Structure-Based Drug Design for Tuberculosis: Challenges Still Ahead
Abstract
1. Introduction
2. Protein Ser/Thr Kinases as Drug Targets
3. DNA Gyrase: A Validated Drug Target
4. General Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perutz, M.F. Fundamental research in molecular biology: Relevance to medicine. Nature 1976, 262, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Wlodawer, A. Rational approach to AIDS drug design through structural biology. Annu. Rev. Med. 2002, 53, 595–614. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.; Erickson, J.W.; Baldwin, J.J.; Varney, M.D. Application of the three-dimensional structures of protein target molecules in structure-based drug design. J. Med. Chem. 1994, 37, 1035–1054. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.-P.; Chari, A.; Ciferri, C.; Liu, W.-T.; Rémigy, H.-W.; Stark, H.; Wiesmann, C. Cryo-EM in drug discovery: Achievements, limitations and prospects. Nat. Rev. Drug. Discov. 2018, 17, 471–492. [Google Scholar] [CrossRef] [PubMed]
- Kuehlbrandt, W. The Resolution Revolution. Science 2014, 343, 1443–1444. [Google Scholar] [CrossRef]
- Mitra, A.K. Visualization of biological macromolecules at near-atomic resolution: Cryo-electron microscopy comes of age. Acta Crystallogr. F Struct. Biol. Commun. 2019, 75, 3–11. [Google Scholar] [CrossRef]
- Muhammed, M.T.; Aki-Yalcin, E. Homology modeling in drug discovery: Overview, current applications, and future perspectives. Chem. Biol. Drug. Des. 2019, 93, 12–20. [Google Scholar] [CrossRef]
- Kuenemann, M.A.; Sperandio, O.; Labbé, C.M.; Lagorce, D.; Miteva, M.A.; Villoutreix, B.O. In silico design of low molecular weight protein-protein interaction inhibitors: Overall concept and recent advances. Prog. Biophys. Mol. Biol. 2015, 119, 20–32. [Google Scholar] [CrossRef]
- Bosc, N.; Muller, C.; Hoffer, L.; Lagorce, D.; Bourg, S.; Derviaux, C.; Gourdel, M.-E.; Rain, J.-C.; Miller, T.W.; Villoutreix, B.O.; et al. Fr-PPIChem: An academic compound library dedicated to protein–protein interactions. ACS Chem. Biol. 2020. [Google Scholar] [CrossRef]
- Yuan, Y.; Pei, J.; Lai, L. Binding site detection and druggability prediction of protein targets for structure-based drug design. Curr. Pharm. Des. 2013, 19, 2326–2333. [Google Scholar] [CrossRef]
- Acharya, C.; Coop, A.; Polli, J.E.; Mackerell, A.D. Recent advances in ligand-based drug design: Relevance and utility of the conformationally sampled pharmacophore approach. Curr. Comput. Aided. Drug. Des. 2011, 7, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Neves, B.J.; Braga, R.C.; Melo-Filho, C.C.; Moreira-Filho, J.T.; Muratov, E.N.; Andrade, C.H. QSAR-based virtual screening: Advances and applications in drug discovery. Front. Pharmacol. 2018, 9, 1275. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.J.; Hubbard, R.E. Fragment-Based Ligand Discovery. In Structural Biology in Drug Discovery Methods, Techniques, and Practices; Renaud, J.-P., Ed.; Wiley: Hoboken, NJ, USA, 2020; Volume 10, pp. 79–98. [Google Scholar]
- Mendes, V.; Blundell, T.L. Targeting tuberculosis using structure-guided fragment-based drug design. Drug Discov. Today 2017, 22, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Kana, B.D.; Karakousis, P.C.; Parish, T.; Dick, T. Future target-based drug discovery for tuberculosis? Tuberculosis 2014, 94, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Koul, A.; Arnoult, E.; Lounis, N.; Guillemont, J.; Andries, K. The challenge of new drug discovery for tuberculosis. Nature 2011, 469, 483–490. [Google Scholar] [CrossRef]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E.; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef]
- Holton, S.J.; Weiss, M.S.; Tucker, P.A.; Wilmanns, M. Structure-based approaches to drug discovery against tuberculosis. Curr. Protein Pept. Sci. 2007, 8, 365–375. [Google Scholar] [CrossRef]
- Terwilliger, T.C.; Park, M.S.; Waldo, G.S.; Berendzen, J.; Hung, L.W.; Kim, C.Y.; Smith, C.V.; Sacchettini, J.C.; Bellinzoni, M.; Bossi, R.; et al. The TB structural genomics consortium: A resource for Mycobacterium tuberculosis biology. Tuberculosis 2003, 83, 223–249. [Google Scholar] [CrossRef]
- Ehebauer, M.T.; Wilmanns, M. The progress made in determining the Mycobacterium tuberculosis structural proteome. Proteomics 2011, 11, 3128–3133. [Google Scholar] [CrossRef]
- Huang, L.; Nazarova, E.V.; Russell, D.G. Mycobacterium tuberculosis: Bacterial Fitness within the Host Macrophage. Microbiol. Spectr. 2019, 7, PMC6459685. [Google Scholar] [CrossRef] [PubMed]
- Sia, J.K.; Rengarajan, J. Immunology of Mycobacterium tuberculosis Infections. Microbiol. Spectr. 2019, 7, PMC6636855. [Google Scholar] [CrossRef] [PubMed]
- Woong Park, S.; Klotzsche, M.; Wilson, D.J.; Boshoff, H.I.; Eoh, H.; Manjunatha, U.; Blumenthal, A.; Rhee, K.; Barry, C.E.; Aldrich, C.C.; et al. Evaluating the sensitivity of Mycobacterium tuberculosis to biotin deprivation using regulated gene expression. PLoS Pathog. 2011, 7, e1002264. [Google Scholar] [CrossRef]
- Wei, J.-R.; Krishnamoorthy, V.; Murphy, K.; Kim, J.-H.; Schnappinger, D.; Alber, T.; Sassetti, C.M.; Rhee, K.Y.; Rubin, E.J. Depletion of antibiotic targets has widely varying effects on growth. Proc. Natl. Acad. Sci. USA 2011, 108, 4176–4181. [Google Scholar] [CrossRef]
- Aleksandrov, A.; Myllykallio, H. Advances and challenges in drug design against tuberculosis: Application of in silico approaches. Expert Opin. Drug Discov.. 2019, 14, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Waman, V.P.; Vedithi, S.C.; Thomas, S.E.; Bannerman, B.P.; Munir, A.; Skwark, M.J.; Malhotra, S.; Blundell, T.L. Mycobacterial genomics and structural bioinformatics: Opportunities and challenges in drug discovery. Emerg. Microbes Infect. 2019, 8, 109–118. [Google Scholar] [CrossRef]
- Pedelacq, J.D.; Nguyen, M.C.; Terwilliger, T.C.; Mourey, L. A Comprehensive Review on Mycobacterium tuberculosis Targets and Drug Development from a Structural Perspective. In Structural Biology in Drug Discovery Methods, Techniques, and Practices; Renaud, J.-P., Ed.; Wiley: Hoboken, NJ, USA, 2020; Volume 3, pp. 545–566. [Google Scholar]
- Cohen, P. Protein kinases--the major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 2002, 1, 309–315. [Google Scholar] [CrossRef]
- Knapp, S. New opportunities for kinase drug repurposing and target discovery. Br. J. Cancer 2018, 118, 936–937. [Google Scholar] [CrossRef]
- Schoijet, A.C.; Sternlieb, T.; Alonso, G.D. Signal Transduction Pathways as Therapeutic Target for Chagas Disease. Curr. Med. Chem. 2019, 26, 6572–6589. [Google Scholar] [CrossRef]
- Lima, M.N.N.; Cassiano, G.C.; Tomaz, K.C.P.; Silva, A.C.; Sousa, B.K.P.; Ferreira, L.T.; Tavella, T.A.; Calit, J.; Bargieri, D.Y.; Neves, B.J.; et al. Integrative Multi-Kinase Approach for the Identification of Potent Antiplasmodial Hits. Front. Chem. 2019, 7, 773. [Google Scholar] [CrossRef]
- Av-Gay, Y.; Everett, M. The eukaryotic-like Ser/Thr protein kinases of Mycobacterium tuberculosis. Trends Microbiol. 2000, 8, 238–244. [Google Scholar] [CrossRef]
- Kang, C.-M.; Abbott, D.W.; Park, S.T.; Dascher, C.C.; Cantley, L.C.; Husson, R.N. The Mycobacterium tuberculosis serine/threonine kinases PknA and PknB: Substrate identification and regulation of cell shape. Genes Dev. 2005, 19, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Wehenkel, A.; Bellinzoni, M.; Graña, M.; Duran, R.; Villarino, A.; Fernandez, P.; Andre-Leroux, G.; England, P.; Takiff, H.; Cerveñansky, C.; et al. Mycobacterial Ser/Thr protein kinases and phosphatases: Physiological roles and therapeutic potential. Biochim. Biophys. Acta Proteins Proteom. 2008, 1784, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Alber, T. Signaling mechanisms of the Mycobacterium tuberculosis receptor Ser/Thr protein kinases. Curr. Opin. Struct. Biol. 2009, 19, 650–657. [Google Scholar] [CrossRef][Green Version]
- Mieczkowski, C.; Iavarone, A.T.; Alber, T. Auto-activation mechanism of the Mycobacterium tuberculosis PknB receptor Ser/Thr kinase. EMBO J. 2008, 27, 3186–3197. [Google Scholar] [CrossRef]
- Lombana, T.N.; Echols, N.; Good, M.C.; Thomsen, N.D.; Ng, H.-L.; Greenstein, A.E.; Falick, A.M.; King, D.S.; Alber, T. Allosteric activation mechanism of the Mycobacterium tuberculosis receptor Ser/Thr protein kinase, PknB. Structure 2010, 18, 1667–1677. [Google Scholar] [CrossRef]
- Greenstein, A.E.; Echols, N.; Lombana, T.N.; King, D.S.; Alber, T. Allosteric activation by dimerization of the PknD receptor Ser/Thr protein kinase from Mycobacterium tuberculosis. J. Biol. Chem. 2007, 282, 11427–11435. [Google Scholar] [CrossRef]
- Wagner, T.; Andre-Leroux, G.; Hindie, V.; Barilone, N.; Lisa, M.-N.; Hoos, S.; Raynal, B.; Vulliez-Le Normand, B.; O′Hare, H.M.; Bellinzoni, M.; et al. Structural insights into the functional versatility of an FHA domain protein in mycobacterial signaling. Sci. Signal. 2019, 12, eaav9504. [Google Scholar] [CrossRef]
- Young, T.A.; Delagoutte, B.; Endrizzi, J.A.; Falick, A.M.; Alber, T. Structure of Mycobacterium tuberculosis PknB supports a universal activation mechanism for Ser/Thr protein kinases. Nat. Struct. Biol. 2003, 10, 168–174. [Google Scholar] [CrossRef]
- Ortiz-Lombardía, M.; Pompeo, F.; Boitel, B.; Alzari, P.M. Crystal structure of the catalytic domain of the PknB serine/threonine kinase from Mycobacterium tuberculosis. J. Biol. Chem. 2003, 278, 13094–13100. [Google Scholar] [CrossRef]
- Sassetti, C.M.; Boyd, D.H. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 2003, 48, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, P.; Saint-Joanis, B.; Barilone, N.; Jackson, M.; Gicquel, B.; Cole, S.T.; Alzari, P.M. The Ser/Thr protein kinase PknB is essential for sustaining mycobacterial growth. J. Bacteriol. 2006, 188, 7778–7784. [Google Scholar] [CrossRef] [PubMed]
- Chawla, Y.; Upadhyay, S.; Khan, S.; Nagarajan, S.N.; Forti, F.; Nandicoori, V.K. Protein kinase B (PknB) of Mycobacterium tuberculosis is essential for growth of the pathogen in vitro as well as for survival within the host. J. Biol. Chem. 2014, 289, 13875. [Google Scholar] [CrossRef] [PubMed]
- Bellinzoni, M.; Wehenkel, A.M.; Duran, R.; Alzari, P.M. Novel mechanistic insights into physiological signaling pathways mediated by mycobacterial Ser/Thr protein kinases. Genes Immun. 2019, 20, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Drews, S.J.; Hung, F.; Av-Gay, Y. A protein kinase inhibitor as an antimycobacterial agent. FEMS Microbiol. Lett. 2001, 205, 369–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wehenkel, A.; Fernandez, P.; Bellinzoni, M.; Catherinot, V.; Barilone, N.; Labesse, G.; Jackson, M.; Alzari, P.M. The structure of PknB in complex with mitoxantrone, an ATP-competitive inhibitor, suggests a mode of protein kinase regulation in mycobacteria. FEBS Lett. 2006, 580, 3018–3022. [Google Scholar] [CrossRef]
- Bais, V.S.; Mohapatra, B.; Ahamad, N.; Boggaram, S.; Verma, S.; Prakash, B. Investigating the inhibitory potential of 2-Aminopurine metal complexes against serine/threonine protein kinases from Mycobacterium tuberculosis. Tuberculosis 2018, 108, 47–55. [Google Scholar] [CrossRef]
- Székely, R.; Waczek, F.; Szabadkai, I.; Németh, G.; Hegymegi-Barakonyi, B.; Eros, D.; Szokol, B.; Pato, J.; Hafenbradl, D.; Satchell, J.; et al. A novel drug discovery concept for tuberculosis: Inhibition of bacterial and host cell signalling. Immunol. Lett. 2008, 116, 225–231. [Google Scholar] [CrossRef]
- Chapman, T.M.; Bouloc, N.; Buxton, R.S.; Chugh, J.; Lougheed, K.E.A.; Osborne, S.A.; Saxty, B.; Smerdon, S.J.; Taylor, D.L.; Whalley, D. Substituted aminopyrimidine protein kinase B (PknB) inhibitors show activity against Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 2012, 22, 3349–3353. [Google Scholar] [CrossRef]
- Lougheed, K.E.A.; Osborne, S.A.; Saxty, B.; Whalley, D.; Chapman, T.; Bouloc, N.; Chugh, J.; Nott, T.J.; Patel, D.; Spivey, V.L.; et al. Effective inhibitors of the essential kinase PknB and their potential as anti-mycobacterial agents. Tuberculosis 2011, 91, 277–286. [Google Scholar] [CrossRef]
- Xu, J.; Wang, J.-X.; Zhou, J.-M.; Xu, C.-L.; Huang, B.; Xing, Y.; Wang, B.; Luo, R.; Wang, Y.-C.; You, X.-F.; et al. A novel protein kinase inhibitor IMB-YH-8 with anti-tuberculosis activity. Sci. Rep. 2017, 7, 5093–5110. [Google Scholar] [CrossRef] [PubMed]
- Appunni, S.; Rajisha, P.M.; Rubens, M.; Chandana, S.; Singh, H.N.; Swarup, V. Targeting PknB, an eukaryotic-like serine/threonine protein kinase of Mycobacterium tuberculosis with phytomolecules. Comput. Biol. Chem. 2017, 67, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Bemis, G.; Hanzelka, B.; Zuccola, H.; Wynn, M.; Moody, C.S.; Green, J.; Locher, C.; Liu, A.; Gao, H.; et al. Mtb PKNA/PKNB Dual Inhibition Provides Selectivity Advantages for Inhibitor Design To Minimize Host Kinase Interactions. ACS Med. Chem. Lett. 2017, 8, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Wlodarchak, N.; Teachout, N.; Beczkiewicz, J.; Procknow, R.; Schaenzer, A.J.; Satyshur, K.; Pavelka, M.; Zuercher, W.; Drewry, D.; Sauer, J.-D.; et al. In Silico Screen and Structural Analysis Identifies Bacterial Kinase Inhibitors which Act with β-Lactams To Inhibit Mycobacterial Growth. Mol. Pharm. 2018, 15, 5410–5426. [Google Scholar] [CrossRef] [PubMed]
- Walburger, A.; Koul, A.; Ferrari, G.; Nguyen, L.; Prescianotto-Baschong, C.; Huygen, K.; Klebl, B.; Thompson, C.; Bacher, G.; Pieters, J. Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science 2004, 304, 1800–1804. [Google Scholar] [CrossRef] [PubMed]
- Scherr, N.; Honnappa, S.; Kunz, G.; Mueller, P.; Jayachandran, R.; Winkler, F.; Pieters, J.; Steinmetz, M.O. Structural basis for the specific inhibition of protein kinase G, a virulence factor of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2007, 104, 12151–12156. [Google Scholar] [CrossRef]
- Lisa, M.-N.; Gil, M.; Andre-Leroux, G.; Barilone, N.; Duran, R.; Biondi, R.M.; Alzari, P.M. Molecular Basis of the Activity and the Regulation of the Eukaryotic-like S/T Protein Kinase PknG from Mycobacterium tuberculosis. Structure 2015, 23, 1039–1048. [Google Scholar] [CrossRef]
- van der Woude, A.D.; Stoop, E.J.M.; Stiess, M.; Wang, S.; Ummels, R.; van Stempvoort, G.; Piersma, S.R.; Cascioferro, A.; Jiménez, C.R.; Houben, E.N.G.; et al. Analysis of SecA2-dependent substrates in Mycobacterium marinum identifies protein kinase G (PknG) as a virulence effector. Cell Microbiol. 2014, 16, 280–295. [Google Scholar] [CrossRef]
- Zulauf, K.E.; Sullivan, J.T.; Braunstein, M. The SecA2 pathway of Mycobacterium tuberculosis exports effectors that work in concert to arrest phagosome and autophagosome maturation. PLoS Pathog. 2018, 14, e1007011. [Google Scholar] [CrossRef]
- Pradhan, G.; Shrivastva, R.; Mukhopadhyay, S. Mycobacterial PknG targets the Rab7l1 signaling pathway to inhibit phagosome-lysosome fusion. J. Immunol. 2018, 201, 1421–1433. [Google Scholar] [CrossRef]
- Bussi, C.; Gutierrez, M.G. Mycobacterium tuberculosis infection of host cells in space and time. FEMS Microbiol. Rev. 2019, 43, 341–361. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.; Niebisch, A.; Gebel, L.; Bott, M. Glutamate production by Corynebacterium glutamicum: Dependence on the oxoglutarate dehydrogenase inhibitor protein OdhI and protein kinase PknG. Appl. Microbiol. Biotechnol. 2007, 76, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Niebisch, A.; Kabus, A.; Schultz, C.; Weil, B.; Bott, M. Corynebacterial protein kinase G controls 2-oxoglutarate dehydrogenase activity via the phosphorylation status of the OdhI protein. J. Biol. Chem. 2006, 281, 12300–12307. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Rieck, B.; Boldrin, F.; Degiacomi, G.; Bellinzoni, M.; Barilone, N.; Alzaidi, F.; Alzari, P.M.; Manganelli, R.; O′Hare, H.M. GarA is an essential regulator of metabolism in Mycobacterium tuberculosis. Mol. Microbiol. 2013, 90, 356–366. [Google Scholar]
- O’Hare, H.M.; Duran, R.; Cerveñansky, C.; Bellinzoni, M.; Wehenkel, A.M.; Pritsch, O.; Obal, G.; Baumgartner, J.; Vialaret, J.; Johnsson, K.; et al. Regulation of glutamate metabolism by protein kinases in mycobacteria. Mol. Microbiol. 2008, 70, 1408–1423. [Google Scholar] [CrossRef]
- Rieck, B.; Degiacomi, G.; Zimmermann, M.; Cascioferro, A.; Boldrin, F.; Lazar-Adler, N.R.; Bottrill, A.R.; Le Chevalier, F.; Frigui, W.; Bellinzoni, M.; et al. PknG senses amino acid availability to control metabolism and virulence of Mycobacterium tuberculosis. PLoS Pathog. 2017, 13, e1006399. [Google Scholar] [CrossRef]
- Wolff, K.A.; de la Peña, A.H.; Nguyen, H.T.; Pham, T.H.; Amzel, L.M.; Gabelli, S.B.; Nguyen, L. A redox regulatory system critical for mycobacterial survival in macrophages and biofilm development. PLoS Pathog. 2015, 11, e1004839. [Google Scholar] [CrossRef]
- Khan, M.Z.; Bhaskar, A.; Upadhyay, S.; Kumari, P.; Rajmani, R.S.; Jain, P.; Singh, A.; Kumar, D.; Bhavesh, N.S.; Nandicoori, V.K. Protein kinase G confers survival advantage to Mycobacterium tuberculosis during latency-like conditions. J. Biol. Chem. 2017, 292, 16093–16108. [Google Scholar] [CrossRef]
- Cowley, S.; Ko, M.; Pick, N.; Chow, R.; Downing, K.J.; Gordhan, B.G.; Betts, J.C.; Mizrahi, V.; Smith, D.A.; Stokes, R.W.; et al. The Mycobacterium tuberculosis protein serine/threonine kinase PknG is linked to cellular glutamate/glutamine levels and is important for growth in vivo. Mol. Microbiol. 2004, 52, 1691–1702. [Google Scholar] [CrossRef]
- Chao, J.; Wong, D.; Zheng, X.; Poirier, V.; Bach, H.; Hmama, Z.; Av-Gay, Y. Protein kinase and phosphatase signaling in Mycobacterium tuberculosis physiology and pathogenesis. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 620–627. [Google Scholar] [CrossRef]
- Chen, D.; Ma, S.; He, L.; Yuan, P.; She, Z.; Lu, Y. Sclerotiorin inhibits protein kinase G from Mycobacterium tuberculosis and impairs mycobacterial growth in macrophages. Tuberculosis 2017, 103, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, S.; Bouzeyen, R.; Chakraborti, S.; Khare, N.; Das, S.; Priya Gosain, T.; Behura, A.; Meena, C.L.; Dhiman, R.; Essafi, M.; et al. NU-6027 Inhibits Growth of Mycobacterium tuberculosis by Targeting Protein Kinase D and Protein Kinase G. Antimicrob. Agents Chemother. 2019, 63, 39. [Google Scholar] [CrossRef] [PubMed]
- Kanehiro, Y.; Tomioka, H.; Pieters, J.; Tatano, Y.; Kim, H.; Iizasa, H.; Yoshiyama, H. Identification of Novel Mycobacterial Inhibitors Against Mycobacterial Protein Kinase G. Front. Microbiol. 2018, 9, 1517. [Google Scholar] [CrossRef] [PubMed]
- Schoeffler, A.J.; Berger, J.M. DNA topoisomerases: Harnessing and constraining energy to govern chromosome topology. Quart. Rev. Biophys. 2008, 41, 41–101. [Google Scholar] [CrossRef] [PubMed]
- Gellert, M.; Mizuuchi, K.; O′Dea, M.H.; Nash, H.A. DNA gyrase: An enzyme that introduces superhelical turns into DNA. Proc. Natl. Acad. Sci. USA 1976, 73, 3872–3876. [Google Scholar] [CrossRef]
- Sissi, C.; Palumbo, M. In front of and behind the replication fork: Bacterial type IIA topoisomerases. Cell. Mol. Life Sci. 2010, 67, 2001–2024. [Google Scholar] [CrossRef]
- Peng, H.; Marians, K.J. Decatenation activity of topoisomerase IV during oriC and pBR322 DNA replication in vitro. Proc. Natl. Acad. Sci. USA 1993, 90, 8571–8575. [Google Scholar] [CrossRef]
- Aubry, A.; Fisher, L.M.; Jarlier, V.; Cambau, E. First functional characterization of a singly expressed bacterial type II topoisomerase: The enzyme from Mycobacterium tuberculosis. Biochem. Biophys. Res. Commun. 2006, 348, 158–165. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025320. [Google Scholar] [CrossRef]
- Blower, T.R.; Williamson, B.H.; Kerns, R.J.; Berger, J.M. Crystal structure and stability of gyrase–fluoroquinolone cleaved complexes from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2016, 113, 1706–1713. [Google Scholar] [CrossRef]
- Fu, G.; Wu, J.; Liu, W.; Zhu, D.; Hu, Y.; Deng, J.; Zhang, X.-E.; Bi, L.; Wang, D.-C. Crystal structure of DNA gyrase B’ domain sheds lights on the mechanism for T-segment navigation. Nucleic Acids Res. 2009, 37, 5908–5916. [Google Scholar] [CrossRef] [PubMed]
- Tretter, E.M.; Schoeffler, A.J.; Weisfield, S.R.; Berger, J.M. Crystal structure of the DNA gyrase GyrA N-terminal domain from Mycobacterium tuberculosis. Proteins 2010, 78, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Roue, M.; Spitzfaden, C.; Petrella, S.; Aubry, A.; Hann, M.; Bax, B.; Mayer, C. Mycobacterium tuberculosis DNA gyrase ATPase domain structures suggest a dissociative mechanism that explains how ATP hydrolysis is coupled to domain motion. Biochem. J. 2013, 456, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Bouige, A.; Darmon, A.; Piton, J.; Roue, M.; Petrella, S.; Capton, E.; Forterre, P.; Aubry, A.; Mayer, C. Mycobacterium tuberculosis DNA gyrase possesses two functional GyrA-boxes. Biochem. J. 2013, 455, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Piton, J.; Petrella, S.; Delarue, M.; Andre-Leroux, G.; Jarlier, V.; Aubry, A.; Mayer, C. Structural Insights into the Quinolone Resistance Mechanism of Mycobacterium tuberculosis DNA Gyrase. PLoS ONE 2010, 5, e12245. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2019. Available online: https://www.who.int/tb/publications/global_report/en/ (accessed on 19 June 2020).
- Nagaraja, V.; Godbole, A.A.; Henderson, S.R.; Maxwell, A. DNA topoisomerase I and DNA gyrase as targets for TB therapy. Drug Discov. Today 2017, 22, 510–518. [Google Scholar] [CrossRef]
- Vanden Broeck, A.; McEwen, A.G.; Chebaro, Y.; Potier, N.; Lamour, V. Structural Basis for DNA Gyrase Interaction with Coumermycin A1. J. Med. Chem. 2019, 62, 4225–4231. [Google Scholar] [CrossRef]
- Mizuuchi, K.; O’Dea, M.H.; Gellert, M. DNA gyrase: Subunit structure and ATPase activity of the purified enzyme. Proc. Natl. Acad. Sci. USA 1978, 75, 5960–5963. [Google Scholar] [CrossRef]
- Grossman, T.H.; Bartels, D.J.; Mullin, S.; Gross, C.H.; Parsons, J.D.; Liao, Y.; Grillot, A.-L.; Stamos, D.; Olson, E.R.; Charifson, P.S.; et al. Dual targeting of GyrB and ParE by a novel aminobenzimidazole class of antibacterial compounds. Antimicrob. Agents Chemother. 2007, 51, 657–666. [Google Scholar] [CrossRef]
- Charifson, P.S.; Grillot, A.-L.; Grossman, T.H.; Parsons, J.D.; Badia, M.; Bellon, S.; Deininger, D.D.; Drumm, J.E.; Gross, C.H.; LeTiran, A.; et al. Novel dual-targeting benzimidazole urea inhibitors of DNA gyrase and topoisomerase IV possessing potent antibacterial activity: Intelligent design and evolution through the judicious use of structure-guided design and structure-activity relationships. J. Med. Chem. 2008, 51, 5243–5263. [Google Scholar] [CrossRef]
- Holdgate, G.A.; Tunnicliffe, A.; Ward, W.H.; Weston, S.A.; Rosenbrock, G.; Barth, P.T.; Taylor, I.W.; Pauptit, R.A.; Timms, D. The entropic penalty of ordered water accounts for weaker binding of the antibiotic novobiocin to a resistant mutant of DNA gyrase: A thermodynamic and crystallographic study. Biochemistry 1997, 36, 9663–9673. [Google Scholar] [CrossRef] [PubMed]
- Grillot, A.-L.; Le Tiran, A.; Shannon, D.; Krueger, E.; Liao, Y.; O′Dowd, H.; Tang, Q.; Ronkin, S.; Wang, T.; Waal, N.; et al. Second-generation antibacterial benzimidazole ureas: Discovery of a preclinical candidate with reduced metabolic liability. J. Med. Chem. 2014, 57, 8792–8816. [Google Scholar] [CrossRef] [PubMed]
- Brown-Elliott, B.A.; Rubio, A.; Wallace, R.J., Jr. In Vitro Susceptibility Testing of a Novel Benzimidazole, SPR719, against Nontuberculous Mycobacteria. Antimicrob. Agents Chemother. 2018, 62, 545. [Google Scholar] [CrossRef] [PubMed]
- Locher, C.P.; Jones, S.M.; Hanzelka, B.L.; Perola, E.; Shoen, C.M.; Cynamon, M.H.; Ngwane, A.H.; Wiid, I.J.; van Helden, P.D.; Betoudji, F.; et al. A Novel Inhibitor of Gyrase B Is a Potent Drug Candidate for Treatment of Tuberculosis and Nontuberculosis Mycobacterial Infections. Antimicrob. Agents Chemother. 2015, 59, 1455–1465. [Google Scholar] [CrossRef]
- Shoen, C.M.; DeStefano, M.; Pucci, M.; Cynamon, M.H. Evaluating the Sterilizing Activity of SPR720 in Combination Therapy against Mycobacterium tuberculosis Infection in Mice. In Proceedings of the Conference of ASM Microbes 2019, San Francisco, CA, USA, 20–24 June 2019. [Google Scholar]
- Coates, W.J.; Gwynn, M.N.; Hatton, I.K.; Masters, P.J.; Pearson, N.D.; Rahman, S.S.; Slocombe, B.; Warrack, J.D.; SmithKline Beecham Ltd. Quinoline Derivatives as Antibacterials. European Patent EP1051413, 4 June 2003. [Google Scholar]
- Bax, B.D.; Chan, P.F.; Eggleston, D.S.; Fosberry, A.; Gentry, D.R.; Gorrec, F.; Giordano, I.; Hann, M.M.; Hennessy, A.; Hibbs, M.; et al. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature 2010, 466, 935–940. [Google Scholar] [CrossRef]
- Blanco, D.; Perez-Herran, E.; Cacho, M.; Ballell, L.; Castro, J.; González del Río, R.; Lavandera, J.L.; Remuiñán, M.J.; Richards, C.; Rullas, J.; et al. Mycobacterium tuberculosis Gyrase Inhibitors as a New Class of Antitubercular Drugs. Antimicrob. Agents Chemother. 2015, 59, 1868–1875. [Google Scholar] [CrossRef]
- Gibson, E.G.; Blower, T.R.; Cacho, M.; Bax, B.; Berger, J.M.; Osheroff, N. Mechanism of Action of Mycobacterium tuberculosis Gyrase Inhibitors: A Novel Class of Gyrase Poisons. ACS Infect. Dis. 2018, 4, 1211–1222. [Google Scholar] [CrossRef]
- Tanner, L.; Denti, P.; Wiesner, L.; Warner, D.F. Drug permeation and metabolism in Mycobacterium tuberculosis: Prioritising local exposure as essential criterion in new TB drug development. IUBMB Life 2018, 70, 926–937. [Google Scholar] [CrossRef]
- Van Wijk, R.C.; Ayoun Alsoud, R.; Lennernäs, H.; Simonsson, U.S.H. Model-Informed Drug Discovery and Development Strategy for the Rapid Development of Anti-Tuberculosis Drug Combinations. Appl. Sci. 2020, 10, 2376. [Google Scholar] [CrossRef]
- Mori, M.; Sammartino, J.C.; Costantino, L.; Gelain, A.; Meneghetti, F.; Villa, S.; Chiarelli, L.R. An Overview on the Potential Antimycobacterial Agents Targeting Serine/Threonine Protein Kinases from Mycobacterium tuberculosis. Curr. Top. Med. Chem. 2019, 19, 646–661. [Google Scholar] [CrossRef]
- Prisic, S.; Husson, R.N. Mycobacterium tuberculosis Serine/Threonine Protein Kinases. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Ehrt, S.; Schnappinger, D.; Rhee, K.Y. Metabolic principles of persistence and pathogenicity in Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018, 16, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Sala, C.; Hartkoorn, R.C. Tuberculosis drugs: New candidates and how to find more. Future Microbiol. 2011, 6, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Villemagne, B.; Machelart, A.; Tran, N.C.; Flipo, M.; Moune, M.; Leroux, F.; Piveteau, C.; Wohlkönig, A.; Wintjens, R.; Li, X.; et al. Fragment-Based Optimized EthR Inhibitors with in Vivo Ethionamide Boosting Activity. ACS Infect. Dis. 2020, 6, 366–378. [Google Scholar] [CrossRef]
- Maveyraud, L.; Mourey, L. Protein X-ray Crystallography and Drug Discovery. Molecules 2020, 25, 1030. [Google Scholar] [CrossRef]
- Rout, M.P.; Sali, A. Principles for Integrative Structural Biology Studies. Cell 2019, 177, 1384–1403. [Google Scholar] [CrossRef]
- Petrella, S.; Capton, E.; Raynal, B.; Giffard, C.; Thureau, A.; Bonneté, F.; Alzari, P.M.; Aubry, A.; Mayer, C. Overall Structures of Mycobacterium tuberculosis DNA Gyrase Reveal the Role of a Corynebacteriales GyrB-Specific Insert in ATPase Activity. Structure 2019, 27, 579–589.e5. [Google Scholar] [CrossRef]
- Vanden Broeck, A.; Lotz, C.; Ortiz, J.; Lamour, V. Cryo-EM structure of the complete E. coli DNA gyrase nucleoprotein complex. Nat. Commun. 2019, 10, 4935. [Google Scholar] [CrossRef]
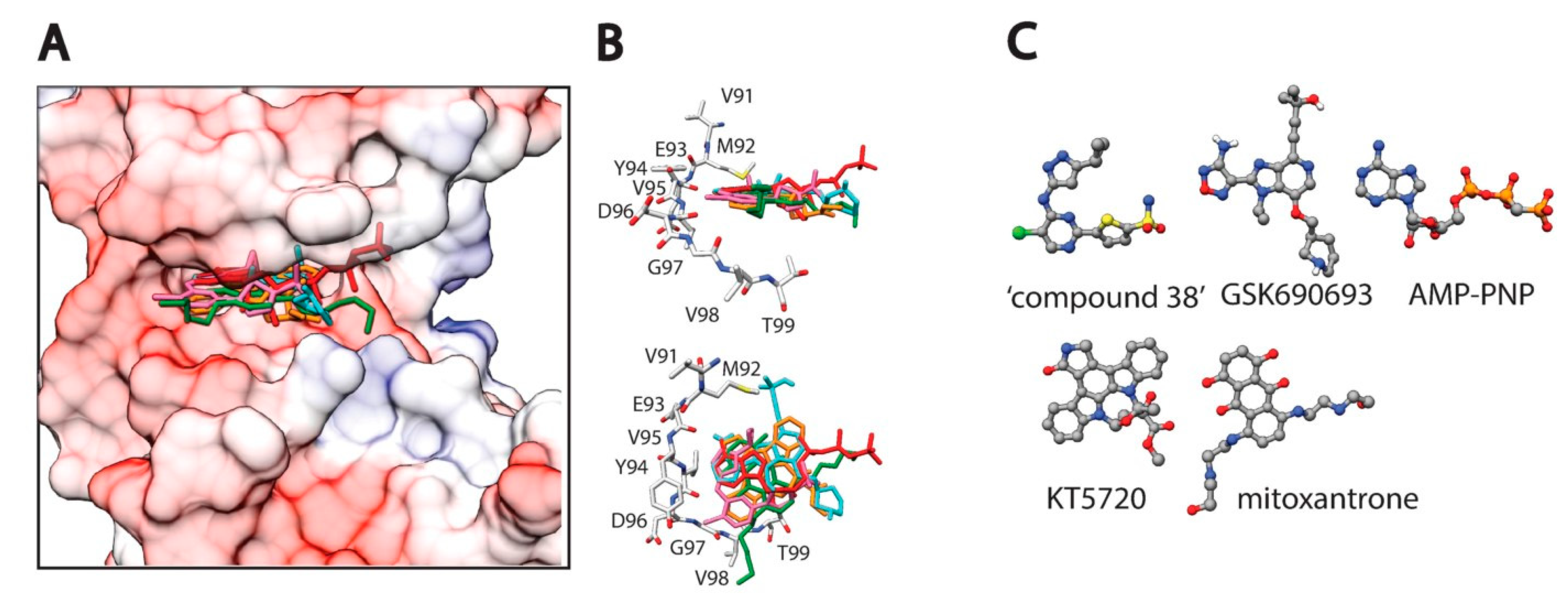

| Family | Name | Structure | IC50 (μM) | Reference |
|---|---|---|---|---|
| Isoquinolines | H-7 | 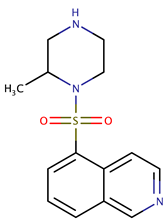 | ND | [47] |
| Staurosporine analogues | Staurosporine | 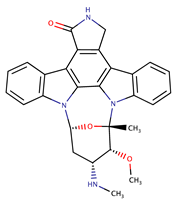 | 0.6 | [44] |
| K-252-a | 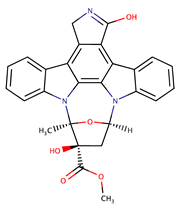 | 0.096 | ||
| K-252-b |  | 0.106 | ||
| KT5720 | 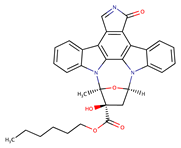 | ~1 a | [37] | |
| Anthracenediones | Mitoxantrone | 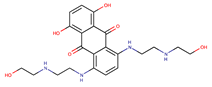 | 0.8 | [48] |
| Aminopurines | 2-A9P | 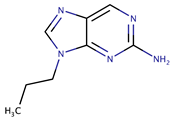 | 1300 | [49] |
| Quinazolines | Disubstituted series |  | ≤ 1.1 | [51] |
| Pyrimidines | Disubstituted (-R1 also as -NHR1) | 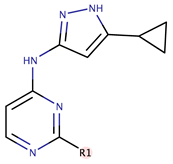 | ≤ 0.4 | [51] |
| ‘Compound 38’ | 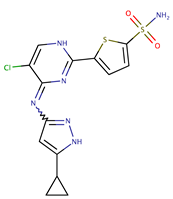 | Ki ~ 1 nM | [55] | |
| Phytocompounds | Demethylcalabaxanthone |  | ND | [54] |
| 4-oxo-crotonic acid derivatives | IMB-YH-8 | 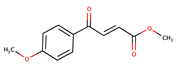 | 20.2 | [53] |
| Imidazopyridine aminofurazans | GSK690693 |  | 0.34 | [56] |
| Family | Name | Structure | IC50 (μM) | Reference |
|---|---|---|---|---|
| Tetrahydrobenzothiophenes | AX20017 | 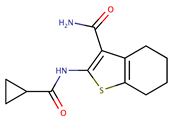 | 0.39 5.5 | [57] [75] |
| Azaphilones | Sclerotiorin | 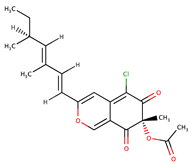 | 76 | [73] |
| Pyrimidines | NU-6027 | 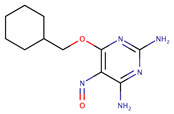 | ND | [74] |
| CYC116 | 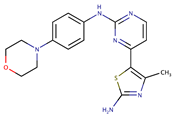 | 35.1 | [75] | |
| Thiophenes | AZD7762 | 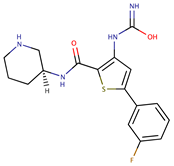 | 30.3 | [75] |
| Methoxybenzenes | R406 (benzenesulfonate) and its free base R406f (also known as Tamatinib) | 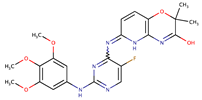 | 8.0 (R406) 16.1 (R406f) | [75] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruch, E.M.; Petrella, S.; Bellinzoni, M. Structure-Based Drug Design for Tuberculosis: Challenges Still Ahead. Appl. Sci. 2020, 10, 4248. https://doi.org/10.3390/app10124248
Bruch EM, Petrella S, Bellinzoni M. Structure-Based Drug Design for Tuberculosis: Challenges Still Ahead. Applied Sciences. 2020; 10(12):4248. https://doi.org/10.3390/app10124248
Chicago/Turabian StyleBruch, Eduardo M., Stéphanie Petrella, and Marco Bellinzoni. 2020. "Structure-Based Drug Design for Tuberculosis: Challenges Still Ahead" Applied Sciences 10, no. 12: 4248. https://doi.org/10.3390/app10124248
APA StyleBruch, E. M., Petrella, S., & Bellinzoni, M. (2020). Structure-Based Drug Design for Tuberculosis: Challenges Still Ahead. Applied Sciences, 10(12), 4248. https://doi.org/10.3390/app10124248