Abstract
Exosomes are extracellular vesicles (EVs) belonging to the nanovesicles family that function as signaling molecules between cells. After their first description in the late 1960s, interest in their potential as a research target has steadily increased. They are small secreted organelles with a single membrane that are well enriched in lipids, proteins, nucleic acids, and glycoconjugates. Exosomes take part in a larger communication network in which cells communicate between one another by DNA shuttling, proteins, RNA, and membrane-bound factors. The machinery of protein quality control occurs through the process termed “exosome biogenesis”. Furthermore, the pathway involved in intercellular movement of vesicles is vital in various aspects of human health and diseases. Due to their inherent properties, exosomes are currently being developed as potential therapeutic agents in a wide range of diseases including infectious and non-infectious diseases. Exosomes and other EVs sourced from Mesenchymal stem cells (MSCs) have been shown in different studies to possess therapeutic effects in diverse disease models either in vivo or in vitro. Some mechanisms and/or pathways that MSC-derived exosomes use to illustrate their therapeutic effect against some diseases have also been summarized. This review aims to highlight the recent findings and potential therapeutic application of exosomes in different diseases such as autoimmune, cardiovascular, obesity, neural, soft tissues, bone, and cartilage.
1. Introduction
Before the late 1960s, little was known about exosomes until their first description by two investigators namely Bonucci and Anderson [1,2]. Right from these early studies, they were known to be the initiators of hydroxyapatite crystals which were formed as a result of direct budding of the plasma membrane [2]. Furthermore, in the 1980s, the term, exosome biogenesis was revealed following a study conducted by Trams et al. [3] who discovered that exosomes in the seminal fluid were derived from prostrate/epididymis.
Exosomes are extracellular vesicles (EVs) that function as signaling molecules between cells. These exosomes are secreted organelles with a single membrane usually small in size with a diameter of ∼30 to 200 nm. They are well enriched in lipids, proteins, nucleic acids, and glycoconjugates. Structurally, exosomes are a series of membrane-associated, oligomeric complexes of protein with a marked molecular diversity formed by budding in endosome and plasma membranes [4]. The term EV includes exosomes, prostasomes, ectosomes, microvesicles, microparticles, tolerosomes, apoptotic bodies, and nanovesicles. Currently, available technology cannot separate exosomes from the other EVs mentioned earlier. Thus, exosomes isolated from cells and used for research related activities are best regarded as EVs, as stated in the guidelines formulated by the International Society of Extracellular Vesicles (ISEV) [5]. Cell-based membrane vesicles deliver microRNA (miRNA), messenger RNA (mRNA), and proteins through intracellular organelles. Microparticles, microvesicles (100–1000 nm), and exosomes (20–200 nm) are three examples of membrane vesicles [6]. Exosomes undergo a cycle called exosome biogenesis to ensure quality control. Once they are released, exosomes have diverse activities such as extracellular matrix remodeling and signal transmission. Intercellular vesicles play vital roles in various health and disease aspects including immunity, cancer, tissue homeostasis, and neurodegenerative diseases. Additionally, pathogens such as viruses utilize exosome biogenesis pathways viz-a-viz by establishing host permissiveness and by assembling infectious particles [7]. Exosome isolation from diverse body fluids such as plasma, cerebrospinal fluid, semen, blood, amniotic fluid, saliva, urine, epididymal and synovial fluid, breast milk, and bronchoalveolar lavage makes them an ideal model for the development of therapeutic agents against multiple diseases [8].
Exosomes are particularly regulated indifferent organisms during stresses [9,10], and they participate in a larger communication network in which cell connects to each other by DNA shuttling, proteins, RNA, and membrane-bound factors. Exosomes derived from tumors, commonly known as Tumor-derived exosomes (TEX), affect the surrounding tumor microenvironment (TME). As a result of the deep distribution of TEX in the blood and lymph, they have a big role in remote tissue sites and in the creation of their pre-metastatic niche referred to as the tumor macroenvironment (TMaE) [11]. TEX delivers tolerogenic signals to immune cells, prevents the proliferation of immune cells, interferes with the differentiation of monocyte, and induces apoptosis in activated CD8+ T lymphocytes leading to immune suppression via paracrine effect [12]. TEX exhibit some ligands (e.g., programmed death-ligand 1 (PD-L1)) to produce beneficial endocrine signals which provide pre-metastatic TMaE, expanding far from the primary tumor [11]. Furthermore, it was shown that the expression of PD-L1 is enhanced in melanoma cells exposed to interferon-γ (IFNγ), leading to the expression of PD-L1 in circulating melanoma exosomes derived from humans (HMEX) [13].
2. Biogenesis of Exosomes
According to the International Society for Extracellular Vesicles (ISEV), extracellular vesicle (EV) is the generic name used to refer to particles naturally released from a cell that is composed of a lipid bilayer and is unable to replicate (lack of functional nucleus). It is difficult to assign an EV to a particular pathway of biogenesis, except that the process was observed using live imaging techniques [5]. The nomenclature of exosomes is derived in 3 different ways: based on their biogenesis, physiological functions within cells, and lastly, empirical definition based on isolation [14]. In this review, exosomes will be defined by their biogenesis. Cells are professionals in producing and exporting molecular products, for instance, transporting insulin to the bloodstream [15]. Before transportation, these molecules are sent first for cell packaging, and they are the exosomes that are first released intracellularly. Multivesicular endosomes (MVEs) also encompass endosomes which are then formed. There are three ways in which exosome biogenesis is formed. First, vesicular budding into discrete endosomes with multivesicular body (MVB) maturation, which releases exosomes after plasma membrane fusion. Second, their immediate release from the plasma membrane by vesicular budding, and lastly, its release is delayed due to budding at the intracellular plasma membrane-connected compartments (IPMCs) after which there is a deconstruction of IPMC necks [16], as shown in Figure 1. Several molecules—such as endosomal sorting complexes required for transport (ESCRT) machinery, tetraspanins, and lipids—were involved in intraluminal vesicle (ILV) biogenesis, [17]. Whereas two different mechanisms, namely inducible release and trans-Golgi network, were assumed to participate in exosome secretion [18].
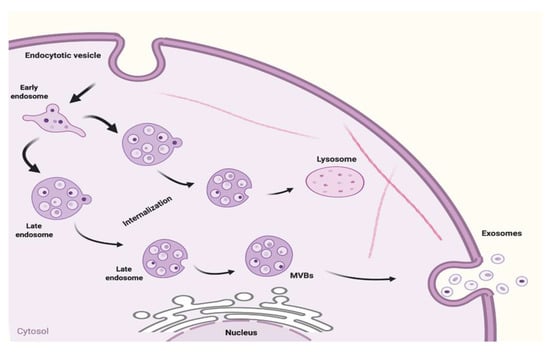
Figure 1.
Biogenesis of exosomes.
Intracellular exosomes are fused into MVEs in the cytoplasm. The mechanisms for exosome generation and release have been well described [19]. Interestingly, exosomes are released into the extracellular matrix after the fusion of the MVEs with the cell membrane [20]. The peripheral membrane proteins (Rab GTPases) were found to facilitate the fusion of the MVEs with plasma membranes including RAB11 and RAB35 after which flotillin- and other cell-specific protein-rich exosomes are released [21], while the second mechanism facilitating the release of exosomes loaded with CD63, TSG1010, and ALIX involves RAB27A and RAB27B [19]. Microvesicles undergo a different mode of biogenesis in contrast to exosomes. They are formed by direct blebbing from the plasma membrane. In summary, diverse complex pathways are used to generate exosomes from endosomes. The formation of the exosomes also varies considerably depending on the type as well as the physiological state of the origin of the cell [22].
Limited MVB is subjected to internal budding leading to late endosome formation, which in turn generates exosomes in a constitutive way. The formation of intraluminal vesicles (ILVs) within MVBs from late endosomal membranes is caused by invagination [23]. During invagination, some proteins are incorporated into the membrane and the cytosolic components are engulfed and enclosed in ILVs. The release of ILVs is indicated in the extracellular space after fusion with the exosome’s plasma membrane. Degradation of the components occurs in the lysosomes. Canonical exosomes are biconcave in shape produced by artificial drying, but they appeared spherical when viewed using transmission electron microscopy [4].
Recent scientific evidence indicates the role of the alternative pathway in exosomal cargo into MVBs. This process occurs in an ESCRT-independent manner dependent upon microdomains based on raft. These microdomains are enriched by sphingomyelinases that form ceramides [24]. Hydrolytic removal of phosphocholine moiety results in the formation of ceramides which is known to promote lateral phase separation and microdomains coalescence. Tetraspanins are proteins that play a key role in protein loading and exosome biogenesis. In the plasma membrane, tetraspanin-enriched microdomains (TEMs) are specialized platforms for protein signaling and receptor compartmentalization. Tetraspanin CD8+ and TEM together play a key role in sorting target receptors and intracellular components into the exosomes [25]. Bioactive molecules sorting into exosomes occurs either through ESCRT-dependent or ESCRT-independent mechanisms depending on the cell type origin. Plasma membrane-budded microvesicles (MVs) are a heterogeneous group of membrane vesicles that are produced with exosomes. They are generated by external budding of the plasma membrane and are of variable shapes, 100–1000 nm in size and are products of endothelial cells, red blood cells, and platelets. The small size and shape of exosomes make it easier for them to escape from the mononuclear phagocytic system cells, allowing them to stay longer in the general circulation [26].
3. Therapeutic Potential of Exosomes in Different Diseases
Attention is currently focused on the clinical application of exosomes for the treatment of disease. Exosomes may be included in useful applications for managing some diseases, including cardiac regeneration, obesity, soft tissues, bone/cartilage injury, brain/neural injury, etc. Figure 2 presents a pictorial presentation of certain conditions of disease in which exosomes were used. Exosomes and other EVs have been also used in stem cell therapy. Several reports have documented the application of Mesenchymal stem cells (MSCs) derived from secretome in various diseases such as skeletal, cardiac, nervous, ovarian, renal, hepatic, infectious, pulmonary, and soft tissue diseases. A list of some of the commonly used stem cells, their application, and mechanisms of actions are presented in Table 1.
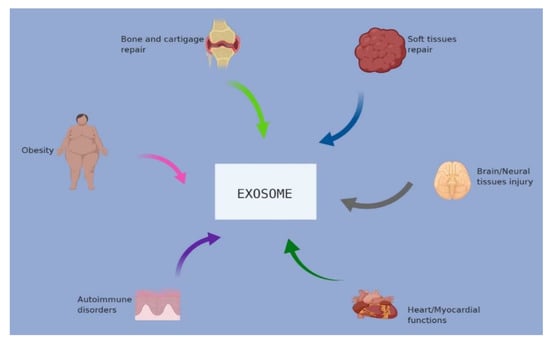
Figure 2.
Applications and use of exosomes in different disease conditions.

Table 1.
A summary of some therapeutic applications of Mesenchymal stem cells (MSC)-derived exosomes and other extracellular vesicles in different diseases.
Nucleic acids and proteins are naturally carried by exosomes making them potential vehicles for the intentional incorporation of proteins and nucleic acids for therapeutic purposes [49]. Synthetic nanoparticles, numerous small chemotherapeutic molecules, and natural compounds are other therapeutic molecules that are efficiently delivered via exosomes [49]. Altogether, these stacks of therapeutic cargos loaded into exosomes are delivered efficiently to host cells with diverse effects [50]. However, concerns are that exosomes may generate immune responses. Therefore, to overcome this challenge, certain factors must be taken into consideration such as the cell source and site of formation. Exosomes should be carefully selected from the donor cells. In recent times, clinical application and use of exosomes in therapeutics have grown rapidly. The targeted use of exosomes is one of the promising methods for treating cancer [51], and these applications have also been extended to other disease conditions such as soft tissues [52], cardiovascular disease [53], obesity [54], autoimmune diseases [55], and others. In summary, we can say that exosomes are potential carriers of minor interfering RNAs and have a great potential for clinical uses in treatments, and some of these useful applications will be discussed as follows.
3.1. Therapeutic Potential of Exosomes in Bone and Cartilage
Skeletal health is dependent on the fact that successful blood vessels and muscle generation are physiological processes that also include exosomal signals. Exosomes are composed of significant amounts of transforming growth factor-β1 (TGF-β1), promyogenic molecules (VEGF), proangiogenic, human T-cell factor 4 (TCF4), miR-494, interleukin-8 (IL-8), IL-6, miR-181, multiple miRNAs, and hepatocyte growth factor (HGF), most of which were thrown by Mesenchymal stem cells (MSCs) [56]. MSC-derived exosomes arising either from bone marrow, adipose tissue umbilical cord, or stimulating stem cells can enhance the bone differentiation of MSCs or primary bone cells. This is illustrated by the osteogenic genes and osteocalcin upregulation and elevated osteoblast multiplication and migration [57]. The changes in gene expression in MSCs, following MSC-derived exosomes absorption, indicates cell adhesion activation, PI3K-Akt signaling, and ECM-receptor interaction pathways, which are finally related to bone differentiation [53]. Exosomes originated from MSCs can also be absorbed immediately by osteoblasts, enhancing their multiplication and stimulating protein synthesis associated with the GLUT3 and MAPK pathways [58]. Adaptation to MSC-derived exosomes leads to tissue changes, such as increased matrix vascularization and mineralization along with bone restoration in rats with bone disorders [59]. Strikingly, MSC-derived exosomes are directly bound to fibronectin and type I collagen proteins [60]. The development of matrix mineralization shows a feature of late exosomes rather than the early stage of bone differentiation. Tendon stem cell (TSC) and exosome injections in rodents led to a decrease in the matrix metalloproteinases (MMP)-3 expression and an increase in the collagenase I (1a1) expression as well as the tissue metalloproteinase-3 (TIMP-3) inhibitor [20]. Furthermore, in vitro studies using 2D-Exo and 3D-Exo umbilical Mesenchymal stem cell (MSC) cultures indicated that both cultures stimulated the proliferation and migration of chondrocytes as well as matrix synthesis and apoptosis inhibition [25]. Finally, exosomes also appear to be part of cartilage growth. Exosomes have an altered miRNA cargo produced by MSCs derived from bone marrow with an activated chondrogenic phenotype (marked by a reduction in miR-6891-5p and miR-377-3p and an elevation in miR-1290, miR-92a, miR-1246, miR-320c, and miR-193a-5p levels), and hence promote chondrogenesis [60]. The most obvious chondrogenic event due to miR-320c exosomal is controlled by the up-regulation of SOX9 and the down-regulation of MMP13 metalloproteinase [61,62].
3.2. Therapeutic Potential of Exosomes in Myocardial Diseases
Exosomes play a crucial participatory role in the pathophysiological pathway of the cardiovascular system, and can become effective targets for clinical applications [63]. Vascular endothelial dysfunction is a critical primary mechanism at the onset of atherosclerosis (AS), and intercellular communication has been identified as a significant part of the pathological process [64,65]. First, endothelial cells (ECs) can express vesicles, as well as absorbing vesicles derived from EC under oxidative pressure, reducing the production of nitric oxide (NO) that has a protective role [66]. Furthermore, it was observed in a previous study that endothelial microbes of coronary artery disease (CAD) were diffused in patients containing miR-92a-3p; a fine vesicle in selected CAD patients, largely controlled by vesicular performance and internal integrity [67]. Similarly, atherosclerotic stimuli as IL-6 or ox-LDL (oxidized low-density lipoprotein) that specifically enhance miR-92a-3p encapsulation in EMV, and EMVs mediated with EMV-3p eventually strengthen the vascular responses in targeted ECs through a THBS1-based mechanism (thrombospondin 1) [68]. Additionally, the information exchange between cracker and plaque cells involves the modification of the endothelial balance. In a previous study, for example, it was observed that AS endothelial apoptosis was caused by growth mass specificity following exosomal lncRNA GAS5 transfer from macrophages to ECs. Furthermore, endothelial apoptosis is initiated by exosomal lncRNA GAS5-overexpressing THP-1 cells while exosomal lncRNA GAS5 knockout cells inhibit this pathway [69]. Meanwhile, the krüppel-like factor 5 (KLF5) up-regulates ox-LDL in vascular smooth muscle cells (SMCs), resulting in endothelial dysfunction. Vascular remodeling is regulated by zinger fingers and KLF5, a known transcription factor. Studies have found that SMCs expressing KLF5 affect the endothelial functions by controlling multiple microRNAs. Of these multiple microRNAs, the most important of them is miR-155 (a known proinflammatory factor). The resulting exosomes produced by KLF5-overexpressing SMCs are well enriched with miR-155. Poor barrier function and weak reproductive capacity are expressed by the ECs [70,71]. Nevertheless, these effects can be prevented by miR-155 suppression or KLF5 disability. In addition, exosomes resulting from SMC (SMC-exos) enriched with miR-221/222 have been identified to interrupt autophagy of endothelial cells through the PTEN/Akt signaling mechanism making ECs more likely to stimulate atherosclerosis. Exosomes derived from platelets help to maintain the function of endothelial vasculature, but whether the modulatory effect is harmful or beneficial is still contested [72]. Some results suggest that the absorption of external platelets can lead to endothelial adhesion and programmed cell death, while others draw inconsistent conclusions [73]. For instance, platelet-exos enriched with miR-320 or miR-223 inhibited inflammation and EC mobility by reducing the appearance of ICAM-1 in EC recipients [74,75]. The reason for the discrepancies is related to the complexity of the packed charges in the platelets, especially the ubiquitin/protease system found in the outer platelets that stimulate the degradation of the inflammatory substances [76,77]. Exosomes from adipose-derived stem cells have been shown to express CD9 and CD29 proteins that protect cardiomyocytes during oxidative stress [22]. Furthermore, exosomes obtained from adipose tissue-derived Mesenchymal stem cells (ADMSCs) promoted cardiac function, particularly myocardium, by inhibiting cardiomyocytes apoptosis and promoting angiogenesis [32]. Finally, induced pluripotent stem cells (iPSCs) obtained from EVs ensure the cardioprotection of the cardiac cells with reference to the left ventricular function in vivo [33]. Prolonged cholesterol precipitation in macrophages leads to atheroma. Exosomes are believed to be incorporated in lipid modulation. Studies on neurodegenerative diseases have shown that the reproductive mechanism of cholesterol-enriched exosomes serves as a balance regulating the homeostasis of intracellular cholesterol. Finally, macrophage foam mediated by exosomes has gained a lot of attention in recent times [78,79].
3.3. Therapeutic Potential of Exosomes in Neural Diseases
Peripheral nerve injury (PNI) is a well-known neurological disorder that adversely affects human health. It affects about 2.8%–5% of patients with polytrauma resulting in sensory-motor dysfunction [80]. Many factors such as immune cell deposition and conversion of Schwann cell (SC) phenotypes lead to axonal development following PNI. It is estimated that the daily growth of regenerating axons is only 1 mm, which could be difficult to manage clinically [81]. Additionally, proximal nerves extended denervation may prolong the possibility of nervous organ irreversible atrophy [82]. Furthermore, nerve tissue damage associated with PNI increases the complexity of nerve regeneration [83,84]. Although the gold standard in the management of PNI is the transplantation of autologous nerves, this technique is rarely performed because there is a shortage of donor sources and possible neurological damage to the donor site [85]. Many studies have incorporated new techniques for starting axon rejuvenation without sacrificing the functions of healthy nerves. Another approach is to use synthetic nerve ducts with numerous stem cells that can be beneficial, but the results are far from ideal [86]. Interestingly, another strategy for the management of peripheral nerve regeneration is the use of exosomes derived from MSC. Another study examined MSC use by modifying them to increase bioactive molecular levels which will promote PNI recovery. Furthermore, the in vitro internalization of MSC exosomes by SCs leads to a significant increase in SC proliferation and subsequently leading to axonal regeneration [87]. The nerve growth factor (NGF), fibroblast growth factor-1 (FGF-1), glial cell-derived neurotrophic factor (GDNF), insulin-like growth factor-1 (IGF-1), and brain-derived neurotrophic factor (BDNF) are some of the multiple neurotrophic causes of MSC exosomes. Nevertheless, MSC exosomes have beneficial effects which include promoting recovery from neural survival, denervation muscle atrophy, and axonal development by the use of bioactive molecules [88].
It has been demonstrated previously that axonal regeneration is controlled by macrophages and not by SCs [89]. Immune cells, primarily phagocytic neutrophils, and macrophages are the first to migrate to the degenerative site within hours or days after peripheral nerve damage, whether caused by a neurological disorder or infection. The critical role of neuroinflammation in PNI recovery cannot be overemphasized, and it starts with a process called Wallerian degeneration (WD). In this process, myelinating SCs undergo differentiation and are mainly seen in the distal portion of the nerve [90]. Thereafter, proinflammatory cytokines and chemokines are produced by differentiated SCs resulting in activation of the neuroinflammatory event. Neuroinflammatory response leads to the hiring of peripheral immune cells and circulating macrophages to the affected site [91]. The combination of resident and circulating macrophages activate the clearance of axonal debris and myelin. It is worthwhile to note here that damaged axons must be cleared of debris and myelin because they contain molecules that exert an inhibitory effect on the development of axons, most especially at the late stages of WD [92]. At the distal stump and neuronal cell body, macrophages and other phagocytic cells precipitate to promote lesion response which is the mechanism adopted by neurons to activate regeneration. Nonetheless, neuroinflammation is a double-edged sword in this scenario as it is a critical response for nerve regeneration, but the excess of it prevents nerve regeneration as well as the incorporation of pain in the neuropathic events. An important goal in PNI management is the creation of a microenvironment allowing a reasonable level of inflammation and regeneration [93]. During peripheral nerve repair, the rebuilding of the vascular network is a vital microenvironment that promotes regeneration and axonal development. Therefore, the integrity of the vascular network plays a key role in peripheral nerve regeneration and a therapeutic option in PNI management [94]. Insults to the neural tissue caused by spinal cord injury increase the number of complement factors. Bone Mesenchymal stem cells (BMSCs) inhibit the levels of C4–C6 as well as C4 binding proteins and complement factor H in rats [21]. Furthermore, the application and use of neural stem cell-derived extracellular vesicles significantly reduce the severity and extent of spinal cord injury by reducing neuronal apoptosis, activation of microglia, and neuroinflammation in rats [23].
Interestingly, MSC exosomes promote angiogenesis through paracrine activation leading to vascular remodeling. Through the P13K/AKT signaling pathway, exosomes are induced by the pluripotent Mesenchymal stem cells which activate local angiogenesis. [95]. Furthermore, vascular plasticity is through MSC exosomes and endothelial cell-transferred proangiogenic miRNAs. Additionally, other studies observed activation of angiogenesis by MSC-derived exosomes decreasing neurological deficits [96]. Intravenous injection of MSC-derived exosomes activates neurite remodeling, neurogenesis, and angiogenesis leading to functional recovery [52]. The same effects were explained in another study in which MSC exosomes decreased inflammation in rat models by activating the endogenous angiogenesis including neurogenesis [95]. In summary, reports from all studies revealed that MSC-derived exosomes are likely to be regulators by promoting communication with vascular endothelial cells thereby enhancing vascular plasticity after nerve injury [96]. Nanoparticles provide beneficial therapeutic effects such as those enhanced by MSC and may be a promising adjuvant for treatment due to the inherent advantages they possessed over the MSCs [97]. MSC-derived exosomes play an important role in the intercellular communication [80] by utilizing a number of neurotrophic factors, protein, and genetic materials to axons. The introduction of MSC-derived exosomes can inhibit the problems arising from stem cell transplantation [86,98].
3.4. Therapeutic Potential of Exosomes in Soft Tissue
Adipose-derived stem cell released exosomes (ADSC-Exos) have a wide range of potential therapeutic potentials and have applications in regenerative engineering, tumor disorders, and skin repairs. Previous studies have reported the applications of naturally derived exosomes in diverse disease models with modifications [54,99]. However, recent researches have brought to light the contents and characteristics of exosomes with adjustments achieved with the use of other substances. The ADSC-Exos have important functions in biological and pathological pathways [100]. However, their applications in clinics are yet to be identified and research is currently ongoing, including the therapeutic doses used. Nevertheless, it was found that the ideal dose was 50 μg/mL when using exosomes for wound healing, but in a separate experiment, ADSC-Exos were used at a dose of 40 μg/mL and effectively enabled adipogenic differentiation [101]. It was observed that several intra-articular injections of human MSC-derived exosome have been shown to promote cartilage repair and regeneration [32]. After 12 weeks of treatment, accelerated tissue filling and increased collagen synthesis were observed in osteoarthritis rat lesions leading to the restoration of cartilage and bone [32].
Regeneration and cutaneous healing are complex mechanisms and need a well-orchestrated integration of many molecular and biological processes, including differentiation, proliferation, cell migration, extracellular matrix deposition, and apoptosis [102]. ADSC-Exos have been found to solve problems mainly resulting from excessive scar formation and delayed cutaneous healing [103]. The internalization of ADSC-Exos by fibroblasts leads to proliferation and migration of collagen type I and III increasing via the PI3K/Akt signaling process. Furthermore, ADSC-Exos could induce migration, proliferation as well as suppressing HaCaT cell apoptosis through the signaling of Wnt/β-catenin [104]. All findings explained the promising potentials of ADSC-Exos in promoting cutaneous wound healing [105]. In ADSC-Exo treated mice, scar formation and clear zone of re-epithelialization were promoted, and the scar areas became narrower in the wounds. Furthermore, ADSC-Exosome could enhance the reconstruction of the extracellular matrix in the regeneration of cutaneous wound by modulating collagen type I: type III proportions, and also transforming growth factor-beta 3 (TGF-β3): TGF-β1 and MMP3: metalloproteinases 1 (TIMP1) tissue inhibitor, and inhibiting the fibroblasts differentiation into myofibroblasts decreasing scar formation [106]. Furthermore, it was also observed that the use of ADSC-Exos leads to the attenuation of the necrotic flap, neovascularization activation, and alleviation of inflammatory reaction after I/R disorder skin flap apoptosis [103]. In summary, it highlights the potentials of ADSC-Exo use in promoting cutaneous regeneration and complete healing. However, the molecular mechanism will need to be evaluated for clinical applications [36].
3.5. Therapeutic Potential of Exosomes in Obesity
In obese individuals, inflammation resulting from adipose tissue has been documented as the main factor in the progression of type 2 diabetes and insulin resistance [107]. Researchers have identified that ADSCs have key roles in metabolic diseases and inflammation resulting from obesity. ADSC-Exos were explained to affect diabetes and obesity. In white adipose tissue (WAT), ADSC-Exos were shown to dominate the anti-inflammatory (M2) macrophage phenotype polarization and regulation of immune homeostasis. In WAT obese mice, exosomes have been observed to promote polarization of the M2 macrophage and inflammation prevention [55,108]. Of note, M2 macrophages resulted from ADSC-Exos exerting high levels of tyrosine hydroxylase potentiating the production of catecholamine, which promotes brown adipose tissue-specific uncoupling protein 1 expression in WAT which activates fat burning leading to energy reserve dissipation. This observation highlights the indispensable exosome-mediated crosstalk existing between macrophages and ADSCs and attracts attention to the potential role of an exosome-based treatment approach to treat obesity [109].
3.6. Therapeutic Potential of Exosomes in Autoimmune Disease
The beneficial roles of exosomes in immune tolerance and stimulation cannot be overemphasized as they are involved in several pathways such as angiogenesis, inflammation, and immune signaling. Exosomes have also acted as good tools for drug delivery due to their inherent biological features such as biocompatibility and stability including the capacity for permeation [110]. Sjögren’s syndrome (SS) is an important autoimmune disorder characterized by lymphocytic infiltration in the salivary and lacrimal glands as well as the existence of numerous autoantibodies. These autoantibodies include anti-La (SS-B) or anti-Ro (SS-A)) with clinical syndromes such as ocular and oral dryness [111]. SS pathogenesis involves both innate and adaptive immune mechanisms such as NF-kB signaling, interferon (IFN) signatures, and B cell-activating factor (BAFF)/BAFF receptor axis [112]. Furthermore, salivary gland epithelial cells (SGECs) play an important role in both autoimmune and inflammatory responses in SS by inducible expression of different immunoreactive factors such as autoantigenic ribonucleoproteins (RNPs), many Toll-like receptors (TLRs), and BAFF. Lymphocytic infiltration, mainly B and CD4+ T cells, occurs and invades epithelial cells. This highlights the correlation between immune and epithelial cells [110]. A previous study documented the continuous release of autoantigenic exosomes La/SS-B, Sm RNPs, and Ro/SS-A by SGECs, documenting the transfer of intracellular autoantigens to autoreactive lymphocytes within RNP-containing exosomes [60]. It was also observed that EBV-miRBART13-3p in exosomes is transferrable from B cells to SGECs in EBV infected B cells. The stromal interacting molecule 1 (STIM1) and aquaporin 5 (AQP5) targeted by functional miRNA could significantly affect salivary secretion [12]. Recently, it was observed that subconjunctival administration of MSC-Exos to rabbits inhibited lacrimal gland inflammation in the affected group compared with the control group administered normal saline. It was concluded that MSC-Exo effects were partly due to the polarization of the lacrimal macrophage as well as the responses of Treg and Th2 by NF-kB signaling pathways. Thus, MSC-Exos may be useful in the treatment of dry eye [113]. Experimental autoimmune uveoretinitis (EAU) could be effectively resolved by the use of bone marrow-derived MSC-Exos [114]. It was observed that in experimentally induced autoimmune uveitis in rats, human-derived Mesenchymal stem cell-derived exosomes inhibited the migration of inflammatory cells, mainly T cell subsets, in the eyes. Additionally, the MSC-Exo inhibited the chemo attractive effects of CCL2 and CCL21 on inflammatory cells [45]. Another experimental study conducted on EAU found that human umbilical cord-derived MSC-Exos (hUC-MSC-Exos) did not inhibit the progression of conA-stimulated T cells, but suppressed inflammatory cell migration [115]. The suppressive effect of hUC-MSC-Exos on the interphotoreceptor retinoid-binding protein (IRBP)-specific Th17 responses via the regulation of DC-derived Th17-polarizing cytokines IL-1β, IL-6, and IL-23 led to the suppression of DC-driven Th17 responses [116]. Thus, it is clear that MSC-Exos can play a role in the treatment of auto-immune uveitis, however, further trials are essential to identify their immunomodulatory and anti-inflammatory effects [112].
3.7. Therapeutic Potential of Exosomes in Infectious (Parasitic and Bacterial) Conditions
Parasites secrete exosomes that interact effectively, as a medium of cell to cell communication, within their host. Logically, it is most probable that the host utilizes this pathway of cellular communication as a defense mechanism [117]. Using plasma cell-derived microvesicles, it was observed that Plasmodium berghei induces the production of CD40 in antigen-presenting cells, leading to generation of a potent inflammatory response and subsequent clearance of the parasite by macrophage activation [118]. Furthermore, immune cell-derived microvesicles from Plasmodium vivax, one of the species of Plasmodium responsible for human malaria infection, was associated with acute inflammation in the course of parasite eradication [119]. Additionally, a massive increase and release of antimicrobial peptide-containing exosomes was observed in intestinal epithelial cells in response to infection with Cryptosporidium [120].
Exosomes are also useful in vaccines and vaccination. In a vaccine trial using Leishmania major-pulsed DC exosomes, it was observed that the DC-derived exosomes mediate protective Th1 immunity against cutaneous leishmaniasis [121]. Similarly, avian coccidiosis in poultry caused by Eimeria species (E. tenella, E. acervulina, E. maxima) can be alleviated using Eimeria parasite antigen-loaded DC exosomes which successfully alleviated clinical signs in poultry thereby reducing mortality rates [122].
With regards to bacterial infection, exosomes derived from the causative agent of bovine tuberculosis, Mycobacterium bovis-infected macrophages promote dendritic cell (DC) activation and the generation of antibacterial T cell response in vivo [47]. Similarly, Mycobacterium tuberculosis (human tuberculosis), induces the release of exosomes from infected macrophages with consequent recruitment of lymphocytes through the secretion of chemokine such as RANTES and MIP- 1α [123].
3.8. Therapeutic Potential of Exosomes in Lung Diseases
Bronchopulmonary dysplasia (BPD) is one of several conditions that could affect the lungs. BPD is a chronic disorder of the lungs in preterm infants [124]. Current therapeutic approaches using conventional therapy have only modest outcomes. The use of MDC-exosome therapeutics appears to be promising in clinical management, as shown in experimental models by alleviating neonatal lung injury [124]. Transplantation of various stem cell types—such as human amnion epithelial cells and endothelial colony-forming cells—appears to be promising with results from the preclinical models. MSC treatment of blunt hyperoxia-induced lung inflammation ameliorates vascular remodeling and improves exercise capacity with higher survival rates [46,125]. Furthermore, using allogeneic human umbilical cord blood-derived MSC in phase 1 clinical trials, it was observed that MSC administration brought about a reduction in inflammatory markers and lowered BPD severity [126]. The mechanism for now remains unknown. Furthermore, using bone marrow stromal cells (BMSCs) in a murine model with neonatal chronic lung disease, the BMSCs release immunomodulatory factors which ameliorated parenchymal and vascular injury of BPD in vivo [46]. Additionally, intratracheal distribution of BMSCs in a murine model with BPD, improved survival and exercise tolerance by attenuating alveolar and lung vascular injury as well as pulmonary hypertension in vivo, while in vitro, the BMSC-derived conditioned medium prevented O2-induced AEC2 apoptosis, boosted endothelial cord formation, and enhanced AEC2 wound healing [125].
3.9. Therapeutic Potential of Exosomes in Liver Diseases
Exosome-based therapeutic methods for the management of different kind of diseases of the liver, including liver tumor, is progressing. Exosomes obtained from adult human liver stem cells (HLSC) were shown to inhibit the growth and survival of HepG2 and primary hepatocellular carcinoma (HCC) in vitro via the use of antitumor miRNAs [127]. Furthermore, an in vivo experiment in a murine model showed accelerated morphological and functional gains of the liver despite 70% hepatectomy as well as proliferation and apoptosis resistance of rat and human hepatocytes in vitro [128]. Additionally, exosomes obtained from human umbilical cord Mesenchymal stem cells ameliorated fibrosis of the liver induced by carbon tetrachloride (CCl4) [129]. It was demonstrated in vivo in mice that immunodeficient NOD/SCID mice with their liver engrafted with human hepatoma cells produce exosomes containing exogenous siRNA which shuttles between hepatic cells and subsequently produces CD81 siRNA [129].
4. Conclusions
Recently, exosomes are considered as significant mediators in intercellular communication. The exosomes’ ability to transport DNA, proteins, non-coding RNAs, and mRNA, makes them an attractive target of research on the pathogenesis of several illnesses, including cardiovascular and autoimmune disease, cancer, obesity, etc. MSC and other cell type-derived exosomes play an important role in intercellular communication by using several neurotrophic factors, protein, and genetic materials to axons, and they can inhibit the issues resulting from stem cell transplantation. The involvement of exosomes in both biological and pathobiological functions makes them an interesting organelle, and hence it is interesting to study their potential biological applications in the treatment of diverse diseases and offer new possibilities in the amelioration and possible cure of some infectious and non-infectious diseases. Furthermore, results from both clinical and experimental studies on MSC-derived exosomes have shown their potential in the treatment of diverse diseases. However, future research must concentrate on improving the methods for the isolation of exosomes and other EVs to distinguish them appropriately, as well as their mechanism of action, before they are used in clinical studies. Success in this regard will promote their prompt application in the diagnosis, prevention, and treatment of diseases.
Author Contributions
Conceptualization, A.M.B., G.E.-S.B., and T.E.O.; methodology, A.M.B., G.E.-S.B., N.R.-P., and A.Z.-B.; software, A.M.B., and G.E.-S.B.; validation, A.M.B., G.E.-S.B., T.E.O., N.R.-P., H.F.H., M.Z., S.A., and A.Z.-B.; formal analysis G.E.-S.B., and T.E.O.; investigation, A.M.B., G.E.-S.B., T.E.O., N.R.-P., H.F.H., M.Z., S.A., and A.Z.-B..; data curation, A.M.B., G.E.-S.B., M.A.G., and A.K.M.; writing—original draft preparation, A.M.B., T.E.O., and G.E.-S.B.; writing—review and editing, A.M.B., T.E.O., G.E.-S.B., N.R.-P., and A.Z.-B.; visualization, A.M.B., G.E.-S.B., N.R.-P., A.Z.-B., M.A.E.-E.; supervision, A.M.B., G.E.-S.B., and H.F.H.; funding acquisition, S.A., H.F.H., and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Graphical abstract and Figure 1 were made using biorender.com.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| EV: | extracellular vesicle |
| miRNA: | microRNA |
| mRNA: | messenger RNA |
| TEX: | Tumor-derived exosomes |
| PD-L1: | programmed death-ligand 1 |
| IFN-γ: | interferon-γ |
| MVEs: | multivesicular endosomes |
| MVB: | multivesicular body |
| ILVs: | intraluminal vesicles |
| TEMs: | tetraspanin-enriched microdomains |
| HGF: | hepatocyte growth factor |
| MSCs: | mesenchymal stem cells |
| TGF-β1: | transforming growth factor-β1 |
| TCF4: | human T-cell factor 4 |
| IL-8: | interleukin-8 |
| ECs: | endothelial cells |
| NF-κB: | nuclear factor-kappa B |
| CAD: | coronary artery disease |
| ox-LDL: | oxidized low-density lipoprotein |
| NO: | nitric oxide |
| KLF5: | krüppel-like factor 5 |
| SMCs: | smooth muscle cells |
| PNI: | Peripheral nerve injury |
| SCs: | Schwann cells |
| NGF: | nerve growth factor |
| FGF-1: | fibroblast growth factor-1 |
| GDNF: | glial cell-derived neurotrophic factor |
| IGF-1: | insulin-like growth factor-1 |
| BDNF: | brain-derived neurotrophic factor |
| WD: | Wallerian degeneration |
| TGF-β3: | transforming growth factor-beta 3 |
| TIMP1: | tissue inhibitor metalloproteinases 1 |
| WAT: | white adipose tissue; IFN: interferon |
| BAFF: | B cell-activating factor |
| SGECs: | salivary gland epithelial cells |
| RNPs: | ribonucleoproteins |
| TLRs: | Toll-like receptors |
| STIM1: | Stromal interacting molecule 1 |
| AQP5: | aquaporin 5 |
| hUC-MSC-Exos: | human umbilical cord-derived MSC-Exos |
| IRBP: | interphotoreceptor retinoid-binding protein |
References
- Bonucci, E. Fine structure of early cartilage calcification. J. Ultrastruct. 1967, 20, 33–50. [Google Scholar] [CrossRef]
- Anderson, H.C. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J. Cell Boil. 1969, 41, 59–72. [Google Scholar] [CrossRef]
- Trams, E.G.; Lauter, C.J.; Salem, J.N.; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta Biomembr. 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Lawson, C.; Vicencio, J.M.; Yellon, D.M.; Davidson, S.M. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J. Endocrin. 2016, 228, R57–R71. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, B.J.; Gu, L.; Sims, B.; Matthews, Q.L. Exosome biogenesis and biological function in response to viral infections. Open Virol. J. 2018, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Qasim, M.; Kim, J.-H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Ali, H.M.; Alayafi, A.A.; Witczak, J.; Ahmad, M. Genetic variation and alleviation of salinity stress in barley (Hordeum vulgare L.). Molecules 2018, 23, 2488. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A.; Witczak, J.; Ahmad, M. Analysis of genetic variation and enhancement of salt tolerance in French pea (Pisum Sativum L.). Intern. J. Molec. Sci. 2018, 19, 2433. [Google Scholar] [CrossRef]
- Tung, K.H.; Ernstoff, M.S.; Allen, C.; La Shu, S. A Review of Exosomes and their Role in The Tumor Microenvironment and Host–Tumor “Macroenvironment”. J. Immunol. Sci. 2019, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Exosomes and tumor-mediated immune suppression. J. Clin. Investig. 2016, 126, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Raposo, G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles 2013, 2, 20389. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, F.; Zhang, J.; Zhang, Q.; Lin, J. Exosome analysis: a promising biomarker system with special attention to saliva. J. Membr. Boil. 2014, 247, 1129–1136. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Boil. 2014, 29, 116–125. [Google Scholar] [CrossRef]
- Record, M.; Subra, C.; Silvente-Poirot, S.; Poirot, M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem. Pharmacol. 2011, 81, 1171–1182. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Hsu, C.; Morohashi, Y.; Yoshimura, S.-i.; Manrique-Hoyos, N.; Jung, S.; Lauterbach, M.A.; Bakhti, M.; Grønborg, M.; Möbius, W.; Rhee, J. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A–C. J. Cell Biol. 2010, 189, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Babst, M. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr. Opin. Cell Boil. 2011, 23, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, D.; Gutiérrez-Vázquez, C.; Jorge, I.; López-Martín, S.; Ursa, A.; Sánchez-Madrid, F.; Vázquez, J.; Yáñez-Mó, M. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J.Biol.Chem. 2013, 288, 11649–11661. [Google Scholar] [CrossRef]
- Tancini, B.; Buratta, S.; Sagini, K.; Costanzi, E.; Delo, F.; Urbanelli, L.; Emiliani, C. Insight into the role of extracellular vesicles in lysosomal storage disorders. Genes 2019, 10, 510. [Google Scholar] [CrossRef]
- Wang, Y.; He, G.; Guo, Y.; Tang, H.; Shi, Y.; Bian, X.; Zhu, M.; Kang, X.; Zhou, M.; Lyu, J.; et al. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J. Cell Mol. Med. 2019, 23, 5475–5485. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, W.; Wang, J.; Fan, J.; Luo, Y.; Li, L.; Kong, F.; Chen, J.; Tang, P.; Cai, W. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019, 10, 340. [Google Scholar] [CrossRef]
- Xiong, L.; Sun, L.; Zhang, Y.; Jin, P.; Yan, J.; Liu, X. Exosomes from bone marrow mesenchymal stem cells can alleviate early brain injury following subarachnoid hemorrhage through miRNA129-5p-HMGB1 pathway. Stem Cells Dev. 2019. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, X.; Qiu, J.; Xin, D.; Li, T.; Chu, X.; Yuan, H.; Wang, H.; Wang, Z.; Wang, D. Exosomes Derived From Bone Marrow Mesenchymal Stem Cells Inhibit Complement Activation In Rats With Spinal Cord Injury. Drug Des. Dev. Ther. 2019, 13, 3693–3704. [Google Scholar] [CrossRef]
- Lankford, K.L.; Arroyo, E.J.; Nazimek, K.; Bryniarski, K.; Askenase, P.W.; Kocsis, J.D. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS ONE 2018, 13, e0190358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chu, W.; Lai, R.; Lim, S.; Hui, J.; Toh, W. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, Y.; Wan, Y.; Gao, J.; Chu, Y.; Li, J. Exosomes from adipose-derived mesenchymal stem cells prevent cardiomyocyte apoptosis induced by oxidative stress. Cell Death Discov. 2019, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ma, Y.; Wang, F.; Hu, L.; Sun, Y. miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res. Ther. 2019, 10, 360. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Z.; Liu, L.; Zhang, B.; Li, B. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J. Cell. Biochem. 2019. [Google Scholar] [CrossRef]
- Atay, S.; Banskota, S.; Crow, J.; Sethi, G.; Rink, L.; Godwin, A.K. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc. Natl. Acad. Sci. USA 2014, 111, 711–716. [Google Scholar] [CrossRef]
- Thomi, G.; Surbek, D.; Haesler, V.; Joerger-Messerli, M.; Schoeberlein, A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res. Ther. 2019, 10, 105. [Google Scholar] [CrossRef]
- Yan, L.; Wu, X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Boil. Toxicol. 2019. [Google Scholar] [CrossRef]
- Yu, T.; Zhao, C.; Hou, S.; Zhou, W.; Wang, B.; Chen, Y. Exosomes secreted from miRNA-29b-modified mesenchymal stem cells repaired spinal cord injury in rats. Braz. J. Med Boil. Res. 2019, 52, e8735. [Google Scholar] [CrossRef]
- Li, J.; Ju, Y.; Liu, S.; Fu, Y.; Zhao, S. Exosomes derived from lipopolysaccharide-preconditioned human dental pulp stem cells regulate Schwann cell migration and differentiation. Connect. Tissue Res. 2019, 1–10. [Google Scholar] [CrossRef]
- Bruno, S.; Tapparo, M.; Collino, F.; Chiabotto, G.; Deregibus, M.C.; Soares Lindoso, R.; Neri, F.; Kholia, S.; Giunti, S.; Wen, S. Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng. Part A 2017, 23, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Liu, L.; Chen, J.; Liu, F. Exosomes derived from human umbilical cord blood mesenchymal stem cells improve hepatic ischemia reperfusion injury via delivering miR-1246. Cell Cycle. 2019, 18, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- El Harane, N.; Kervadec, A.; Bellamy, V.; Pidial, L.; Neametalla, H.J.; Perier, M.-C.; Lima Correa, B.; Thiébault, L.; Cagnard, N.; Duché, A. Acellular therapeutic approach for heart failure: in vitro production of extracellular vesicles from human cardiovascular progenitors. Europ. Heart J. 2018, 39, 1835–1847. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.-T.; Skupien-Rabian, B.; Chen, X. Induced pluripotent stem cell (iPSC)–derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Shao, H.; Wang, H.; Zhang, Z.; Su, C.; Dong, L.; Yu, B.; Chen, X.; Li, X.; Zhang, X. Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci.Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Aslam, M.; Baveja, R.; Liang, O.D.; Fernandez-Gonzalez, A.; Lee, C.; Mitsialis, S.A.; Kourembanas, S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Amer. J. Resp. Crit. Care Med. 2009, 180, 1122–1130. [Google Scholar] [CrossRef]
- Giri, P.K.; Schorey, J.S. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS ONE 2008, 3, e2461. [Google Scholar] [CrossRef]
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013, 22, 845–854. [Google Scholar] [CrossRef]
- Das, C.K.; Jena, B.C.; Banerjee, I.; Das, S.; Parekh, A.; Bhutia, S.K.; Mandal, M. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol. Pharm. 2018, 16, 24–40. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- Pullan, J.E.; Confeld, M.I.; Osborn, J.K.; Kim, J.; Sarkar, K.; Mallik, S. Exosomes as drug carriers for cancer therapy. Mol. Pharmac. 2019, 16, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Merino-González, C.; Zuñiga, F.A.; Escudero, C.; Ormazabal, V.; Reyes, C.; Nova-Lamperti, E.; Salomón, C.; Aguayo, C. Mesenchymal stem cell-derived extracellular vesicles promote angiogenesis: potencial clinical application. Front. Physiol. 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Rao, S.-S.; Wang, Z.-X.; Cao, J.; Tan, Y.-J.; Luo, J.; Li, H.-M.; Zhang, W.-S.; Chen, C.-Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Heldring, N.; Mäger, I.; Wood, M.J.; Le Blanc, K.; Andaloussi, S.E. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum. Gene Ther. 2015, 26, 506–517. [Google Scholar] [CrossRef]
- Burke, J.; Hunter, M.; Kolhe, R.; Isales, C.; Hamrick, M.; Fulzele, S. Therapeutic potential of mesenchymal stem cell based therapy for osteoarthritis. Clin. Transl. Med. 2016, 5, 27. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef]
- Toh, W.S.; Lai, R.C.; Hui, J.H.P.; Lim, S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin. Cell Dev. Boil. 2017, 67, 56–64. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Xiao, K.; Xiang, S.; Li, Z.; Weng, X. Emerging role of exosomes in the joint diseases. Cell. Physiol. Biochem. 2018, 47, 2008–2017. [Google Scholar] [CrossRef]
- Huang, L.; Ma, W.; Ma, Y.; Feng, D.; Chen, H.; Cai, B. Exosomes in mesenchymal stem cells, a new therapeutic strategy for cardiovascular diseases? Int. J. Boil. Sci. 2015, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Park, S.-R.; Jung, B.-K.; Jeon, Y.-K.; Lee, Y.-S.; Kim, M.-K.; Kim, Y.-G.; Jang, J.-Y.; Kim, C.-W. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PloS ONE 2013, 8, e84256. [Google Scholar] [CrossRef] [PubMed]
- Ailawadi, S.; Wang, X.; Gu, H.; Fan, G.-C. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochem. Biophys. Res. Commun. 2015, 1852, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Y.; Pan, Y.; Zhang, L.; Shen, C.; Qin, G.; Ashraf, M.; Weintraub, N.; Ma, G.; Tang, Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem. Biophys. Res. Commun. 2013, 431, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiang, M.; Meng, D.; Sun, N.; Chen, S. Inhibition of myocardial ischemia/reperfusion injury by exosomes secreted from mesenchymal stem cells. Stem Cells Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- de Jong, O.G.; Verhaar, M.C.; Chen, Y.; Vader, P.; Gremmels, H.; Posthuma, G.; Schiffelers, R.M.; Gucek, M.; van Balkom, B.W. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J. Extracell. Vesicles 2012, 1, 18396. [Google Scholar] [CrossRef]
- Hu, G.; Drescher, K.M.; Chen, X. Exosomal miRNAs: biological properties and therapeutic potential. Front. Genet. 2012, 3, 56. [Google Scholar] [CrossRef]
- Gambim, M.H.; Do Carmo, A.D.O.; Marti, L.; Veríssimo-Filho, S.; Lopes, L.R.; Janiszewski, M. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit. Care 2007, 11, R107. [Google Scholar] [CrossRef]
- Suzuki, E.; Fujita, D.; Takahashi, M.; Oba, S.; Nishimatsu, H. Stem cell-derived exosomes as a therapeutic tool for cardiovascular disease. World J. Stem Cells 2016, 8, 297. [Google Scholar] [CrossRef]
- Bakogiannis, C.; Sachse, M.; Stamatelopoulos, K.; Stellos, K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine 2019, 122, 154157. [Google Scholar] [CrossRef]
- Hafiane, A.; Daskalopoulou, S.S. Extracellular vesicles characteristics and emerging roles in atherosclerotic cardiovascular disease. Metabolism 2018, 85, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, W.; Guo, Y.; Chen, W.; Zheng, P.; Zeng, J.; Tong, W. Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. PLoS ONE 2017, 12, e0185406. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.-R.; Liao, M.-F.; Wang, M.-h.; Cheng, C.-M.; Chen, C.-H. Mesenchymal stem cell derived exosomes: a new hope for the treatment of cardiovascular disease? Acta Cardiol. Sin. 2014, 30, 395. [Google Scholar] [PubMed]
- Vella, L.J.; Sharples, R.A.; Nisbet, R.M.; Cappai, R.; Hill, A.F. The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur. Biophys. J. 2008, 37, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.; Sun, H.; Sakai, S.; Mitsutake, S.; Okada, M.; Tahara, H.; Furukawa, J.-i.; Fujitani, N.; Shinohara, Y.; Igarashi, Y. Decreased amyloid-β pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J. Biol. Chem. 2014, 289, 24488–24498. [Google Scholar] [CrossRef]
- Wang, X.; Huang, W.; Liu, G.; Cai, W.; Millard, R.W.; Wang, Y.; Chang, J.; Peng, T.; Fan, G.-C. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J. Mol. Cell. Cardiol. 2014, 74, 139–150. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.-j.; Fine, D.; Schmulen, J.; Hu, Y.; Godin, B.; Zhang, J.X.; Liu, X. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab. Chip. 2013, 13, 2879–2882. [Google Scholar] [CrossRef]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2011, 40, D1241–D1244. [Google Scholar] [CrossRef]
- Chivet, M.; Hemming, F.; Fraboulet, S.; Sadoul, R. Emerging role of neuronal exosomes in the central nervous system. Front. Physiol. 2012, 3, 145. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Usuki, S.; Sakai, S.; Hanamatsu, H.; Mioka, T.; Kimura, N.; Okada, M.; Tahara, H.; Furukawa, J.-i. A potential function for neuronal exosomes: Sequestering intracerebral amyloid-β peptide. FEBS lett. 2015, 589, 84–88. [Google Scholar] [CrossRef]
- Frühbeis, C.; Fröhlich, D.; Krämer-Albers, E.-M. Emerging roles of exosomes in neuron–glia communication. Front. Physiol. 2012, 3, 119. [Google Scholar] [CrossRef] [PubMed]
- An, K.; Klyubin, I.; Kim, Y.; Jung, J.H.; Mably, A.J.; T O’Dowd, S.; Lynch, T.; Kanmert, D.; Lemere, C.A.; Finan, G.M. Exosomes neutralize synaptic-plasticity-disrupting activity of Aβ assemblies in vivo. Molec. Brain. 2013, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Pusic, A.D.; Kraig, R.P. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia 2014, 62, 284–299. [Google Scholar] [CrossRef]
- Hamlett, E.D.; Goetzl, E.J.; Ledreux, A.; Vasilevko, V.; Boger, H.A.; LaRosa, A.; Clark, D.; Carroll, S.L.; Carmona-Iragui, M.; Fortea, J. Neuronal exosomes reveal Alzheimer’s disease biomarkers in Down syndrome. Alzheimer’s Dement. 2017, 13, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Katakowski, M.; Wang, F.; Qian, J.-Y.; Liu, X.S.; Ali, M.M.; Buller, B.; Zhang, Z.G.; Chopp, M. MicroRNA-17–92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 2017, 48, 747–753. [Google Scholar] [CrossRef]
- Ching, R.C.; Kingham, P.J. The role of exosomes in peripheral nerve regeneration. Neural Regen. Res. 2015, 10, 743. [Google Scholar]
- Dong, R.; Liu, Y.; Yang, Y.; Wang, H.; Xu, Y.; Zhang, Z. MSC-Derived Exosomes-Based Therapy for Peripheral Nerve Injury: A Novel Therapeutic Strategy. BioMed Res. Int. 2019, 2019, 6458237–6458312. [Google Scholar] [CrossRef]
- Mahmood, A.; Lu, D.; Chopp, M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J. Neurotrauma. 2004, 21, 33–39. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflamm. 2011, 8, 110. [Google Scholar] [CrossRef]
- Glenn, T.D.; Talbot, W.S. Signals regulating myelination in peripheral nerves and the Schwann cell response to injury. Curr. Opin. Neurol. 2013, 23, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, L.I.; Popovich, P.G. Inflammation and axon regeneration. Curr. Opin. Neurol. 2011, 24, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Fugleholm, K. The surgery of peripheral nerves (including tumors). In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 115, pp. 781–802. [Google Scholar]
- De Albornoz, P.M.; Delgado, P.J.; Forriol, F.; Maffulli, N. Non-surgical therapies for peripheral nerve injury. Br. Med. Bull. 2011, 100, 73–100. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Niu, X.; Hu, B.; Chen, S.; Song, W.; Ding, J.; Zhang, C.; Wang, Y. Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int. J. Boil. Sci. 2017, 13, 232. [Google Scholar] [CrossRef]
- Hu, G.-w.; Li, Q.; Niu, X.; Hu, B.; Liu, J.; Zhou, S.-m.; Guo, S.-c.; Lang, H.-l.; Zhang, C.-q.; Wang, Y. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res. Ther. 2015, 6, 10. [Google Scholar] [CrossRef]
- Lai, R.C.; Chen, T.S.; Lim, S.K. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen. Med. 2011, 6, 481–492. [Google Scholar] [CrossRef]
- Ludwig, A.-K.; Giebel, B. Exosomes: small vesicles participating in intercellular communication. Int. J. Biochem. Cell Boil. 2012, 44, 11–15. [Google Scholar] [CrossRef]
- Huang, C.-C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as biomimetic tools for stem cell differentiation: applications in dental pulp tissue regeneration. Biomaterials. 2016, 111, 103–115. [Google Scholar] [CrossRef]
- Qiu, H.; Liu, S.; Wu, K.; Zhao, R.; Cao, L.; Wang, H. Prospective application of exosomes derived from adipose-derived stem cells in skin wound healing: A review. J. Cosmet. Dermatol. 2019. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Orgill, D.; Murphy, G. The pathophysiologic basis for wound healing and cutaneous regeneration. In Biomaterials for Treating Skin Loss; Elsevier: Amsterdam, The Netherlands, 2009; pp. 25–57. [Google Scholar]
- Hong, P.; Yang, H.; Wu, Y.; Li, K.; Tang, Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res. Ther. 2019, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Fu, B.; Yang, X.; Xiao, Y.; Pan, M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J. Cell. Biochem. 2019, 120, 10847–10854. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Yu, M.; Zhang, Y.; Tian, W. Exosome-like vesicles derived from adipose tissue provide biochemical cues for adipose tissue regeneration. Tissue Eng. Part A. 2017, 23, 1221–1230. [Google Scholar] [CrossRef]
- Thoene; Koh; Nishida-Aoki; Sims; Heijnen; Arraud; Brisson; Yoshioka, Y.; Im; Ishihara; et al. Abstract Book: ISEV2017. J. Extracell. Vesicles. 2017, 6, 77–227. [Google Scholar]
- Wisse, B.E. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J. Am. Soc. Nephrol. 2004, 15, 2792–2800. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, M.; Tian, W. Physiological and pathological impact of exosomes of adipose tissue. Cell Prolif. 2016, 49, 3–13. [Google Scholar] [CrossRef]
- Lazar, I.; Clement, E.; Dauvillier, S.; Milhas, D.; Ducoux-Petit, M.; LeGonidec, S.; Moro, C.; Soldan, V.; Dalle, S.; Balor, S. Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: a novel mechanism linking obesity and cancer. Cancer Res. 2016, 76, 4051–4057. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, W.; Yang, F.; Yu, L.; Yu, Z.; Pan, J.; Wang, L.; Cao, X.; Wang, J. Immunosuppressive exosomes from TGF-β1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells. Cell Res. 2012, 22, 607–610. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Theander, E.; Baldini, C.; Seror, R.; Retamozo, S.; Quartuccio, L.; Bootsma, H.; Bowman, S.J.; Dörner, T.; Gottenberg, J.-E. Early diagnosis of primary Sjögren’s syndrome: EULAR-SS task force clinical recommendations. Expert Rev. Clin. Immunol. 2016, 12, 137–156. [Google Scholar] [CrossRef]
- Yang, C.; Robbins, P.D. Immunosuppressive exosomes: a new approach for treating arthritis. Int. J. Rheumatol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, L.; Wei, Y.; Ea, V.L.; Nian, H.; Wei, R. Recent advances of exosomes in immune-mediated eye diseases. Stem Cell Res. Ther. 2019, 10, 278. [Google Scholar] [CrossRef]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef]
- Tran, T.-H.; Mattheolabakis, G.; Aldawsari, H.; Amiji, M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin. Immun. 2015, 160, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, M.; Nagaeva, O.; Kargl, D.; Baranov, V.; Mincheva-Nilsson, L. Thermal-and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS ONE 2011, 6, e16899. [Google Scholar] [CrossRef]
- Coakley, G.; Maizels, R.M.; Buck, A.H. Exosomes and other extracellular vesicles: the new communicators in parasite infections. Trends Parasitol. 2015, 31, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Barnes, T.; Hafalla, J.C.; Combes, V.; Ryffel, B.; Secher, T.; Grau, G.E.; Riley, E.M.; de Souza, J.B. Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog. 2010, 6, e1000744. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.M.; Franklin, B.S.; Teixeira-Carvalho, A.; Agnaldo Filho, L.; de Paula, S.C.; Fontes, C.J.; Brito, C.F.; Carvalho, L.H. Augmented plasma microparticles during acute Plasmodium vivax infection. Malaria J. 2010, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Gong, A.-Y.; Roth, A.L.; Huang, B.Q.; Ward, H.D.; Zhu, G.; LaRusso, N.F.; Hanson, N.D.; Chen, X.-M. Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog. 2013, 9, e1003261. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, J.K.; Berzel, S.; Fajardo-Moser, M.; Remer, K.A.; Moll, H. Fragments of antigen-loaded dendritic cells (DC) and DC-derived exosomes induce protective immunity against Leishmania major. Vaccine 2010, 28, 5785–5793. [Google Scholar] [CrossRef]
- del Cacho, E.; Gallego, M.; Lee, S.H.; Lillehoj, H.S.; Quilez, J.; Lillehoj, E.P.; Sánchez-Acedo, C. Induction of protective immunity against Eimeria tenella, Eimeria maxima, and Eimeria acervulina infections using dendritic cell-derived exosomes. Infect. Immun. 2012, 80, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.P.; Smith, V.L.; Karakousis, P.C.; Schorey, J.S. Exosomes isolated from mycobacteria-infected mice or cultured macrophages can recruit and activate immune cells in vitro and in vivo. J. Immun. 2012, 189, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Willis, G.R.; Mitsialis, S.A.; Kourembanas, S. “Good things come in small packages”: application of exosome-based therapeutics in neonatal lung injury. Ped. Res. 2018, 83, 298–307. [Google Scholar] [CrossRef] [PubMed]
- van Haaften, T.; Byrne, R.; Bonnet, S.; Rochefort, G.Y.; Akabutu, J.; Bouchentouf, M.; Rey-Parra, G.J.; Galipeau, J.; Haromy, A.; Eaton, F. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am. J. Respir. Crit. Care Med. 2009, 180, 1131–1142. [Google Scholar] [CrossRef]
- Chang, Y.S.; Ahn, S.Y.; Yoo, H.S.; Sung, S.I.; Choi, S.J.; Oh, W.I.; Park, W.S. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J. Pediatrics 2014, 164, 966–972. e966. [Google Scholar] [CrossRef]
- Fonsato, V.; Collino, F.; Herrera, M.B.; Cavallari, C.; Deregibus, M.C.; Cisterna, B.; Bruno, S.; Romagnoli, R.; Salizzoni, M.; Tetta, C. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells 2012, 30, 1985–1998. [Google Scholar] [CrossRef]
- Herrera, M.; Fonsato, V.; Gatti, S.; Deregibus, M.C.; Sordi, A.; Cantarella, D.; Calogero, R.; Bussolati, B.; Tetta, C.; Camussi, G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J. Cell. Mol. Med. 2010, 14, 1605–1618. [Google Scholar] [CrossRef]
- Masyuk, A.I.; Masyuk, T.V.; LaRusso, N.F. Exosomes in the pathogenesis, diagnostics and therapeutics of liver diseases. J. Hepat. 2013, 59, 621–625. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).