Influence of Heterointerfaces on the Kinetics of Oxygen Surface Exchange on Epitaxial La1.85Sr0.15CuO4 Thin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Thin Film Deposition
2.2. Thin Film Characteristics
2.3. Oxygen Surface Exchange Kinetics
2.4. Optical Conductivity
3. Results
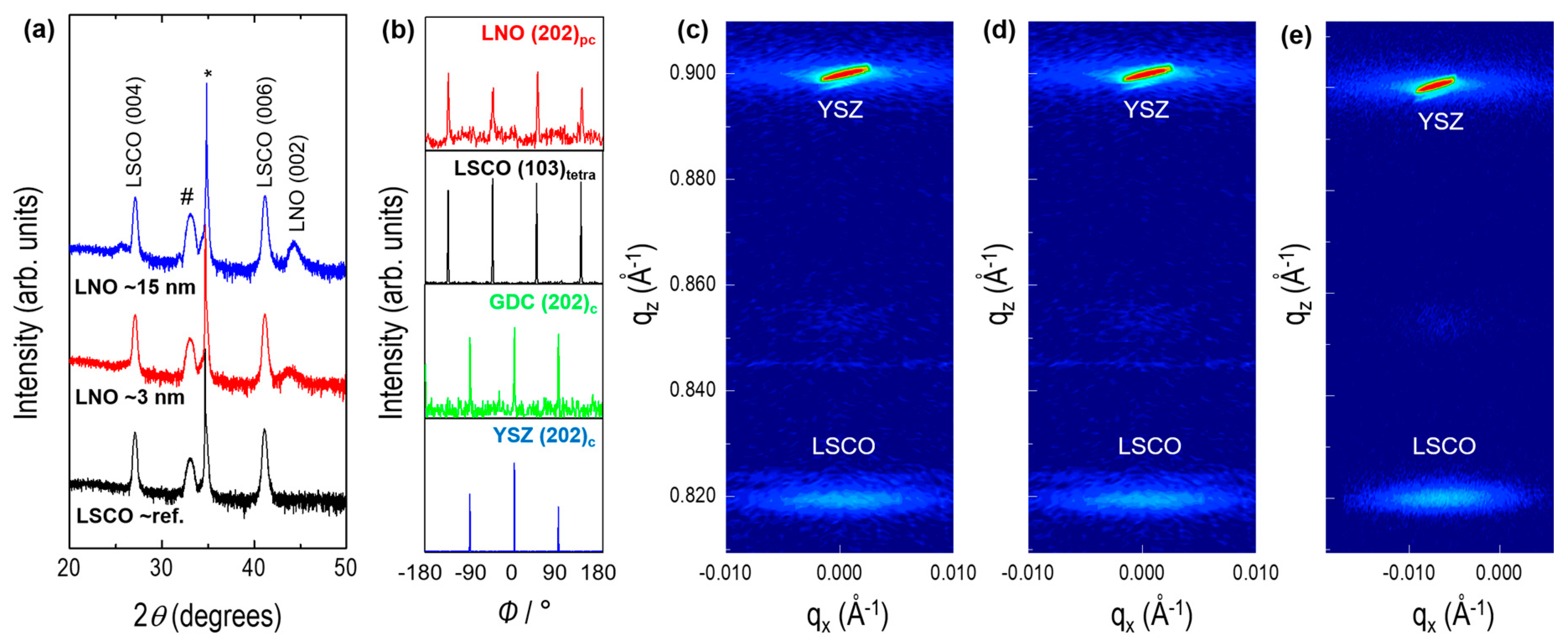
3.1. Thin Film Characteristics
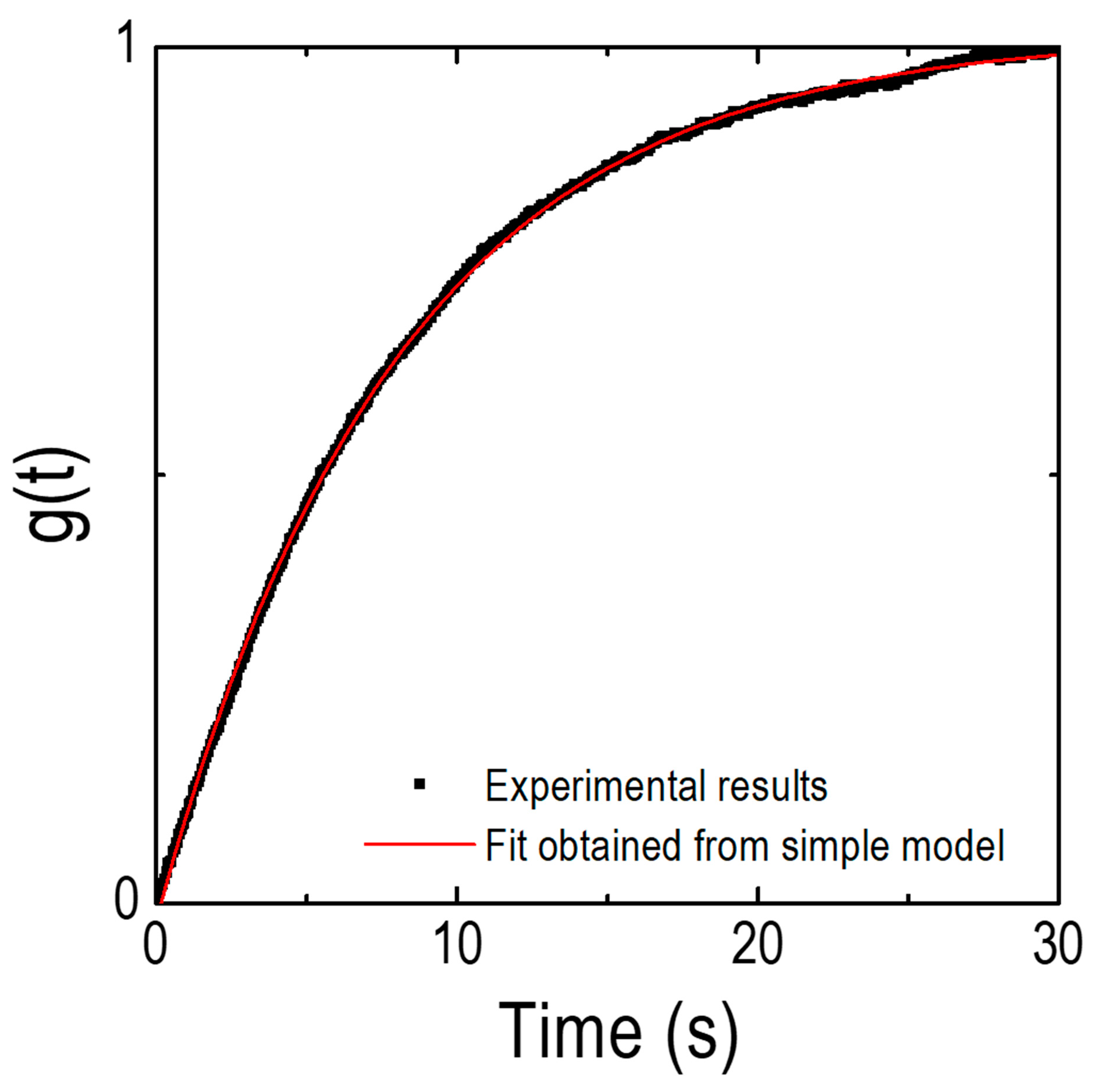
3.2. Oxygen Surface Exchange Kinetics
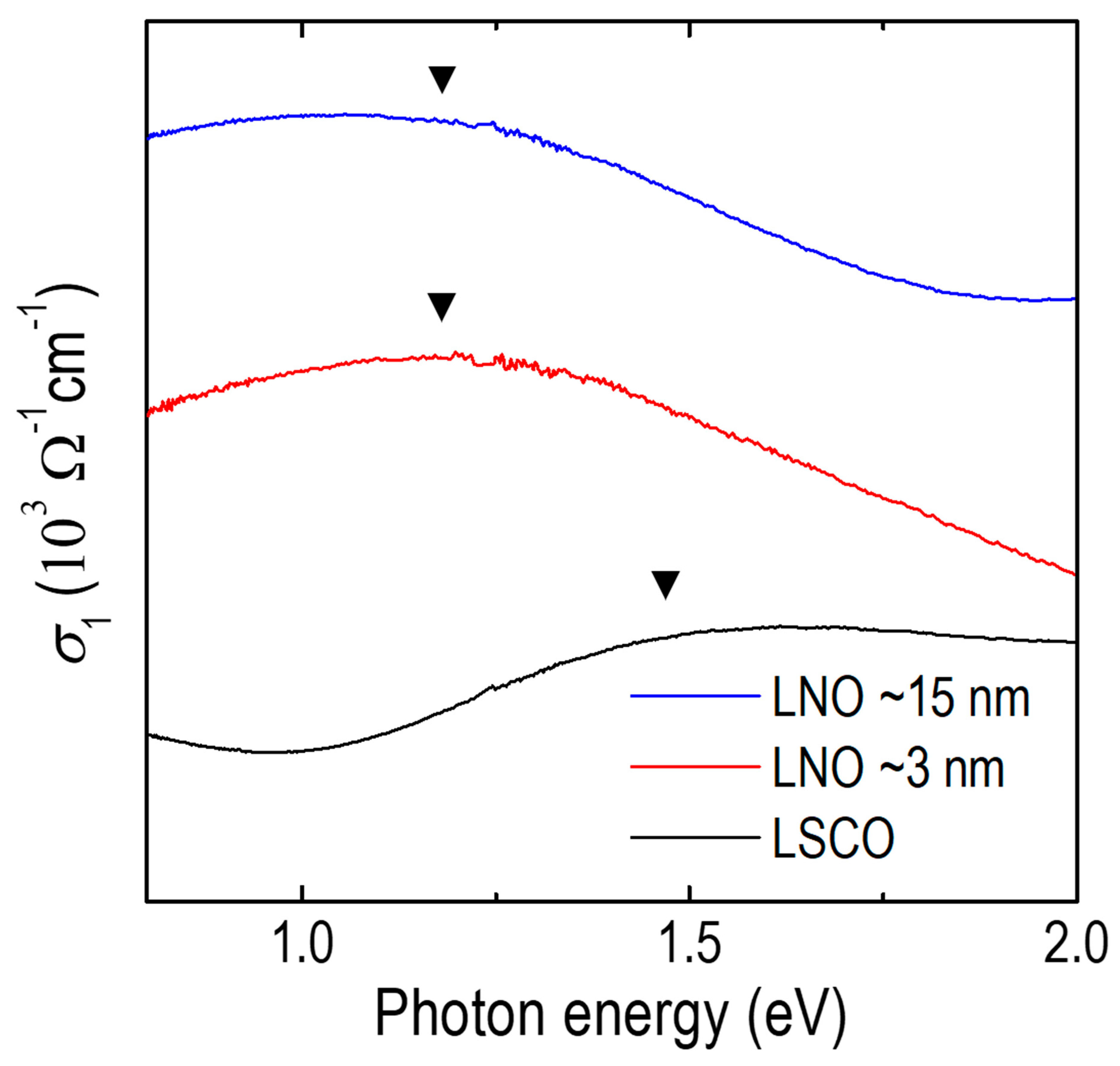
3.3. Optical Conductivity
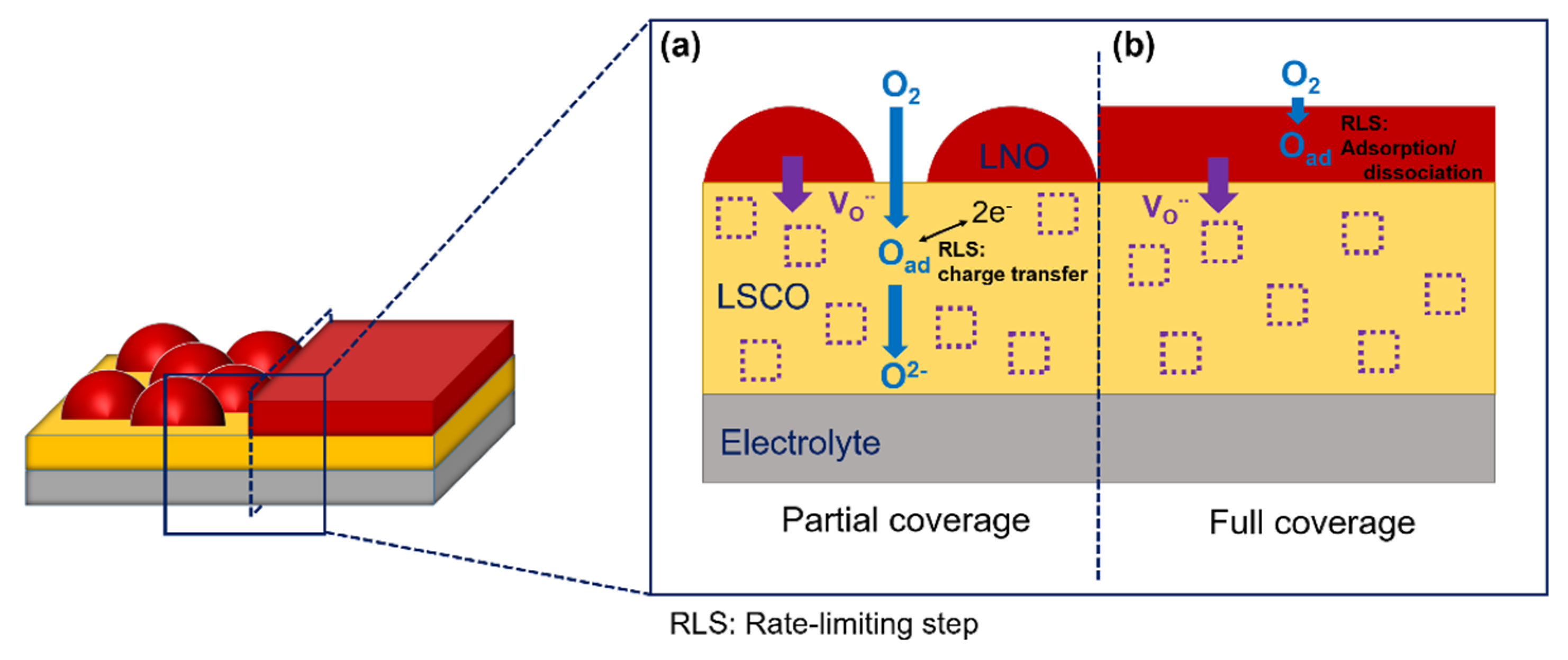
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, Z.P.; Haile, S.M. A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 2004, 431, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M.; Steele, B.C.H. Properties of La0.6Sr0.4Co0.2Fe0.8O3−δ (LSCF) double layer cathodes on gadolinium-doped cerium oxide (CGO) electrolytes: I. Role of SiO2. Solid State Ion. 1998, 106, 247–253. [Google Scholar] [CrossRef]
- Esquirol, A.; Brandon, N.P.; Kilner, J.A.; Mogensen, M. Electrochemical characterization of La 0.6Sr0.4Co0.2Fe0.8O3 cathodes for intermediate-temperature SOFCs. J. Electrochem. Soc. 2004, 151, A1847. [Google Scholar] [CrossRef]
- Yang, G.; Jung, W.; Ahn, S.J.; Lee, D. Controlling the oxygen electrocatalysis on perovskite and layered oxide thin films for solid oxide fuel cell cathodes. Appl. Sci. 2019, 9, 1030. [Google Scholar] [CrossRef]
- Chen, J.; Liang, F.L.; Chi, B.; Pu, J.; Jiang, S.P.; Jian, L. Palladium and ceria infiltrated La0.8Sr0.2Co0.5Fe0.5O3−δ cathodes of solid oxide fuel cells. J. Power Sources 2009, 194, 275–280. [Google Scholar] [CrossRef]
- Crumlin, E.J.; Ahn, S.J.; Lee, D.; Mutoro, E.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Oxygen electrocatalysis on epitaxial La0.6Sr0.4CoO3−δ perovskite thin films for solid oxide fuel cells. J. Electrochem. Soc. 2012, 159, F219–F225. [Google Scholar] [CrossRef]
- Liu, M.F.; Ding, D.; Blinn, K.; Li, X.X.; Nie, L.F.; Liu, M. Enhanced performance of LSCF cathode through surface modification. Int. J. Hydrogen Energy 2012, 37, 8613–8620. [Google Scholar] [CrossRef]
- Lou, X.; Wang, S.; Liu, Z.; Yang, L.; Liu, M. Improving La0.6Sr0.4Co0.2Fe0.8O3−δ cathode performance by infiltration of a Sm0.5Sr0.5CoO3−δ coating. Solid State Ion. 2009, 180, 1285–1289. [Google Scholar] [CrossRef]
- Mutoro, E.; Crumlin, E.J.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Enhanced oxygen reduction activity on surface-decorated perovskite thin films for solid oxide fuel cells. Energy Environ. Sci. 2011, 4, 3689–3696. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.L.; Grimaud, A.; Hong, W.T.; Biegalski, M.D.; Morgan, D.; Shao-Horn, Y. Enhanced oxygen surface exchange kinetics and stability on epitaxial La0.8Sr0.2CoO3−δ Thin Films by La0.8Sr0.2MnO3−δ Decoration. J. Phys. Chem. C 2014, 118, 14326–14334. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.L.; Hong, W.T.; Biegalski, M.D.; Morgan, D.; Shao-Horn, Y. Oxygen surface exchange kinetics and stability of (La,Sr)2CoO4±δ/La1−xSrxMO3−δ (M = Co and Fe) hetero-interfaces at intermediate temperatures. J. Mater. Chem. A 2015, 3, 2144–2157. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.L.; Wang, X.R.; Morgan, D.; Shao-Horn, Y. Enhancement of oxygen surface exchange on epitaxial La0.6Sr0.4Co0.2Fe0.8O3−δ thin films using advanced heterostructured oxide interface engineering. MRS Commun. 2016, 6, 204–209. [Google Scholar] [CrossRef]
- Sase, M.; Hermes, F.; Yashiro, K.; Sato, K.; Mizusaki, J.; Kawada, T.; Sakai, N.; Yokokawa, H. Enhancement of oxygen surface exchange at the hetero-interface of (La,Sr)CoO3/(La,Sr)2CoO4 with PLD-layered films. J. Electrochem. Soc. 2008, 155, B793–B797. [Google Scholar] [CrossRef]
- Yashiro, K.; Nakamura, T.; Sase, M.; Hermes, F.; Sato, K.; Kawada, T.; Mizusaki, J. Composite cathode of perovskite-related oxides, (La,Sr)CoO3−δ/(La,Sr)2CoO4−δ, for solid oxide fuel cells. Electrochem. Solid State Lett. 2009, 12, B135–B137. [Google Scholar] [CrossRef]
- Crumlin, E.J.; Mutoro, E.; Ahn, S.J.; la O’, G.J.; Leonard, D.N.; Borisevich, A.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Oxygen reduction kinetics enhancement on a heterostructured oxide surface for solid oxide fuel cells. J. Phys. Chem. Lett. 2010, 1, 3149–3155. [Google Scholar] [CrossRef]
- Amow, G.; Skinner, S.J. Recent developments in Ruddlesden-Popper nickelate systems for solid oxide fuel cell cathodes. J. Solid State Electrochem. 2006, 10, 538–546. [Google Scholar] [CrossRef]
- Burriel, M.; Garcia, G.; Santiso, J.; Kilner, J.A.; Chater, R.J.; Skinner, S.J. Anisotropic oxygen diffusion properties in epitaxial thin films of La2NiO4+δ. J. Mater. Chem. A 2008, 18, 416–422. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.L.; Grimaud, A.; Hong, W.T.; Biegalski, M.D.; Morgan, D.; Shao-Horn, Y. Strontium influence on the oxygen electrocatalysis of La2−xSrxNiO4±δ (0.0 ≤ xSr ≤ 1.0) thin films. J. Mater. Chem. A 2014, 2, 6480–6487. [Google Scholar] [CrossRef]
- Lee, D.; Grimaud, A.; Crumlin, E.J.; Mezghani, K.; Habib, M.A.; Feng, Z.; Hong, W.T.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Strain influence on the oxygen electrocatalysis of the (100)-oriented epitaxial La2NiO4+δ thin films at elevated temperatures. J. Phys. Chem. C 2013, 117, 18789–18795. [Google Scholar] [CrossRef]
- Oh, D.; Gostovic, D.; Wachsman, E.D. Mechanism of La0.6Sr0.4Co0.2Fe0.8O3 cathode degradation. J. Mater. Res. 2012, 27, 1992–1999. [Google Scholar] [CrossRef]
- Simner, S.P.; Anderson, M.D.; Engelhard, M.H.; Stevenson, J.W. Degradation mechanisms of La–Sr–Co–Fe–O3 SOFC Cathodes. Electrochem. Solid-State Lett. 2006, 9, A478–A481. [Google Scholar] [CrossRef]
- Lee, Y.L.; Lee, D.; Wang, X.R.; Lee, H.N.; Morgan, D.; Shao-Horn, Y. Kinetics of oxygen surface exchange on epitaxial Ruddlesden–Popper phases and correlations to first-principles descriptors. J. Phys. Chem. Lett. 2016, 7, 244–249. [Google Scholar] [CrossRef]
- Meyer, T.L.; Jacobs, R.; Lee, D.; Jiang, L.; Freeland, J.W.; Sohn, C.; Egami, T.; Morgan, D.; Lee, H.N. Strain control of oxygen kinetics in the Ruddlesden-Popper oxide La1.85Sr0.15CuO4. Nat. Commun. 2018, 9, 92. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Greene, L.H.; Mckinnon, W.R.; Hull, G.W.; Geballe, T.H. Superconductivity at 40 K in the oxygen-defect perovskites La2−xSrxCuO4−y. Science 1987, 235, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Sato, H. Thickness dependence of superconductivity and resistivity in La1.85Sr0.15CuO4 films. Phys. C Supercond. 2008, 468, 991–995. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Ding, D.; Ding, Y.; Choi, Y.; Zhang, L.; Yoo, S.; Chen, D.; deGlee, B.; Xu, H.; et al. A robust and active hybrid catalyst for facile oxygen reduction in solid oxide fuel cells. Energy Environ. Sci. 2017, 10, 964–971. [Google Scholar] [CrossRef]
- Chen, Y.; Yoo, S.; Pei, K.; Chen, D.; Zhang, L.; deGlee, B.; Murphy, R.; Zhao, B.; Zhang, Y.; Chen, Y.; et al. An in situ formed, dual-phase cathode with a highly active catalyst coating for protonic ceramic fuel cells. Adv. Funct. Mater. 2018, 28, 1704907. [Google Scholar] [CrossRef]
- Lee, Y.L.; Morgan, D.; Kleis, J.; Rossmeisl, J. Ab initio defect energetics in LaBO3 perovskite solid oxide fuel cell materials. ECS Trans. 2019, 25, 2761–2767. [Google Scholar] [CrossRef]
- Pan, C.C.; Chen, Y.H.; Wu, N.; Zhang, M.L.; Yuan, L.H.; Zhang, C.R. First-principle study of O vacancy on LaNiO3 (001) surface. Int. J. Hydrogen Energy 2016, 41, 15756–15763. [Google Scholar] [CrossRef]
- Lu, L.; Guo, Y.; Zhang, H.; Jin, J. Electrochemical performance of La2NiO4+δ–La0.6Sr0.4Co0.2Fe0.8O3−δ composite cathodes for intermediate temperature solid oxide fuel cells. Mater. Res. Bull. 2010, 45, 1135–1140. [Google Scholar] [CrossRef]
- Moreno, R.; García, P.; Zapata, J.; Roqueta, J.; Chaigneau, J.; Santiso, J. Chemical strain kinetics induced by oxygen surface exchange in epitaxial films explored by time-resolved X-ray diffraction. Chem. Mater. 2013, 25, 3640–3647. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H.N. Controlling oxygen mobility in Ruddlesden-Popper oxides. Materials 2017, 10, 368. [Google Scholar] [CrossRef]
- Mitterdorfer, A.; Gauckler, L.J. La2Zr2O7 formation and oxygen reduction kinetics of the La0.85Sr0.15MnyO3, O2(g)|YSZ system. Solid State Ion. 1998, 111, 185–218. [Google Scholar] [CrossRef]
- la O’, G.J.; Ahn, S.J.; Crumlin, E.; Orikasa, Y.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Catalytic activity enhancement for oxygen reduction on epitaxial perovskite thin films for solid-oxide fuel cells. Angew. Chem. Int. Ed. 2010, 49, 5344–5347. [Google Scholar] [CrossRef]
- Ma, B.; Balachandran, U.; Park, J.H.; Segre, C.U. Determination of chemical diffusion coefficient of SrFeCo0.5Ox by the conductivity relaxation method. Solid State Ion. 1996, 83, 65–71. [Google Scholar] [CrossRef]
- Murugaraj, R.; Govindaraj, G.; Suganthi, R.; George, D. Electrical conductivity studies of sodium borate system based on diffusion controlled relaxation model. J. Mater. Sci. 2003, 38, 107–112. [Google Scholar] [CrossRef]
- Chen, X.J.; Soltan, S.; Zhang, H.; Habermeier, H.U. Strain effect on electronic transport and ferromagnetic transition temperature in La0.9Sr0.1MnO3 thin films. Phys. Rev. B 2002, 65, 174402. [Google Scholar] [CrossRef]
- Mosleh, M.; Søgaard, M.; Hendriksen, P.V. Kinetics and mechanisms of oxygen surface exchange on La0.6Sr0.4FeO3−δ thin films. J. Electrochem. Soc. 2009, 156, B441–B457. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Garcia, G.; Burriel, M.; Bonanos, N.; Santiso, J. Electrical conductivity and oxygen exchange kinetics of La2NiO4+δ thin films grown by chemical vapor deposition. J. Electrochem. Soc. 2008, 155, P28–P32. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Steil, M.C.; Dordor, P.; Mauvy, F.; Grenier, J.C. Oxygen transport properties of La2Ni1−xCuxO4+δ mixed conducting oxides. Solid State Sci. 2003, 5, 973–981. [Google Scholar] [CrossRef]
- De Souza, R.A. A universal empirical expression for the isotope surface exchange coefficients (k*) of acceptor-doped perovskite and fluorite oxides. Phys. Chem. Chem. Phys. 2006, 8, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.B.; Chen, X.Y.; Wilson, J.R. Mechanisms and rate laws for oxygen exchange on mixed-conducting oxide surfaces. J. Catal. 2007, 245, 91–109. [Google Scholar] [CrossRef]
- Adler, S.B. Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Rev. 2004, 104, 4791–4844. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lynch, M.E.; Lin, M.C.; Liu, M. Prediction of O2 dissociation kinetics on LaMnO3-based cathode materials for solid oxide fuel cells. J. Phys. Chem. C 2009, 113, 7290–7297. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, X.; Zhang, W.; Zheng, Y.; Li, Y.; Yu, B.; Wang, J.; Chen, J. Measurement of oxygen reduction/evolution kinetics enhanced (La,Sr)CoO3/(La,Sr)2CoO4 hetero-structure oxygen electrode in operating temperature for SOCs. Int. J. Hydrogen Energy 2018, 44, 19102–19112. [Google Scholar] [CrossRef]
- Choi, W.S.; Kwon, J.H.; Jeen, H.; Hamann-Borrero, J.E.; Radi, A.; Macke, S.; Sutarto, R.; He, F.; Sawatzky, G.A.; Hinkov, V.; et al. Strain-induced spin states in atomically ordered cobaltites. Nano Lett. 2012, 12, 4966–4970. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Jacobs, R.; Jee, Y.; Seo, A.; Sohn, C.; Ievlev, A.V.; Ovchinnikova, O.S.; Huang, K.; Morgan, D.; Lee, H.N. Stretching epitaxial La0.6Sr0.4CoO3−δ for fast oxygen reduction. J. Phys. Chem. C 2017, 121, 25651–25658. [Google Scholar] [CrossRef]
- Uchida, S.; Ido, T.; Takagi, H.; Arima, T.; Tokura, Y.; Tajima, S. Optical spectra of La2−xSrxCuO4: Effect of carrier doping on the electronic structure of the CuO2 plane. Phys. Rev. B 1991, 43, 7942–7954. [Google Scholar] [CrossRef]
- Comanac, A.; de’ Medici, L.; Capone, M.; Millis, A.J. Optical conductivity and the correlation strength of high-temperature copper-oxide superconductors. Nat. Phys. 2008, 4, 287–290. [Google Scholar] [CrossRef]
- Höfer, H.E.; Kock, W.F. Crystal chemistry and thermal behavior in the La(Cr, Ni)O3 perovskite system. J. Electrochem. Soc. 1993, 140, 2889–2894. [Google Scholar] [CrossRef]
- Fierro, J.L.G.; Tascón, J.M.D.; Tejuca, L.G. Surface properties of LaNiO3: Kinetic studies of reduction and of oxygen adsorption. J. Catal. 1985, 93, 83–91. [Google Scholar] [CrossRef]
- Retuerto, M.; Pereira, A.G.; Pérez-Alonso, F.J.; Peña, M.A.; Fierro, J.L.G.; Alonso, J.A.; Fernández-Díaz, M.T.; Pascual, L.; Rojas, S. Structural effects of LaNiO3 as electrocatalyst for the oxygen reduction reaction. Appl. Catal. B 2017, 203, 363–371. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Kim, S.-Y.; Sohn, C.; Keum, J.K.; Lee, D. Influence of Heterointerfaces on the Kinetics of Oxygen Surface Exchange on Epitaxial La1.85Sr0.15CuO4 Thin Films. Appl. Sci. 2021, 11, 3778. https://doi.org/10.3390/app11093778
Yang G, Kim S-Y, Sohn C, Keum JK, Lee D. Influence of Heterointerfaces on the Kinetics of Oxygen Surface Exchange on Epitaxial La1.85Sr0.15CuO4 Thin Films. Applied Sciences. 2021; 11(9):3778. https://doi.org/10.3390/app11093778
Chicago/Turabian StyleYang, Gene, So-Yeun Kim, Changhee Sohn, Jong K. Keum, and Dongkyu Lee. 2021. "Influence of Heterointerfaces on the Kinetics of Oxygen Surface Exchange on Epitaxial La1.85Sr0.15CuO4 Thin Films" Applied Sciences 11, no. 9: 3778. https://doi.org/10.3390/app11093778
APA StyleYang, G., Kim, S.-Y., Sohn, C., Keum, J. K., & Lee, D. (2021). Influence of Heterointerfaces on the Kinetics of Oxygen Surface Exchange on Epitaxial La1.85Sr0.15CuO4 Thin Films. Applied Sciences, 11(9), 3778. https://doi.org/10.3390/app11093778






