Microwave Assisted Surfactant-Thermal Synthesis of Metal-Organic Framework Materials
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis
2.3. Characterization
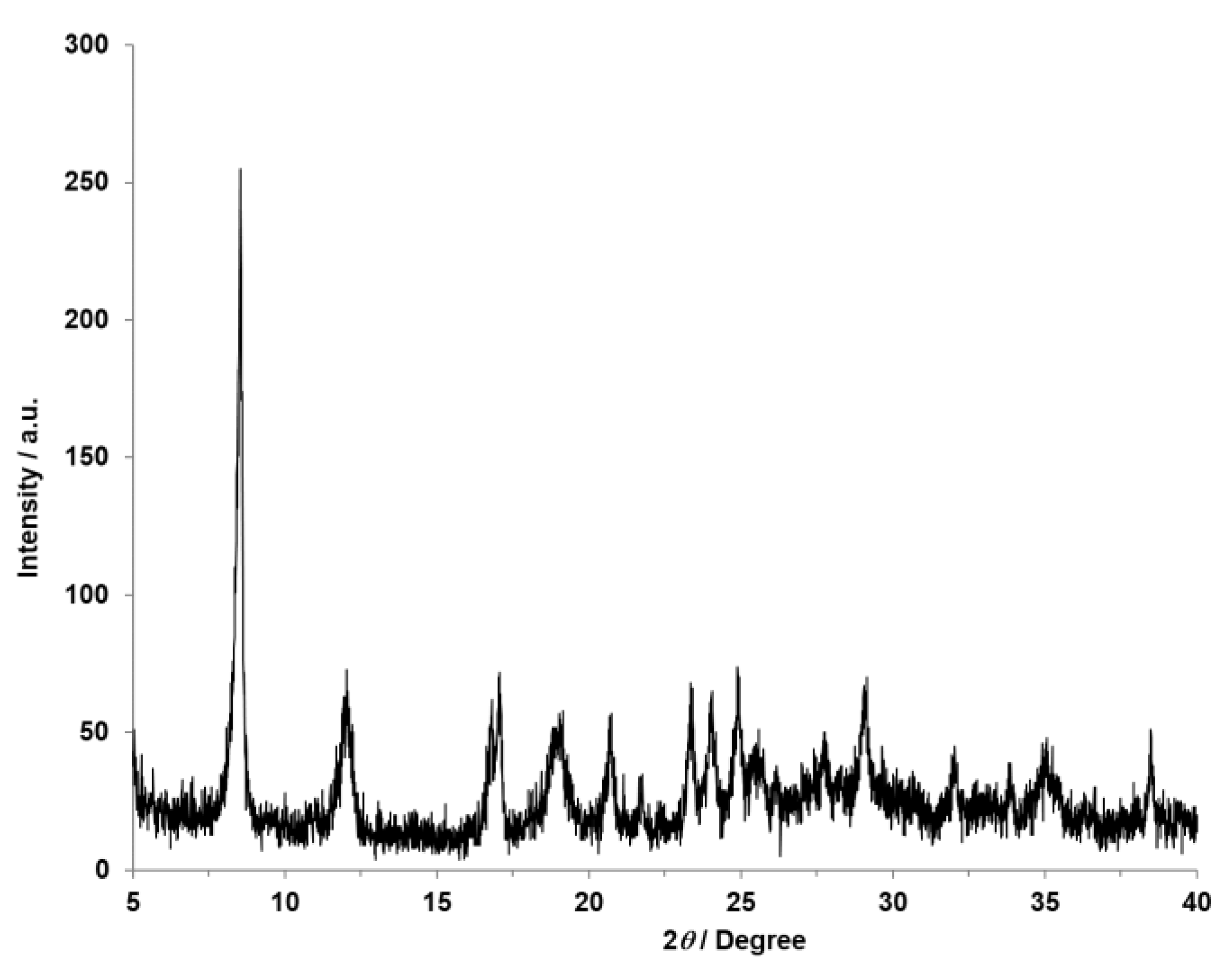
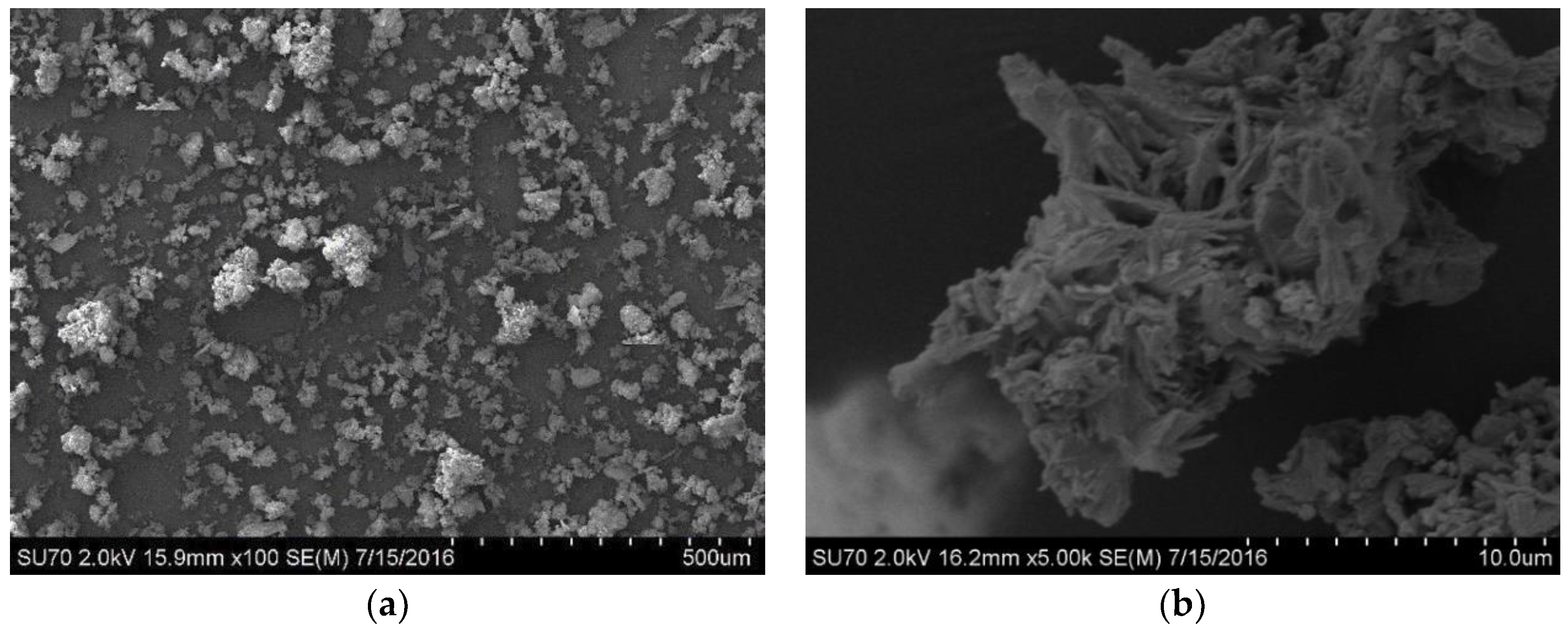
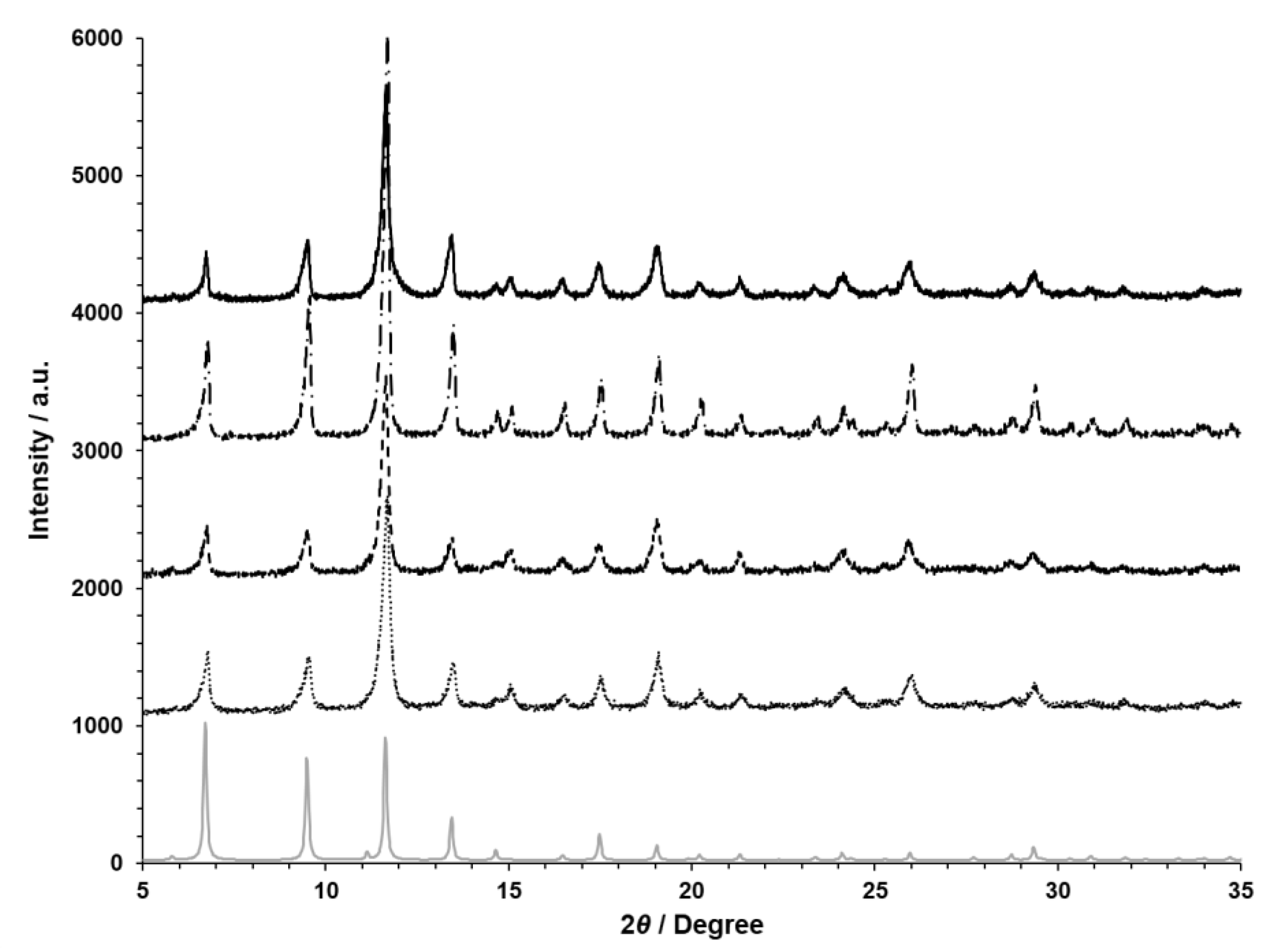
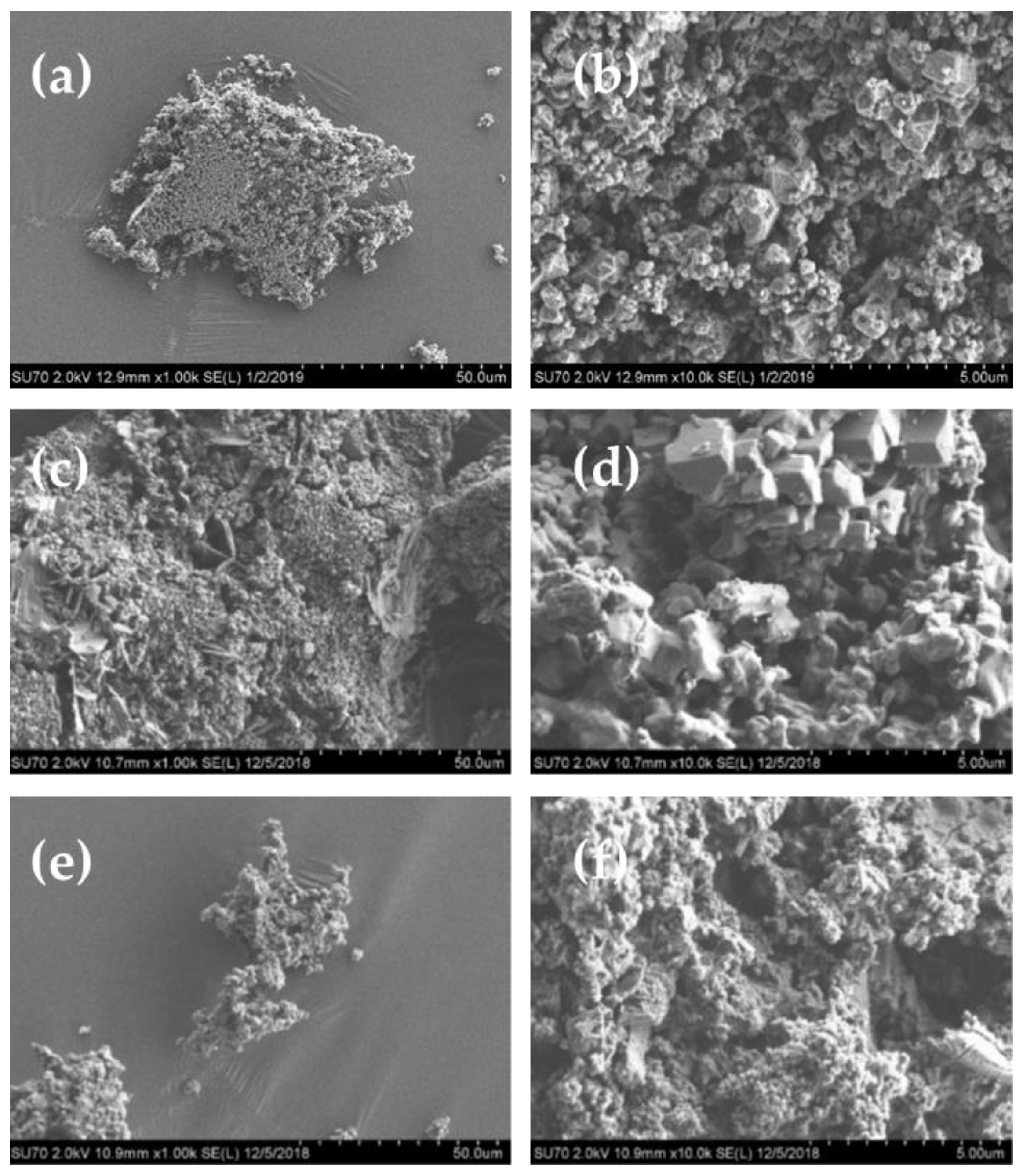
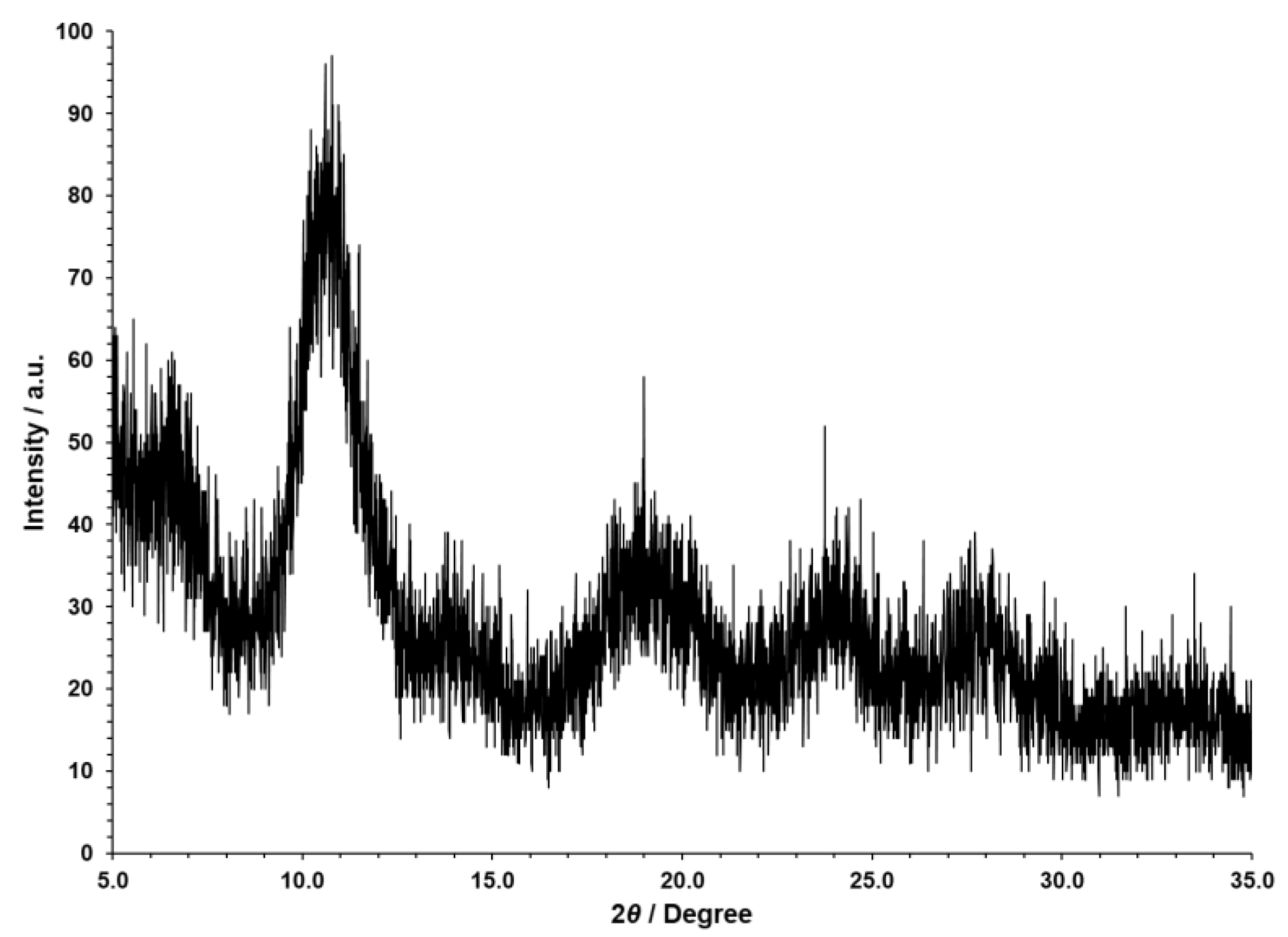
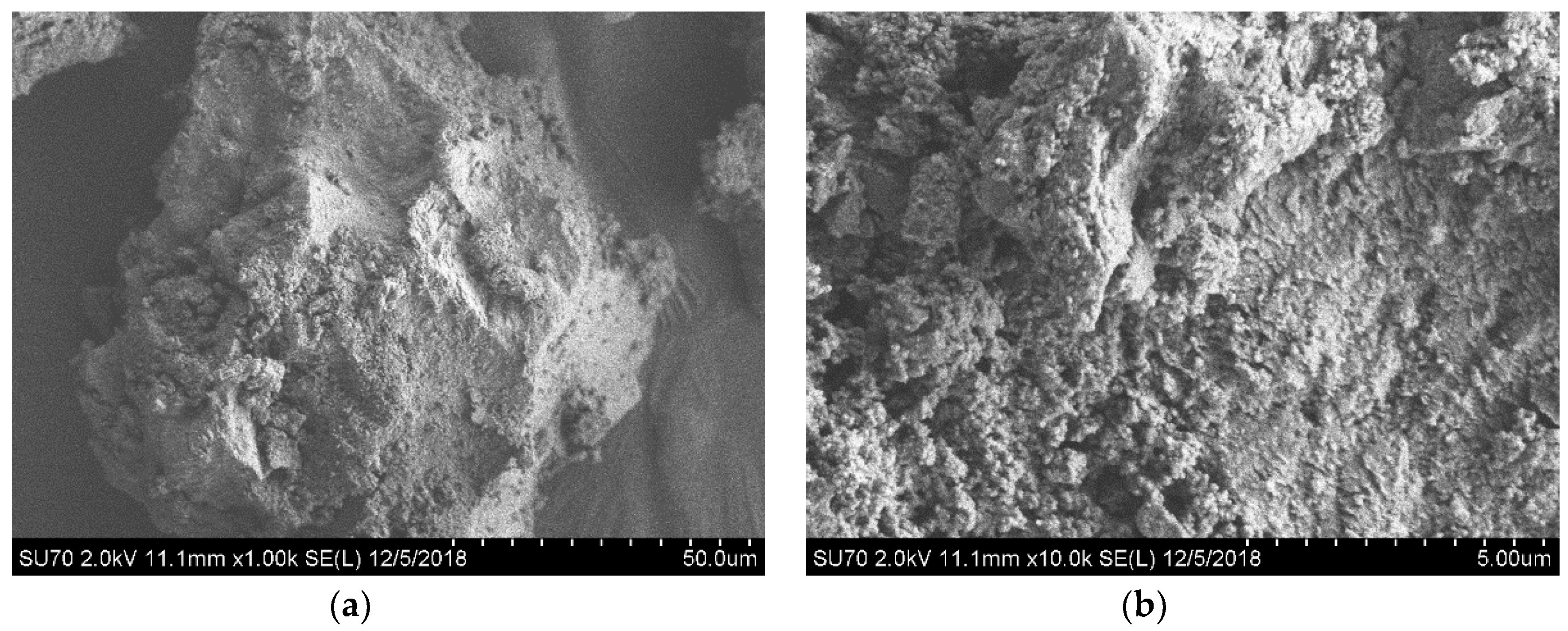
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, N.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Hu, Z.; Wang, G.; Uvdal, K. Coordination polymers for energy transfer: Preparations, properties, sensing applications, and perspectives. Coord. Chem. Rev. 2015, 284, 206–235. [Google Scholar] [CrossRef]
- Kathalikkattil, A.C.; Babu, R.; Tharun, J.; Roshan, R.; Park, D.W. Advancements in the Conversion of Carbon Dioxide to Cyclic Carbonates Using Metal Organic Frameworks as Catalysts. Catal. Surv. Asia 2015, 19, 223–235. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal–Organic Frameworks as Catalysts for Oxidation Reactions. Chem. Eur. J. 2016, 22, 8012–8024. [Google Scholar] [CrossRef]
- Seetharaj, R.; Vandana, P.V.; Arya, P.; Mathew, S. Dependence of solvents, pH, molar ratio and temperature in tuning metal organic framework architecture. Arab. J. Chem. 2016. [Google Scholar] [CrossRef]
- Dimethylamine; Aldrich: St. Louis, MO, USA, 2020; Available online: https://www.merckmillipore.com/JP/en/product/Dimethylamine,MDA_CHEM-822033 (accessed on 12 June 2020).
- N,N-Diethylformamide; Aldrich: St. Louis, MO, USA, 2020. Available online: https://www.merckmillipore.com/HK/en/product/NN-Diethylformamide,MDA_CHEM-821752 (accessed on 12 June 2020).
- N,N-Dimethylformamide; Aldrich: St. Louis, MO, USA, 2020. Available online: https://www.merckmillipore.com/JP/en/product/NN-Dimethylformamide,MDA_CHEM-100397 (accessed on 12 June 2020).
- Aidoudi, F.H.; Morris, R.E. CHAPTER 10 Ionothermal Synthesis. In Catalysis in Ionic Liquids: From Catalyst Synthesis to Application; The Royal Society of Chemistry: London, UK, 2014; pp. 508–536. ISBN 978-1-84973-603-9. [Google Scholar]
- Zhang, B.; Zhang, J.; Han, B. Assembling Metal–Organic Frameworks in Ionic Liquids and Supercritical CO2. Chem. Asian J. 2016, in press. [Google Scholar] [CrossRef]
- Gao, J.; Ye, K.; He, M.; Xiong, W.W.; Cao, W.; Lee, Z.Y.; Wang, Y.; Wu, T.; Huo, F.; Liu, X.; et al. Tuning metal–carboxylate coordination in crystalline metal–organic frameworks through surfactant media. J. Solid State Chem. 2013, 206, 27–31. [Google Scholar] [CrossRef]
- Xiong, W.W.; Zhang, Q. Surfactants as Promising Media for the Preparation of Crystalline Inorganic Materials. Angew. Chem. Int. Ed. 2015, 54, 11616–11623. [Google Scholar] [CrossRef]
- Xu, H.; Gao, J.; Wang, J.; Qian, X.; Song, R.; Cui, Y.; Yang, Y.; Qian, G. Surfactant-thermal method to synthesize a new Zn(II)-trimesic MOF with confined Ru(bpy)32+ complex. J. Solid State Chem. 2015, 226, 295–298. [Google Scholar] [CrossRef]
- Yu, X.; Toh, Y.S.; Zhao, J.; Nie, L.; Ye, K.; Wang, Y.; Li, D.; Zhang, Q. Surfactant-thermal method to prepare two new cobalt metal-organic frameworks. J. Solid State Chem. 2015, 232, 14–18. [Google Scholar] [CrossRef]
- Gao, J.; He, M.; Lee, Z.Y.; Cao, W.; Xiong, W.W.; Li, Y.; Ganguly, R.; Wu, T.; Zhang, Q. A surfactant-thermal method to prepare four new three-dimensional heterometal–organic frameworks. Dalton Trans. 2013, 42, 11367. [Google Scholar] [CrossRef]
- Lu, H.S.; Bai, L.; Xiong, W.W.; Li, P.; Ding, J.; Zhang, G.; Wu, T.; Zhao, Y.; Lee, J.M.; Yang, Y.; et al. Surfactant Media To Grow New Crystalline Cobalt 1,3,5-Benzenetricarboxylate Metal–Organic Frameworks. Inorg. Chem. 2014, 53, 8529–8537. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ye, K.; Yang, L.; Xiong, W.W.; Ye, L.; Wang, Y.; Zhang, Q. Growing Crystalline Zinc-1,3,5-benzenetricarboxylate Metal–Organic Frameworks in Different Surfactants. Inorg. Chem. 2014, 53, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Dong, W.; Wu, Y.; Li, D.; Liu, B.; Zhang, Q. A new surfactant-introduction strategy for separating the pure single-phase of metal–organic frameworks. Chem. Commun. 2015, 51, 9479–9482. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lu, Q.; Ma, Q.; Zhang, H. Two-Dimensional Metal–Organic Framework Nanosheets. Small Methods 2017, 1, 1600030. [Google Scholar] [CrossRef]
- Alves, C.K. Environmentally Benign Solvents in Organic Synthesis: Current Topics. Curr. Org. Chem. 2005, 9, 195–218. [Google Scholar] [CrossRef]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005, 7, 64–82. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, J.; Liu, J.; Xue, S.; Li, Y.; Wang, C. Four-component reaction between naphthols, substituted β-nitrostyrenes, substituted benzaldehydes and ammonium acetate in water-PEG-400: An approach to construct polysubstituted naphthofuranamines. RSC Adv. 2015, 5, 48580–48585. [Google Scholar] [CrossRef]
- Hese, S.V.; Kamble, R.D.; Mogle, P.P.; Kadam, S.S.; Hebade, M.J.; Ambhore, A.N.; Dawane, B.S. Green synthesis and antimicrobial evaluation of pyrido[1,2-a]pyrimidine-3-carbonitrile derivatives. Pharma Chem. 2015, 7, 249–256. [Google Scholar]
- Wang, Z.; Cao, X.; Yang, Y.; Lu, M. Green and Efficient Methods for One-Pot Aerobic Oxidative Synthesis of Benzimidazoles from Alcohols with TEMPO-PEG4000-NHC-Cu(II) Complex in Water. Synth. Commun. 2015, 45, 1476–1483. [Google Scholar] [CrossRef]
- Vivekanand, T.; Vinoth, P.; Agieshkumar, B.; Sampath, N.; Sudalai, A.; Menendez, J.C.; Sridharan, V. Highly efficient regioselective synthesis of pyrroles via a tandem enamine formation-Michael addition-cyclization sequence under catalyst- and solvent-free conditions. Green Chem. 2015, 17, 3415–3423. [Google Scholar] [CrossRef]
- Kumar, T.V.; Rao, C.R.K.; Mukkanti, K.; Mainkar, P.S. Polyethylene glycol (PEG-400) as an efficient and recyclable reaction medium for one-pot synthesis of substituted pyrroles under catalyst-free conditions. Asian J. Chem. 2015, 27, 1457–1461. [Google Scholar] [CrossRef]
- Rajeswari, M.; Sindhu, J.; Singh, H.; Khurana, J.M. An efficient, green synthesis of novel regioselective and stereoselective indan-1,3-dione grafted spirooxindolopyrrolizidine linked 1,2,3-triazoles via a one-pot five-component condensation using PEG-400. RSC Adv. 2015, 5, 39686–39691. [Google Scholar] [CrossRef]
- Khan, M.N.; Karamthulla, S.; Choudhury, L.H.; Faizi, M.S. Ultrasound assisted multicomponent reactions: A green method for the synthesis of highly functionalized selenopyridines using reusable polyethylene glycol as reaction medium. RSC Adv. 2015, 5, 22168–22172. [Google Scholar] [CrossRef]
- Kidwai, M.; Chauhan, R. A Rapid and an Efficient Route to the One-pot, Multicomponent Synthesis of 1H-Pyrazolo[1,2-b]phthalazine-5,10-dione Ring Systems. J. Heterocycl. Chem. 2014, 51, 1689–1696. [Google Scholar] [CrossRef]
- Wang, Z.G.; Xia, Y.G.; Jin, Y.; Lu, M. Green and reusable homogeneous oxidative system with ceric ammonium nitrate/[Imim-PEG1000-TEMPO] for efficient aerobic oxidation of alcohols and one-pot synthesis of benzimidazoles from alcohols under ambient conditions. Appl. Organomet. Chem. 2015, 29, 109–112. [Google Scholar] [CrossRef]
- Mogle, P.P.; Kamble, R.D.; Hese, S.V.; Dawane, B.S. Bleaching earth clay (pH 12.5)/PEG-400: An efficient recyclable catalytic system for synthesis of 5,6,7,8-tetrahydroquinoline-3-carbonitrile derivatives. Res. Chem. Intermed. 2014, 41, 4673–4678. [Google Scholar] [CrossRef]
- Liang, C.; Tang, Z.; Qian, W.; Shi, C.; Song, H. Ultrasound-promoted synthesis of 2-aminothiophenes accelerated by DABCO utilizing PEG-200 as solvent. J. Chem. Pharm. Res. 2014, 6, 798–802. [Google Scholar]
- Nagaraju, A.; Ramulu, B.J.; Shukla, G.; Srivastava, A.; Verma, G.K.; Raghuvanshi, K.; Singh, M.S. Catalyst-free one-pot four-component domino reactions in water-PEG-400: Highly efficient and convergent approach to thiazoloquinoline scaffolds. Green Chem. 2015, 17, 950–958. [Google Scholar] [CrossRef]
- Reddy, M.V.; Son, S.M.; Jeong, Y.T. TiO2-SiO2 in PEG: A Highly Efficient Green Catalytic System for One-Pot Synthesis of Benzo[a]xanthen-11-ones. J. Heterocycl. Chem. 2014, 51, 1246–1250. [Google Scholar] [CrossRef]
- Shen, M.; Wang, J.; Shang, S.; Song, Z. A simple and efficient procedure for the one-pot three-component synthesis of spiro[indoline-3,9′-tetrazolo[5,1-b]quinazoline]-2,8′(5′H)-dione in PEG-H2O medium. Chem. Lett. 2014, 43, 1239–1241. [Google Scholar] [CrossRef]
- Reddy, A.S.; Reddy, M.N.; Swamy, K.C.K. A simple copper-catalyzed tandem cyclization of ynamides leading to triazolo-1,2,4-benzothiadiazine-1,1-dioxides in PEG-400 medium. RSC Adv. 2014, 4, 28359–28367. [Google Scholar] [CrossRef]
- Kadam, A.; Dawane, B.; Pawar, M.; Shegokar, H.; Patil, K.; Meshram, R.; Gacche, R. Development of novel pyrazolone derivatives as inhibitors of aldose reductase: An eco-friendly one-pot synthesis, experimental screening and in silico analysis. Bioorg. Chem. 2014, 53, 67–74. [Google Scholar] [CrossRef]
- Sindhu, J.; Singh, H.; Khurana, J.M. A green, multicomponent, regio- and stereo-selective 1,3-dipolar cycloaddition of azides and azomethine ylides generated in situ with bifunctional dipolarophiles using PEG-400. Mol. Divers. 2014, 18, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, H.; Iranpoor, N.; Samadi, A. One-pot synthesis of aryl alkyl thioethers and diaryl disulfides using carbon disulfide as a sulfur surrogate in the presence of diethylamine catalyzed by copper(I) iodide in polyethylene glycol (PEG200). Tetrahedron Lett. 2014, 55, 1212–1217. [Google Scholar] [CrossRef]
- Piltan, M.; Moradi, L.; Zarei, S.A.; Rostami, H. One-pot multicomponent synthesis of novel tricyclic pyrrolo[2,1-c][1,4]benzoxazines. Chin. Chem. Lett. 2014, 25, 234–236. [Google Scholar] [CrossRef]
- Acharya, A.P.; Kamble, R.D.; Patil, S.D.; Hese, S.V.; Yemul, O.S.; Dawane, B.S. Green method for synthesis of 3-[2-(substituted-phenyl)-2-oxo ethylidene]-1,3-dihydro-indol-2-one and their in vitro antimicrobial activity. Res. Chem. Intermed. 2015, 41, 2953–2959. [Google Scholar] [CrossRef]
- McGarvey, P.W.; Hoffmann, M.M. Solubility of Some Mineral Salts in Polyethylene Glycol and Related Surfactants. TSD 2018, 55, 203–209. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Seo, Y.K.; Hundal, G.; Jang, I.T.; Hwang, Y.K.; Jun, C.H.; Chang, J.S. Microwave synthesis of hybrid inorganic–organic materials including porous Cu3(BTC)2 from Cu(II)-trimesate mixture. Microporous Mesoporous Mater. 2009, 119, 331–337. [Google Scholar] [CrossRef]
- Schlesinger, M.; Schulze, S.; Hietschold, M.; Mehring, M. Evaluation of synthetic methods for microporous metal–organic frameworks exemplified by the competitive formation of [Cu2(btc)3(H2O)3] and [Cu2(btc)(OH)(H2O)]. Microporous Mesoporous Mater. 2010, 132, 121–127. [Google Scholar] [CrossRef]
- Blanita, G.; Borodi, G.; Lazar, M.D.; Biris, A.R.; Barbu-Tudoran, L.; Coldea, I.; Lupu, D. Microwave assisted non-solvothermal synthesis of metal–organic frameworks. RSC Adv. 2016, 6, 25967–25974. [Google Scholar] [CrossRef]
- Diring, S.; Furukawa, S.; Takashima, Y.; Tsuruoka, T.; Kitagawa, S. Controlled Multiscale Synthesis of Porous Coordination Polymer in Nano/Micro Regimes. Chem. Mater. 2010, 22, 4531–4538. [Google Scholar] [CrossRef]
- Zou, F.; Yu, R.; Li, R.; Li, W. Microwave-Assisted Synthesis of HKUST-1 and Functionalized HKUST-1-@H3PW12O40: Selective Adsorption of Heavy Metal Ions in Water Analyzed with Synchrotron Radiation. ChemPhysChem 2013, 14, 2825–2832. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Zhang, J.; Peng, L.; Han, B.; Mu, T.; Li, J.; Yang, G. Poly(ethylene glycol) Stabilized Mesoporous Metal–Organic Framework Nanocrystals: Efficient and Durable Catalysts for the Oxidation of Benzyl Alcohol. ChemPhysChem 2014, 15, 85–89. [Google Scholar] [CrossRef]
- Hoffmann, M.M.; Bothe, S.; Gutmann, T.; Hartmann, F.F.; Reggelin, M.; Buntkowsky, G. Directly vs Indirectly Enhanced 13C in Dynamic Nuclear Polarization Magic Angle Spinning NMR Experiments of Nonionic Surfactant Systems. J. Phys. Chem. C 2017, 121, 2418–2427. [Google Scholar] [CrossRef]
- Zagari, J.; Hoffmann, M.M. Polydispersity Analysis of Polyethylene Glycol by Gas and Liquid Chromatography. Chem. Educ. 2019, 24, 30–34. [Google Scholar]
- Sanchez-Sanchez, M.; de Asua, I.; Ruano, D.; Diaz, K. Direct Synthesis, Structural Features, and Enhanced Catalytic Activity of the Basolite F300-like Semiamorphous Fe-BTC Framework. Cryst. Growth Des. 2015, 15, 4498–4506. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Liu, L.; Han, Z.B.; Wang, S.M.; Yuan, D.Q.; Ng, S.W. Robust Molecular Bowl-Based Metal–Organic Frameworks with Open Metal Sites: Size Modulation To Increase the Catalytic Activity. Inorg. Chem. 2015, 54, 3719–3721. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forsyth, C.; Taras, T.; Johnson, A.; Zagari, J.; Collado, C.; Hoffmann, M.M.; Reed, C.R. Microwave Assisted Surfactant-Thermal Synthesis of Metal-Organic Framework Materials. Appl. Sci. 2020, 10, 4563. https://doi.org/10.3390/app10134563
Forsyth C, Taras T, Johnson A, Zagari J, Collado C, Hoffmann MM, Reed CR. Microwave Assisted Surfactant-Thermal Synthesis of Metal-Organic Framework Materials. Applied Sciences. 2020; 10(13):4563. https://doi.org/10.3390/app10134563
Chicago/Turabian StyleForsyth, Cory, Tyler Taras, Adam Johnson, Jessica Zagari, Crystal Collado, Markus M. Hoffmann, and Carly R. Reed. 2020. "Microwave Assisted Surfactant-Thermal Synthesis of Metal-Organic Framework Materials" Applied Sciences 10, no. 13: 4563. https://doi.org/10.3390/app10134563
APA StyleForsyth, C., Taras, T., Johnson, A., Zagari, J., Collado, C., Hoffmann, M. M., & Reed, C. R. (2020). Microwave Assisted Surfactant-Thermal Synthesis of Metal-Organic Framework Materials. Applied Sciences, 10(13), 4563. https://doi.org/10.3390/app10134563





