Subpopulations of Organoid-Forming Cells Have Different Motility
Abstract
1. Introduction
2. Materials and Methods
2.1. Organoid Culture
2.2. Holographic Phase-Contrast Microscopy
2.3. Flow Cytometry and Phenotypic Study
3. Results
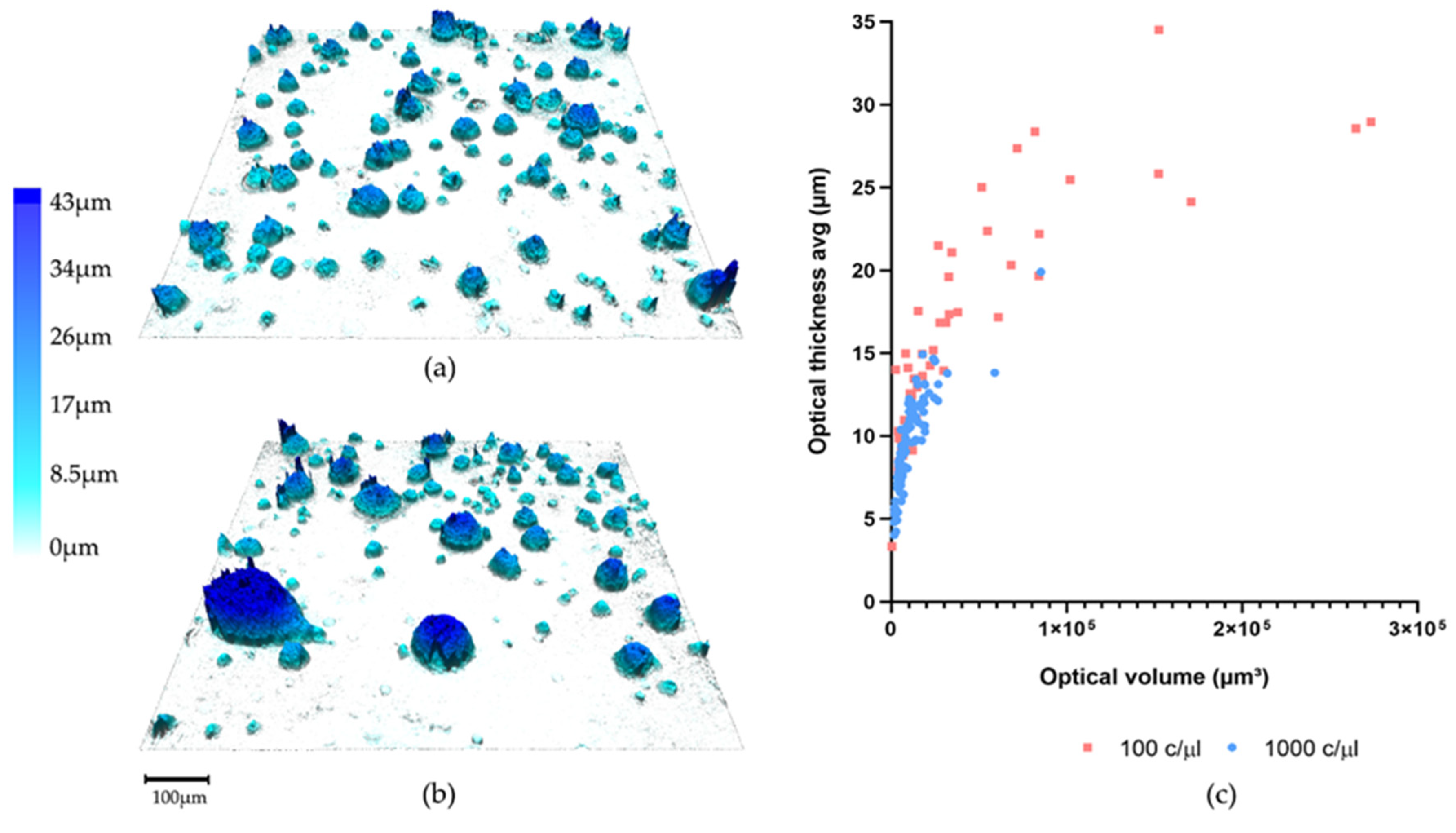
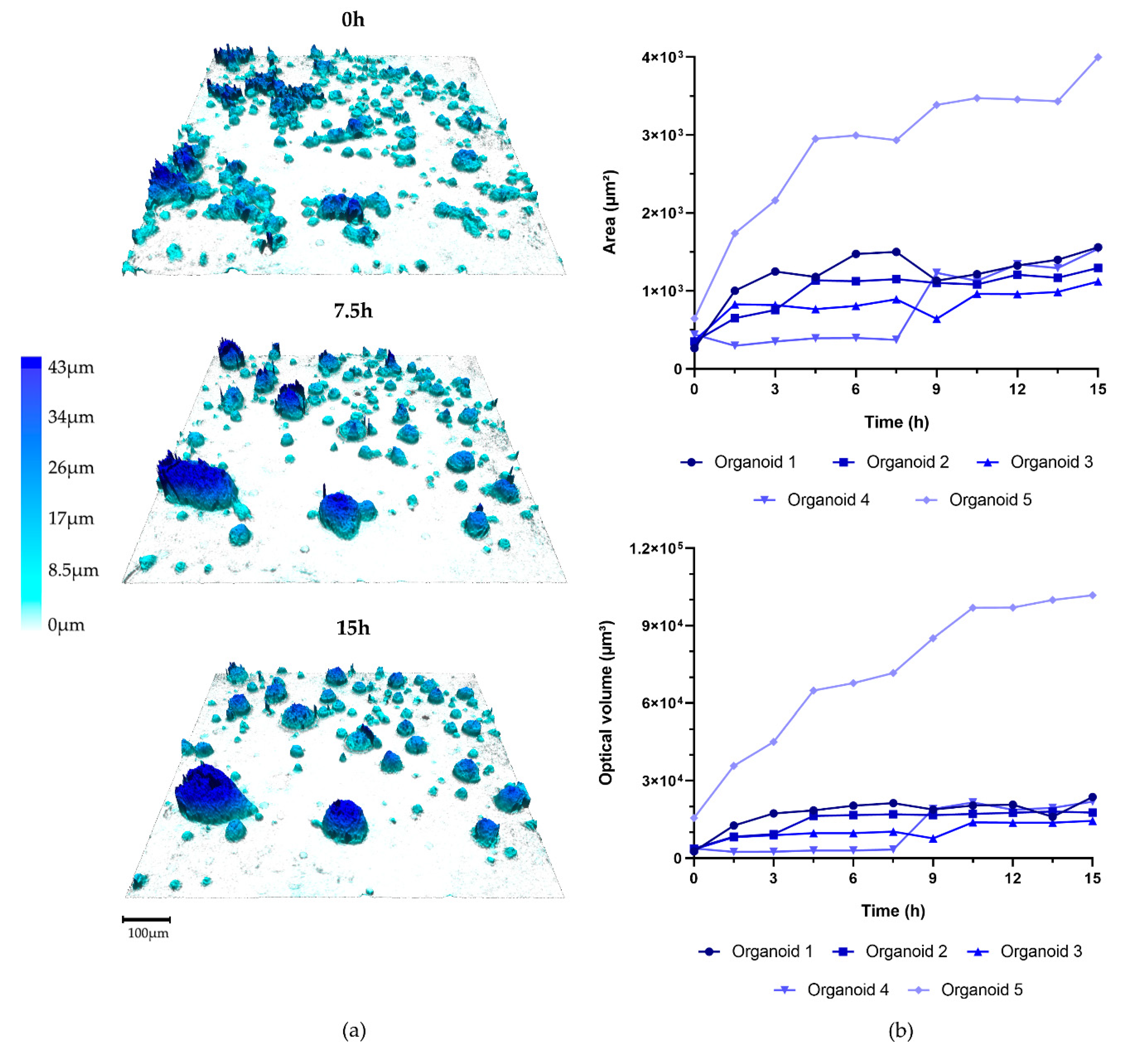
3.1. Organoid Formation from OPSCC Cells
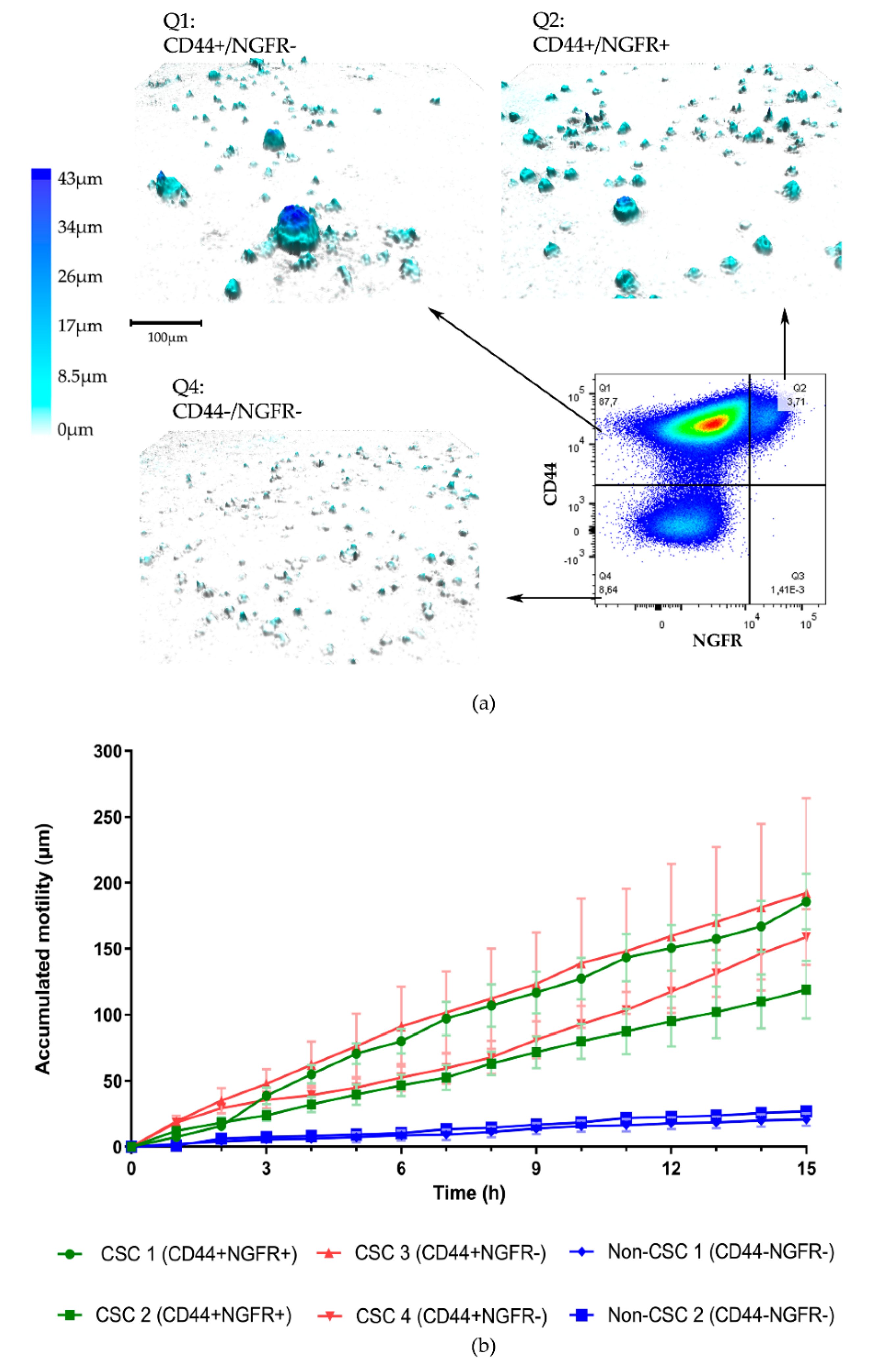
3.2. Organoid Formation from Sorted Cells
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monroe, M.M.; Anderson, E.C.; Clayburgh, D.R.; Wong, M.H. Cancer stem cells in head and neck squamous cell carcinoma. J. Oncol. 2011, 2011, 762780. [Google Scholar] [CrossRef] [PubMed]
- Grønhøj, C.; Jakobsen, K.K.; Jensen, D.H.; Rasmussen, J.; Andersen, E.; Friborg, J.; von Buchwald, C. Pattern of and survival following loco-regional and distant recurrence in patients with HPV+ and HPV− oropharyngeal squamous cell carcinoma: A population-based study. Oral Oncol. 2018, 83, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Nör, J.E. Orosphere assay: A method for propagation of head and neck cancer stem cells. Head Neck 2013, 35, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Vela, I.; Sboner, A.; Iaquinta, P.J.; Karthaus, W.R.; Gopalan, A.; Dowling, C.; Wanjala, J.N.; Undvall, E.A.; Arora, V.K.; et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014, 159, 176–187. [Google Scholar] [CrossRef]
- Panek, M.; Grabacka, M.; Pierzchalska, M. The formation of intestinal organoids in a hanging drop culture. Cytotechnology 2018, 70, 1085–1095. [Google Scholar] [CrossRef]
- Mehta, P.; Novak, C.; Raghavan, S.; Ward, M.; Mehta, G. Self-Renewal and CSCs In Vitro Enrichment: Growth as Floating Spheres. Methods Mol. Biol. 2018, 1692, 61–75. [Google Scholar] [CrossRef]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef]
- Ghuwalewala, S.; Ghatak, D.; Das, P.; Dey, S.; Sarkar, S.; Alam, N.; Panda, C.K.; Roychoudhury, S. CD44(high)CD24(low) molecular signature determines the Cancer Stem Cell and EMT phenotype in Oral Squamous Cell Carcinoma. Stem Cell Res. 2016, 16, 405–417. [Google Scholar] [CrossRef]
- Kang, S.Y.C.; Kannan, N.; Zhang, L.; Martinez, V.; Rosin, M.P.; Eaves, C.J. Characterization of Epithelial Progenitors in Normal Human Palatine Tonsils and Their HPV16 E6/E7-Induced Perturbation. Stem Cell Rep. 2015, 5, 1210–1225. [Google Scholar] [CrossRef]
- Murillo-Sauca, O.; Chung, M.K.; Shin, J.H.; Karamboulas, C.; Kwok, S.; Jung, Y.H.; Oakley, R.; Tysome, J.R.; Farnebo, L.O.; Kaplan, M.J.; et al. CD271 is a functional and targetable marker of tumor-initiating cells in head and neck squamous cell carcinoma. Oncotarget 2014, 5, 6854–6866. [Google Scholar] [CrossRef]
- Wu, V.; Auchman, M.; Mollica, P.A.; Sachs, P.C.; Bruno, R.D. ALDH1A1 positive cells are a unique component of the tonsillar crypt niche and are lost along with NGFR positive stem cells during tumourigenesis. Pathology 2018, 50, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Pytynia, M.; Pelizzari, C.; Spiotto, M. Bioluminescent imaging of HPV-positive oral tumor growth and its response to image-guided radiotherapy. Cancer Res. 2014, 74, 2073–2081. [Google Scholar] [CrossRef]
- Forslund, O.; Sugiyama, N.; Wu, C.; Ravi, N.; Jin, Y.; Swoboda, S.; Andersson, F.; Bzhalava, D.; Hultin, E.; Paulsson, K.; et al. A novel human in vitro papillomavirus type 16 positive tonsil cancer cell line with high sensitivity to radiation and cisplatin. BMC Cancer 2019, 19, 265. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, M.; Sebesta, M.; Bengtsson, B.; Pettersson, S.G.; Egelberg, P.; Lenart, T. High-resolution digital transmission microscopy—A Fourier holography approach. Opt. Lasers Eng. 2004, 41, 553–563. [Google Scholar] [CrossRef][Green Version]
- Kim, M. Principles and techniques of digital holographic microscopy. SPIE Rev. 2010, 1, 018005. [Google Scholar] [CrossRef]
- Sebesta, M.; Egelberg, P.; Langberg, A.; Lindskov, J.-H.; Alm, K.; Janicke, B. HoloMonitor M4: Holographic imaging cytometer for real-time kinetic label-free live-cell analysis of adherent cells. Proc. SPIE. 2016, 971813. [Google Scholar] [CrossRef]
- Hiraga, T.; Ito, S.; Nakamura, H. Cancer Stem–like Cell Marker CD44 Promotes Bone Metastases by Enhancing Tumorigenicity, Cell Motility, and Hyaluronan Production. Cancer Res. 2013, 73, 4112–4122. [Google Scholar] [CrossRef]
- Wingren, A.G. Moving into a new dimension: Tracking migrating cells with digital holographic cytometry in 3D. Cytom. A 2019, 95, 144–146. [Google Scholar] [CrossRef]
- Azharuddin, M.; Roberg, K.; Dhara, A.K.; Jain, M.V.; Darcy, P.; Hinkula, J.; Slater, N.K.H.; Patra, H.K. Dissecting multi drug resistance in head and neck cancer cells using multicellular tumor spheroids. Sci. Rep. 2019, 9, 20066. [Google Scholar] [CrossRef]
- Kasprowicz, R.; Suman, R.; O’Toole, P. Characterising live cell behaviour: Traditional label-free and quantitative phase imaging approaches. Int. J. Biochem. Cell Biol. 2017, 84, 89–95. [Google Scholar] [CrossRef]
- Laissue, P.P.; Alghamdi, R.A.; Tomancak, P.; Reynaud, E.G.; Shroff, H. Assessing phototoxicity in live fluorescence imaging. Nat. Methods 2017, 14, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Logg, K.; Bodvard, K.; Blomberg, A.; Kall, M. Investigations on light-induced stress in fluorescence microscopy using nuclear localization of the transcription factor Msn2p as a reporter. FEMS Yeast Res. 2009, 9, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Divi, V.; Owen, J.; Bradford, C.; Carey, T.; Papagerakis, S.; Prince, M. Metastatic Potential of Cancer Stem Cells in Head and Neck Squamous Cell Carcinoma. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Le, P.N.; Keysar, S.B.; Miller, B.; Eagles, J.R.; Chimed, T.S.; Reisinger, J.; Gomez, K.E.; Nieto, C.; Jackson, B.C.; Somerset, H.L.; et al. Wnt signaling dynamics in head and neck squamous cell cancer tumor-stroma interactions. Mol. Carcinog. 2019, 58, 398–410. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez Jimenez, D.; Carreira Santos, S.; Greiff, L.; Alm, K.; Lindstedt, M. Subpopulations of Organoid-Forming Cells Have Different Motility. Appl. Sci. 2020, 10, 4673. https://doi.org/10.3390/app10134673
Gomez Jimenez D, Carreira Santos S, Greiff L, Alm K, Lindstedt M. Subpopulations of Organoid-Forming Cells Have Different Motility. Applied Sciences. 2020; 10(13):4673. https://doi.org/10.3390/app10134673
Chicago/Turabian StyleGomez Jimenez, David, Sofia Carreira Santos, Lennart Greiff, Kersti Alm, and Malin Lindstedt. 2020. "Subpopulations of Organoid-Forming Cells Have Different Motility" Applied Sciences 10, no. 13: 4673. https://doi.org/10.3390/app10134673
APA StyleGomez Jimenez, D., Carreira Santos, S., Greiff, L., Alm, K., & Lindstedt, M. (2020). Subpopulations of Organoid-Forming Cells Have Different Motility. Applied Sciences, 10(13), 4673. https://doi.org/10.3390/app10134673





