Abstract
To evaluate peri-implant bone formation following single or combined systemic administration of alendronate and simvastatin in healthy and osteoporotic rats, eighty female Wistar rats were ovariectomized (n = 40) or sham-operated (n = 40). At six weeks, implants were placed in femoral condyles. Then, ovariectomized (OVX) and sham-operated (SHAM) animals received daily subcutaneous alendronate (50 µg/kg), simvastatin (5 mg/kg), or both, for three weeks. Control animals received subcutaneous saline. Thereafter, specimens were retrieved for biomechanical testing, histological evaluation, and bone area (BA%) and bone-to-implant contact (BIC%). In healthy and osteoporotic rats, similar (p > 0.05) push-out values were observed for all groups. For BA% analysis, control rats showed similar results for OVX (9.2% ± 2.4%) and SHAM (11.1% ± 3.5%) animals. In contrast, single or combined drug therapy significantly increased BA% compared to controls in both healthy and osteoporotic conditions (p < 0.05). In osteoporotic conditions, alendronate alone showed a superior effect on BA% compared to simvastatin alone, or their combination. Systemic alendronate, simvastatin, or both showed a similar BIC% compared to controls (p > 0.05). The present study demonstrates that single or combined systemic alendronate and simvastatin increases bone formation around implants (i.e., distance osteogenesis) in healthy and osteoporotic bone conditions. However, these drugs showed no beneficial effect on direct bone-to-implant contact or implant fixation.
1. Introduction
Loss of teeth (i.e., edentulism) is regarded as a major health problem in dentistry [1,2]. Consequently, titanium implants have become the treatment of choice for replacing missing teeth [3]. Contemporary titanium implant therapy in healthy patients has resulted in significant long-term success following treatment [4]. However, complications related to the healing process of titanium implants may occur in patients with systemic impaired bone metabolism and turnover, e.g., osteoporosis [5]. This is a major systemic condition affecting bone physiology and might lead to increased risk of implant failure [6,7,8].
In the past, several approaches have been used to enhance titanium implant success in patients with impaired bone condition [9]. For instance, different surgical techniques, such as the undersized drilling technique and osteotome, have been used to improve the primary stability of titanium implants in low-quality bone. Further, titanium implant design has been changed to enhance initial implant stability and surface modifications have been applied to stimulate osseointegration [10,11]. These implant surface treatments aim to stimulate bone formation at the implant surface (i.e., contact osteogenesis) [11,12], including implant surface roughness at the nano-level or the deposition of calcium-phosphate coatings. However, such surface modifications do not offer a beneficial effect on bone formation at a distance up to 1 mm (i.e., distance osteogenesis) from the implant surface [13,14]. In principle, both contact and distance osteogenesis are needed to promote the osseointegration of titanium implants. In view of this, one suggested method is to provide the implant surface with bioactive molecules, like growth factors, but the reported data are not consistent [11,15]. In addition, safety issues hamper the application of growth factors [16]. Therefore, the use of bone-targeting agents and drugs, which do not trigger a toxic or adverse side effect and have a confirmed beneficial effect on bone formation, has been proposed [17]. As a consequence, therapeutic pharmacological agents are used that are already widely applied for the treatment of bone disorders like osteoporosis. The additional advantage of such pharmacological agents (e.g., bisphosphonates and statins) is that they are not limited to applications for improving peri-implant bone formation in impaired bone conditions, but can also be used in healthy patients with bone of poor quality, i.e., very trabecular, spongy bone [10,18,19,20].
The principle of treatment with bisphosphonates and statins is based on their systemic or local administration [10]. Bisphosphonate drugs (BPs) exhibit a potent anti-resorptive effect on bone through inhibition of osteoclasts and have been postulated to enhance bone healing through reduction of undesirable bone remodeling around titanium implants [21]. However, BPs might be less able to control bone metabolism compared to anabolic agents (e.g., statins, parathyroid hormone), which are capable of osteoblastic stimulation [10]. Therefore, anabolic and anti-resorptive therapies have been used individually or in combination to treat patients with osteoporotic bone conditions [21,22]. It is alluring to hypothesize that the administration of a combination of anti-resorptive BPs and anabolic statins has a synergistic effect on peri-implant healing [23,24,25,26]. In view of this, it has to be noticed that anabolic and anti-resorptive pharmacological agents have been evaluated in several studies [13,18,19,23,24,27,28] for their effects on bone implant osseointegration. However, there is a lack of evidence as to whether combined administration of anabolic and anti-resorptive pharmacological agents at the time of implant placement has a synergistic effect on the peri-implant bone responses and implant osseointegration. Consequently, the aim of the present study was to evaluate peri-implant bone formation in healthy and osteoporotic rats following single or combined administration of alendronate and simvastatin via daily subcutaneous injections for three weeks from the moment of implant installation.
2. Materials and Methods
2.1. Preparation of Mini-implants
A total of 80 cylindrical implants (2 mm in diameter and 4 mm in length) were fabricated from commercially pure titanium (Aries Alloys, Mumbai, India). All implants were grit-blasted (roughness, Ra = 0.5µm) and cleaned ultrasonically in 10% nitric acid, followed by 100% acetone and 70% ethanol (15 minutes in each). Thereafter, all implants were air-dried and sterilized by autoclaving.
2.2. Animal Model
The present study was approved by the Animal Ethics Committee of King Saud University, College of Dentistry, Kingdom of Saudi Arabia (Approval # 4/67/389683). A total of 80 skeletally mature female Wistar rats (12 weeks of age and weight of ~ 250 g) were included in this study. Among them, 40 rats were ovariectomized (OVX) to induce osteoporotic bone conditions and the remaining 40 rats were subjected to sham surgery (SHAM) to serve as controls with healthy bone conditions. The animals were housed in standardized rat cages (4–5 animals per cage) maintained in a laboratory environment with controlled temperature (22 °C–24 °C) and humidity (45%–55%) and 12-hourly light and dark cycles. All the animals had ad libitum access to a standard rat chow diet and water.
2.3. Implantation Procedures
All surgical procedures were performed in sterile conditions under general anesthesia administered through a single intraperitoneal injection of 0.2 mg/kg xylazine (Chanazine, Chanelle Pharmacuetical, Dublin, Ireland) and 0.5 mg/kg ketamine hydrochloride (Ketamine, Pharmazeutische Präparate, Giessen, Germany). The implantation procedure was performed under general anesthesia, six weeks after ovariectomy and sham operations as previously described [29]. In brief, the right leg of the animal was shaved and disinfected with 10% Povidone-iodine solution (Alphadin, MedicScience Life Care Pvt. Ltd., Haryana, India). A 2 cm long midline longitudinal parapedicular incision was made over the right femoral condyle. The knee joint capsule was incised longitudinally, and complete exposure of the joint was achieved by elevating the patellar ligament gently and retracting it laterally. A cylindrical hole measuring 2 mm in diameter and 4 mm in depth was drilled in the right femoral intercondylar notch, using surgical burs on a rotary handpiece (800 r/min) along with coolant saline irrigation. Finally, one implant per rat was placed press-fit into the implant bed. The surgical wound was then closed in layers using resorbable 4-0 VICRYL® sutures (polyglactin 910, Ethicon, Johnson & Johnson, New Brunswick, NJ, USA).
2.4. Administration of Anti-Osteoporotic Drugs
From the day following implantation, OVX and SHAM animals were subcutaneously administered alendronate (50 µg/kg of body weight) and simvastatin (5 mg/kg of bodyweight), alone or in combination, daily for 3 weeks, as previously [30] shown to promote bone formation in rats and near the human dose. The experimental groups and timetable are illustrated in Figure 1, and were:
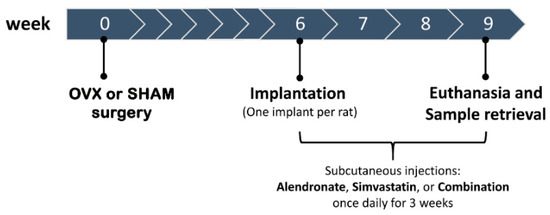
Figure 1.
Schematic representation of study design and timetable.
- Alendronate treatment (ALN; Fosamax® 70 mg, Merck Sharp & Dohme Ltd, Hoddesdon, UK) was used for 20 animals (10 OVX, 10 SHAM).
- Simvastatin treatment (SIM; Zocor®, Merck Sharp & Dohme B.V, Haarlem, The Netherlands) was used for 20 animals (10 OVX, 10 SHAM).
- Combined treatment (ALN+SIM) was used for 20 animals (10 OVX, 10 SHAM).
- Saline administration (10 mL) was used for 20 animals (10 OVX, 10 SHAM) as non-treated controls.
2.5. Sample Retrieval, Preparation, and Analyses
Three weeks post-implantation, all rats were euthanized in a CO2 chamber. Femoral condyles, including titanium implants, were dissected and sectioned out from the adhering tissues. Specimens (n = 3) were randomly selected and transported immediately on ice for biomechanical testing. The remaining specimens (n = 7 samples per protocol in each group) were fixed in 10% neutral buffered formalin solution for 48 hours. After fixation, samples were stored in 70% ethanol and subsequently prepared for histological and histomorphometric analyses.
2.6. Mechanical Push-out Testing
Push-out testing was performed as described previously [29], using the Instron Universal test machine (Instron Ltd, High Wycombe, Buckinghamshire, UK). In brief, specimens were ground to expose both sides of the implant, after which they were placed on a jig hole 0.5 mm larger than the implant diameter. The crosshead was moved as close to the specimens as possible without touching it with the push-out rod. Thereafter, a vertical force (parallel to the long-axis of implant) with a displacement speed of 0.5 mm/min was applied on the implant. The test was stopped when the peak force was reached (representing implant loosening) and was recorded in Newtons (N).
2.7. Histological Preparation and Evaluation
After fixation in formalin and storage in ethanol, the harvested bone specimens were dehydrated in ascending concentrations of ethyl alcohol from 70% to 100% and subsequently embedded in poly(methylmethacrylate) (pMMA) resin. After polymerization, ~10-µm-thick serial cross-sections (perpendicular to the long axis of the implant) of the resin embedded specimens were made using a diamond-coated hard-tissue microtome. The sections were stained with methylene blue and basic fuchsin. The sectioning was started 1 mm from the top of the implant. Histological and histomorphometric evaluation were carried out using a light microscope (Aperio ImageScope, Leica Biosystems, Buffalo Grove, IL, USA). The histomorphometric analyses were performed using a computer-based image analysis system (IMAGE-J 1.4, National Institute of Health, Bethesda, MD, USA). Blinded histomorphometric measurements were performed for three histological sections per implant (at x20 objective magnification). First, the bone area (BA%) was determined in a region of interest (ROI), i.e., a circle that included the implant and a 1 mm circumferential area around it. Second, bone-to-implant contact (BIC%), the relative length of the implant circumference in direct contact with bone tissue, was measured in three different sections per implant. The average of these measurements was used for statistical analysis.
2.8. Statistical Analysis
Statistical analysis of the acquired data was done using InStat Statistical Program (Version 3.05, GraphPad Software, San Diego, CA, USA). Descriptive statistics of the variables (push-out force, histomorphometric BA% and BIC%) were expressed as mean and standard deviation (SD). First, an unpaired Student’s t-test was conducted to evaluate differences in the mean values between the saline groups in OVX compared to SHAM rats. Second, one-way analysis of variance (ANOVA) with Dunnett post-hoc test was used for comparison of the variables in each treatment group with saline group as control. Third, a non-parametric Mann–Whitney test was used for statistical comparison between OVX and SHAM rats of the pooled BIC% and BA% data of the drug-receiving groups. The level of statistical significance was set at p < 0.05.
3. Results
3.1. Animal Model
The total number of implants placed and retrieved in each study group and the numbers of specimens used for push-out testing and histomorphometric analysis (BA% and BIC%) are shown in Table 1. Four rats died intra-operatively during the implantation procedure as a result of respiratory complications related to anesthesia (two OVX rats: ALN and combined, two SHAM rats: ALN and saline). The rest of the study animals showed uneventful post-operative healing and remained healthy during the entire experimental period. No undesirable clinical complications (e.g., infections) were observed.

Table 1.
Number of implants placed, retrieved, and used for push-out testing, histological evaluation, and histomorphometric measurements.
3.2. Biomechanical Push-Out Test
The biomechanical push-out force (N) for the implants in healthy and osteoporotic animals treated using different drug protocols are listed in Table 2. Quantitative data on biomechanical push-out force showed similar results for all groups, irrespective of bone condition or pharmacological treatment. Statistical testing could not confirm the presence of statistically significant differences (p > 0.05) in the mean push-out force values between the various study groups. The force required to push out the implant from adjacent bone tissue ranged from 10 to 25 N.

Table 2.
Mean values of measured variables in different study groups and drug treatment protocols.
3.3. Histological and Histomorphometric Evaluation
Successful induction of osteoporosis was confirmed by light microscopical examination of the histological sections at low magnification, which indicated that overall the bone trabeculae were decreased and the intertrabecular space increased for the rats receiving saline administration after OVX versus SHAM surgery (Figure 2).
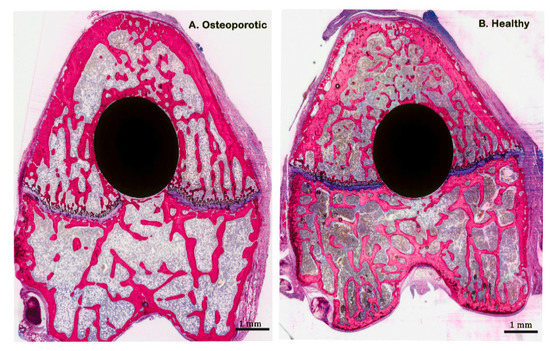
Figure 2.
Representative histological images stained with methylene blue and basic fuchsin, showing non-decalcified (pMMA embedded) cross-sections of saline group in both osteoporotic and healthy conditions.
Assessment at higher magnification of the histological sections of the rats that received anti-osteoporotic drugs demonstrated that no gross differences in bone formation existed adjacent to the implants as treated with alendronate and simvastatin, alone or in combination, in both osteoporotic and healthy animals (Figure 3 and Figure 4). On the other hand, bone formation in these rats was considerably increased in the 1 mm peri-implant area compared with the osteoporotic and healthy rats that received saline treatment.
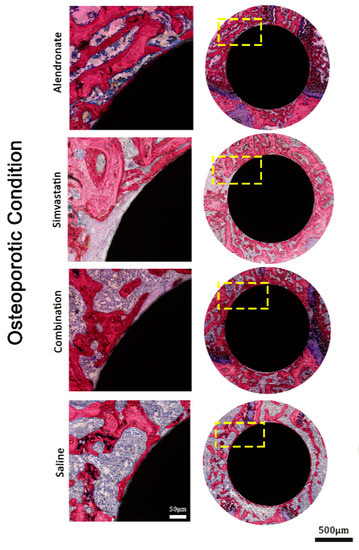
Figure 3.
Representative histological images stained with methylene blue and basic fuchsin, showing non-decalcified (pMMA embedded) cross-sections in osteoporotic bone conditions.
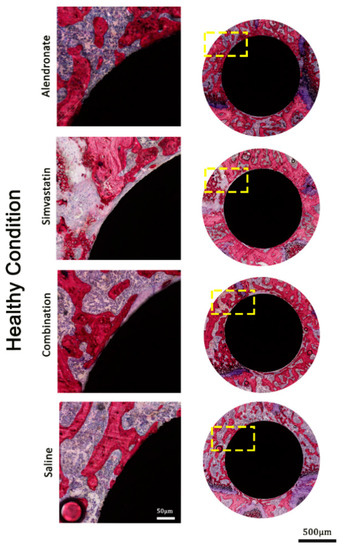
Figure 4.
Representative histological images stained with methylene blue and basic fuchsin, showing non-decalcified (pMMA embedded) cross-sections in healthy bone conditions.
At the implant–bone interface, the bone was observed to be in close contact with the implant surface. The bone–implant contact was characterized by intermittent sites, where the bone was deposited without an intervening fibrous tissue layer, while in other areas a small gap was seen between bone and implant. This gap was filled with bone marrow-like tissue. In each group a wide variation was found to exist in the amount of tight bone contact between rats. In some specimens, a lot of bone–implant contact was seen, while much less direct bone contact was found in other specimens (Figure 5). Observations were very similar for osteoporotic and healthy animals.
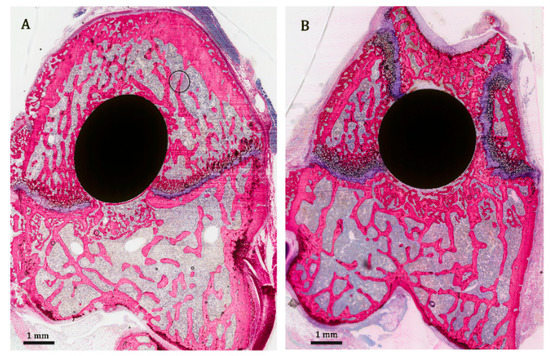
Figure 5.
Representative histological images stained with methylene blue and basic fuchsin, showing non-decalcified (pMMA embedded) cross-sections displaying the varying amount of tight bone contact to implant in different treatment groups. (A) osteoporotic condition, alendronate group. (B) healthy condition, combination group.
The data of the histomorphometric BA% and BIC% analysis are given in Table 2, Figure 6 and Figure 7. Statistical testing revealed that BA% in OVX (9.2% ± 2.4%) and SHAM (11.1% ± 3.5%) rats for the saline controls did not differ significantly (p > 0.05) between rats (Table 2 and Figure 6). On the other hand, as presented in Table 2 and Figure 7A, three weeks of daily subcutaneous injection of alendronate, simvastatin, or combination led to a significant increase (p < 0.05) in bone area (BA%) around titanium implants compared to the saline controls in healthy as well as in osteoporotic animals. In addition, in osteoporotic conditions, alendronate administration resulted in an enhanced BA% around implants compared to simvastatin and the combination of alendronate and simvastatin treatment. In SHAM rats, no significant differences in BA% existed between the various treatment groups. Statistical analysis of the BIC% data demonstrated that systemic administration of alendronate and simvastatin, alone or in combination, did not alter bone–implant contact (BIC%) compared to saline controls in both osteoporotic and healthy conditions (Table 2 and Figure 7B. Finally, using a non-parametric Mann–Whitney rank sum test for the pooled data of the various drug treatment groups, a significant difference (p < 0.01) in BA% between OVX and SHAM rats (16.6% ± 6.6% vs 20.1% ± 5.1%) was observed, while similar BIC% between OVX and SHAM rats (50.1% ± 27.2% vs. 49.3% ± 25.8%) were observed (Figure 8).
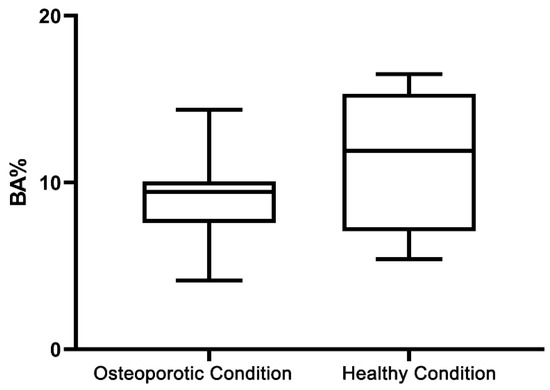
Figure 6.
Box-and-whisker plot shows the histomorphometric bone area (BA%) in saline groups in both osteoporotic and healthy conditions.
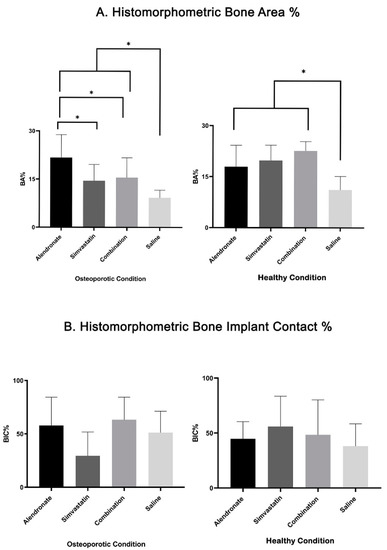
Figure 7.
Bar-chart with mean and standard deviation, based on histomorphometric assessment, showing (A) bone area (BA%) and (B) bone-to-implant contact (BIC%) for all study groups. (* indicates p < 0.05).
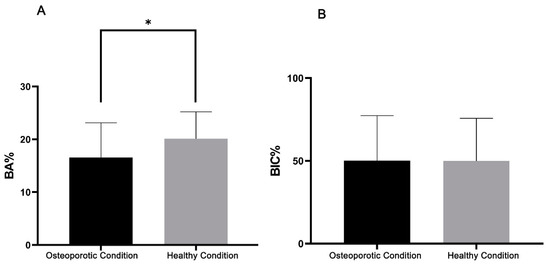
Figure 8.
Bar-chart with mean and standard deviation, based on histomorphometric assessment, showing (A) bone area (BA%) and (B) bone-to-implant contact (BIC%) for the pooled data of the various drug treatment groups between osteoporotic and healthy conditions. (* indicates p < 0.01).
4. Discussion
This study aimed to evaluate whether the systemic administration of alendronate, simvastatin, or their combination is capable to promote peri-implant bone responses in healthy and osteoporotic animal models. Results showed no effects of pharmacological treatment on biomechanical push-out testing, irrespective of bone condition. Further, similar histomorphometric bone area (BA%) results were observed for saline controls in osteoporotic and healthy bone conditions. In contrast, three weeks of daily subcutaneous administration of alendronate, simvastatin, or their combination led to a significant increase (p < 0.05) in BA% around titanium implants in osteoporotic as well as healthy bone conditions, compared to saline controls. In osteoporotic bone conditions, alendronate treatment was shown to significantly enhance BA% around implants compared to simvastatin or the combination of alendronate and simvastatin. Overall, the BA% after treatment with pharmacological agents was significantly higher in healthy compared to osteoporotic bone conditions. However, systemic treatment with alendronate, simvastatin, or their combination did not alter BIC% levels compared to saline controls in either osteoporotic and healthy bone conditions.
In clinical practice, several anti-osteoporotic drugs are used for the treatment of osteoporosis [21]. Recommendations and guidelines for the use of anti-osteoporotic drugs are based on clinical evidence and trials [31]. Additionally, improving the treatment protocols, using powerful drugs, and selecting the optimal route of administration are necessary to control impaired bone metabolism in patients who suffer from osteoporosis. For example, various treatment options have been suggested, mostly comprising treatment with anti-osteoporotic drugs, such as BPs [32]. Pre-clinical studies have already been performed to study the effect of such treatments on peri-implant bone regeneration in both healthy and osteoporotic animal models [9,18,19,33,34,35]. Despite existing evidence that single treatment with BPs or simvastatin can enhance bone formation around dental implants in healthy and osteoporotic animal models, only limited work has been dedicated toward the effect of combined administration of anti-resorptive and anabolic agents [22,23,24,25,36,37]. In addition, there is a wide variation in the route of administration of such pharmacological agents, the timing in relation to osteoporosis induction and subsequent implant placement, as well as implantation time. Consequently, the current study was conducted as part of a consecutive series of studies focused on the effect of anti-resorptive agents on bone healing around dental implants in healthy and osteoporotic bone conditions [20].
The data of our study corroborate those from other studies, although minor differences are apparent. For the evaluation of the osseointegration of titanium implants, histomorphometric BA% and BIC% are the most traditional analytic methods to determine the amount of contact osteogenesis (0–500 µm) and distance osteogenesis (500–1000 µm), as previously defined and described [14]. Distance osteogenesis originates from the existing bone or from the blood of the bone marrow and periosteum. In relation to this, we hypothesized that systemic administration of anti-osteoporotic drugs (alendronate, simvastatin or their combination) treats the existing osteoporotic bone tissue surrounding titanium implants and triggers peri-implant bone formation. Comparisons between treatments of individual administration of alendronate or simvastatin and their combination showed that alendronate had a major increasing effect on peri-implant bone area (BA%) in the osteoporotic bone conditions. In healthy bone conditions, this effect was not observed; here, all treatments enhanced peri-implant BA% compared to saline controls. These findings in healthy bone conditions corroborate those reported by others, which demonstrated improvement of the peri-implant bone formation upon daily systemic treatment with BPs [30,38]. On the other hand, no additive effect of the combined treatment was observed. In addition, in our study the effect of simvastatin in osteoporotic bone conditions was less. An et al. [39] performed a systematic review of the efficacy of statin treatment for osteoporosis and its efficacy toward fracture rates and bone mineral density (BMD) in osteoporotic patients in clinical studies. Their meta-analysis indicated that statins decrease fracture risk and increase BMD, but this effect was more significant in male over female patients. We used female rats in our work, which can explain the lesser efficacy of simvastatin treatment.
Further, it has to be emphasized that no difference in BA% was observed for saline controls in osteoporotic and healthy bone conditions. We assume that this is due to the rather short implantation time and the implant shape used. All rats were euthanized three weeks after implant installation. Bone healing after creation of the implant bed starts with blood clot formation and an inflammatory response. Subsequently, callus tissue is deposited, which remodels into lamellar bone. Bone remodeling is associated with a decrease in bone amount, as bone trabeculae become thinner and intertrabecular voids increase in size. The start of bone formation and remodeling in rats is usually completed within three weeks [40]. In the current study, skeletally mature female rats with an age of three months were used to mimic elderly females, which inherently have slower bone formation rates compared to young rats. Apparently, bone remodeling was not completed yet three weeks after implant installation and resulted in similar BA% for the osteoporotic and healthy rats without pharmacological treatment. This effect could have even been enhanced by the implant design used. The implants in the current study had a cylindrical shape without screw threads and were placed press-fit in the implant bed, which had the same diameter as the implant. In clinical practice, threaded dental implants are used, which are installed in the bone using an undersized surgical technique. It has been shown that the implant installation technique has a significant effect on bone formation around implants [41,42].
Our data showed that treatment with anti-osteoporotic drugs (alendronate, simvastatin or their combination) did not affect bone-to-implant contact (BIC) levels, which were comparable for osteoporotic and healthy bone conditions. It has to be noted that published data are inconsistent regarding the BIC% outcomes. Some studies have shown a significant increase in BIC% level related to BP therapy, whereas others have not. For instant, Eberhardt et al. [38] showed that daily subcutaneous treatment with 25 μg/kg body weight ibandronate for 28 days improved bone-to-implant contact of uncoated titanium implants. In contrast, Giro et al. [30] found that the systemic administration of alendronate resulted in BIC% levels comparable to the non-treated controls. Further, Kurth et al. [43], assessed BIC% in ovariectomized (OVX) and sham-operated rats assigned to daily subcutaneous injections of either saline or ibandronate (1 or 25 μg/kg body weight) and found no difference between experimental groups. These inconsistent observations can be partly explained by differences in the experimental design, type of drugs, administration route, dose, and evaluation periods. He et al [23] performed a preclinical meta-analysis to the effect of zolendronate administration on implant osseointegration, quantitatively assessing BIC% and BA%. They concluded that zolendronate administration generally increases BA%, but that for short implantation times (< eight weeks) the effect is less compared to long implantation times (> eight weeks). In view of this meta-analysis and our short implantation time (three weeks), shape of the implants (cylindrical press-fit) as well as age of the rats, this explains our findings.
The similarity in BIC% for all different pharmacological treatments in osteoporotic versus healthy bone conditions clarifies the results of the push-out test. A push-out test measures the maximal force needed to separate an implant from its surrounding bone, and is affected by parameters such as thickness of bone, bone defect angulation, and lever position [44]. A push-out test is particularly applicable for cylindrical implants without any screw threads, because it provides information about the attachment of the bone to the implant surface. As no differences in BIC% were found, it is not remarkable that our push-out data did not differ between the various experimental groups, which further confirms the reliability of push-out testing.
Although our results suggest that particularly anti-osteoporotic treatment with alendronate might reverse the negative impact of postmenopausal osteopenia on the peri-implant bone at a distance from the implant surface, the data about the effect on the bone directly adjacent to the implant surface are not convincing. Consequently, more information is required from long-term implantation studies using standardized protocols for administration route, dosage, and anti-osteoporotic drug before translational steps toward osteoporotic patients requiring dental implants are taken.
5. Conclusions
Based on the histomorphometric analyses, single or combined pharmacological treatment via systemic administration (i.e., daily subcutaneous injection for three weeks) of alendronate or simvastatin can promote peri-implant bone formation around implants (i.e., distance osteogenesis) in healthy and osteoporotic bone conditions. However, these systemic therapies showed no beneficial effect on the biomechanical implant fixation or the direct bone–implant contact.
Author Contributions
Conceptualization, A.M.B., M.Y.S., A.A.N., J.J.J.P.v.d.B., J.A.J. and H.S.A.; data curation, A.M.B.; formal analysis, M.Y.S.; funding acquisition, H.S.A.; investigation, A.M.B. and M.Y.S.; methodology, A.M.B., M.Y.S., A.A.N., J.J.J.P.v.d.B. and H.S.A.; project administration, A.A.N.; resources, A.A.N. and J.J.J.P.v.d.B.; validation, J.J.J.P.v.d.B. and J.A.J.; visualization, J.A.J.; writing – original draft, A.M.B.; writing – review and editing, J.J.J.P.v.d.B., J.A.J. and H.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work (grant: RG-1441-026).
Acknowledgments
The authors are grateful for the technical support of Vincent Cuijpers, Martijn Martens, and Natasja van Dijk in histological preparation and sectioning. The authors are also grateful to Marygrace Baysa Vigilla and Rhodanne Lambarte for their support during animal experimental work. Finally, the authors would like to thank Terrence Sumeng for his assistance during the histomorphometric measurements.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Heydecke, G.; Locker, D.; Awad, M.A.; Lund, J.P.; Feine, J. Oral and general health-related quality of life with conventional and implant dentures. Community Dent. Oral Epidemiol. 2003, 31, 161–168. [Google Scholar] [CrossRef]
- Petersen, P.E. The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century--the approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2003, 31 (Suppl. 1), 3–23. [Google Scholar] [CrossRef]
- Henry, P.J. Tooth loss and implant replacement. Aust. Dent. J. 2000, 45, 150–172. [Google Scholar] [CrossRef]
- Abtahi, J.; Tengvall, P.; Aspenberg, P. Bisphosphonate coating might improve fixation of dental implants in the maxilla: A pilot study. Int. J. Oral Maxillofac. Surg. 2010, 39, 673–677. [Google Scholar] [CrossRef]
- Von Wowern, N. General and oral aspects of osteoporosis: A review. Clin. Oral Investig. 2001, 5, 71–82. [Google Scholar] [CrossRef]
- Dennison, E.M.; Cole, Z.; Cooper, C. Diagnosis and epidemiology of osteoporosis. Curr. Opin. Rheumatol. 2005, 17, 456–461. [Google Scholar] [CrossRef]
- Garg, A.K.; Winkler, S.; Bakaeen, L.G.; Mekayarajjananonth, T. Dental implants and the geriatric patient. Implant. Dent. 1997, 6, 168–173. [Google Scholar] [CrossRef]
- Gaetti-Jardim, E.C.; Junior, J.S.; Goiato, M.C.; Pellizer, E.P.; Magro-Filho, O.; Gaetti-Jardim, E.C. Dental Implants in Patients With Osteoporosis. J. Craniofacial Surg. 2011, 22, 1111–1113. [Google Scholar] [CrossRef]
- Dundar, S.; Yaman, F.; Gecor, O.; Cakmak, O.; Kirtay, M.; Yildirim, T.T.; Karaman, T.; Benlidayi, M.E. Effects of Local and Systemic Zoledronic Acid Application on Titanium Implant Osseointegration. J. Craniofac. Surg. 2017, 28, 935–938. [Google Scholar] [CrossRef]
- Alghamdi, H.; A Jansen, J. Bone Regeneration Associated with Nontherapeutic and Therapeutic Surface Coatings for Dental Implants in Osteoporosis. Tissue Eng. Part B Rev. 2013, 19, 233–253. [Google Scholar] [CrossRef]
- Junker, R.; Dimakis, A.; Thoneick, M.; A Jansen, J. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 185–206. [Google Scholar] [CrossRef]
- Le Guehennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, H.; Van Oirschot, B.A.; Bosco, R.; Beucken, J.J.V.D.; Aldosari, A.A.F.; Anil, S.; A Jansen, J. Biological response to titanium implants coated with nanocrystals calcium phosphate or type 1 collagen in a dog model. Clin. Oral Implant. Res. 2012, 24, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E. Understanding Peri-Implant Endosseous Healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.-W.; Chien, E.Y.; Chien, H.-H. Dental implant bioactive surface modifications and their effects on osseointegration: A review. Biomark. Res. 2016, 4, 24. [Google Scholar] [CrossRef]
- James, A.W.; Lachaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Nadar, R.A.; Beucken, J.J.V.D.; Leeuwenburgh, S.C. Chapter 10—Pharmacological Interventions Targeting Bone Diseases in Adjunction with Bone Grafting; Chapter 10; Alghamdi, H., Jansen, J.A., Eds.; Dental Implants and Bone Grafts, Woodhead Publishing, Elsevier: Amsterdam, The Netherlands, 2020; pp. 251–280. [Google Scholar]
- Ayukawa, Y.; Ogino, Y.; Moriyama, Y.; Atsuta, I.; Jinno, Y.; Kihara, M.; Tsukiyama, Y.; Koyano, K. Simvastatin enhances bone formation around titanium implants in rat tibiae. J. Oral Rehabil. 2010, 37, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Back, D.A.; Pauly, S.; Rommel, L.; Haas, N.P.; Schmidmaier, G.; Wildemann, B.; Greiner, S. Effect of local zoledronate on implant osseointegration in a rat model. BMC Musculoskelet. Disord. 2012, 13, 42. [Google Scholar] [CrossRef]
- Basudan, A.M.; Shaheen, M.Y.; De Vries, R.B.; Beucken, J.J.V.D.; Jansen, J.A.; Alghamdi, H.S. Antiosteoporotic Drugs to Promote Bone Regeneration Related to Titanium Implants: A Systematic Review and Meta-Analysis. Tissue Eng. Part B Rev. 2019, 25, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Cerocchi, I.; Celi, M.; Scialdoni, A.; Saturnino, L.; Gasbarra, E. Pharmacological agents and bone healing. Clin. Cases Miner. Bone Metab. 2009, 6, 144–148. [Google Scholar] [PubMed]
- Moraschini, V.; Almeida, D.; Calasans-Maia, J.; Calasans-Maia, M.D. The ability of topical and systemic statins to increase osteogenesis around dental implants: A systematic review of histomorphometric outcomes in animal studies. Int. J. Oral Maxillofac. Surg. 2018, 47, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Bao, W.; Wu, X.-D.; Huang, W.; Chen, H.; Li, Z. Effects of Systemic or Local Administration of Zoledronate on Implant Osseointegration: A Preclinical Meta-Analysis. BioMed Res. Int. 2019, 2019, 9541485. [Google Scholar] [CrossRef] [PubMed]
- Abumoussa, S.; Ruppert, D.S.; Lindsay, C.; Dahners, L.; Weinhold, P.; Abumoussa, S. Local delivery of a zoledronate solution improves osseointegration of titanium implants in a rat distal femur model. J. Orthop. Res. 2018, 36, 3294–3298. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Lee, R.S.; Du, Z.; Hamlet, S.; Vaquette, C.; Ivanovski, S. The influence of high-dose systemic zoledronate administration on osseointegration of implants with different surface topography. J. Periodontal Res. 2019, 54, 633–643. [Google Scholar] [CrossRef]
- Kellesarian, S.; Al Amri, M.; Al-Kheraif, A.; Ghanem, A.; Malmstrom, H.; Javed, F. Efficacy of Local and Systemic Statin Delivery on the Osseointegration of Implants: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2017, 32, 497–506. [Google Scholar] [CrossRef]
- Ji, W.; Wang, H.; Beucken, J.J.V.D.; Yang, F.; Walboomers, X.F.; Leeuwenburgh, S.; A Jansen, J. Local delivery of small and large biomolecules in craniomaxillofacial bone. Adv. Drug Deliv. Rev. 2012, 64, 1152–1164. [Google Scholar] [CrossRef]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef]
- Alghamdi, H.; Beucken, J.J.V.D.; Jansen, J.A. Osteoporotic Rat Models for Evaluation of Osseointegration of Bone Implants. Tissue Eng. Part C Methods 2014, 20, 493–505. [Google Scholar] [CrossRef]
- Giro, G.; Coelho, P.G.; Pereira, R.; Jorgetti, V.; Marcantonio-Junior, E.; Orrico, S.R.P. The effect of oestrogen and alendronate therapies on postmenopausal bone loss around osseointegrated titanium implants. Clin. Oral Implant. Res. 2010, 22, 259–264. [Google Scholar] [CrossRef]
- Orimo, H.; Nakamura, T.; Hosoi, T.; Iki, M.; Uenishi, K.; Endo, N.; Ohta, H.; Shiraki, M.; Sugimoto, T.; Suzuki, T.; et al. Japanese 2011 guidelines for prevention and treatment of osteoporosis—Executive summary. Arch. Osteoporos. 2012, 7, 3–20. [Google Scholar] [CrossRef]
- Orimo, H.; Nakamura, T.; Fukunaga, M.; Ohta, H.; Hosoi, T.; Uemura, Y.; Kuroda, T.; Miyakawa, N.; Ohashi, Y.; Shiraki, M.; et al. Effects of alendronate plus alfacalcidol in osteoporosis patients with a high risk of fracture: The Japanese Osteoporosis Intervention Trial (JOINT)–02. Curr. Med. Res. Opin. 2011, 27, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Bobyn, J.D.; McKenzie, K.; Karabasz, D.; Krygier, J.J.; Tanzer, M. Locally Delivered Bisphosphonate for Enhancement of Bone Formation and Implant Fixation. J. Bone Jt. Surg. Am. 2009, 91, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Toker, H.; Ozdemir, H.; Ozer, H.; Eren, K. Alendronate enhances osseous healing in a rat calvarial defect model. Arch. Oral Biol. 2012, 57, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Chen, J.; Yan, F.; Doan, N.; Ivanovski, S.; Xiao, Y. Serum bone formation marker correlation with improved osseointegration in osteoporotic rats treated with simvastatin. Clin. Oral Implant. Res. 2011, 24, 422–427. [Google Scholar] [CrossRef]
- Jakobsen, T.; Bechtold, J.E.; Søballe, K.; Jensen, T.; Greiner, S.; Vestermark, M.T.; Baas, J. Local delivery of zoledronate from a poly (D,L-lactide)-Coating increases fixation of press-fit implants. J. Orthop. Res. 2015, 34, 65–71. [Google Scholar] [CrossRef]
- Vohra, F.; Al-Rifaiy, M.Q.; Almas, K.; Javed, F. Efficacy of systemic bisphosphonate delivery on osseointegration of implants under osteoporotic conditions: Lessons from animal studies. Arch. Oral Biol. 2014, 59, 912–920. [Google Scholar] [CrossRef]
- Eberhardt, C.; Stumpf, U.; Brankamp, J.; Schwarz, M.; Kurth, A. Osseointegration of Cementless Implants with Different Bisphosphonate Regimens. Clin. Orthop. Relat. Res. 2006, 447, 195–200. [Google Scholar] [CrossRef]
- An, T.; Hao, J.; Sun, S.; Li, R.; Yang, M.; Cheng, G.; Zou, M. Efficacy of statins for osteoporosis: A systematic review and meta-analysis. Osteoporos. Int. 2016, 28, 47–57. [Google Scholar] [CrossRef]
- Handool, K.O.; Ibrahim, S.; Kaka, U.; Omar, M.A.; Abu, J.; Sabri, M.Y.; Yusof, L.M. Optimization of a closed rat tibial fracture model. J. Exp. Orthop. 2018, 5, 13. [Google Scholar] [CrossRef]
- Shalabi, M.M.; Wolke, J.G.C.; De Ruijter, A.J.E.; A Jansen, J. Histological evaluation of oral implants inserted with different surgical techniques into the trabecular bone of goats. Clin. Oral Implant. Res. 2007, 18, 489–495. [Google Scholar] [CrossRef]
- Tabassum, A.; Meijer, G.J.; Walboomers, F.X.; A Jansen, J. Biological limits of the undersized surgical technique: A study in goats. Clin. Oral Implant. Res. 2010, 22, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kurth, A.; Eberhardt, C.; Muller, S.; Steinacker, M.; Schwarz, M.; Bauss, F. The bisphosphonate ibandronate improves implant integration in osteopenic ovariectomized rats. Bone 2005, 37, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Dhert, W.J.A.; Klein, C.P.A.T.; Wolke, J.G.C.; Van Der Velde, E.A.; De Groot, K.; Rozing, P.M. A mechanical investigation of fluorapatite, magnesiumwhitlockite, and hydroxylapatite plasma-sprayed coatings in goats. J. Biomed. Mater. Res. 1991, 25, 1183–1200. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).