Fabrication of Ceramic Moulds Using Recycled Shell Powder and Sand with Geopolymer Technology in Investment Casting
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Experimental
2.2.1. Geopolymer Slurry Preparation
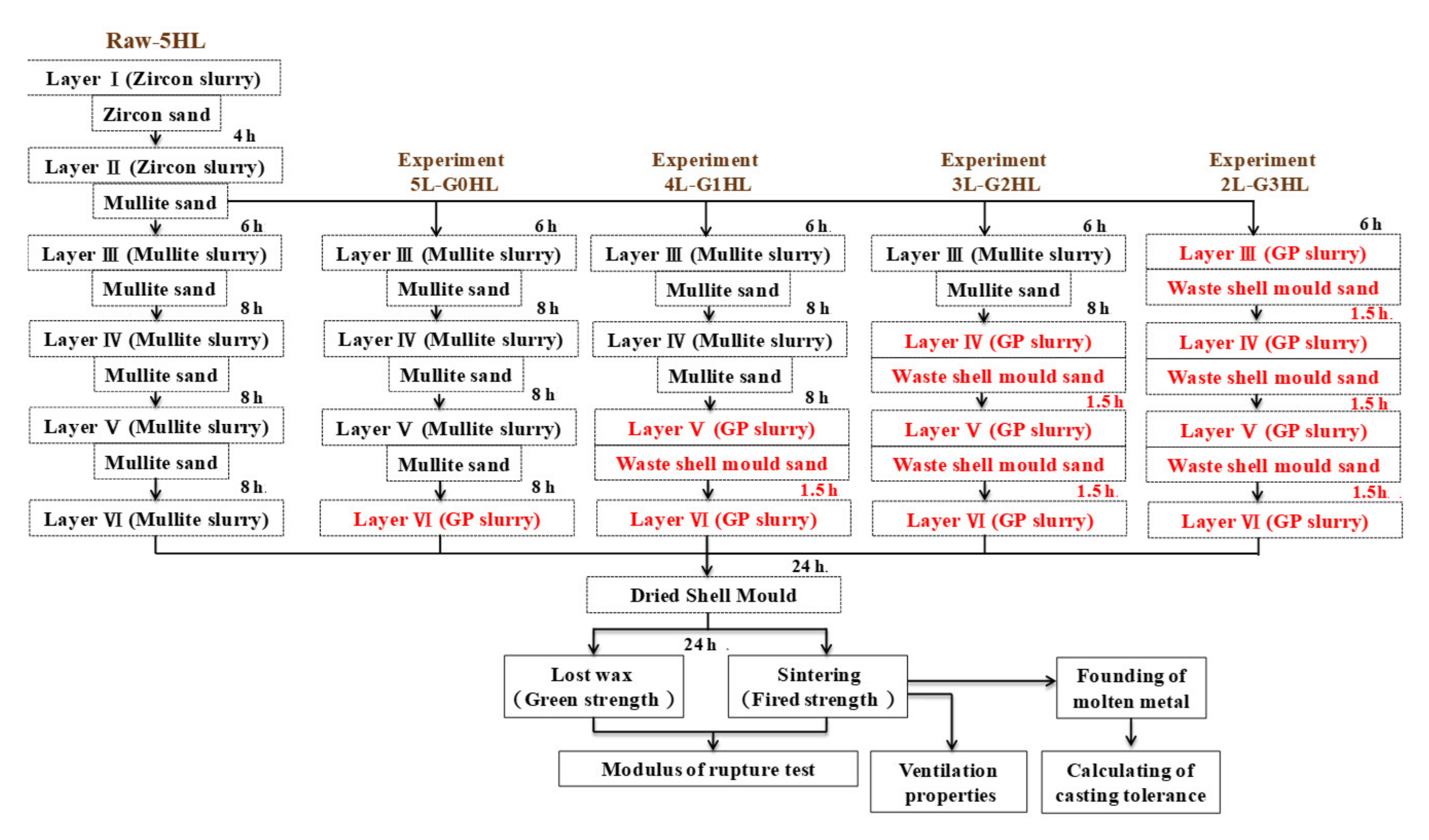
2.2.2. Preparation of Ceramic Shell with Geopolymer Slurry
2.3. Tests
3. Results and Discussion
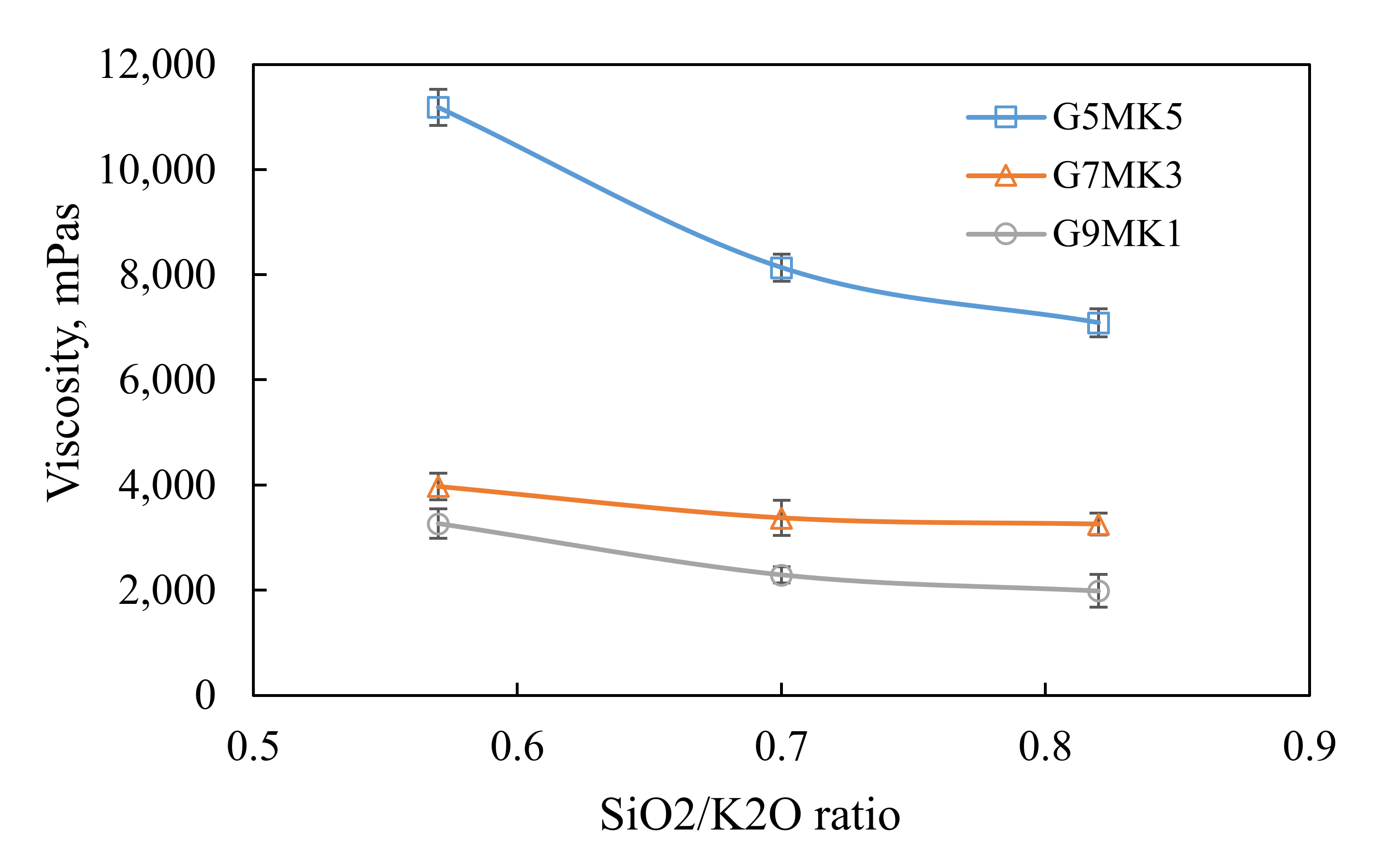
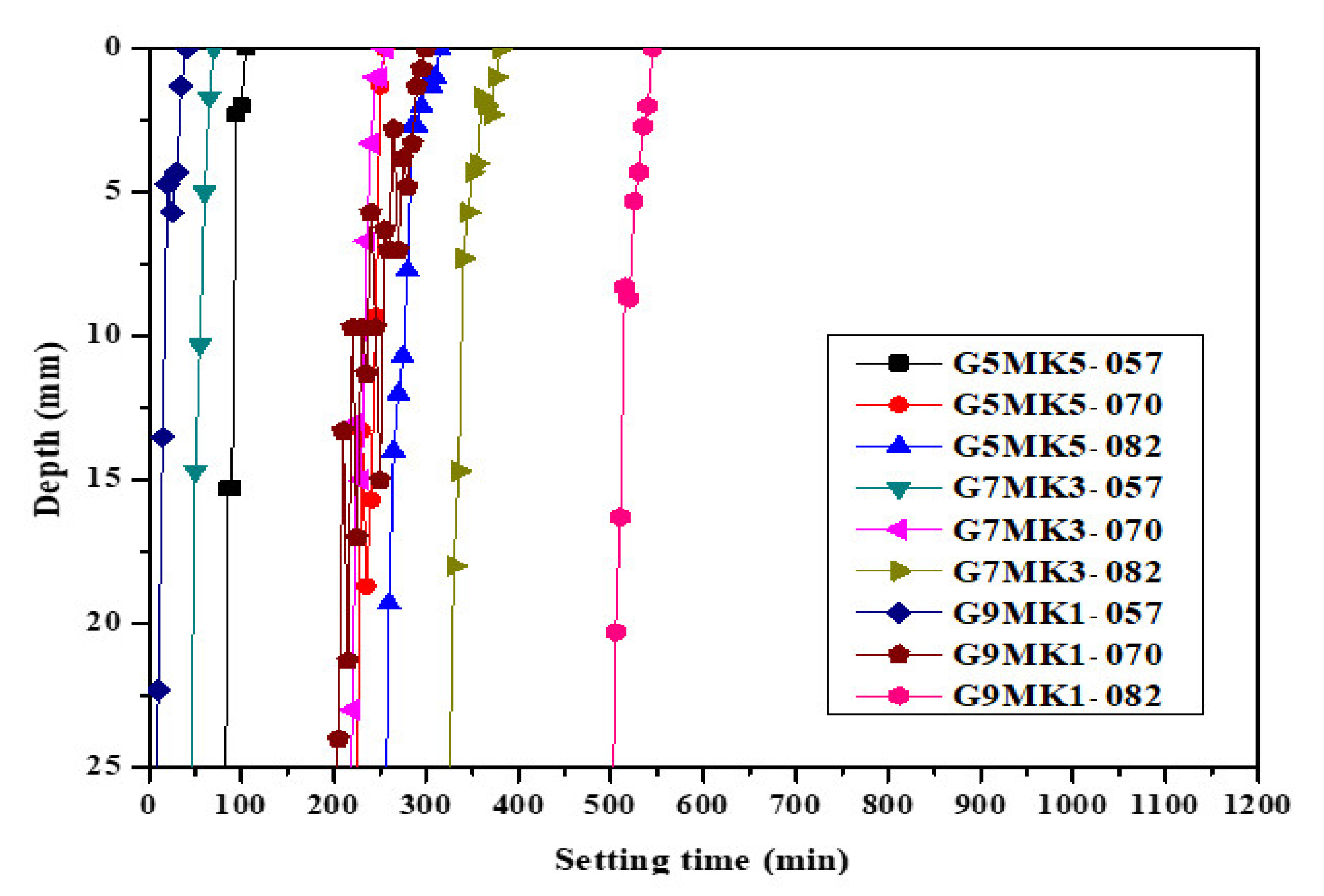
3.1. The Influence of Mixtures and SiO2/K2O Modulus on the Viscosity, Setting Time and Flexural Strength of Geopolymer Slurry
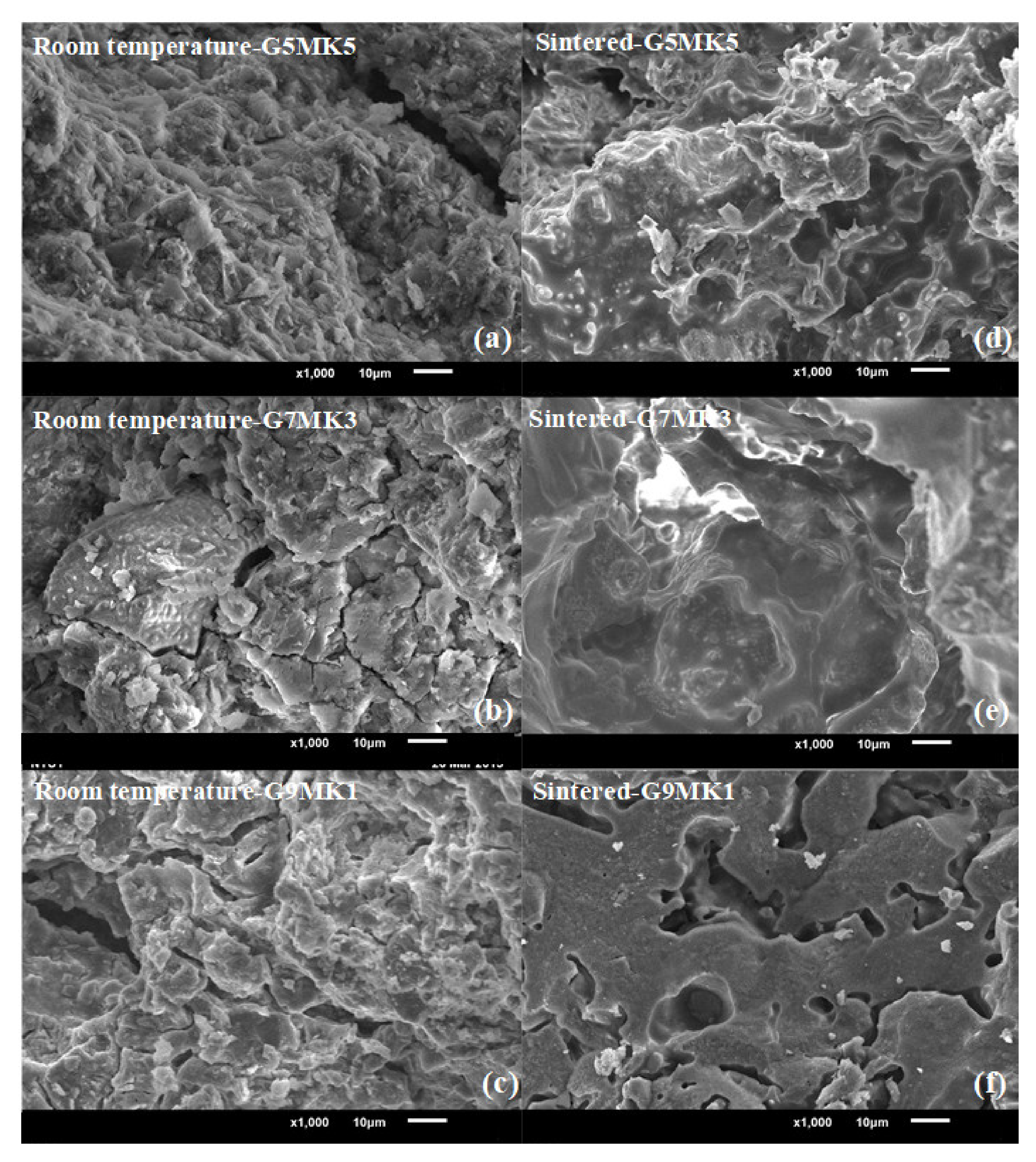
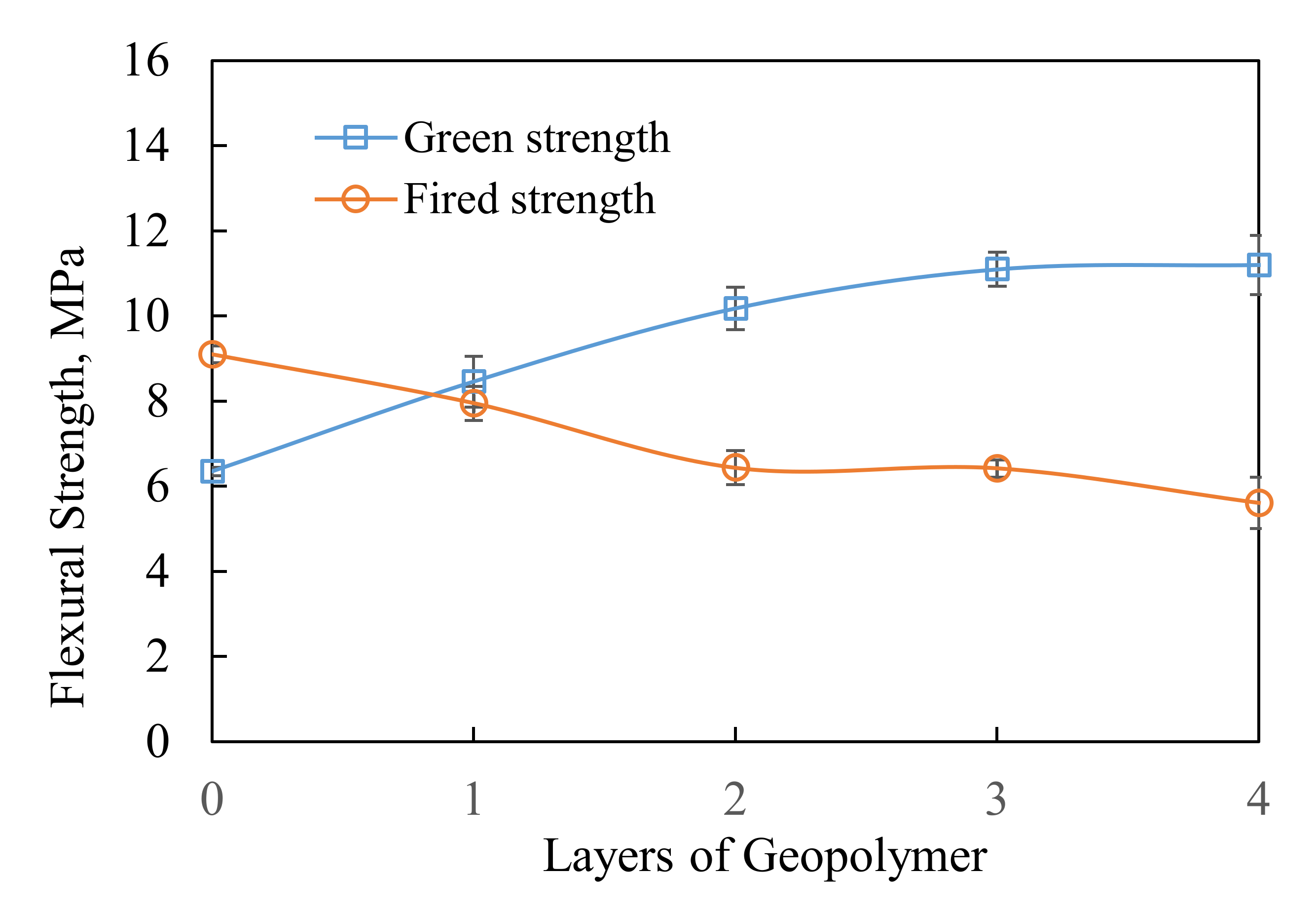
3.2. Strength and Permeability of Geopolymer Ceramic Shell
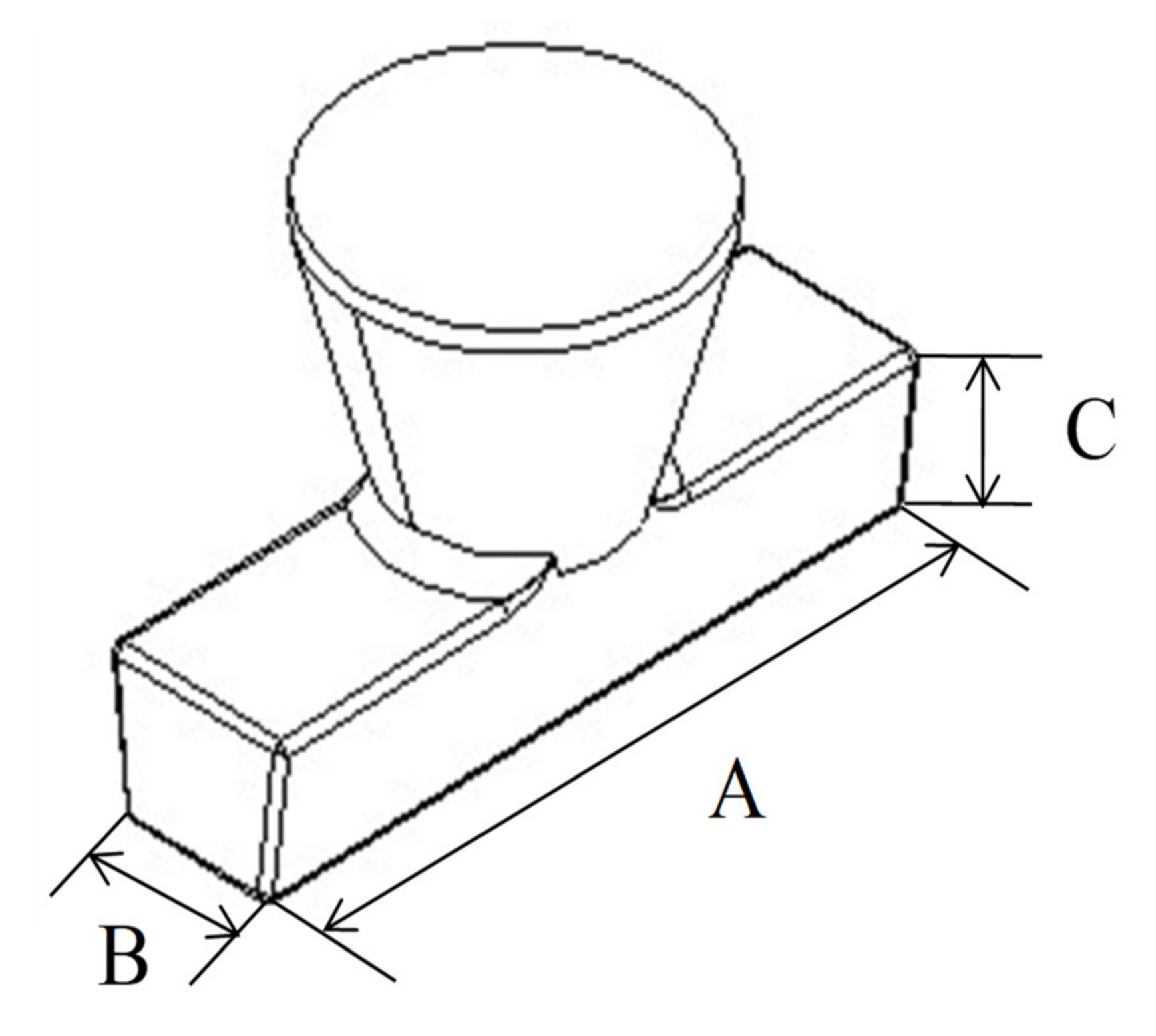
3.3. Casting Practice of Geopolymer Shell Mould and Tolerance Evaluation
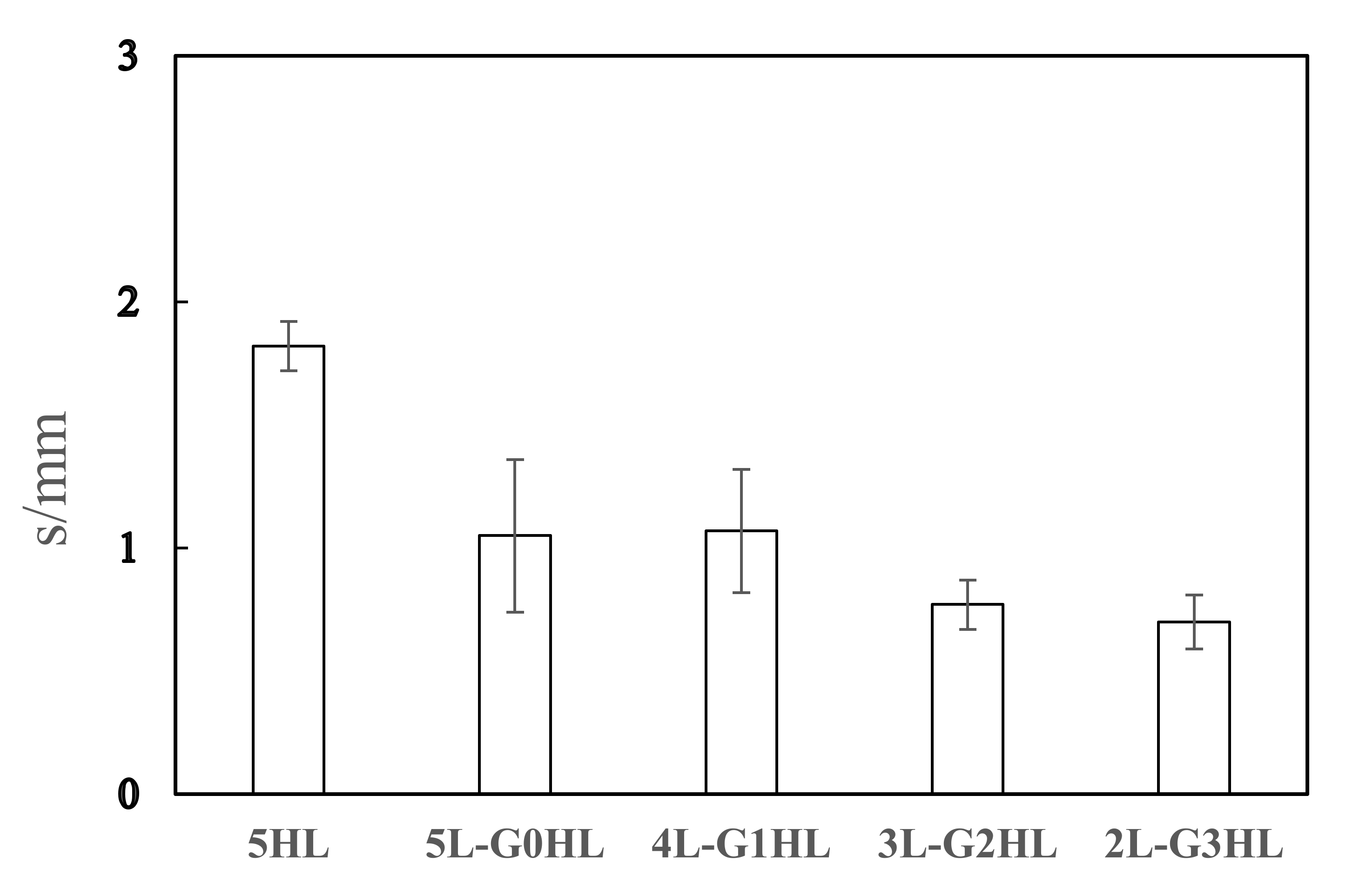
3.4. Evaluation of Drying Time Saving for Preparation Shell Moulds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jones, S.; Jolly, M.R.; Lewis, K. Development of techniques for predicting ceramic shell properties for investment casting. Br. Ceram. Trans. 2002, 101, 106–113. [Google Scholar] [CrossRef]
- Lin, K.L.; Cheng, C.J.; Cheng, A.; Chao, S.J. Study on recycled waste foundry sand as raw materials of cement additives. Sustain. Environ. Res. 2012, 22, 91–97. [Google Scholar]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Davisovits, J. Geopolymer Chemistry and Applications, 3rd ed.; Institute Géopolymère, Geopolymer Institute: Saint-Quentin, France, 2011. [Google Scholar]
- Li, Q.; Xu, H.; Li, F.; Li, P.; Shen, L.; Zhai, J.P. Synthesis of geopolymer composites from blends of CFBC fly and bottom ashes. Fuel 2012, 97, 366–372. [Google Scholar] [CrossRef]
- Holtzer, M.; Drozyński, D.; Bobrowski, A.; Plaza, W. Influence of binding rates on strength properties of moulding sands with the geopol binder. Arch. Foundry Eng. 2014, 14, 37–40. [Google Scholar] [CrossRef]
- Bobrowski, A.; Holtzer, M.; Zymankowska-Kumon, S.; Dańko, R. Harmfulness assessment of moulding sands with a geopolymer binder and a new hardener, in an aspect of the emission of substances from the BTEX group. Arch. Metall. Mater. 2015, 60, 341–344. [Google Scholar] [CrossRef]
- Drozyński, D.; Bobrowski, A.; Holtzer, M. Influence of the reclaim addition on properties of moulding sands with the geopol binder. Arch. Foundry Eng. 2015, 15, 138–142. [Google Scholar] [CrossRef]
- Bobrowski, A.; Drozyhski, D.; Grabowska, B.; Kaczmarska, K. Easy knock-out moulding and core sands-The future for metal casting. Foundry Trade J. Int. 2019, 193, 278–281. [Google Scholar]
- Vykoukal, M.; Burian, A.; Přerovská, M. GEOPOL®. The innovated environment friendly inorganic binder system. Arch. Foundry Eng. 2019, 19, 109–116. [Google Scholar]
- Zivica, V.M.; Palou, T.; Bágel, T.I.L. High strength metahalloysite based geopolymer. Compos. Part B-Eng. 2014, 57, 155–165. [Google Scholar] [CrossRef]
- Yahya, Z.; Abdullah, M.M.A.; Hussin, K.; Ismai, K.N.; Sandu, A.V.; Vizureanu, P.; Abd Razak, P.R. Chemical and Physical Characterization of Boiler Ash from Palm Oil Industry Waste for Geopolymer Composite. Rev. Chim. (Buchar.) 2013, 64, 1408–1412. [Google Scholar]
- Ahmad, R.; Abdullah, M.M.A.B.; Hussin, K.; Sandu, A.V.; Binhussain, M.; Jaya, N.A. Processing and properties of polymer filled geopolymer ceramics fabricated via powder metallurgy method: A review. Rev. Adv. Mater. Sci. 2016, 44, 26–32. [Google Scholar]
- Zych, J.; Kolczyk, J.; Snopkiewicz, T. New Investigation Method of the Permeability of Ceramic Moulds Applied in the Investment Casting Technology. Arch. Foundry Eng. 2013, 13, 107–112. [Google Scholar] [CrossRef]
- Patankar, S.V.; Jamkar, S.S.; Ghuga, Y.M. Effect of water-to-geopolymer binder ratio on the production of fly ash based geopolymer concrete. Int. J. Adv. Technol. Civ. Eng. 2013, 2, 79–83. [Google Scholar]
- Keller, D.S.; Keller, D.V., Jr. The effect of particle size distribution on the antithixotropic and shear thickening properties of coal–water dispersions. J. Rheol. 1991, 35, 1583–1607. [Google Scholar] [CrossRef]
- Konijn, B.J.; Sanderink, O.B.J.; Kruyt, N.P. Experimental study of the viscosity of suspensions: Effect of solid fraction, particle size and suspending liquid. Powder Technol. 2014, 266, 61–69. [Google Scholar] [CrossRef]
- Senapati, P.K.; Mishra, B.K.; Parida, A. Modeling of viscosity for power plant ash slurry at higher concentrations: Effect of solids volume fraction, particle size and hydrodynamic interactions. Powder Technol. 2010, 197, 1–8. [Google Scholar] [CrossRef]
- Gao, K.; Lin, K.L.; Wang, D.Y.; Hwang, C.L.; Shiu, H.S.; Chang, Y.M.; Cheng, T.W. Effects SiO2/Na2O molar ratio on mechanical properties and the microstructure of nano-SiO2 metakaolin-based geopolymers. Constr. Build. Mater. 2014, 52, 503–510. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, A.; Palomo, G.C.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Granizo, M.L.; Blanco, M.T. Alkaline activation of metakaolin. J. Therm. Anal. 1998, 52, 957–965. [Google Scholar] [CrossRef]
- Singh, P.S.; Trigg, M.; Burgar, I.; Barstow, T. Geopolymer formation processes at room temperature studied by 29Si and 27Al MASNMR. Mater. Sci. Eng. A 2005, 396, 392–402. [Google Scholar] [CrossRef]
- Granizo, M.L.; Alonso, S.; Blanco-Varela, M.T.; Palomo, A. Alkaline Activation of Metakaolin: Effect of Calcium Hydroxide in the Products of Reaction. J. Am. Ceram. Soc. 2002, 85, 225–231. [Google Scholar] [CrossRef]
- Granizo, M.L.; Blanco, M.T.; Puertas, F.; Palomo, A. Alkaline activation of metacaolin: Influence of synthesis parameters. In Proceedings of the Tenth International Congress on Chemistry of Cement, Goteborg, Sweden, 2–6 June 1997. [Google Scholar]
- Lecomte, I.; Henrist, C.; Liégeoisa, M.; Maserib, F.; Rulmonta, A.; Clootsa, R. (Micro)-structural comparison between geopolymers, alkali-activated slag cement and Portland cement. J. Eur. Ceram. Soc. 2006, 26, 3789–3797. [Google Scholar] [CrossRef]
- Feng, D.; Provis, J.L.; van Deventer, J.S.J. Thermal activation of albite for the synthesis of one-part mix geopolymers. J. Am. Ceram. Soc. 2012, 95, 565–572. [Google Scholar] [CrossRef]
- Pimraksaa, K.; Chindaprasirt, P.; Rungchet, A.; Sagoe-Crentsil, K.; Sato, T. Lightweight geopolymer made of highly porous siliceous materials with various Na2O/Al2O3 and SiO2/Al2O3 ratios. Mat. Sci. Eng. A 2011, 528, 6616–6623. [Google Scholar] [CrossRef]
- Bell, J.L.; Driemeyer, P.E.; Kriven, W.M. Formation of Ceramics from Metakaolin-Based Geopolymers: Part I—Cs-Based Geopolymer. J. Am. Ceram. Soc. 2009, 92, 1–8. [Google Scholar] [CrossRef]
- Jonghe, L.C.D.; Rahaman, M.N. Sintering of Ceramics. In Handbook of Advanced Ceramics; Elsevier Inc.: Philadelphia, PA, USA, 2013. [Google Scholar]
- Chang, K.T. A Study of Carbonate Mineral Powders Additives on the Collapsibility of Ceramic Shell Mould for Investment Casting. Master’s Thesis, National Taipei University of Technology, Taipei, Taiwan, 2009. [Google Scholar]
- Bell, J.L.; Driemeyer, P.E.; Kriven, W.M. Formation of Ceramics from Metakaolin-Based Geopolymers. Part II: K-Based Geopolymer. J. Am. Ceram. Soc. 2009, 92, 607–615. [Google Scholar] [CrossRef]
- Halikia, I.; Zoumpoulakis, L.; Christodoulou, E.; Prattis, D. Kinetic study of the thermal decomposition of calcium carbonate by isothermal methods of analysis. Eur. J. Miner. Process. Environ. Prot. 2001, 1, 89–102. [Google Scholar]

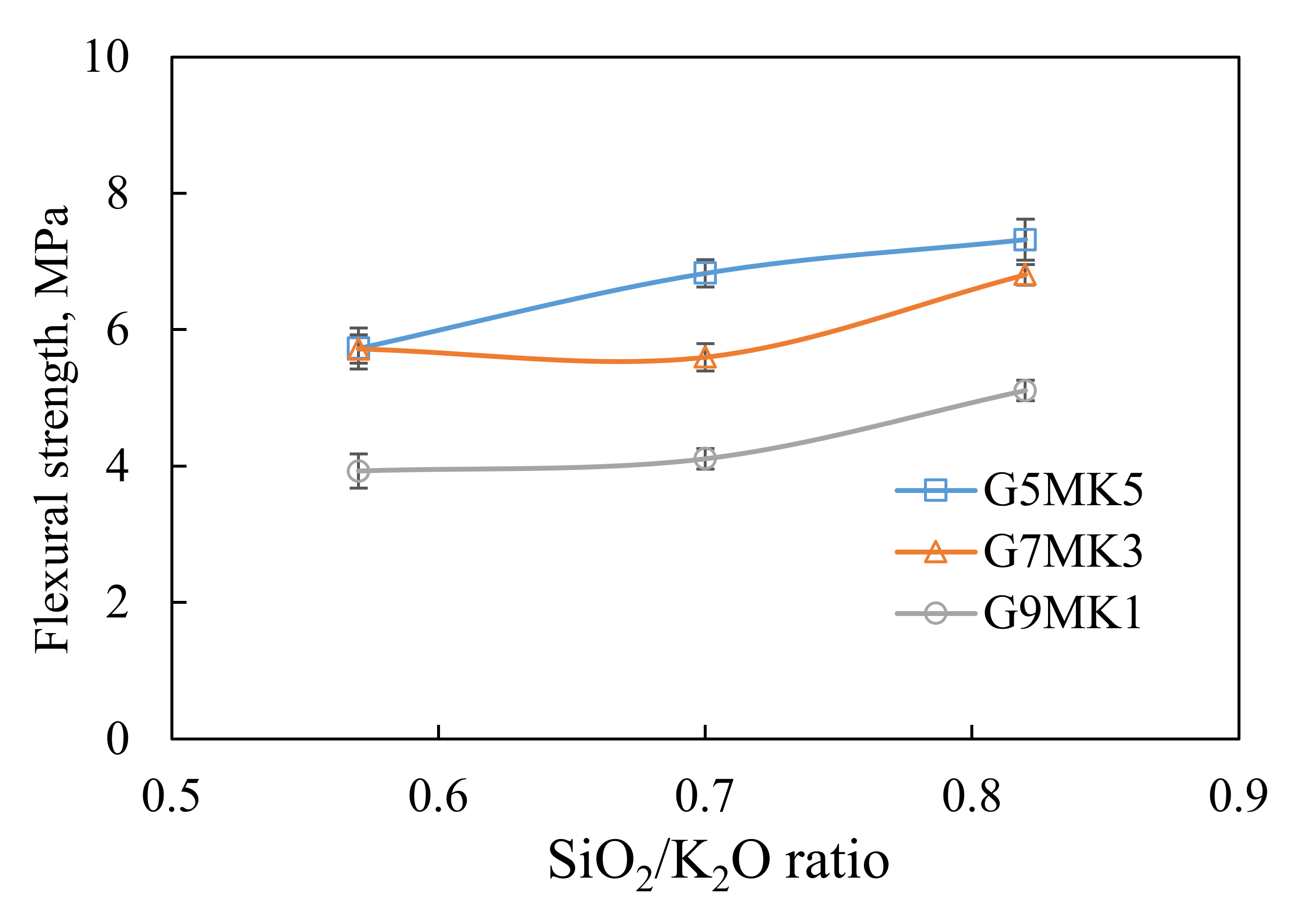
| Composition (wt%) | SiO2 | Al2O3 | ZrO2 | CaO | Others | Ignition Loss |
|---|---|---|---|---|---|---|
| Waste shell powder | 48.9 | 21.7 | 28.9 | N.D. | 0.5 | N.D. |
| Metakaolin | 54.7 | 39.8 | N.D. | N.D. | 3.6 | 1.9 |
| Calcium Carbonate | 2.2 | 1.4 | N.D. | 56.8 | 1.1 | 38.5 |
| Specimens | Waste Shell Powder (wt%) | Calcium Carbonate (wt%) | Metakaolin (wt%) | SiO2/K2O Molar Ratio | Solid/Liquid Ratio |
|---|---|---|---|---|---|
| G5MK5 | 80 | 10 | 10 | 0.57 | 3.46 |
| G7MK3 | 14 | 6 | 0.70 | ||
| G9MK1 | 18 | 2 | 0.82 |
| Dimensions, mm | Wax Pattern | 5HL | 5L-G0HL | 4L-G1HL | 3L-G2HL | 2L-G3HL |
|---|---|---|---|---|---|---|
| A | 172.5 ± 0.9 | 172.2 ± 0.1 | 172.1 ± 0.2 | 172.2 ± 0.1 | 172.6 ± 0.1 | - |
| B | 38.6 ± 0.4 | 38.7 ± 0.1 | 38.8 ± 0.2 | 38.8 ± 0.1 | 38.6 ± 0.1 | - |
| C | 35.9 ± 0.4 | 35.6 ± 0.1 | 35.7 ± 0.2 | 35.7 ± 0.2 | 35.9 ± 0.1 | - |
| Specimens | 5HL | 5L-G0HL | 4L-G1HL | 3L-G2HL | 2L-G3HL |
|---|---|---|---|---|---|
| Drying Time (h) | 34 | 34 | 27.5 | 21 | 14.5 |
| Saving Time (h) | - | 0 | 6.5 | 13 | 19.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-H.; Wu, Y.-F.; Ding, Y.-C.; Cheng, T.-W. Fabrication of Ceramic Moulds Using Recycled Shell Powder and Sand with Geopolymer Technology in Investment Casting. Appl. Sci. 2020, 10, 4577. https://doi.org/10.3390/app10134577
Lee W-H, Wu Y-F, Ding Y-C, Cheng T-W. Fabrication of Ceramic Moulds Using Recycled Shell Powder and Sand with Geopolymer Technology in Investment Casting. Applied Sciences. 2020; 10(13):4577. https://doi.org/10.3390/app10134577
Chicago/Turabian StyleLee, Wei-Hao, Yi-Fong Wu, Yung-Chin Ding, and Ta-Wui Cheng. 2020. "Fabrication of Ceramic Moulds Using Recycled Shell Powder and Sand with Geopolymer Technology in Investment Casting" Applied Sciences 10, no. 13: 4577. https://doi.org/10.3390/app10134577
APA StyleLee, W.-H., Wu, Y.-F., Ding, Y.-C., & Cheng, T.-W. (2020). Fabrication of Ceramic Moulds Using Recycled Shell Powder and Sand with Geopolymer Technology in Investment Casting. Applied Sciences, 10(13), 4577. https://doi.org/10.3390/app10134577






