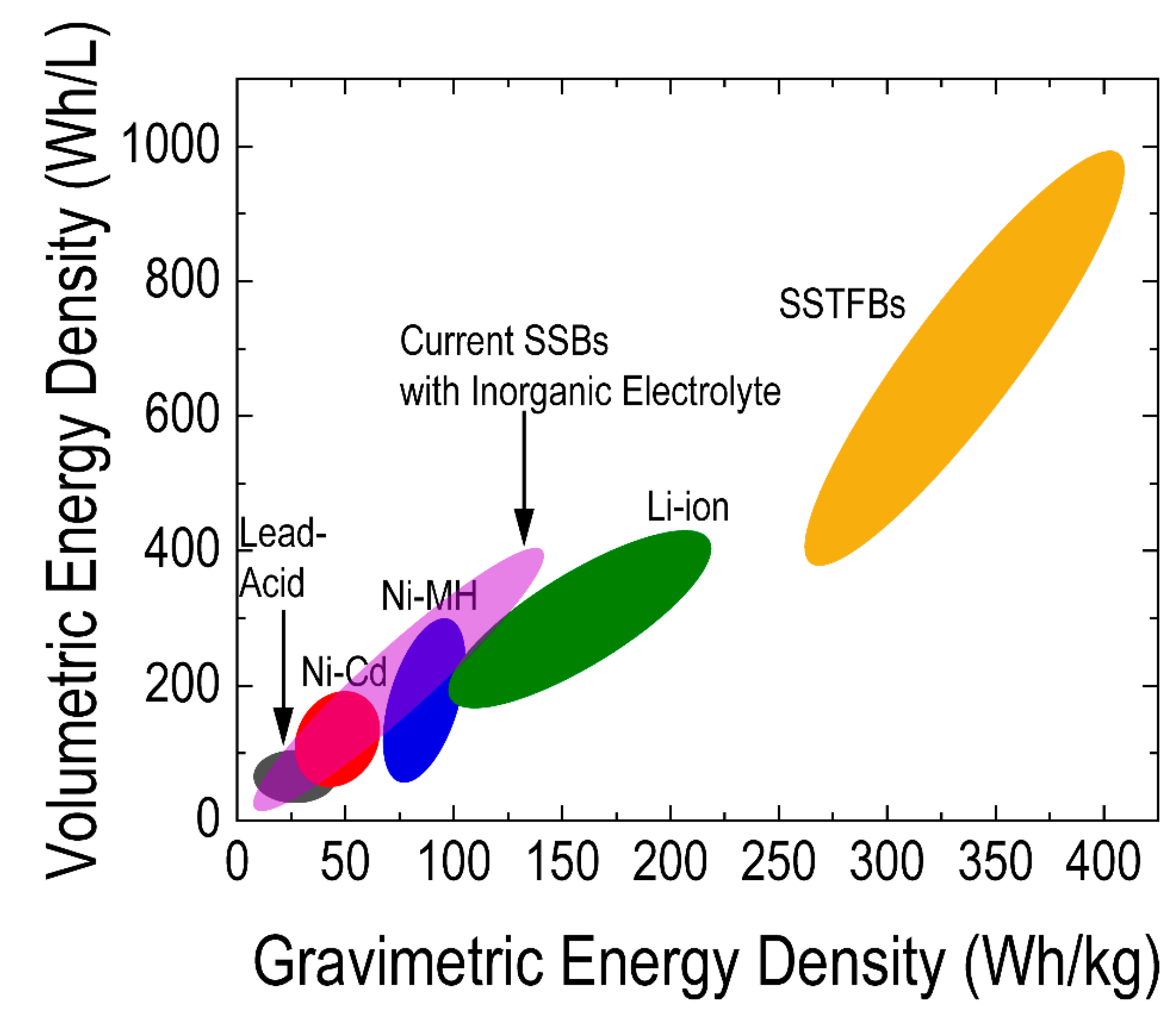
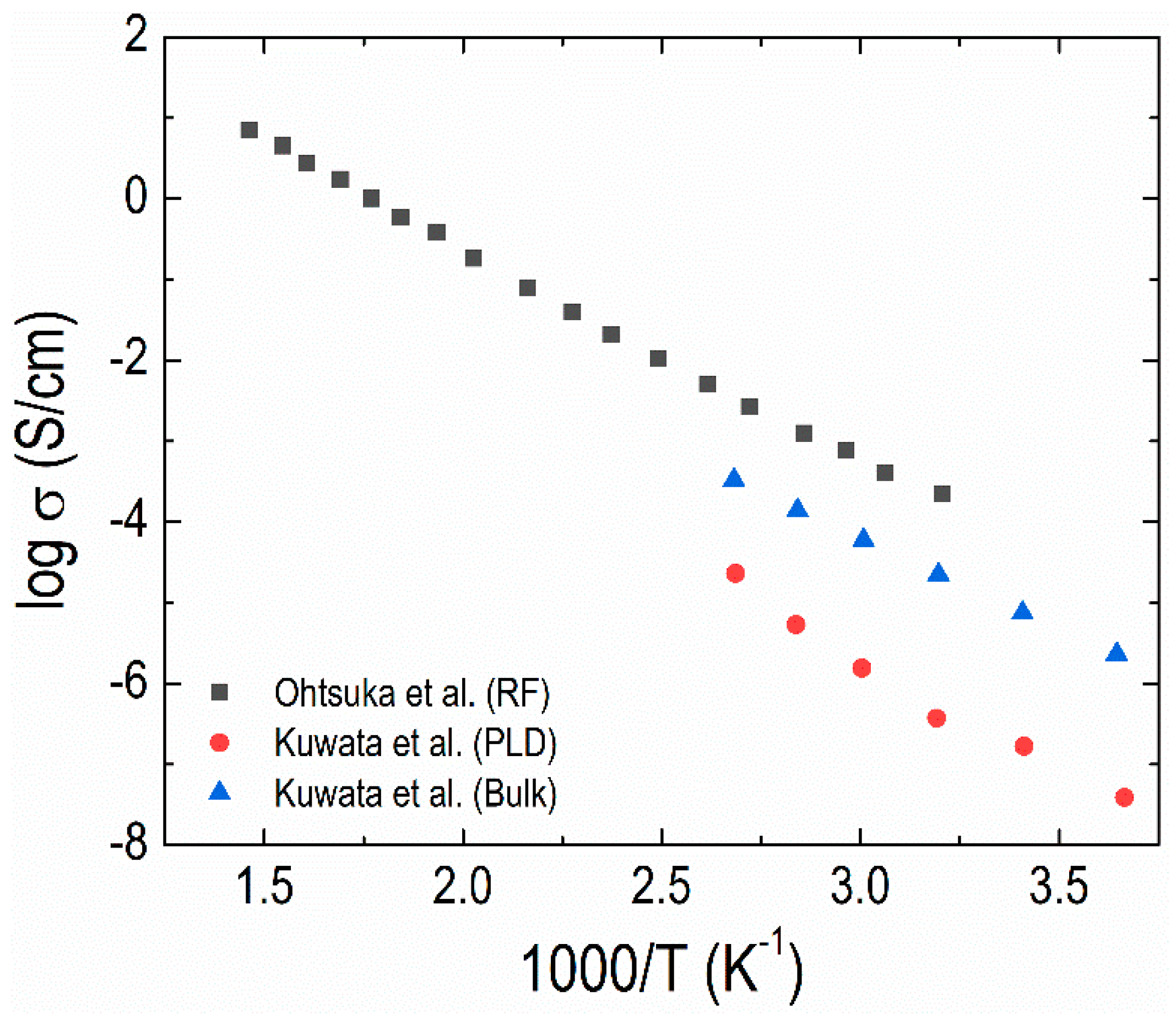
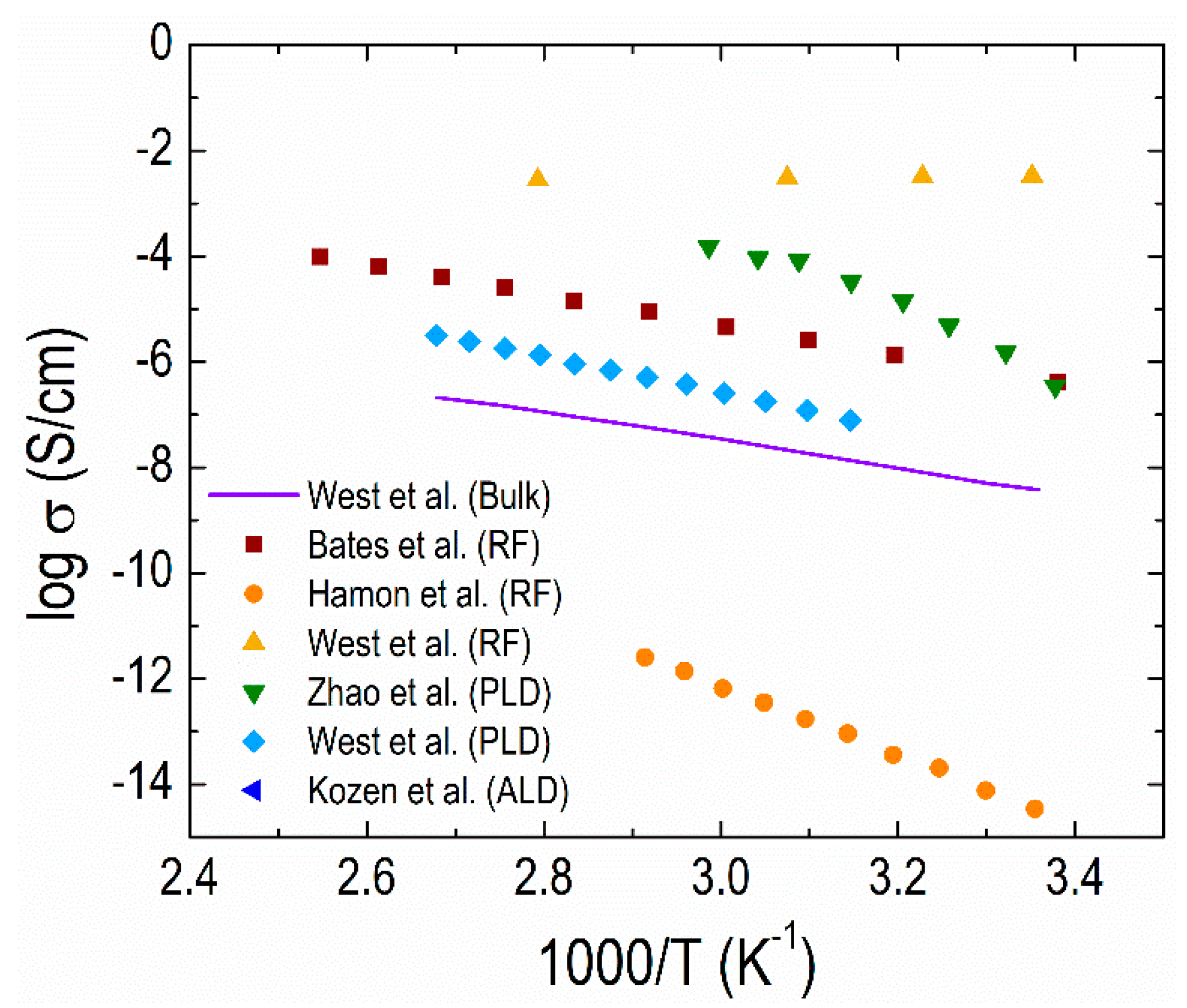
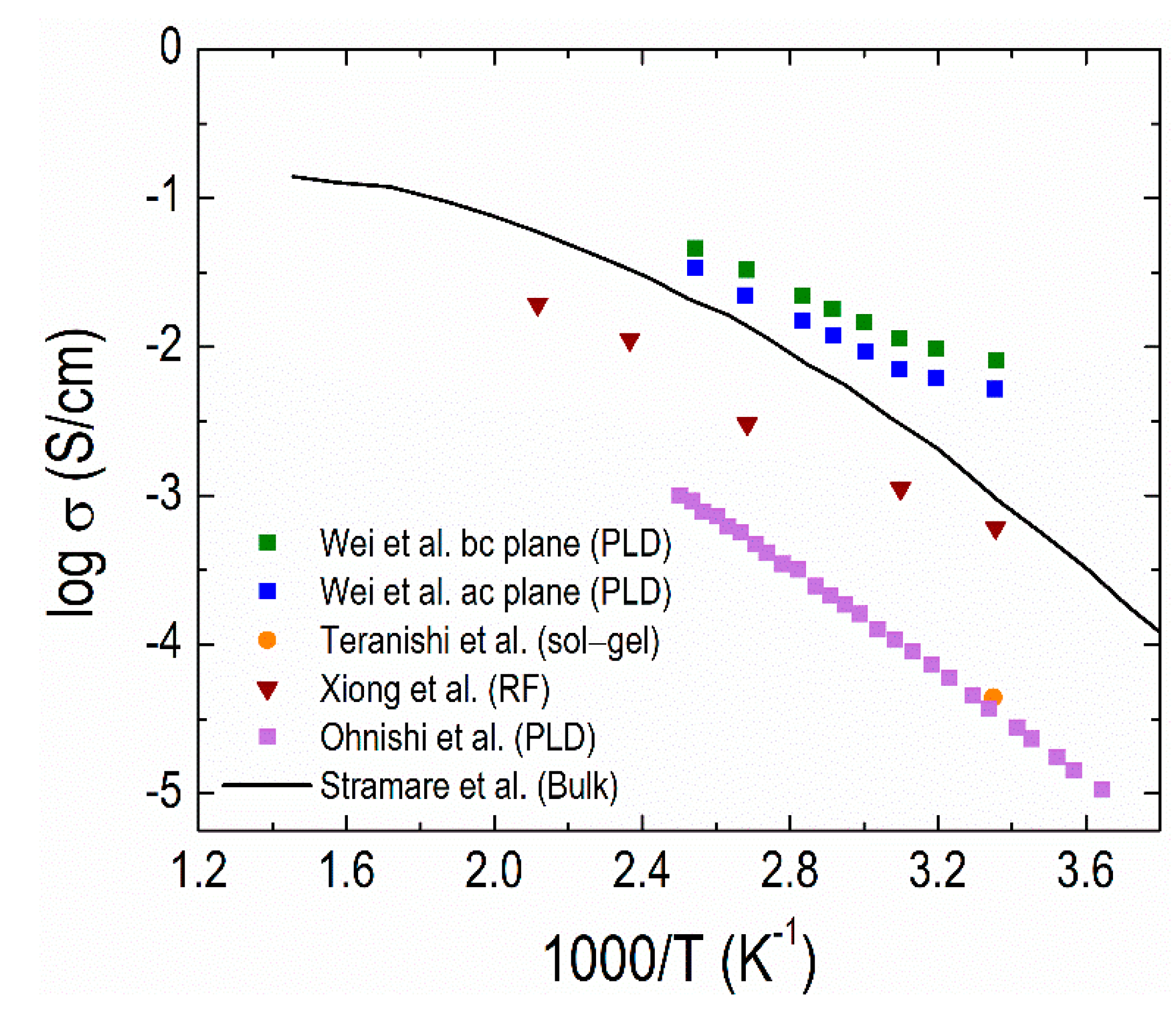
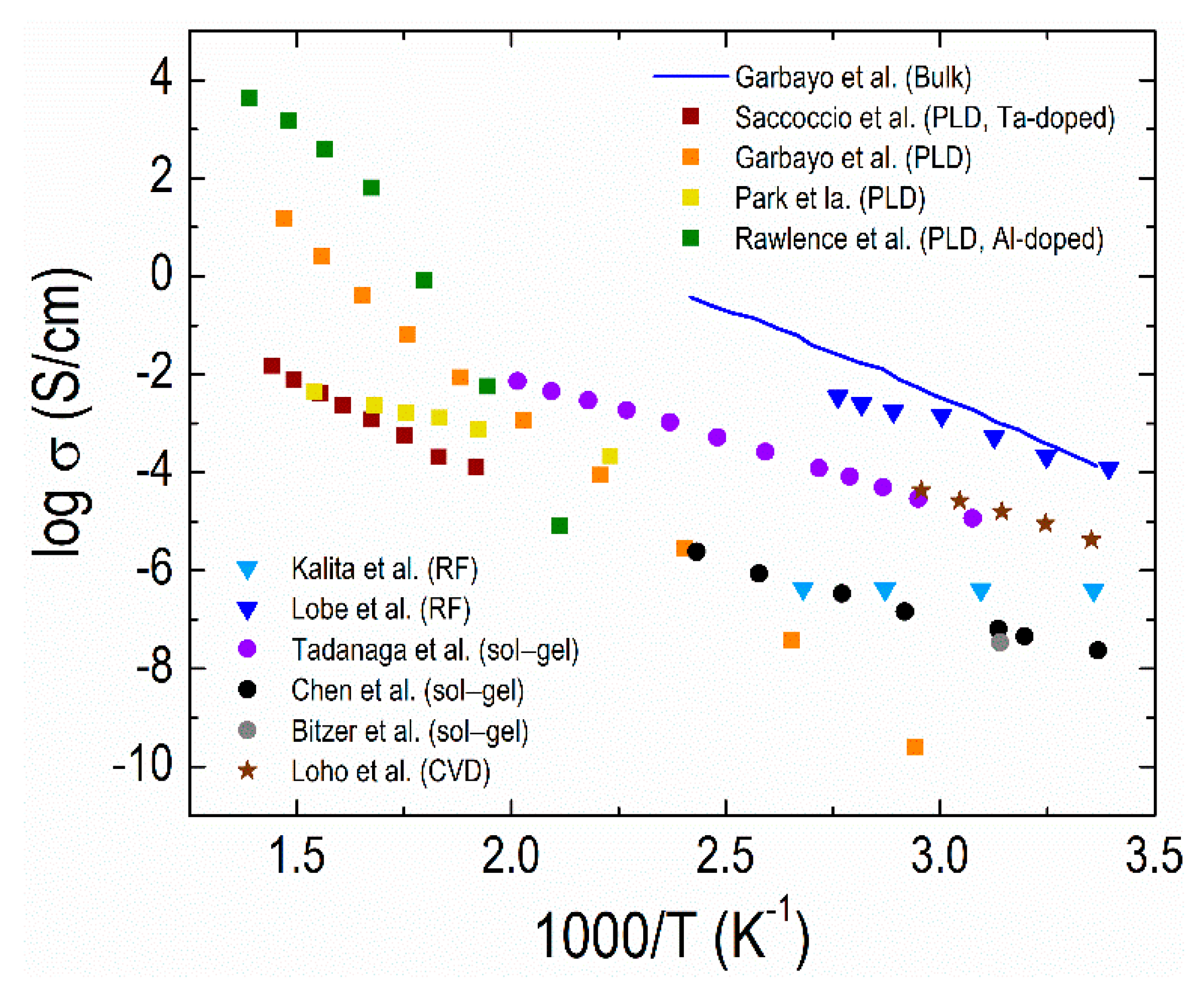
| Material | Discharge Rate (C) | Current Density (mA/cm2) | Initial Discharge Capacity (mAh/g) | Ref. |
|---|---|---|---|---|
| Si | 0.05 | 2834 | [93] | |
| Si | 0.2 | 2433 | [95] | |
| Si | 0.2 | 3000 | [96] | |
| Si | 200 | 4122 | [98] | |
| Si | 0.5 | 4021 | [100] | |
| Si | 0.05 | 3400 | [101] | |
| Li1.25H1.63Ti2O5.44−σ | 35 | 130 | [113] | |
| TiO2 | 10 | 131 | [109] | |
| TiO2 | 0.1 | 343 | [112] | |
| TiO2 | 0.1 | 320 | [115] | |
| TiO2@α–Fe2O3 | 100 | 800 | [116] | |
| Cu0.8LTO | 0.1 | 209 | [110] | |
| TNO | 100 | 184 | [119] | |
| TNO | 1 | 195 | [121] | |
| TNO | 50 | 230 | [122] | |
| TNO | 0.1 | 281 | [123] | |
| TNO | 0.1 | 289 | [124] |
| Material | Doping | Discharge Rate (C) | Initial Discharge Capacity (mAh/g) | Ref. |
|---|---|---|---|---|
| LCO | Ba, Ti | 0.2 | 190.5 | [128] |
| LCO | La, Al | 0.1 | 190 | [129] |
| LMO | Y | 0.5 | 120 | [138] |
| LMO | Ce | 1 | 101 | [139] |
| LNVO | 0.02 | 80 | [141] | |
| LNVO | 0.1 | 30 | [142] | |
| NMC-811 | Ta | 0.067 | 212 | [157] |
| LiNi0.83Co0.11Mn0.6O2 | 1 | 200 | [158] | |
| NMC-811 | LiPO4-AlPO4-Al(PO3)3 | 0.1 | 218 | [171] |
| Li1.14Ni0.14Co0.14Mn0.54O2 | La(PO3)3 | 0.1 | 286 | [172] |
| NMC-811 | LiTa2PO8 | 0.1 | 231 | [173] |
| Category | Electrolyte | Ionic Conductivity (S/cm) | Activation Energy (eV) | Temperature (°C) | Ref. |
|---|---|---|---|---|---|
| NASICON | Li1+xAlxTi2−x(PO4)3 (x = 0.2) | 3.4 × 10−3 | 0.28 | RT | [174] |
| NASICON | Li1+xAlxGe2−x(PO4)3 (x = 0.2) | 10−4 | 0.38 | RT | [175] |
| NASICON | Li1+xAlxGe2−x(PO4)3 (x = 0.5) | 4.22 × 10−3 | 0.51 | 27 | [176] |
| LISICON | Li3.6Ge0.6C0.4O4 | 4 × 10−5 | 0.44 | RT | [176] |
| LISICON | Li10.42Ge1.5P1.5Cl0.8O11.92 | 3.7 × 10−5 | 0.39 | 27 | [177] |
| Thio-LISICON | Li3.25Ge0.25P0.75S4 | 2.2 × 10−3 | 0.21 | RT | [178] |
| LIPON | Li3.3PO3.9N0.17 | 2.6 × 10−6 | 0.56 | 25 | [179] |
| LIPON | Li3.2PO3.0N1.0 | 3.1 × 10−6 | 0.57 | RT | [180] |
| LIPON | Li3.13PO1.69N1.39 | 4.9 × 10−6 | 0.55 | 22 | [181] |
| Perovskite | Li0.34La0.51TiO2.94 | 2 × 10−5 | 0.42 | RT | [182] |
| Perovskite | Li0.27Sr0.063La0.54TiO3 | 4.84 × 10−4 | 0.29 | RT | [183] |
| Perovskite | Li0.43La0.56Ti0.95Ge0.05O3 | 1.2 × 10−5 | RT | [184] | |
| Perovskite | Li0.38Sr0.44Ta0.75Zr0.25O3 | 2.0 × 10−4 | 0.26 | 30 | [185] |
| Perovskite | Li3/8Sr7/16Ta3/4Zr1/4O3 | 2.7 × 10−4 | 0.36 | 27 | [186] |
| Perovskite | Li3/8Sr7/16Hf1/4Ta3/4O3 | 3.8 × 10−4 | 0.36 | 25 | [187] |
| Perovskite | Li3/8Sr7/16Ta3/4Hf1/4O3 | 5.2 × 10−4 | 0.33 | 25 | [188] |
| Garnet | Li7La3Zr2O12 | 2 × 10−5 | 0.26 | 30 | [189] |
| Garnet | Li5La3Nb2O12 | 10−6 | 0.43 | 25 | [190] |
| Garnet | Li5La3Ta2O12 | 10−6 | 0.56 | 25 | [191] |
| Garnet | Li7La2Zr2O12 | 7.74 × 10−4 | 0.32 | RT | [55] |
| Garnet | Li6.75La3Zr1.75Ta0.25O12 | 8.7 × 10−4 | 0.22 | 25 | [191] |
| Garnet | La3Zr2Li6.55Ga0.15O12 | 1.3 × 10−3 | 0.3 | 24 | [192] |
| LiRAP | Li3OCl0.5Br0.5 | 1.94 × 10−3 | 0.2 | RT | [193] |
| LiRAP | Li2(OH)0.9F0.1Cl | 3.5 × 10−5 | 0.52 | RT | [194] |
| Sulfide | Li10GeP2S12 | 1.7 × 10−2 | 0.18 | RT | [51] |
| Sulfide | Li9.54Si1.74P1.44S11.7Cl0.3 | 2.5 × 10−2 | 0.24 | 25 | [27] |
| Sulfide | Li6PS5I | 2.2 × 10−4 | 0.26 | RT | [195] |
| Sulfide | Li22SiP2S18 | 3 × 10−3 | [196] | ||
| Argyrodite | Li5.3PS4.3Br1.7 | 1.1 × 10−2 | 0.18 | 25 | [197] |
| Argyrodite | Li5.5PS4.5Cl1.5 | 10−2 | 0.27 | 25 | [198] |
| Electrode | Electrolyte | Buffer Layer | Coating Method | Interface Resistance w/ Buffer Layer (Ω·cm2) | Interface Resistance w/o Buffer Layer (Ω·cm2) | Ref. |
|---|---|---|---|---|---|---|
| LCO | LLZO–Nb | Li3BO3 | Screen printing | 230 | [238] | |
| LCO | Li3.25Ge0.25P0.75S4 | LTO | Spray coating | 34.56 | 714.71 | [256] |
| LCO | Li10SnP2S12 | Li3NbO4 | ALD | 790 | [302] | |
| LCO | LiBH4 | Li3PO4 | PLD | 8 | 3850 | [303] |
| LCO | LiBH4 | LiNbO3 | PLD | 47.36 | [303] | |
| LCO | LiBH4 | Al2O3 | PLD | 599.45 | [303] | |
| LCO | 80Li2S·20P2S5 | Li3PO4 | PLD | 250 | [305] | |
| LMO | Li3.25Ge0.25P0.75S4 | LiNbO3 | Spray coating | 157.08 | 7854 | [301] |
| NMC-532 | Li7(Al0.1)La3Zr2O12 | LCO | Sputtering | 1004.53 | [298] | |
| NMC-811/LCO | Li10GeP2S12 | LiNbO3 | Solution coating | 39.82 | 95.93 | [307] |
| NMC-532 | Li6.75La3Zr1.75Ta0.25O12 | Li–Ti–O | Solution coating | 7461.3 | 9817.5 | [296] |
| NMC-811 | Li10GeP2S12 | LiNbO3 | Solution coating | 125.6 | 204.2 | [308] |
| Electrode | Electrolyte | Buffer Layer | Coating Method | Interface Resistance w/ Buffer Layer (Ω·cm2) | Interface Resistance w/o Buffer Layer (Ω·cm2) | Ref. |
|---|---|---|---|---|---|---|
| Li metal | Li7La2.75Ca0.25Zr1.75Nb0.25O12 | Al2O3 | ALD | 1 | 1710 | [255] |
| Li metal | Li5La3Ta2O12 | ZnO | ALD | 20 | 2000 | [88] |
| Li metal | Li1.3Al0.3Ti1.7(PO4)3 | Al2O3 | ALD | 117,810 | 314,160 | [311] |
| Li metal | Li6.28Al0.24La3Zr2O12 | LiNbO3 | sputtering | 91 | 1078 | [312] |
| Li metal | Li6.4La3Zr1.4Ta0.6O12 | ITO | sputtering | 32 | 1192 | [314] |
| Materials | Crystalline Structure | Discharge Rate (C) | Current Density (μA/cm2) | Discharge Capacity (mAh/g) | Density (g/cm3) | Discharge Capacity (μAh/cm2 μm) | Ref. |
|---|---|---|---|---|---|---|---|
| LTO a | (111)-Oriented | 10 | 157 | 3.38 | 53 | [348] | |
| LTO a | (111)-Oriented | 60 | 146 | 3.38 | 49 | [348] | |
| LTO a | (100) Epitaxial | 3 | 313 | 3.38 | 106 | [349] | |
| LTO a | (110) Epitaxial | 3 | 277 | 3.38 | 94 | [349] | |
| LTO a | (111) Epitaxial | 3 | 283 | 3.38 | 96 | [349] | |
| LTO b | (111)-Oriented | 0.5 | 7 | 3.38 | 25 | [350] | |
| LTO b | (111)-Oriented | 95 | 62 | 3.38 | 21 | [350] | |
| LTO c | (111)-Oriented | 10 | 110 | 3.38 | 37 | [354] | |
| LNVO a | Amorphous | 100 | 979 | 4.19 | 410 | [365] | |
| LNVO b | Amorphous | 100 | 1878 | 4.19 | 787 | [359] | |
| LNVO b | Amorphous | 75 | 1100 | 4.19 | 461 | [358,360] | |
| LNVO b | Polycrystalline | 75 | 850 | 4.19 | 356 | [358,360] | |
| TNO a | Polycrystalline | 35 | 398 | 4.42 | 176 | [361] | |
| TNO a | Polycrystalline | 50 | 324 | 4.42 | 143 | [361] |
| Materials | Crystalline Structure | Discharge Rate (C) | Current Density (μA/cm2) | Discharge Capacity (mAh/g) | Density (g/cm3) | Discharge Capacity (μAh/cm2 μm) | Ref. |
|---|---|---|---|---|---|---|---|
| LCO a | (104) Epitaxial | 100 | 23 | 5.03 | 13 | [373] | |
| LCO a | (104) Epitaxial | 0.01 | 90 | 5.03 | 45 | [373] | |
| LCO b | Amorphous | 30 | 49 | 5.03 | 25 | [375] | |
| LCO b | (100)-Oriented | 30 | 120 | 5.03 | 61 | [375] | |
| LCO c | Polycrystalline | 0.5 | 50 | 5.03 | 25 | [377] | |
| LiNi0.5Mn0.25Co0.25O2 b | Polycrystalline | 117 | 4.9 | 58 | [394] | ||
| LiNi0.3Mn0.3Co0.3O2 b | Polycrystalline | 0.1 | 127 | 4.9 | 62 | [392] | |
| LiNi0.5Mn0.3Co0.2O2 a | Polycrystalline | 0.5 | 125 | 4.9 | 61 | [393] | |
| LMO a | (100) Epitaxial | 0.7 | 129 | 4.32 | 56 | [384] | |
| LMO a | (100) Epitaxial | 13 | 100 | 4.32 | 43 | [384] | |
| LMO a | (100) Epitaxial | 33 | 84 | 4.32 | 36 | [384] | |
| LMO a | (110) Epitaxial | 0.7 | 113 | 4.32 | 49 | [384] | |
| LMO a | (110) Epitaxial | 13 | 50 | 4.32 | 22 | [384] | |
| LMO a | (111) Epitaxial | 0.1 | 95 | 4.32 | 41 | [384] | |
| LMO a | (111) Epitaxial | 13 | 50 | 4.32 | 22 | [384] | |
| LMO d | Amorphous | 0.1 | 61 | 4.32 | 26 | [101] | |
| LMO d | Polycrystalline | 0.1 | 70 | 4.32 | 30 | [101] | |
| LMO e | Polycrystalline | 100 | 88 | 4.32 | 38 | [390] | |
| LMO f | Polycrystalline | 50 | 80 | 4.32 | 35 | [391] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Abraham, C.; Ma, Y.; Lee, M.; Helfrick, E.; Oh, D.; Lee, D. Advances in Materials Design for All-Solid-state Batteries: From Bulk to Thin Films. Appl. Sci. 2020, 10, 4727. https://doi.org/10.3390/app10144727
Yang G, Abraham C, Ma Y, Lee M, Helfrick E, Oh D, Lee D. Advances in Materials Design for All-Solid-state Batteries: From Bulk to Thin Films. Applied Sciences. 2020; 10(14):4727. https://doi.org/10.3390/app10144727
Chicago/Turabian StyleYang, Gene, Corey Abraham, Yuxi Ma, Myoungseok Lee, Evan Helfrick, Dahyun Oh, and Dongkyu Lee. 2020. "Advances in Materials Design for All-Solid-state Batteries: From Bulk to Thin Films" Applied Sciences 10, no. 14: 4727. https://doi.org/10.3390/app10144727
APA StyleYang, G., Abraham, C., Ma, Y., Lee, M., Helfrick, E., Oh, D., & Lee, D. (2020). Advances in Materials Design for All-Solid-state Batteries: From Bulk to Thin Films. Applied Sciences, 10(14), 4727. https://doi.org/10.3390/app10144727






